Abstract
Background
Over the last months, during the COVID‐19 pandemic, a growing number of chilblain‐like lesions were reported mainly in children and rarely in young adults. The relationship with SARS‐CoV‐2 infection was postulated, often without any laboratory, instrumental or clinical confirmation. The disclosure of information about chilblain‐like lesions as a COVID‐19 manifestation in social media has created concern in children’s families and paediatricians.
Objectives
To verify whether the chilblain‐like lesions were caused by SARS‐CoV‐2 infection.
Methods
Prospective study on a case series including children who presented with acral lesions at the Pediatric Dermatology Outpatient and Pediatric Emergency Unit of the University of Bologna, from 1 April to 30 April 2020. We reported demographical, laboratory and clinical features, history of close contact with COVID‐19 patients, presence of similar skin lesions in other family members, precipitating and risk factors for chilblain onset.
Results
We evaluated eight patients (five females, three males) aged between 11 and 15 years. We excluded acute or previous SARS‐CoV‐2 infection with RT‐PCR nasopharyngeal swab, serum antibody levels using chemiluminescent immunoassays. Other acute infections causing purpuric lesions at the extremities were negative in all patients. Skin lesion biopsy for histological and immunohistochemical evaluation was made in two cases and was consistent with chilblain. PCR assay on skin lesion biopsy for parvovirus B19, Mycoplasma pneumoniae and SARS‐CoV‐2 was performed in a patient and resulted negative. We identified common precipitating and risk factors: physical (cold and wet extremities, low BMI), cold and wet indoor and outdoor environment, behaviours, habits and lifestyle. We therefore reached a diagnosis of primary chilblains.
Conclusions
During the COVID‐19 pandemic, a ‘cluster’ of primary chilblains developed in predisposed subjects, mainly teenagers, due to cold exposure in the lockdown period. Laboratory findings support our hypothesis, although it is also possible that an unknown infectious trigger may have contributed to the pathogenesis.
Introduction
Global public health is still challenged by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pandemic, the causative agent of coronavirus disease 2019 (COVID‐19).
The Emilia Romagna region, as well as Lombardy, is one of the largest and most serious clusters of COVID‐19 in Italy. The transmission of SARS‐CoV‐2 occurs through airborne spread, droplets, aerosol, hand contact and faecal shedding. The main paths of transmission in paediatric age are through close contact with other infected family members (89%), exposure to epidemic areas (33%) or both. 1
Clinical manifestations in the paediatric age range from mild to critical; however, most patients are asymptomatic or present a mild disease with fever, fatigue, dry cough, myalgia and diarrhoea. Severe and critical forms are rarer if compared with adults or the elderly, although bilateral interstitial pneumonia and late‐onset neonatal sepsis have been reported. 2 , 3
Skin findings associated with SARS‐CoV‐2 infection represent a new hot topic. The dermatological manifestations range from viral exanthema‐like rashes to vasculopathy‐related lesions. 4
Although this debate is very lively in adults, little is known about the dermatological manifestations of the virus in the paediatric age. Genovese and Morey‐Olivé reported different types of rash, such as varicella‐like, maculopapular and urticaria‐like eruptions, in children with confirmed or highly suspected COVID‐19. 5 , 6
In the last month, an ‘outbreak’ of chilblain‐like lesions was reported, mainly in the paediatric population. 7 , 8 The relationship with SARS‐CoV‐2 infection was postulated by several authors. The chilblain‐like lesions were variably associated with the presence of respiratory and systemic symptoms or with the history of close contact with positive cases, though lacking the criteria for testing for SARS‐CoV‐2 in the majority of cases. 8
In April 2020, we observed a growing number of chilblain‐like manifestations similar to cold‐induced lesions during the pandemic, with the opportunity to study eight cases, two children and six adolescents, and report here our results.
The aim of this study was to verify whether the chilblain‐like lesions were a cutaneous clue for SARS‐CoV‐2 infection or due to other causes.
Material and methods
Eight children with chilblain‐like eruptions on their hands and/or feet were evaluated in the Pediatric Dermatology Outpatient and Pediatric Emergency Unit of Sant’Orsola Malpighi University Hospital in Bologna, the main city of Emilia‐Romagna region, between 1 April 2020 and 30 April 2020. Data collected were age and gender of patients, time at onset, localization and morphology of the skin lesions, presence of respiratory or systemic symptoms, history of close contact with COVID‐19 patients and presence of similar skin lesions in other family members.
