Abstract
Background and purpose
Although the main clinical features of COVID‐19 infection are pulmonary, several associated neurological signs, symptoms and diseases are emerging. The incidence and characteristics of neurological complications are unclear. For this reason, the European Academy of Neurology (EAN) core COVID‐19 Task Force initiated a survey on neurological symptoms observed in patients with COVID‐19 infection.
Methods
A 17‐question online survey was made available on the EAN website and distributed to EAN members and other worldwide physicians starting on 9 April 2020.
Results
By 27 April 2020, proper data were collected from 2343 responders (out of 4199), of whom 82.0% were neurologists, mostly from Europe. Most responders (74.7%) consulted patients with COVID‐19 mainly in emergency rooms and in COVID‐19 units. The majority (67.0%) had evaluated fewer than 10 patients with neurological manifestations of COVID‐19 (neuro COVID‐19). The most frequently reported neurological findings were headache (61.9%), myalgia (50.4%), anosmia (49.2%), ageusia (39.8%), impaired consciousness (29.3%) and psychomotor agitation (26.7%). Encephalopathy and acute cerebrovascular disorders were reported at 21.0%. Neurological manifestations were generally interpreted as being possibly related to COVID‐19; they were most commonly recognized in patients with multiple general symptoms and occurred at any time during infection.
Conclusion
Neurologists are currently and actively involved in the management of neurological issues related to the COVID‐19 pandemic. This survey justifies setting up a prospective registry to better capture the prevalence of patients with neuro COVID‐19, neurological disease characteristics and the contribution of neurological manifestations to outcome.
Keywords: coronavirus, COVID‐19, neurological, survey
Introduction
After an initial rapid outbreak in China at the end of 2019, coronavirus disease 2019 (COVID‐19) started to break out in Europe at the beginning of 2020, infecting millions and killing by now more than 307 000 people worldwide [1]. Exact epidemiological data on the global incidence and prevalence of COVID‐19 are probably underestimated because the use of the reverse transcriptase polymerase chain reaction (RT‐PCR) test to diagnose the infection is restricted in many countries, the results can be false negative [2, 3] and because of the presence of asymptomatic carriers [4]. Serological confirmation is even less available, and there are different serological assays (ELISA, Western blot, CLIA) which vary in specificity and sensibility [5, 6].
The clinical presentation of COVID‐19 is quite heterogeneous with a broad variety of symptoms and a wide spectrum of severity. Indeed, besides classical respiratory symptoms and signs (cough, dyspnoea and pyrexia), renal, gastrointestinal, dermatological and neurological manifestations have been described [7, 8]. Moreover, whilst the clinical course is most commonly mild to moderate [9], severe cases are also frequent, requiring hospitalization and often admission to the intensive care unit (ICU) due to severe acute respiratory syndrome (SARS‐CoV‐2) [10].
Reports of neurological dysfunction of the central and peripheral nervous system in patients with COVID‐19 infection are increasing. Some patients complain of headache, anosmia and ageusia, but a wider range of more severe neurological complications may also occur, especially in patients requiring hospitalization, including stroke, encephalopathy, encephalitis and polyneuritis [8, 11, 12, 13, 14]. It is currently unknown to what extent European neurologists are involved in the diagnosis and management of COVID‐19 related neurological features (‘neuro COVID‐19’). Moreover, the impact of pre‐existing neurological diseases on the onset of neurological dysfunction, the clinical course of patients with neuro COVID‐19, the association of neurological complaints with the viral infection and short‐ and long‐term outcomes are yet to be elucidated [15]. To fill some of these gaps in knowledge, a core COVID‐19 Task Force of the European Academy of Neurology (EAN) promoted a survey amongst its members and the world medical community on neurological signs and symptoms observed in patients with COVID‐19 infection.
Methods
The main aim of the online survey was to rapidly collate core relevant data on the impact of neuro COVID‐19 in various countries through reporting from the physicians involved in the care of patients with COVID‐19 infection.
