Abstract
Background
Patient selection and cannulation arguably represent the key steps for the successful implementation of extracorporeal membrane oxygenation (ECMO) support. Cannulation is traditionally performed in the operating room or the catheterization laboratory for a number of reasons, including physician preference and access to real‐time imaging, with the goal of minimizing complications and ensuring appropriate cannula positioning. Nonetheless, the patients' critical and unstable conditions often require emergent initiation of ECMO and preclude the safe transport of the patient to a procedural suite.
Aims
Therefore, with the objective of avoiding delay with the initiation of therapy and reducing the hazard of transport, we implemented a protocol for bedside ECMO cannulation.
Matherial and Methods
A total of 89 patients required ECMO support at Hennepin County Medical Center between March 2015 and December 2019. Twenty‐eight (31%) required veno‐venous support and were all cannulated at the bedside. Overall survival was 71% with no morbidity or mortality related to the cannulation procedure.
Conclusion
In the current pandemic, the strategy of veno‐venous bedside cannulation may have additional benefits for the care of patients with refractory acute respiratory distress syndrome due to coronavirus disease‐2019, decreasing the risk of exposure of health care worker or other patients to the novel severe acute respiratory syndrome coronavirus‐2 occurring during patient transport, preparation, or during disinfection of the procedural suite and the transportation pathway after ECMO cannulation.
Keywords: bedside cannulation, COVID‐19, ECMO, perfusion
1. INTRODUCTION
Veno‐venous (VV) extracorporeal membrane oxygenation (ECMO) has been recognized as a potentially life‐saving therapy for patients with refractory acute respiratory distress syndrome (ARDS) secondary to pneumonia and its use in adults has increased exponentially following the influenza A (H1N1) pandemic in 2009. 1 , 2 , 3 , 4 , 5 The Extracorporeal Life Support Organization has published comprehensive guidelines defining the appropriate clinical indications for VV‐ECMO use and has established detailed protocols and quality measures to ensure appropriate implementation of therapy. 6 , 7
Extracorporeal circulation in VV‐ECMO is traditionally obtained with the insertion of two venous cannulas either with the internal jugular‐femoral or the femorofemoral configuration. 5 , 8 , 9 In recent years, a technique using a single‐bicaval dual‐lumen catheter (Avalon Elite; Maquet Inc, Rastatt, Germany) with access through the right internal jugular vein (IJV) has become available as an alternative method to the traditional double‐venous cannulation strategy. 5 , 10 , 11 The single insertion site, which may reduce the risk of bleeding and infection, the location of the insertion site in the neck, which may facilitate patients' prone positioning as needed, and possibly the more efficient oxygenation, most likely related to the lesser incidence of the phenomenon of “recirculation,” account for the advantages of using a single dual‐lumen cannula vs a double cannulation strategy. 8 , 9 , 12 , 13 , 14 On the other hand, the perceived technical difficulty of the internal jugular venous cannulation using a large bore cannula (27 or 31 Fr, both with an insertable length of 31 cm) is seen as a potential disadvantage. For this reason, the dual‐lumen catheter IJV placement is traditionally performed in the operating room or the catheterization laboratory with the assistance of fluoroscopy and transesophageal echocardiography. 11 , 15 , 16
In 2015, we implemented a strategy of routine bedside ECMO cannulation at Hennepin County Medical Center. An internal committee was created with the objective of designing protocols and determining the logistics of the cannulation procedure. Dry runs were simulated in the Surgical Intensive Care Unit (ICU) until the optimal cannulation protocol was defined. A total of 89 patients required ECMO support at Hennepin County Medical Center between March 2015 and December 2019. Of these, 28 (31%) patients were cannulated for VV support, all at the bedside. A dual‐lumen bicaval cannula (Avalon Elite; Maquet Inc) was used in 23 cases; a two cannula approach using the right IJV and the femoral vein was selected in 5 cases due to patient‐specific factors, such as a high native cardiac output. Of the 28 VV‐ECMO cases, the average age was 40 years (range, 12‐66), 12 (43%) were women, and the average body surface area (BSA) was 2.04 m2 (range, 1.53‐2.71). Cannula size selection was based on the patient's BSA. The indication for VV‐ECMO was ARDS in all cases; the etiology of ARDS was pneumonia (N = 10; 36%), massive aspiration (N = 7; 25%), blunt trauma (N = 8; 29%), and drowning (N = 3; 10%). Bedside cannulation was successful in 27 of 28 cases (97%), and there was no mortality or morbidity associated with the procedure. The failed cannulation was in the case of a young woman who hanged herself. Percutaneous access to the right IJV failed due to massive subcutaneous emphysema; she was cannulated using a peripheral venoarterial configuration via the femoral vessels. ECMO blood flow achieved was greater than 60% of native cardiac output in all cases. The median days of VV‐ECMO support were 8 (range, 1‐37). A total of 20 of 28 patients (71%) undergoing VV‐ECMO support survived to hospital discharge. There was no occurrence of thrombotic or hemorrhagic complications.