Risk factors for primary chilblain development were collected, including the presence of cold and sweaty extremities, body mass index (BMI) percentiles, family history for chilblains or autoimmune diseases, viral infections, environmental factors and personal habits.
After the first dermatological evaluation, the patients underwent a first series of laboratory tests (time 0 = T0) including the following:
-
‐
routine blood test, C‐reactive protein (CRP)
-
‐
coagulation profile (PT, aPTT, INR, fibrinogen, D‐dimer)
-
‐
lactate dehydrogenase (LDH), ferritin and IL6
-
‐
antinuclear antibody (ANA) reflex
-
‐
cytokines (including IL8, 12p70, 1β, 10, tumour necrosis factor α), homocysteine, lymphocyte subset, anticoagulant lupus like (LAC), anti‐cardiolipin and anti beta2 glycoprotein I antibodies (IgM and IgG)
-
‐
nasopharyngeal swab for SARS‐CoV‐2 RNA real‐time polymerase chain reaction (RT‐PCR)
-
‐
serum IgM and IgG levels for SARS‐CoV‐2 by using a commercially available chemiluminescent immunoassays (CLIA), i.e. iFlash‐SARS‐CoV‐2 IgG and IgM, on the iFlash Immunoassay Analyzer (YHLO BIOTECH, Shenzhen, China)
-
‐
serum IgM and IgG levels for Mycoplasma pneumoniae, Epstein–Barr virus (EBV), cytomegalovirus (CMV)
-
‐
PCR assay on blood samples for parvovirus B19 DNA and enterovirus RNA
-
‐
PCR assay on skin biopsy for parvovirus B19, Mycoplasma pneumoniae and SARS‐CoV‐2 was performed in a single patient (12.5%).
A second evaluation (time 1 = T1) was performed on all patients 8–20 days after T0, repeating routine analysis and serological tests for parvovirus B19, CMV, EBV and Mycoplasma pneumonia.
Skin biopsy for histological and immunohistochemical evaluation was made in two cases on finger and toe lesions, respectively.
Qualitative detection of COVID‐19 was performed through RNA extraction from throat swab and biopsy using QIAsymphony SP/AS instruments with QIAsymphony DSP Virus/Pathogen Midi Kit (Qiagen, Hilden, Germany). Real‐time reverse transcription was carried out according to Corman VM et al. 9 using RNA‐dependent RNA polymerase gene (RdRp) as amplicon target. The limit of detection for the RdRp assay is 3.6 copies per reaction (95%: 2.7–11.2). 9
Results
The patients evaluated were 8, 5 females and 3 males. The age ranged from 11 to 15 years.
The mean time from the lesions onset to the visit was 19.6 days (9–30).
All patients were asymptomatic, and their medical history was unremarkable. None of them, including all family members, referred history of close contact with COVID‐19 patients. Moreover, neither parents nor siblings presented any cutaneous or systemic and respiratory symptoms.
Clinical aspect was the same in all cases, presenting multiple red‐purple macules and/or patches (8/8), nodules (4/8) and bullae (3/8). The lesions are most often multiple and symmetrical, rarely one or a few. Sites involved were the feet (8/8) and hands (3/8), with the following localizations: toes (8/8), soles and heels (3/8) and fingers (3/8) (Figs 1,2). The associated symptoms were itching (5/8), pain (3/8) and heel tingling (1/8). Two morphological variants are identified: (1) patches or nodular type and (2) blistering type (Fig. 1).
Figure 1.
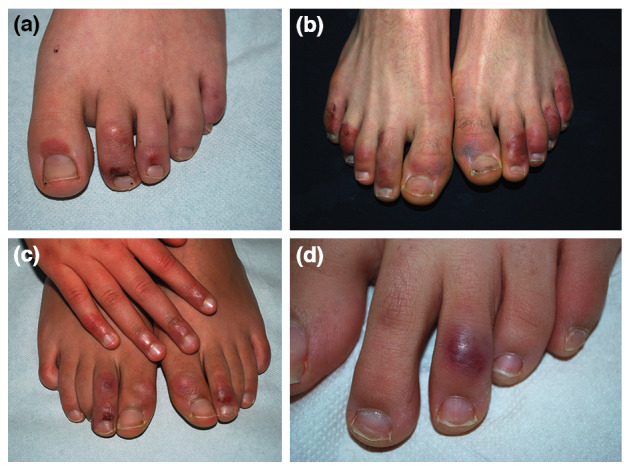
Clinical aspect of the acral lesions. Dusky red purpuric macules and patches and crusted lesion of the 2nd toe (a). Bullous chilblain (type 2). Multiple lesions on the dorsal aspect of toes and dusky red oedematous purpuric macule with central greyish discolouration, affecting the 1th left toe (b). Red dusky patches and nodules, some with a little crust on right hand and feet (c). Dusky red nodule of 3rd left toe (d).