Questionnaire design
The EAN core COVID‐19 Task Force modified an available Italian survey originally developed by investigators at the University of Milan, Italy (AP and LC). The questionnaire was limited to 17 key questions to facilitate rapid responses and increase the completion rate. Questions were formulated to capture (i) the main characteristics of the responder [medical specialty and country of origin, involvement as consultant or primary physician in charge of the care of patients with COVID‐19 infection, site of evaluation of patients (in the emergency room, ER; COVID‐19 intensive care units, ICUs; COVID‐19 wards or neurology wards)]; (ii) number and typical age of patients with COVID‐19 infection evaluated; (iii) number of PCR test positive patients; (iv) type and frequency of general (non‐neurological) symptoms and signs; (v) type and frequency of neurological symptoms and investigations; (vi) an opinion on the association of the observed neurological symptoms with COVID‐19 infection; (vii) number of ICU patients evaluated. A dedicated electronic form was used to distribute the survey online. Concerning the frequency of general and neurological symptoms and signs, percentages were used to define boundaries: absent (0%), low (<25%), moderate (25%–50%) and high (>50%) frequency. Figure 1a, b shows the detailed questionnaire structure.
Figure 1.

(a), (b) Details of the 17‐question online questionnaire.
Survey dissemination
The main target of the survey was all EAN members, but the survey was open to any physician worldwide. EAN used all available communication channels (EAN website, EAN pages, Twitter, Facebook, WhatsApp, other societies’ website) for dissemination. The survey was officially launched on 9 April 2020. Email reminders were sent at intervals during the following 2 weeks to advertise the survey.
Statistical analysis
Descriptive statistics were performed for all questions included in the survey, using counts and percentages for categorical variables and medians with ranges for numerical variables. Neurological clinical features were first analysed for the entire sample of respondents, and then stratified by subgroup for different general clinical features, by continent, by country (only including countries with at least 50 respondents), by role of the respondent (consultant or primary physician), by site of evaluation of patients (ER, COVID‐19 ICU, COVID‐19 ward, neurology ward), by time of onset of the clinical manifestation and by opinion on causality. Differences in neurological manifestations between categories of stratified variables were assessed with the chi‐squared test. Significance level was set at P < 0.05.
All analyses were carried out with the SAS statistical package (version 9.4, SAS Institute, Cary, NC, USA).
Results
Responders’ characteristics
By censorship on 27 April 2020, a total of 4199 physicians participated in the survey. The survey took on average 3 min (median 2.9; range 1–9) to complete. A total of 1856 participating physicians completed only the first five questions (Fig. 1), without providing any further information. Therefore, these physicians were excluded from all the subsequent analyses. The remaining 2343 physicians (55.8%) provided full responses on the country of origin, the approximate number of consultations, the average age of evaluated patients and the percentage of patients with confirmed COVID‐19 infection.
Specialty
Responders were represented by 1921 neurologists (82.0%) and by 422 physicians from other medical specialties (18.0%). Amongst the latter, 50 (2.1%) were internal medicine physicians, 48 (2.0%) family medicine doctors and 48 (2.0%) anaesthesiologists (Table 1).
Table 1.
Distribution of responders by medical specialty
| Medical specialty | Number | Rate (%) |
|---|---|---|
| Neurology | 1921 | 82.0 |
| Internal medicine | 50 | 2.1 |
| Family medicine | 48 | 2.0 |
| Anaesthesiology | 48 | 2.0 |
| Paediatrics | 22 | 0.9 |
| Emergency medicine | 19 | 0.8 |
| Surgery | 17 | 0.7 |
| Infectious diseases | 12 | 0.5 |
| Pulmonary medicine | 10 | 0.4 |
| Allergy and immunology | 3 | 0.1 |
| Other | 193 | 8.2 |
| Total | 2343 | 100.0 |
Physician role
There were 1436 (74.7%) consultant physicians and 487 (25.3%) primary physicians of patients with COVID‐19 infection, whilst 420 did not provide this information. Consultations were mostly in the ER (872) and COVID‐19 wards (779). Most clinicians (1572; 67.1%) had seen fewer than 10 patients with COVID‐19 infection, 461 (19.7%) between 10 and 30 patients, 162 (6.9%) between 30 and 50 patients, and 148 (6.3%) more than 50 patients.
Geographical distribution
Most responders were from Europe (Table 2), mainly from Italy (267), France (191), Turkey (168), Spain (138), Switzerland (105) and Portugal (100). Twelve countries (10 from Europe) contributed with 50 or more responses.
Table 2.
Geographical distribution of responders
| World region | Number of respondents | Rate (%) |
|---|---|---|
| Europe | 1646 | 70.2 |
| Asia | 305 | 13.0 |
| Africa | 58 | 2.5 |
| America | 330 | 14.1 |
| Oceania | 4 | 0.2 |
| Total | 2343 | 100.0 |
General characteristics of patients with COVID‐19
The most typical age of patients ranged from 60 to 79 years (50.7%), followed by 40 to 59 years (32.8%). The age distribution was similar amongst countries except for Central Asia, where patients were younger, mainly aged 40–59 years.