2. STRATEGY AND TIPS FOR CANNULATION
We designed a process of in situ VV‐ECMO cannulation based on the layout of our ICU where patients with refractory respiratory failure are routinely hospitalized (Figure 1). We utilize a portable fluoroscopy bed which is placed to the side of the ICU bed (Figure 2). After moving the patient from the ICU to the fluoroscopy bed, the medical equipment is positioned around the patient to allow convenient access to the right side of the neck as the cannula insertion site (Figure 3). The procedure is completed under sterile conditions with fluoroscopic guidance. Fluoroscopic guidance represents our preferred imaging method since it may offer the highest level of safety. 9 , 11 However, in the absence of conditions allowing routine use of fluoroscopy at the bedside, the procedure can also be safely performed with a transthoracic echocardiogram (TTE) to confirm guidewire and cannula positioning. 9 , 17 , 18 , 19 Appropriate positioning of the guidewire can be confirmed with subcostal views, making sure that the wire is advanced into the retrohepatic inferior vena cava (IVC). 9 , 18 , 19 , 20 , 21 , 22 , 23 Alternatively, imaging by portable chest X‐ray can also be used to spot‐check guidewire and cannula position.
Figure 1.
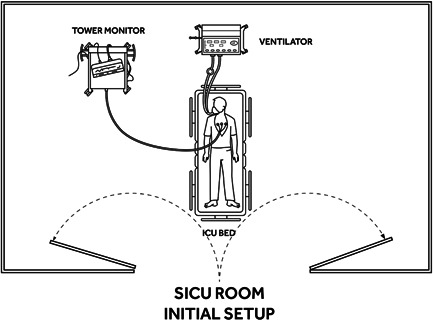
Initial room layout. ICU, intensive care unit
Figure 2.
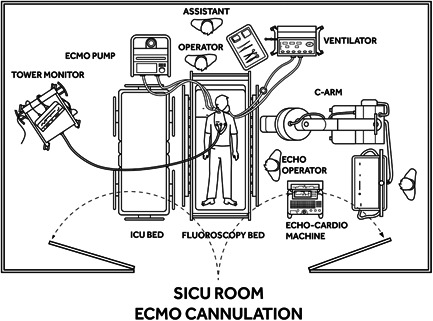
Bedside cannulation with the use of fluoroscopy and transthoracic echocardiogram (TTE). Equipment is positioned around the patient allowing the operator and assistant to be positioned at the head of the bed. In the absence of the availability of fluoroscopy, the procedure can be completed with the use of a portable chest X‐ray in combination with TTE. ECHO, echocardiography; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit
Figure 3.