Figure 2.
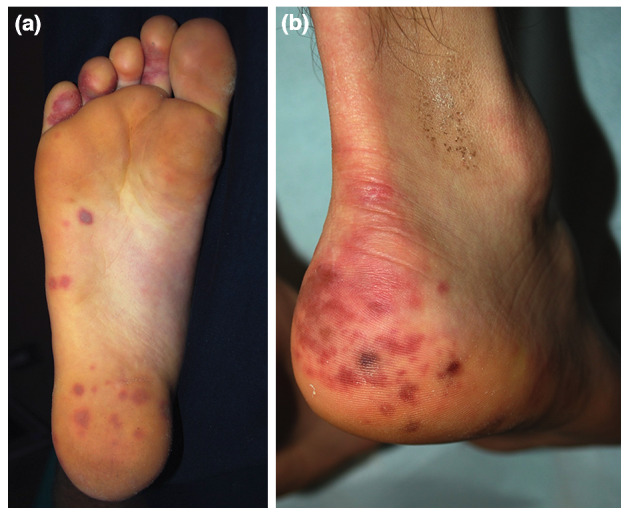
Skin lesions of the feet. Chilblain with unusual involvement. Red dusky round macules on the sole (a). Patches and macules on the heel (b).
RT‐PCR nasopharyngeal swab and serological tests resulted negative in all eight cases.
Also, PCR assay skin biopsy for SARS‐CoV‐2 RNA of the chilblain lesion in one case was negative.
Regarding laboratory findings, all patients had normal blood count and biochemical profile; 3/8 (37.5%) showed only slight lymphocytosis (T0), then returning to normal value (T1).
Furthermore, the immune‐inflammatory parameters and cytokines considered to be related to SARS‐CoV‐2 (IL6, LDH and ferritin) were unaltered. The ANA reflex titre was normal in all patients.
The values of homocysteine, lymphocyte subset, LAC, anti‐cardiolipin and anti‐beta2 glycoprotein I antibodies (IgM and IgG) were within range in all patients.
Concerning the serological tests, four patients (50%) showed an increased level of IgG for Mycoplasma pneumoniae (nr < 12 U/mL) at T0; at T1, IgG level was slightly decreased in two patients (25%), unchanged in one patient (12.5%) and slightly increased in one patient (12.5%). Elevated IgM levels were not observed in any patients at any time.
Seven patients (87.5%) were immune (IgG positive, IgM negative) to EBV; IgM was observed in only one patient (12.5%) in association with IgG and EBNA, both at T0 and T1: these values are relatable to a previous infection. Three patients (37.5%) were immune to CMV, and none showed active infection.
Regarding viral PCR examinations on blood, two patients (25%) showed a low viral load of parvovirus B19 DNA (<250 copies/mL), which corresponds to residual copies from a past infection. Finally, enterovirus RNA was not found in any of the cases.
The precipitating and risk factors for chilblain onset were evaluated. In all eight children, the hands and feet were cold and sweaty. BMI was <25th percentile in 4/8 patients and within 25–50th percentiles in 4/8. A previous family history of chilblains was reported in only 2/8 cases (25%), whereas the personal and family history was unremarkable for connective autoimmune diseases in all patients.
Regarding personal behaviour and habits, the patients referred barefoot walking or thin socks use (8/8), incorrect posture (8/8) and contact with cold floors (7/8). Finally, the environmental factors considered were temperature variation in the month of March (Fig. 3) and home heating turn off (5/8).
Figure 3.