The perceived distribution of general COVID‐19 symptoms is illustrated in Fig. 2a, b. The most common symptom was fever (79.9%), followed by fatigue (76.8%), dry cough (73.9%) and dyspnoea (60.0%).
Figure 2.
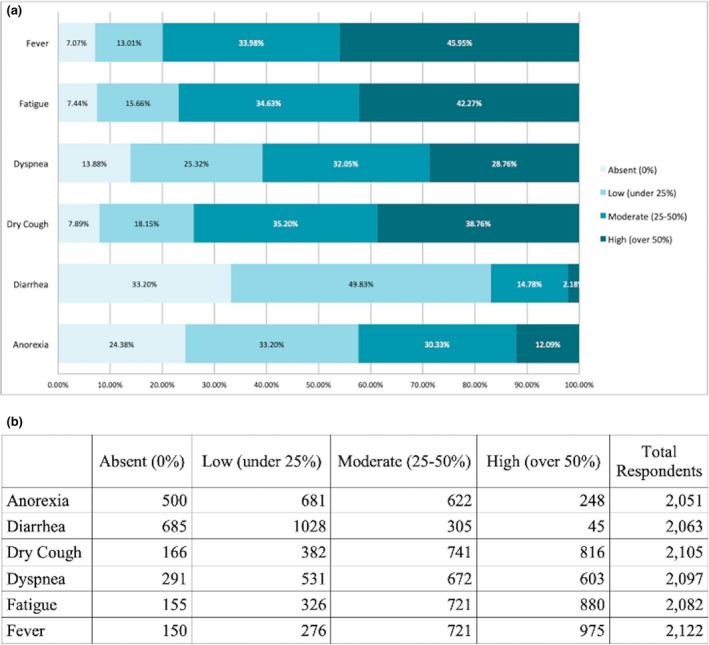
(a) Estimation rates of non‐neurological symptoms in patients with COVID‐19. (b) Number of responders allocated by estimation rate for each symptom.
The median proportion of PCR‐confirmed cases was 52% (range 0%–100%). Laboratory findings included elevated C‐reactive protein (69.8%), lymphopenia (57.7%), elevated lactate dehydrogenase levels (48.6%) and elevated D‐dimer (48.6%) (Fig. 3a, b).
Figure 3.
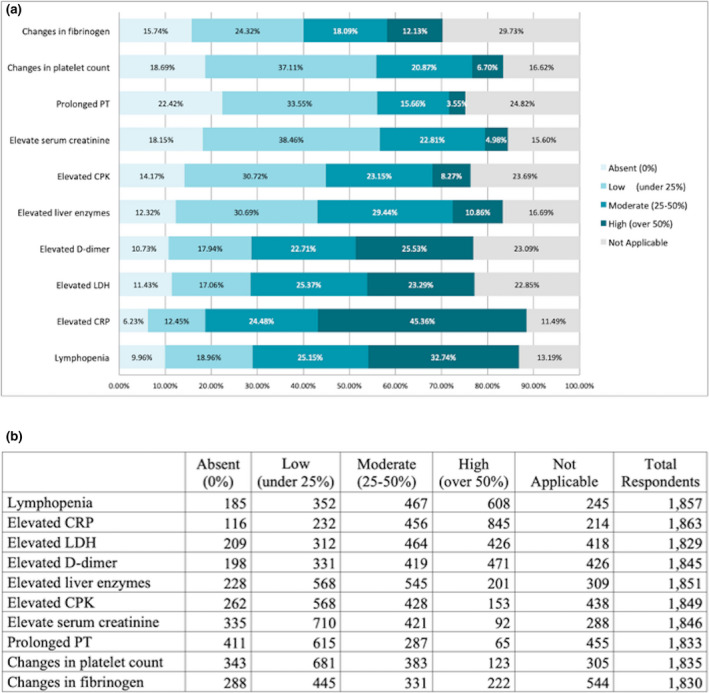
(a) Estimated rates of laboratory findings in patients with COVID‐19. (b) Number of responders allocated by estimation rate for each finding.
