Manufacturing processes of all plasma‐derived medicinal products (PDMPs) include dedicated virus removal or virus inactivation steps to provide a high safety margin against known or emerging viruses. The recent outbreak of the novel coronavirus SARS‐CoV‐2 in China and the subsequent pandemic prompted us to evaluate the safety of selected manufacturing steps of PDMPs focusing specifically on process steps where there is still a lack of published data with regard to coronavirus inactivation.
Coronaviruses have previously been shown to be sensitive to commonly used inactivation methods such as solvent/detergent (S/D) treatment3 and pasteurization. 1 Virus filtration as well as other mechanisms also are highly effective for coronavirus removal or inactivation. As a model virus for the family of coronaviruses we used the transmissible gastroenteritis virus (TGEV), which naturally infects pigs. TGEV was spiked into intermediates derived from the full‐scale manufacturing process, and virus removal was evaluated using scale‐down models. Virus titers were determined using standard procedures, and large‐volume testing was applied at selected time points to increase sensitivity of the assay. Virus titration was performed using FSNi cells, a porcine kidney cell line developed in house. Virus titers are depicted as log CCID50/mL.
In contrast to the respiratory SARS‐CoV‐2, TGEV is a gastrointestinal coronavirus that is known to be relatively stable at low pH due to its physiological mode of transmission via the stomach. 2 Therefore, TGEV is likely to represent a worst‐case model for SARS‐CoV‐2 with regard to sensitivity to low pH treatment.
In an intravenous immunoglobulin (IVIG) solution (50 mg/mL) that is incubated at pH 4.0 ± 0.1 and 25 ± 0.5°C, TGEV was completely inactivated after 21 days of treatment (Fig. 1A), resulting in a final logarithmic reduction factor (LRF) of at least 5.6 log (Fig. 2B). Control inactivation kinetics in IVIG solution at neutral pH (stability control) and in cell culture medium (medium stability control) were slower and thus demonstrated that the inactivation was by a combination of low pH and the inherent virus instability over the 21‐day incubation period. These data demonstrate that the coronavirus TGEV is strongly susceptible to low‐pH treatment under inactivation conditions that meet the Chinese and WHO guidelines and are commonly used in manufacturing of IVIG products worldwide. A different method of low‐pH treatment of an IVIG intermediate (pH 4.0 ± 0.05, 37 ± 1°C, ≥540 min, low concentration of PS80) has previously been shown to inactivate various enveloped viruses. 3 Under these conditions, TGEV was effectively inactivated within 540 minutes of treatment, resulting in a final LRF of 4.0 log (Figs. 1B and 2B). Given the inherent relative stability of TGEV at low‐pH conditions the low level of residual infectivity detected is not unexpected and provides confidence that other coronaviruses that are not gastroenteric would likely be inactivated with greater efficiency.
Fig 1.
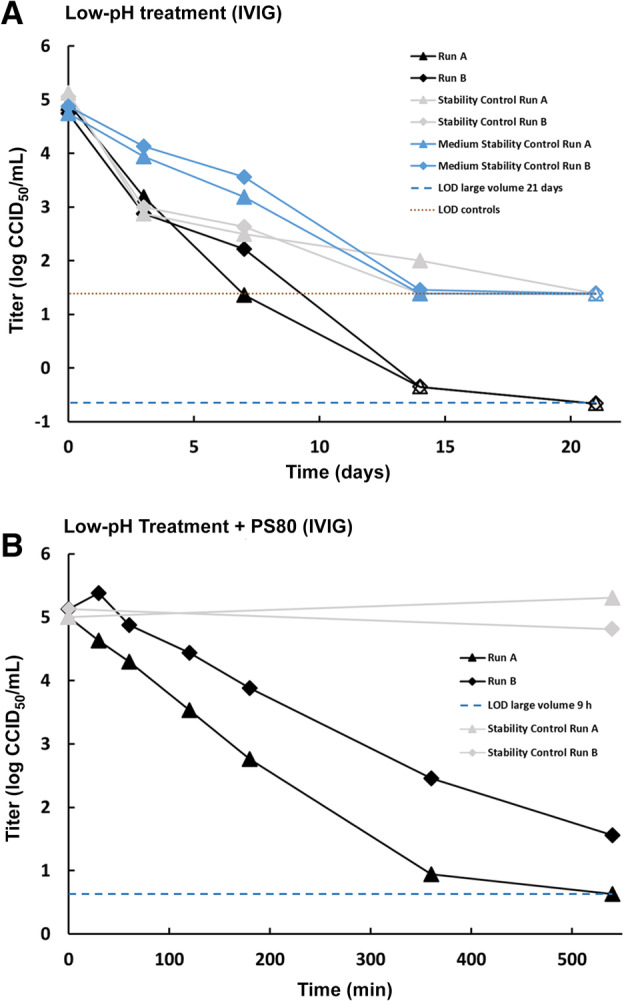
Susceptibility of the coronavirus TGEV to low‐pH treatment. (A) Treatment at pH 4.0 and 25°C in an IVIG solution. Stability controls (virus spiked into IVIG solution at neutral pH [pH 7.0] and held at process temperature) are shown in light gray. Medium stability controls (virus spiked into titration medium and held at process temperature) are shown in blue. Limits of detection (LOD) are shown for 21‐day test samples (based on cumulated volume of samples with no residual infectivity) and controls only. (B) Treatment at pH 4.0 and 37°C in an IVIG intermediate in presence of PS80. Stability controls (virus spiked into IVIG intermediate at neutral pH [pH 7.0] and held at process temperature) are shown in light gray. LOD is shown for 540‐minute test samples. (A, B) Results of two independent replicates are shown (Run A and Run B). Open symbols indicate when the virus titer has reached the detection limit (no residual infectivity detected). [Color figure can be viewed at wileyonlinelibrary.com]
Fig 2.