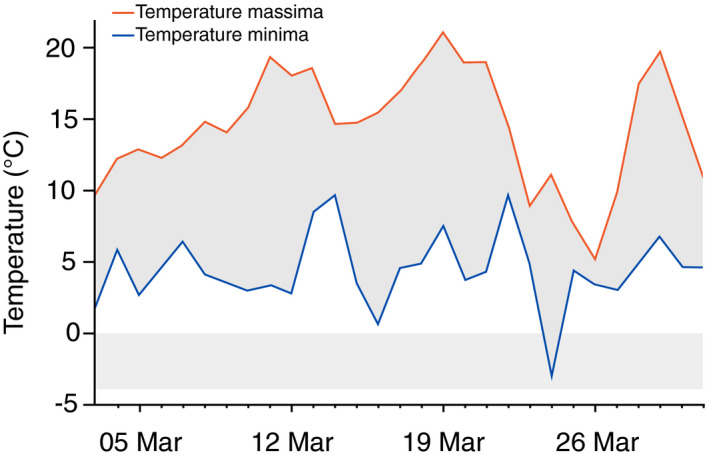
Minimum and maximum temperatures of Bologna in March 2020.
The histology of skin biopsy made in two patients was characterized by superficial and deep perivascular, interface, vacuolar focally lichenoid dermatitis. Dilated vessels, massive oedema and marked infiltrate lymphocytes were present in the papillary dermis. Dense focally lichenoid infiltrate of lymphocytes at the dermoepidermal junction with some necrotic keratinocytes in the epidermis and dense perivascular infiltrate of lymphocytes in the dermis were observed. There was massive pallor (oedema) of the papillary dermis. The dense lymphocytic infiltrate circled the venules in the dermis. No fibrin in the vessels was detected. The histological findings were consistent for chilblain (Fig. 4).
Figure 4.
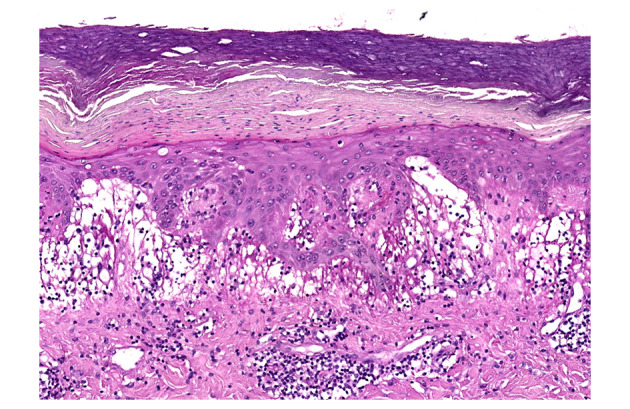
Histology. Dilated vessels, massive oedema, marked infiltrate lymphocytes in the papillary dermis. Dense infiltrate of lymphocytes at the dermoepidermal junction. Some necrotic keratinocytes in the epidermis. H&E 10×.
Since laboratory tests excluded infectious or autoimmune aetiology and considering the habits and behaviour of the patients during the lockdown, a diagnosis of idiopathic or primary chilblains (PC) was made.
We recommended protection from the cold. Topical steroids were prescribed in all patients. After a 4‐ to 5‐week follow‐up, the patients denied any symptoms and the lesions healed.
Discussion
Our primary aim was to investigate a potential link between SARS‐CoV‐2 infection and the occurrence of chilblain‐like lesions in children. Various cutaneous findings were observed in adults infected with COVID‐19 and, simultaneously, a marked increase of chilblain‐like lesions occurred worldwide among children during the COVID‐19 pandemic 10 , 11
In our cases, we exclude SARS‐CoV‐2 infection. The serology was performed in all cases three weeks after the appearance of the chilblains, and both IgM and IgG were negative, and this led us to rule out infection with the virus.
Concerning the CLIA test for SARS‐CoV‐2 serum antibody, datasheet reports show an excellent clinical concordance rate based on large sample evaluation (>2000 patients in >30 hospitals) (up to 2020.3.4): average sensitivity for IgM: >90% (newly diagnosed patients), average specificity for IgM: >95% (negative patients), average sensitivity for IgG: >95% (clinical diagnosed patients) and average specificity for IgG: >95% (negative patients).
Negative SARS‐CoV‐2 infection can also be concluded from the medical history of our patients: all of them and their parents were asymptomatic and denied any previous contact with a suspected or confirmed COVID‐19‐infected patient.
The possible correlation with the COVID‐19 infection derives from the rapid increase of the number of the cases during the concomitant pandemia and from the signalling in seven critical COVID‐19 patients of acroischemia in Wuhans. 12 Hence, the terminological confusion among chilblain‐like or pernio‐like and acroischemia. The chilblain is an acrosyndrome and should not be confused with acroischemia.