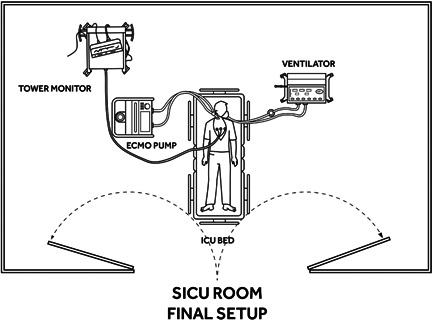
Final arrangement. ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit
Cannulation best practices using our approach are listed in Table 1. We always use real‐time ultrasound visualization for the puncture of the IJV. The patient is maintained in slight Trendelenburg position for the entire duration of the procedure to reduce the risk of venous air embolism. Systemic anticoagulation is established with an initial intravenous bolus of 5000 units of unfractionated heparin (UFH). The guidewire is advanced deep into the retrohepatic IVC and its position is confirmed by imaging. We used the standard packaging of the Avalon Elite Venous Cannula Kit (0.038″ × 210 cm change guidewire) in all cases (Table 2). After serial dilation of the skin and soft tissue at the cannula insertion site, the IJV is cannulated with 10 Fr through 30 Fr dilators. The cannula is inserted under imaging guidance ensuring no resistance is encountered while the catheter is advanced through the right atrium into the IVC. The cannula is connected to the ECMO circuit with meticulous deairing and is secured to the skin once final manual manipulation is made to ensure adequate extracorporeal blood flow and desired arterial oxygenation (Table 1). Anticoagulation during therapy is maintained with an infusion of UFH, titrated for Antifactor Xa activity goal of 0.2 to 0.4. Antifactor Xa monitoring represents the institutional preference, since assessing the common pathway of the coagulation cascade may be the most reliable measure of the anticoagulation status. 24 , 25 , 26
Table 1.
Avalon Elite cannula insertion
|
• Time‐out • Patient in slight Trendelenburg position • Placement of horizontal back roll at the level of the scapulae with modest neck extension • The patient is prepped and draped under sterile conditions • Sterile preparation of the equipment on a back table • Systemic heparinization is administered (5000 IU) • Checklist verification • Puncture of right IJV under US visualization • Advancing guidewire (0.038″ × 210 cm) under fluoroscopy and TTE guidance, alternatively portable chest X‐ray sequence can be used to confirm adequate positioning in absence of fluoroscopy • Guidewire must follow a perfectly straight line, reaching the IVC in the mid‐abdomen (in absence of fluoroscopy, the position can be reliably verified with TTE subcostal views and portable chest X‐ray) • Progressive dilation of the skin entry site with dilators 10‐30 Fr. • Dilators are advance smoothly through the insertion site for a few cm (3‐5) to obtain proper dilatation of skin incision and venipuncture. • When the biggest dilators are utilized, the skin entry site requires to be enlarged with a surgical scalpel to allow smooth sliding of dilators removing any resistance to advancement at the skin entry site • The placement of the guidewire is reconfirmed by imaging to ensure the adequate position of the cannula deep into the IVC. • The Avalon Elite cannula is primed with heparinized saline and advanced over the guidewire under imaging guidance. • The tip of the cannula should be located approximately 5 cm below the right hemidiaphragm to ensure that the inflow port, which is at 10 cm from the tip, is positioned in the right atrium. • Location of the inflow port in the right atrium with jet anteriorly directed towards the tricuspid valve is confirmed by TTE imaging • Once the proper position and orientation of the cannula are obtained, the two attachment ports are connected to the ECMO circuit with meticulous deairing. It is recommended that tubing from the ECMO circuit is marked with blue tape to identify the outflow line carrying deoxygenated blood (from the patient to the oxygenator) and with red tape for the return inflow line carrying oxygenated blood (from the oxygenator to the patient). Red tubing is connected to cannula inflow (marked by an arrow pointing to the patient) and blue tubing is connected to cannula outflow (marked by an arrow pointing away from the patient) • The cannula needs to be positioned to the side of the neck maintaining the inflow port anteriorly to obtain the appropriate orientation of blood inflow in the right atrium towards the tricuspid valve • Blood flow is initiated at a slow rate to avoid sudden intravascular volume shift that would cause sudden hypotension. It is crucial to notice the difference between bright blood flowing in the anterior port compared to dark blood flowing in the posterior port. • Appropriate securing of the cannula to the skin is extremely important to avoid catheter's migration and rotation. • We apply to stitches that after being tied to the skin are looped around each one of cannula ports passing in the “crotch” between the two ports |
Note: Technical tips of cannulation: The procedure requires the presence of two operators familiar with the use of the cannula and its insertion kit. It is important to prepare surgical instruments, tubing clamps, and all the needed items (Table 2). The patient is routinely cross‐matched for two units of packed red blood cells.
Abbreviations: ECMO, extracorporeal membrane oxygenation; IJV, internal jugular vein; IVC, inferior vena cava; TTE, transthoracic echocardiogram; US, ultrasound.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Table 2.