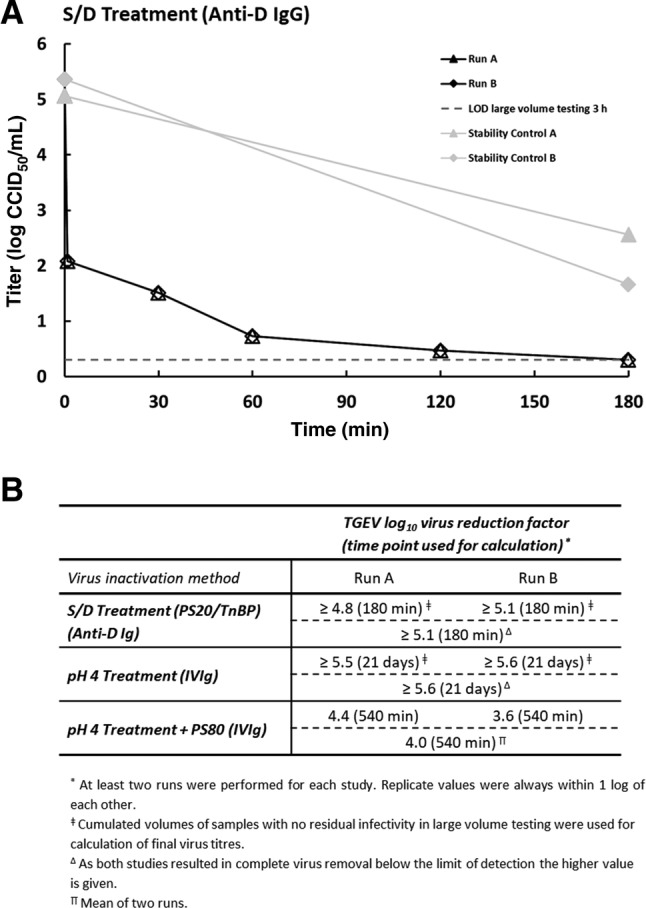
(A) Susceptibility of the coronavirus TGEV to S/D treatment (1.7% wt/wt PS20, 1.0% wt/wt TnBP, 30°C) in an anti‐D IgG intermediate. Results of two independent replicates are shown (Run A and Run B). Stability controls (virus spiked into anti‐D IgG intermediate and held at process temperature) are shown in light gray. Open symbols indicate when the virus titer has reached the detection limit (no residual infectivity detected). Limit of detection (LOD) is shown for 180‐minute test samples (based on cumulated volume of samples with no residual infectivity). (B) Reduction factors for inactivation of TGEV by manufacturing steps of different plasma‐derived products. Individual as well as final reduction factors are given.
Some S/D treatments have been shown to be highly effective at inactivating SARS‐CoV. 4 In the manufacturing of an anti‐D IgG product, S/D treatment is performed early in the process in cryodepleted supernatant of human plasma. Due to the complex composition of this intermediate, including naturally occurring lipid levels, these conditions can be considered a worst case for virus inactivation. Consequently, we evaluated S/D treatment in an anti‐D IgG intermediate using 1.7% wt/wt PS20 (polysorbate 20) and 1.0% wt/wt tri(n‐butyl) phosphate (TnBP) at 30 ± 1°C. TGEV was rapidly inactivated in the anti‐D IgG intermediate (Fig. 2A). S/D treatment resulted in no detectable infectivity at the first time point and a final LRF of at least 5.1 log was demonstrated (Fig. 2B), showing that S/D treatment with PS20 and TnBP is highly effective at inactivating coronavirus (TGEV) even in this complex intermediate.
Together with earlier reports that SARS‐CoV and TGEV are effectively inactivated by pasteurization and standard S/D treatment conditions these studies provide further evidence that various low‐pH incubation and nonstandard S/D treatment steps are also effective at inactivating coronaviruses, which, taken together with other safety measures, provide assurance of a high margin of virus safety against SARS‐CoV‐2 for PDMPs.
CONFLICT OF INTEREST
The authors are employees of CSL Behring, a manufacturer of plasma‐derived biotherapies.
REFERENCES
- 1. Gröner A, Broumis C, Fang R, et al. Effective inactivation of a wide range of viruses by pasteurization. Transfusion 2018;58:41‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Laude H, Gelfi J, Aynaud JM. In vitro properties of low‐ and high‐passaged strains of transmissible gastroenteritis coronavirus of swine. Am J Vet Res 1981;42:447‐9. [PubMed] [Google Scholar]
- 3. Stucki M, Boschetti N, Schäfer W, et al. Investigations of prion and virus safety of a new liquid IVIG product. Biologicals 2008;36:239‐47. [DOI] [PubMed] [Google Scholar]
- 4. Rabenau HF, Biesert L, Schmidt T, et al. SARS‐coronavirus (SARS‐CoV) and the safety of a solvent/detergent (S/D) treated immunoglobulin preparation. Biologicals 2005;33:95‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]