Characteristics of patients with neuro COVID‐19
Neurological findings
Almost all responders who filled the complete list of questions on neurological manifestations (1452/1505) reported at least one neurological finding. The commonest clinical features reported (occurring with moderate or high frequency) were, in decreasing order, headache (61.9%), myalgia (50.4%), anosmia (49.2%) and ageusia (39.8%), impaired consciousness (29.3%), psychomotor agitation (26.7%), day‐time sleepiness (24.3%), encephalopathy (21.3%), cerebrovascular disease (21.0%) and dizziness (20.3%). Less frequent were dysphagia (11.2%), sleep disorders other than hypersomnia (10.7%), peripheral nerve damage (8.5%), seizures (8.1%), ataxia (7.4%), meningeal signs (5.7%), movement disorders (5.2%) and visual abnormalities (5.1%). Further details can be found in Fig. 4a, b.
Figure 4.
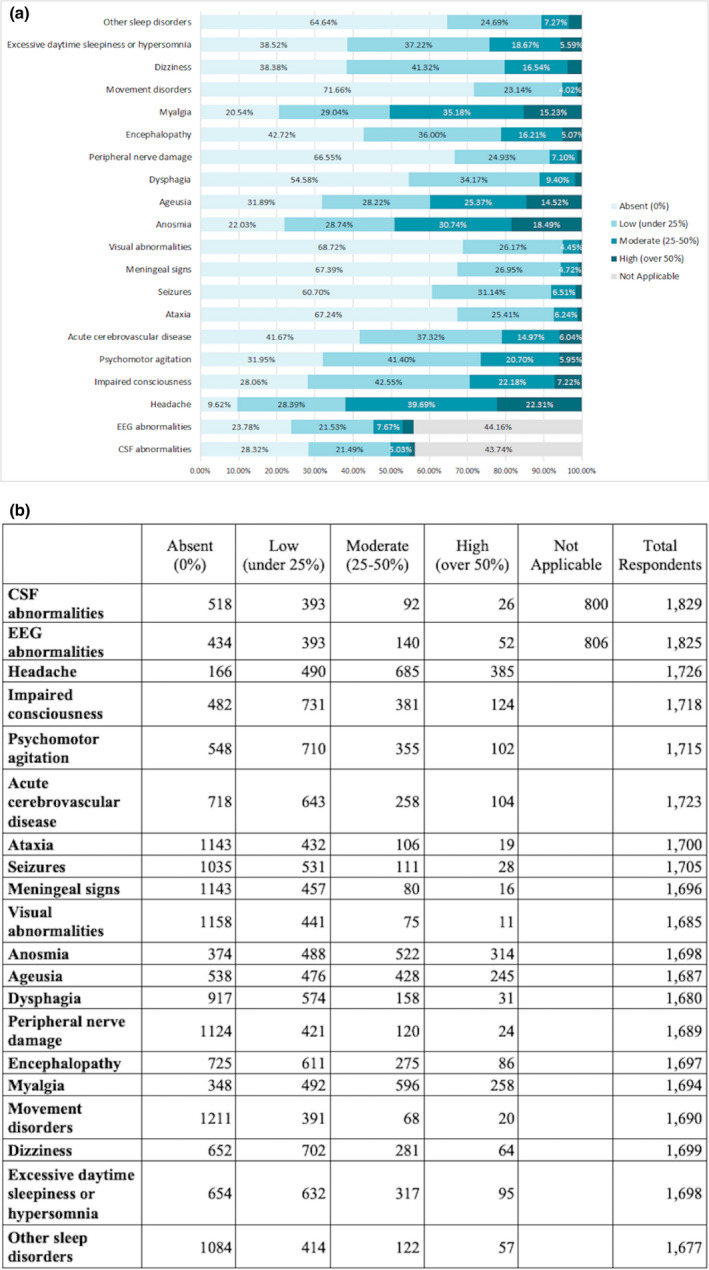
(a) Estimated rates of neurological findings in patients with COVID‐19. (b) Number of responders allocated by estimation rate for each finding.
Cerebrospinal fluid and electroencephalographic abnormalities were detected in 27.4% and 32.0% of cases, respectively (Fig. 4a, b). The estimated frequency of neuroimaging assessments had a bimodal distribution: for 27.9% responders, computed tomography or magnetic resonance imaging was performed in more than 90% of cases, whereas for 19.46%, in less than 10% of patients (median 21; range 3–80).
Time of onset of neurological symptoms
Neurological symptoms were present at the time of admission according to 521/1705 responders (30.6%), appeared during the hospitalization in 350 (20.5%), and were present both at admission and during hospitalization for 834 (48.9%).