The detection of acroischaemic or other skin vascular lesions in critical or severe COVID‐19 affected patients, caused by altered coagulation and vascular damage, has led clinicians to consider the virus responsible for all other acral lesions, including chilblain‐like manifestations. In many of these virologic, serologic, instrumental or clinical confirmation of the infection was missing, and sometimes, the final diagnosis was based only on close contact with COVID‐19‐infected patients. 13 Even in large study, the virus was detected in less than half of patients with pseudo‐chilblains. 7
COVID‐19‐related pseudo‐chilblains has been supposed associated with younger age, less severe disease and late appearance compared to COVID‐19 onset. 7 Pseudo‐chilblain late onset led to hypothesize a late manifestation of vascular damage secondary to antigen–antibody mechanism, 7 , 14 and according to some authors, this might explain the frequently negative PCR results on nasopharyngeal swab. 7 , 8 Few paediatric cases with clinical, instrumental or virologic confirmation were included in these studies. 14 , 15
The disclosure of chilblain information as a COVID‐19 cutaneous sign in social media has created concern in children’s families and paediatricians.
However, many of the recently reported chilblains occurred in children without general symptoms of SARS‐CoV‐2 and coming from asymptomatic families. 16
As chilblains are not frequent in paediatric age and uncommon in ‘Mediterranean’ climate, the recent increasing number of cases could suggest an infectious aetiology. So after SARS‐CoV‐2, we ruled out other pathogens causing purpuric lesions at the extremities, such as in papular purpuric gloves and socks syndrome and/or atypical hand–foot–mouth disease, and viral infections which are rarely reported as precipitating factors of chilblains. 17
We tested for Mycoplasma pneumoniae, Epstein–Barr virus, parvovirus B19, enterovirus and citomegalovirus. None of the 8 children involved in our study was found to be in the acute phase of any of these infections. In all cases, a complete blood count and C‐reactive protein were within normal limits.
Lockdown involving the main gathering places for children (kindergartens, schools, gyms, parks) from the end of February resulted in a reduction in the spread of typical childhood infectious diseases and a lower number of admissions to the paediatric healthcare facilities.
In two cases, biopsy was performed to evaluate the histological characteristics of skin lesions and to exclude the presence of vasculitis or thrombi (reported by Colonna et al. 18 ), whose appearance in the late phase of COVID‐19 has been discussed. 14 Histopathology of skin lesions showed in both the association of dermal oedema with superficial and deep perivascular lymphocytic infiltrate with a perieccrine distribution, the hallmark of primary chilblains 19 , 20 (Fig. 3). Immunohistochemical analysis showed inflammatory infiltrate with predominant CD3+ T cells and a normal CD4/CD4 ratio.
Also, the histology of pernio‐like or chilblain‐like lesions reported in the literature showed findings consistent with a diagnosis of chilblain. 15 , 16 , 18 , 21
Clinical examination, microbiological investigations and laboratory data (including ANA, LAC, fibrinogen, D‐dimer) did not reveal any other underlying diseases (viral infection, lupus, hypercoagulation status). It is also possible that an unknown infectious trigger may have contributed to our cluster.
We therefore reached a diagnosis of idiopathic or primary chilblains.
The primary chilblain (PC), also called pernio, is a self‐limiting inflammatory disorder, caused by prolonged exposure to cold temperatures and wet weather, especially during late winter or early spring. Cold may cause persistent or prolonged vasoconstriction in vulnerable people, with subsequent hypoxaemia and secondary inflammatory reaction. 22 The pathogenesis of PC has been attributed to a defective vasodilatory reflex.
We observed typical PC lesions in bilateral acral areas of the hands and feet, especially on the dorsal aspect of toes and in 3/8 cases also on the heel and plantar surface. PC affects susceptible individuals, mainly young females between the ages of 15 and 30 years, and less frequently children and the old people. 23
At this point, why a ‘cluster’ of chilblains was taking place in children not only in Italy but also in other countries 14 remains to be clarified. Many other published cases were evaluated through teledermatology, 10 often without tests and the evaluation of other possible aetiologies common to various countries, such as the drastic lifestyle changes due to lockdown.
We therefore investigated predisposing factors, including age, common characteristics in affected subjects (such as cold and wet extremities and familiarity for chilblains) and trigger factors in environmental conditions (indoor and outdoor).
In the paediatric age, girls going through puberty seem to be more at risk, especially teenagers with thin body habitus. 19 Our cases were all adolescent, F:M = 5:3 with a mean age of 12.8 years. BMI was <25th percentile in 4/8 patients and within 25–50th percentile in 4/8.