Items needed for the procedure
| Angio Kit |
| Sterile gowns |
| Towels, sponges, and drapes |
| C‐arm cover |
| Vascular US cover and gel |
| Skin prep |
| Local anesthesia |
| Adhesive skin dressing |
| Avalon Elite Catheter |
| Avalon Elite Access Kit |
| Cannulation tray |
| Four sterile tubing clamps |
| Tubing scissors |
| Bulb syringe |
| Basins |
| Saline flushes |
| Surgical instruments |
| Scalpel |
| Scissor |
| Needle holder |
| Sutures |
| Two hemostats |
| Banding gun and zip ties |
Abbreviation: US, ultrasound.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3. DISCUSSION
In our experience, the procedure of bedside VV‐ECMO cannulation was safe and effective. We had only one case of failed IJV single‐cannula insertion which required veno‐arterial cannulation using the common femoral artery and vein due to anatomic constraints. All procedures were performed in the ICU under fluoroscopic and echocardiographic guidance. Our set up allowed the efficient utilization of fluoroscopy by using a mobile, X‐ray compatible bed (Figures 1 and 2). We selected the use of a bicaval dual‐lumen cannula whenever indicated to facilitate adequate ECMO flow and optimize blood oxygenation by reducing “recirculation.” 5 , 12 , 24 The selection of double‐venous cannulation was based on operator preference due to patient characteristics, such as a body mass index above 30, or in situations of patient's hemodynamic conditions, which represented a concern for maintaining adequate extracorporeal circulation, such as a cardiac index (CI) above 2.5 L/min/m2. Although there is a lack of randomized trials comparing the effectiveness of the single dual‐lumen versus a double‐venous cannulation strategy, clinical data and experimental studies show at least comparable flow parameters and clinical results. 27 , 28 , 29 Nonetheless, the location of the cannula on the side of the neck, as compared to the groin, provides a better opportunity for patient mobilization and may offer significant advantages in light of the fact that VV‐ECMO support is often required for a considerable length of time.
The benefits of in situ ECMO cannulation, not only in terms of potentially expediting the timing of initiation of therapy and decreasing the hazard of transporting the patient to the procedural location, appears of crucial importance during the coronavirus disease 2019 (COVID‐19) pandemic in the extreme cases of respiratory decompensation and refractory hypoxemia which may benefit from VV‐ECMO support. Avoidance of transporting the patient out of the ICU to reach the designated cannulation location reduces the risk of severe acute respiratory syndrome coronavirus 2 transmission to other patients and health care providers while also decreasing the risk of environmental contamination inevitably associated with the transportation process, and possibly decreasing unnecessary personal protective equipment usage outside of the ICU. The use of fluoroscopic guidance has represented the standard for our protocol; however, cannulation can also be safely completed using echocardiographic imaging with TTE or even using portable chest X‐ray, which can be both routinely arranged at any health care facility. The use of fluoroscopy at the bedside can be challenging if logistics are not suitable to accommodate an X‐ray compatible bed in the ICU room (Figure 2). On the other hand, the use of TEE, as the primary guiding imaging strategy, 15 , 16 , 17 , 18 requires the presence of another specialized physician assigned to perform the procedure, which may represent a limiting factor to complete cannulation, especially under emergent conditions. For these reasons, if the use of fluoroscopy is not suitable, we favor using TTE and portable chest X‐ray guidance, which in combination ensure appropriate visualization of guidewire and cannula during the cannulation procedure as efficiently and reliably as TEE and may be more easily available. 9 , 16 , 20 Nonetheless, no matter of the imaging technique used, single‐venous cannulation with bicaval dual‐lumen catheter remains a highly demanding procedure with the risk of life‐threatening complications, so that it should be performed by experienced operators in highly specialized centers. 21 , 23 , 30
4. CONCLUSION
Our experience shows that adequate planning of VV‐ECMO cannulation allows safe completion of the procedure at bedside. This strategy can be particularly useful in patients with refractory hypoxemia due to COVID‐19 associated severe ARDS as bedside cannulation can minimize the inadvertent nosocomial transmission of this highly contagious disease. VV‐ECMO management is resource‐intensive and limited to specialized referral centers but can be used as salvage therapy in patients with refractory respiratory failure who have preserved life expectancy and few associated end‐organ failures. Certainly, we acknowledge the ethical dilemma of patient selection for access to life‐sustaining extracorporeal support during this pandemic. Nonetheless, the opportunity of saving even a relatively small number of patients in comparison to the large loss of lives produced by this highly lethal disease cannot be ignored.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS STATEMENT
This study meets the ethical guidelines, including adherence to the legal requirements of the study country.