Association with COVID‐19
Overall, 1292/1705 (75.0%) responders thought there was an association between COVID‐19 and the observed neurological symptoms: 1046 (61.3%) considered neurological findings as possibly related to systemic effects of COVID‐19, and 246 (14.4%) thought the association was definite. Amongst the remaining responders, 210 (12.3%) declared that the association was unknown, and 203 (11.9%) considered the neurological findings incidental.
ICU patients
Patients were not frequently seen in ICU settings (reported < 10% by 83.9% of responders). Only 10.7% of responders had seen between 10 and 30 patients in this setting.
There were no major differences in neurological manifestations on comparing continents and individual countries. However, African responders reported more severe findings compared to European responders (impaired consciousness, 81.1% vs. 74.3%; encephalopathy, 69.4% vs. 59.6%; dysphagia, 58.8% vs. 44.9%; meningeal signs, 50.0% vs. 31.4%) (see Table S1). No differences were found between consultants and primary physicians concerning neurological findings (data not shown). Neurological symptoms, signs and diseases were most commonly reported in people with multiple general symptoms of infection (Table S2). Neurological manifestations could occur at any time during the infection (Table S3). As expected, the most severe neurological manifestations were observed by responders involved in the ICU (Table S4).
Discussion
To our knowledge, so far this is the largest survey on neurological manifestations of SARS‐CoV‐2 infection promoted by an international academic society with almost 4200 responders in less than 3 weeks. This survey captures a broad spectrum of reported manifestations by investigating a variety of different care settings and obtaining data from neurologists and other specialists involved in the management of the outbreak as primary physicians or consultants.
It was found that neurologists were frequently involved in the care of patients with COVID‐19 infection, mainly as consultant physicians in emergency settings and COVID‐19 wards. Remarkably, it was found that the spectrum of neurological changes recognized by physicians is broad and frequent (95.6% of responders recognized at least one neurological manifestation amongst the evaluated patients). This finding seems to be relevant compared with the first observations described in China where more than 35% of 126 affected patients were found to have some neurological features [8]. However, this is not unexpected because the large majority of our responders were neurologists.
Our survey has revealed that a wide array of neurological manifestations is recognized at differing rates, the commonest being headache, myalgia, anosmia, ageusia, impaired consciousness, psychomotor agitation, day‐time sleepiness, encephalopathy, cerebrovascular disease and dizziness. These diverse neurological complaints suggest the possibility of involvement of the entire (central and peripheral) nervous system by this disease [16, 17, 18]. Many viruses, including coronaviruses, can alter the structure and function of the nervous system manifesting as meningitis, encephalitis, toxic encephalopathy and post‐infectious demyelinating [19]. Coronaviruses can invade nervous tissues, involving macrophages, microglia, astrocytes [20], and cause nerve damage not only through direct infection pathways (both circulatory and neuronal) but also through secondary hypoxia, immune‐mediated injuries, attack to enzymes involved in the renin‐angiotensin system, and other mechanisms [16]. Indeed, COVID‐19 viral load has been found in the brain tissue samples of patients who died during the pandemic [17, 21].
Anosmia and ageusia were commonly reported in our survey. These findings are in line with previous reports [22] which have suggested that these symptoms are due to direct effects of the virus on the olfactory system [23] and gustatory receptors [24]. Coronaviruses may also enter the brain through the olfactory tract in the early stages of infection [25]. Interestingly, anosmia and ageusia can be an early sign of infection, a sign of a milder form of infection and can occur during and after the general symptoms [9].
Cerebrovascular events were reported to occur at a frequency of 21% of cases by our responders. Indeed, there is growing evidence that the COVID‐19 pandemic is having several important implications for stroke [26, 27]. Patients with previous stroke appear to be more susceptible to COVID‐19 and to severe forms [28, 29]. Moreover, COVID‐19 infection itself seems to be a risk factor for stroke, probably related to increased predisposition to thrombotic disease [30, 31]. The occurrence of thrombotic complications has been documented in 31% of ICU patients with COVID‐19 [32]. The SARS‐CoV‐2 virus binds to angiotensin‐converting enzyme 2 (ACE2) in brain endothelial and smooth muscle cells. ACE2 is part of the renin‐angiotensin system along with angiotensin‐converting enzyme 1 (ACE1) and angiotensin II. Angiotensin II is pro‐inflammatory, induces vasoconstriction and promotes organ damage. Depletion of ACE2 by SARS‐CoV‐2 may enhance the activity of the ACE1/angiotensin II axis and promote tissue injury, predisposing to occurrence of stroke [33, 34].