Non‐freezing weather and humidity represented triggering factors to be considered. In the late winter–early spring, the climate is generally not rigid at our latitude, whereas pronounced temperature variations were registered in Bologna this March (Fig. 3). A marked humidity characterized the climate in Bologna and in one case also the home environment.
Around this time, also many apartments are cooler because the heating has been turned off.
New behaviours, habits and/or lifestyles arising from the lockdown were evaluated. Toddlers and school‐age children were forced to stay at home all day and changed their habits. We evaluated the clothing, the new behaviours and the characteristics of the home. Some ‘domestic behaviours’ already frequent among adolescents increased during the lockdown period, such as the tendency to isolation in their room with computers due to school needs and entertainment on social network with friends. An accurate personal history showed inadequate clothing in most cases, and in fact, many of them walked barefoot on cold marble or ceramic floors or had prolonged incorrect postural habits such as staying with their legs bent or crossed on the floor or on a chair for many hours. Incorrect postural habits may have contributed to a reduction of blood flow and to lowering skin temperature. These data led us to assume that although the outside temperature was not rigid, these new behaviours, poor clothing, unheated apartments and the characteristics of the indoor environments may have caused the cooling of extremities and feet.
This explains the unusual plantar localization of chilblains in 3 of our cases who walked barefoot, similar to 3 other reported cases who wore shoe boots. 24 In the literature, recreational chilblains are reported with involvement of unusual areas. Unusual localization appears on the hips due to prolonged cold exposure, provoked by tight low‐cut jeans. 25
We have assumed that in predisposed subjects, mainly teenagers, the changes in lifestyle due to the COVID‐19 lockdown, the weather conditions in March and April 2020 and the cold and wet indoor climate may justify the 'cluster' of PC. The longer course of the lesions in our patients compared to typically acute PC 23 might be explained by the persistence of the lockdown.
It would be interesting to verify whether in COVID‐19‐positive young adults, the presence of chilblains is caused by exposure to cold in predisposed subjects and not linked to SARS‐CoV‐2 infection, as already suspected. 7
The major limitation of our study was the small sample size. We were not able to test other patients due to the difficulties to access the hospital. The observation of other cases, with a careful clinical examination and an accurate history, will provide further diagnostic clues and will be necessary to verify our hypothesis.
Conclusion
In the literature, the reasons for the chilblain ‘cluster’ during the COVID‐19 pandemic have so far been not well identified, also on account of the difficulties to perform clinical and diagnostic evaluation.
Negative results of RT‐PCR nasopharyngeal swab, serological tests and PCR assay skin biopsy ruled out a SARS‐CoV‐2 infection in all of our cases.
It is also possible that an unknown infectious trigger may have contributed to our cluster of cases. Nevertheless, the exclusion of other more frequent infectious agents, the normality of laboratory tests and the histological findings led us to the diagnosis of chilblain, a benign acral inflammatory disorder.
We conclude that the chilblains observed in our cases are primary and affect predisposed subjects due to cold exposure in the lockdown period (March–April 2020), during a worldwide emergency.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Acknowledgement
The patients in this manuscript have given written informed consent to the publication of their case details.
Funding: None declared.
Conflict of interest: None declared.
No disclosure of prior presentation of study data as an abstract or poster.