Calcaterra D, Heather B, Kohl LP, Erickson HL, Prekker ME. Bedside veno‐venous ECMO cannulation: A pertinent strategy during the COVID‐19 pandemic. J Card Surg. 2020;35:1180–1185. 10.1111/jocs.14641
REFERENCES
- 1. Davies A, Jones D, Bailey M, et al. Extracorporeal membrane oxygenation for 2009 influenza A (H1N1) acute respiratory distress syndrome. JAMA. 2009;302(17):1888‐1895. [DOI] [PubMed] [Google Scholar]
- 2. Holzgraefe B, Broom M, Kalzen H, Konrad D, Palmer K, Frenckner B. Extracorporeal membrane oxygenation for pandemic H1N1 2009 respiratory failure. Minerva Anestesiol. 2010;76(12):1043‐1051. [PubMed] [Google Scholar]
- 3. Grasselli G, Bombino M, Patroniti N, et al. Management of acute respiratory complications from influenza A (H1N1) infection: experience of a tertiary‐level intensive care unit. Minerva Anestesiol. 2011;77(9):884‐891. [PubMed] [Google Scholar]
- 4. Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A (H1N1). JAMA. 2011;306(15):1659‐1668. [DOI] [PubMed] [Google Scholar]
- 5. Tulman DB, Stawicki SP, Whitson BA, et al. Veno‐venous ECMO: a synopsis of nine key potential challenges, considerations, and controversies. BMC Anesthesiol. 2014;14:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Extracorporeal Life Support Organization Guidelines. http://www.elsonet.org/index. Accessed April 27, 2020.
- 7. The Extracorporeal Life Support Organization (ELSO) . ELSO Anticoagulation Guideline. 2014. https://www.elso.org/portals/0/files/elsoanticoagulationguideline8-2014-table-contents.pdf. Accessed April 20, 2020.
- 8. Jayaraman AL, Cormican D, Shah P, Ramakrishna H. Cannulation strategies in adult veno‐arterial and veno‐venous extracorporeal membrane oxygenation: techniques, limitations, and special considerations. Ann Card Anaesth. 2017;20(suppl):S11‐S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ngai CW, Ng PY, Sin WC. Bicaval dual lumen cannula in adult veno‐venous extracorporeal membrane oxygenation‐clinical pearls for safe cannulation. J Thorac Dis. 2018;10(suppl 5):S624‐S628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bermudez CA, Rocha RV, Sappington PL, Toyoda Y, Murray HN, Boujoukos AJ. Initial experience with single cannulation for venovenous extracorporeal oxygenation in adults. Ann Thorac Surg. 2010;90(3):991‐995. [DOI] [PubMed] [Google Scholar]
- 11. Teman NR, Haft JW, Napolitano LM. Optimal endovascular methods for placement of bicaval dual‐lumen cannulae for venovenous extracorporeal membrane oxygenation. ASAIO J. 2013;59(4):442‐447. [DOI] [PubMed] [Google Scholar]
- 12. Lindholm JA. Cannulation for veno‐venous extracorporeal membrane oxygenation. J Thorac Dis. 2018;10(suppl 5):S606‐S612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Banfi C, Pozzi M, Siegenthaler N, et al. Veno‐venous extracorporeal membrane oxygenation: cannulation techniques. J Thorac Dis. 2016;8(12):3762‐3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011;365:1905‐1914. [DOI] [PubMed] [Google Scholar]
- 15. Griffee MJ, Tonna JE, McKellar SH, Zimmerman JM. Echocardiographic guidance and troubleshooting for venovenous extracorporeal membrane oxygenation using the dual‐lumen bicaval cannula. J Cardiothorac Vasc Anesth. 2018;32(1):370‐378. [DOI] [PubMed] [Google Scholar]