In our survey, neurological symptoms, signs and diseases occurred in people in whom the general manifestations of infection were common and widespread (requiring hospitalization), and appeared at various times during the infection. As expected, the most severe neurological features were reported by physicians consulted in the ICU, as reported recently in a case series of 58 ICU patients with COVID‐19 infection [35]. The tendency of neurological symptoms, signs or diseases to appear at any time during the infection may be explained by differing, perhaps not yet entirely understood, mechanisms of action of coronaviruses, as demonstrated in preclinical models [36]. The occurrence of neurological manifestations after the onset of the acute phase of the disease may imply the presence of post‐infectious immune‐mediated mechanisms [37] or the SARS‐CoV‐2 potential to chronically infect the central nervous system as other CoVs [38]. For this reason, further occurrence of neurological complications of immune‐mediated reactions to the virus at the end of the acute phase of the pandemic or even later may be possible. To support this hypothesis, cases of Guillain–Barré syndrome, a classical example of post‐infectious immune‐mediated disease, have already been reported in the literature [39, 40, 41, 42].
Despite some observed differences, perhaps attributable to the setting and the degree of involvement of the responders during the outbreak, neurological manifestations did not differ greatly across countries and continents. This might suggest that the different genetic characteristics of the host and the environment are not major features in determining whether the virus involves the nervous system.
Most survey responders thought that the neurological manifestations that they observed were associated with the viral infection. Still, 24% of clinicians defined the association as either ‘unknown’ or ‘coincidental’. Indeed, the median proportion of PCR‐confirmed cases was 52% with wide variability amongst responders. This type of study cannot determine whether infection and neurological manifestations are independent or not, although the reported prevalence of neurological symptoms and diseases appear well above general population background rates. In a scoping review of four retrospective studies, a 6.0%–36.4% risk of secondary neurological complications was demonstrated in hospitalized patients with COVID‐19 [13]. In a recent study on seizures occurring during the epidemic, there was no evidence suggesting an additional risk of acute symptomatic seizures in people with COVID‐19 [43]. However, inconsistent reporting and limited statistical analyses amongst these studies prevented accurate assessment of comparative outcomes. Thus, the emergence of a neurological disease during the acute phase of COVID‐19 infection must be assessed in its complex context, and consideration given case‐by‐case as to whether the disease may be a direct or indirect effect of viral invasion or represent a stochastic finding. Causality is difficult to prove. Studies providing dedicated comparisons with the number of cases of each neurological disease expected in the care setting and geographical area in the general population during follow‐up would provide some support.
As any survey, results must be interpreted with caution, and it is acknowledged that ours has some strengths and several limitations. The major strength is the involvement of a large number and wide range of neurologists across multiple countries who contributed to the direct management of patients with COVID‐19 or consulted for the identification of neurological disorders. This contributes to providing a comprehensive picture of the outbreak and its neurological manifestations from the perspective of clinicians dealing with it. Another strength is that the spectrum of the infection and its complications do not differ when described by physicians delivering primary care and those providing only a consultation. Therefore, detection bias does not seem to have affected our results to a significant extent.
The limitations of this survey are the different degree of contribution of the participating countries, with only 11 countries represented by more than 50 interviewees. The continents are not represented in equal proportions either and perhaps the spectrum of neurological manifestations may reflect the different ease of access of affected individuals to local healthcare facilities. This might explain the differing peak age when comparing high‐income to resource‐poor countries. Selection bias cannot be excluded and the results should be interpreted in context, driven mostly by participants from Europe. In addition, limitations intrinsic to the characteristics of survey methodology are reporting and recall bias. However, this survey is not intended to give a precise picture of the pandemic but just to provide an overview based on the impressions of the physicians who were asked to intervene in the management of the disease. A third limitation is the low full completion rate (40%) and the high number of missing variables (see tables). However, even with these limitations, this survey provides valid data on clinician experience, in line with previous reports, and with a large sample size. Most importantly, the study establishes a solid basis to outline the spectrum of COVID‐19 infection and its neurological aspects, and supports the need for further study, such as dedicated case registries.
In conclusion, this survey indicates that neurologists are involved in the management of neurological issues related to the current COVID‐19 pandemic. Overall, our findings underscore the high recognized prevalence of neurological disorders accompanying the COVID‐19 outbreak at a global level, the association of these disorders with more widespread symptoms and signs of COVID‐19 infection, and the observation that involvement of the nervous system can occur at any time during the infection and may provide clues into various underlying pathogenic mechanisms. Nonetheless, the numbers provided by our survey should represent important information for leading the European healthcare systems to consider strengthening neurological services.