References
- 1. Qiu H, Wu J, Hong L, Luo Y, Song Q Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID‐19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020; 20: 689–696. 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu W, Zhang Q, Chen J et al. Detection of COVID‐19 in Children in Early January 2020 in Wuhan. China. N Engl J Med 2020; 382:1370–1371. 10.1056/NEJMc2003717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coronado Munoz A, Nawaratne U, McMann D et al. Late‐Onset Neonatal Sepsis in a Patient with COVID‐19. N Engl J Med 2020; 382: e49. 10.1056/NEJMc2010614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Suchonwanit P, Leerunyakul K Kositkuljorn C. Cutaneous manifestations in COVID‐ 19: Lessons learned from current evidence. J Am Acad Dermatol 2020; 83: e57–e60. 10.1016/j.jaad.2020.04.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Genovese G, Colonna C Marzano AV. Varicella‐like exanthem associated with COVID‐19 in an 8‐year‐old girl: A diagnostic clue? Pediatr Dermatol 2020; Online ahead of print. 10.1111/pde.14201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morey‐Olivé M, Espiau M, Mercadal‐Hally M, Lera‐Carballo E García‐Patos V. Cutaneous manifestations in the current pandemic of coronavirus infection disease (COVID 2019). An Pediatr (Engl Ed) 2020; Online ahead of print. 10.1016/j.anpede.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Galván Casas C, Català A, Carretero Hernández G et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol 2020; Online ahead of print. 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fernandez‐Nieto D, Jimenez‐Cauhe J, Suarez‐Valle A et al. Characterization of acute acro‐ischemic lesions in non‐hospitalized patients: a case series of 132 patients during the COVID‐19 outbreak. J Am Acad Dermatol 2020; 83: e61–e63. 10.1016/j.jaad.2020.04.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Corman VM, Landt O, Kaiser M et al. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Eurosurveillance 2020; 25: 2000045. 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duong TA, Velter C, Rybojad M et al. Did Whatsapp® reveal a new cutaneous COVID‐19 manifestation? J Eur Acad Dermatol Venereol 2020; Online ahead of print. 10.1111/jdv.16534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Piccolo V, Neri I, Filippeschi C et al. Chilblain‐like lesions during COVID‐19 epidemic: a preliminary study on 63 patients. J Eur Acad Dermatol Venereol 2020; Online ahead of print. 10.1111/jdv.16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y, Cao W, Xiao M et al. Clinical and coagulation characteristics of 7 patients with critical COVID‐2019 pneumonia and acro‐ischemia. Zhonghua Xue Ye Xue Za Zhi 2020; 41: E006. 10.3760/cma.j.issn.0253-2727.2020.0006. [DOI] [PubMed] [Google Scholar]
- 13. Bouaziz JD, Duong T, Jachiet M et al. Vascular skin symptoms in COVID‐19: a french observational study. J Eur Acad Dermatol Venereol 2020; Online ahead of print. 10.1111/jdv.16544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Landa N, Mendieta‐Eckert M, Fonda‐Pascual P Aguirre T. Chilblain‐like lesions on feet and hands during the COVID‐19 Pandemic. Int J Dermatol 2020; 59: 739–743. 10.1111/ijd.14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Romani J, Baselga E Mitjà O et al. Chilblain and acral purpuric lesions in Spain during COVID confinement: Retrospective analysis of 12 cases. Actas Dermosifiliograficas 2020; S0001‐7310(20): 30087‐9. 10.1016/j.ad.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Recalcati S, Barbagallo T, Frasin LA et al. Acral cutaneous lesions in the Time of COVID‐19. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Larkins N Murray KJ. Major cluster of chilblain cases in a cold dry Western Australian winter. J Paediatr Child Health 2013; 49: 144–147. [DOI] [PubMed] [Google Scholar]
- 18. Colonna C, Monzani NA, Rocchi A, Gianotti R, Boggio F Gelmetti C. Chilblains‐like lesions in children following suspected COVID‐19 infection. Pediatr Dermatol 2020; Online ahead of print. 10.1111/pde.14210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nyssen A, Benhadou F, Magnée M, André J, Koopmansch C Wautrecht J‐C. Chilblains. Vasa 2020; 49: 133–140. [DOI] [PubMed] [Google Scholar]
- 20. Cribier BA, Djeridi N, Peltre B Grosshans E. A histologic and immunohistochemical study of chilblains. J Am Acad Dermatol 2001; 45: 924–929. [DOI] [PubMed] [Google Scholar]
- 21. Kolivras A, Dehavay F, Delplace D et al. Coronavirus (COVID‐19) infection‐induced chilblains: a case report with histopathological findings. JAAD Case Rep 2020; 6: 489–492. 10.1016/j.jdcr.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prakash S Weisman MH. Idiopathic chilblains. Am J Med 2009; 122: 1152–1155. [DOI] [PubMed] [Google Scholar]
- 23. Simon TD, Soep JB Hollister JR. Pernio in pediatrics. Pediatrics 2005; 116: e472–e475. [DOI] [PubMed] [Google Scholar]
- 24. Coskey RJ Mehregan AH. Shoe boot pernio. Arch Dermatol 1974; 109: 56–57. [PubMed] [Google Scholar]
- 25. Weismann K Larsen FG. Pernio of the hips in young girls wearing tight‐fitting jeans with a low waistband. Acta Derm Venereol 2006; 86: 558–559. [DOI] [PubMed] [Google Scholar]