- 16. Griffee MJ, Zimmerman JM, McKellar SH, Tonna JE. Echocardiography‐guided dual‐lumen venovenous extracorporeal membrane oxygenation cannula placement in the ICU—A retrospective review. J Cardiothorac Vasc Anesth. 2020;34(3):698‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trimlett RH, Cordingley JJ, Griffiths MJ, Price S, Hunter DN, Finney SJ. A modified technique for insertion of dual lumen bicaval cannulae for venovenous extracorporeal membrane oxygenation. Intensive Care Med. 2011;37:1036‐1037. [DOI] [PubMed] [Google Scholar]
- 18. Dolch ME, Frey L, Buerkle MA, Weig T, Wassilowsky D, Irlbeck M. Transesophageal echocardiography‐guided technique for extracorporeal membrane oxygenation dual‐lumen catheter placement. ASAIO J. 2011;57:341‐343. [DOI] [PubMed] [Google Scholar]
- 19. Chimot L, Marqué S, Gros A, et al. Avalon bicaval dual‐lumen cannula for venovenous extracorporeal membrane oxygenation: survey of cannula use in France. ASAIO J. 2013;59:157‐161. [DOI] [PubMed] [Google Scholar]
- 20. Tabak B, Elliott CL, Mahnke CB, Tanaka LY, Ogino MT. Transthoracic echocardiography visualization of bicaval dual lumen catheters for veno‐venous extracorporeal membrane oxygenation. J Clin Ultrasound. 2012;40:183‐186. [DOI] [PubMed] [Google Scholar]
- 21. Yastrebov K, Manganas C, Kapalli T, Peeceeyen S. Right ventricular loop indicating malposition of J‐wire introducer for double lumen bicaval venovenous extracorporeal membrane oxygenation (VV ECMO) cannula. Heart Lung Circ. 2014;23:e4‐e7. [DOI] [PubMed] [Google Scholar]
- 22. Shaheen A, Tanaka D, Cavarocchi NC, Hirose H. Veno‐venous extracorporeal membrane oxygenation (V V ECMO): indications, preprocedural considerations, and technique. J Card Surg. 2016;31:248‐252. [DOI] [PubMed] [Google Scholar]
- 23. Camboni D, Philipp A, Lubnow M, et al. Extracorporeal membrane oxygenation by single‐vessel access in adults: advantages and limitations. ASAIO J. 2012;58:616‐621. [DOI] [PubMed] [Google Scholar]
- 24. Protti A, Iapichino GE, Di Nardo M, Panigada M, Gattinoni L. Anticoagulation management and antithrombin supplementation practice during veno‐venous extracorporeal membrane oxygenation: a worldwide survey. Anesthesiology. 2020;132(3):562‐570. [DOI] [PubMed] [Google Scholar]
- 25. Grecu L. Anticoagulation for extracorporeal membrane oxygenation: between the rock and the hard place. Crit Care Med. 2020;48(2):264‐266. [DOI] [PubMed] [Google Scholar]
- 26. Sklar MC, Sy E, Lequier L, Fan E, Kanji HD. Anticoagulation practices during venovenous extracorporeal membrane oxygenation for respiratory failure. a systematic review. Ann Am Thorac Soc. 2016;13(12):2242‐2250. [DOI] [PubMed] [Google Scholar]
- 27. Napp LC, Kühn C, Hoeper MM, et al. Cannulation strategies for percutaneous extracorporeal membrane oxygenation in adults. Clin Res Cardiol. 2016;105:283‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuhl T, Michels G, Pfister R, Wendt S, Langebartels G, Wahlers T. Comparison of the avalon dual‐lumen cannula with conventional cannulation technique for venovenous extracorporeal membrane oxygenation. Thorac Cardiovasc Surg. 2015;63(08):653‐662. [DOI] [PubMed] [Google Scholar]
- 29. Wang S, Force M, Kunselman AR, Palanzo D, Brehm C, Ündar A. Hemodynamic evaluation of avalon elite bi‐caval dual lumen cannulas and femoral arterial cannulas. Artif Organs. 2019;43(1):41‐53. [DOI] [PubMed] [Google Scholar]
- 30. Hirose H, Yamane K, Marhefka G, Cavarocchi N. Right ventricular rupture and tamponade caused by malposition of the Avalon cannula for venovenous extracorporeal membrane oxygenation. J Cardiothorac Surg. 2012;7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]