The present survey provides the basis to implement surveillance programmes for a more complete assessment of neurological disorders across different countries. Moreover, it is likely that long‐term neurological complications and new post‐infection neurological findings will be recognized.
A registry promoted and endorsed by the EAN has recently been activated (ENERGY, see ean.org) to provide a more comprehensive longitudinal picture of neurological manifestations in various European countries during and after the acute phase of the present COVID‐19 outbreak.
Disclosure of conflict of interest
E. Moro, A. Priori, E. Beghi, R. Helbok, L. Campiglio, C. Bassetti, E. Bianchi, L. Maia, S. Ozturk, F. Cavallieri, M. Zedde, J. Sellner, D. Bereczki, M. Rakusa, G. Di Liberto, A. Sauerbier, A. Pisani, A. Macerollo, R. Soffietti, P. Taba, C. Oreja‐Guevara, B. Bodini, T. Jenkins and TJ von Oertzen have nothing to declare related to this study. M. Crean and A. Twardzik are employed by the EAN.
Supporting information
Table S1. Neurological manifestations distribution by continent.
Table S2. Neurological manifestations distribution by general COVID‐19 symptoms.
Table S3. Neurological manifestations distribution by time of occurrence.
Table S4. Neurological manifestations severity distribution by place of observation.
Acknowledgements
The EAN core COVID‐19 Task Force thanks all the collaborating physicians and centres across the world that have participated in this survey.
[Correction added on 31 July 2020, after first online publication: The middle initial for T. J. Jenkins has been corrected to T. M. Jenkins.]
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. World Health Organization . Coronavirus disease (COVID‐2019) situation reports. 2020. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/situation‐reports/ (accessed 17/05/2020).
- 2. Watson J, Brush JE. Interpreting a COVID‐19 test result. BMJ 2020; 369. doi: 10.1136/bmj.m1808. [DOI] [PubMed] [Google Scholar]
- 3. Kucirka LM, Lauer SA, Laeyendecker O, et al. Variation in false‐negative rate of reverse transcriptase polymerase chain reaction‐based SARS‐CoV‐2 tests by time since exposure. Ann Intern Med 2020; M20‐1495. 10.7326/M20-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS‐CoV‐2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020; 382: 2081–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kirkcaldy RD, King BA, Brooks JT. COVID‐19 and postinfection immunity. JAMA 2020; 323: 2245. 10.1001/jama.2020.7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Theel ES, Slev P, Wheeler S, Couturier MR, Wong SJ, Kadkhoda K. The role of antibody testing for SARS‐CoV‐2: is there one? J Clin Microbiol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lian J, Jin X, Hao S, et al. Epidemiological, clinical, and virological characteristics of 465 hospitalized cases of coronavirus disease 2019 (COVID‐19) from Zhejiang Province in China. Influenza Other Respir Viruses 2020. 10.1111/irv.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mao L, Lin H, Wang M, et al. Neurologic manifestation of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020; 77: 1–9. 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lechien JR, Chiesa‐Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1,420 European patients with mild‐to‐moderate coronavirus disease 2019. J Intern Med 2020. 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang JJY, Lee KS, Ang LW, Leo YS, Young BE. Risk factors of severe disease and efficacy of treatment in patients infected with COVID‐19: a systematic review, meta‐analysis and meta‐regression analysis. Clin Infect Dis 2020. 10.1093/cid/ciaa576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and Neuropsychiatric Complications of COVID‐19 in 153 Patients: A UK‐wide Surveillance Study. Lancet Psychiatry 2020; S2215‐0366(20)30287‐X. 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Whittaker A, Anson M, Harky A. Neurological manifestations of COVID‐19: A systematic review and current update. Acta Neurol Scand 2020; 142: 14–22. 10.1111/ane.13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herman C, Mayer K, Sarwal A. Scoping review of prevalence of neurological comorbidities in patients hospitalized for COVID‐19. Neurology 2020. 10.1212/WNL.0000000000009673. [DOI] [PubMed] [Google Scholar]
- 14. Ellul M, Benjamin L, Singh B, et al. Neurological associations of COVID‐19. Lancet Neurol 2020; S1474‐4422(20)30221‐0. 10.1016/S1474-4422(20)30221-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sellner J, Taba P, Ozturk S, Helbok R. The need for neurologists in the care of COVID‐19 patients. Eur J Neurol 2020. 10.1111/ene.14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, et al. Nervous system involvement after infection with COVID‐19 and other coronaviruses. Brain Behav Immun 2020; 1591: 18–22. 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Puelles VG, Lutgehertmann M, Sperhake MT, et al. Multiorgan and renal tropism of SARS‐CoV‐2. N Engl J Med 2020. 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS‐CoV‐2 may play a role in the respiratory failure of COVID‐19 patients. J Med Virol 2020; 92: 552–555. 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Michalicová A, Bhide K, Bhide M, Kováč A. How viruses infiltrate the central nervous system. Acta Virol 2017; 61: 393–400. 10.4149/av_2017_401. [DOI] [PubMed] [Google Scholar]
- 20. Al‐Obaidi MMJ, Bahadoran A, Wang SM, Manikam R, Raju CS, Sekaran SD. Disruption of the blood brain barrier is vital property of neurotropic viral infection of the central nervous system. Acta Virol 2018; 62: 16–27. 10.4149/av_2018_102. [DOI] [PubMed] [Google Scholar]
- 21. Bernard‐Valnet R, Pizzarotti B, Anichini A, et al. Two patients with acute meningo‐encephalitis concomitant to SARS‐CoV‐2 infection. Eur J Neurol 2020. 10.1111/ene.14298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lechien JR, Chiesa‐Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunction as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): a multicenter European study. Eur Arch Otorhinolaryngol 2020; 6: 1–11. 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bulfamante G, Chiumello D, Canevini MP, et al. First ultrastructural autoptic findings of SARS‐Cov‐2 in olfactory pathways and brainstem. Minerva Anesthesiol 2020; 86: 678–679. 10.23736/S0375-9393.20.14772-2. [DOI] [PubMed] [Google Scholar]
- 24. Vaira LA, Salzano G, Fois AG, Piombino P, De Riu G. Potential pathogenesis of ageusia and anosmia in COVID‐19 patients. Int Forum Allergy Rhinol. 2020. 10.1002/alr.22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Desforges M, Le Coupanec A, Dubeau P, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses 2020; 12: 14. 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Markus HS, Brainin M. COVID‐19 and stroke – a global World Stroke Organization perspective. Int J Stroke. 2020; 29: 1747493020923472. 10.1177/1747493020923472. [DOI] [PubMed] [Google Scholar]
- 27. Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of ischaemic stroke associated with COVID‐19. J Neurol Neurosurg Psychiatry 2020; jnnp‐2020‐323586. 10.1136/jnnp-2010-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aggarwal G, Lippi G, Michael Henry B. Cerebrovascular disease is associated with an increased disease severity in patients with coronavirus disease 2019 (COVID‐19): a pooled analysis of published literature. Int J Stroke. 2020; 20: 1747493020921664. 10.1177/1747493020921664. [DOI] [PubMed] [Google Scholar]
- 29. Pranata R, Huang I, Lim MA, Wahjoepramono PEJ, July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID‐19 – systematic review, meta‐analysis, and meta‐regression. J Stroke Cerebrovasc Dis. 2020; 14: 104949. 10.1016/j.jstrokecerebrovasdis.2020.104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hess DC, Eldahshan W, Rutkowski E. COVID‐19‐related stroke. Transl Stroke Res 2020; 7: 1–4. 10.1007/s12975-020-00818-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oxley TJ, Mocco J, Majidi S, et al. Large‐vessel stroke as a presenting feature of COVID‐19 in the young. NEJM 2020; 382: 60. 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thrombosis Research 2020; 3848: 145–147. doi:10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. South AM, Diz DI, Chappell MC. COVID‐19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020; 318: H1084–H1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID‐19, SARS‐CoV‐1, MERS‐CoV and lessons from the past. J Clin Virol. 2020; 9: 104362. 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS‐CoV‐2 infection. N Engl J Med 2020; 382: 2268–2270. 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Natoli S, Oliveira V, Calabresi P, Maia LF, Pisani A. Does SARS‐Cov‐2 invade the brain? Translational lessons from animal models. Eur J Neurol 2020; 27: 1764–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ng OW, Chia A, Tan AT, et al. Memory T‐cell responses targeting the SARS coronavirus persists up to 11 years post‐infection. Vaccine 2016; 34: 2008–14. 10.1016/j.vaccine.2016.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bergman CC, Lane TE, Stohlman SA. Coronavirus infection of the central nervous system: host‐virus stand‐off. Nat Rev Microbiol 2006; 4: 121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Toscano G, Palmerini F, Ravaglia S, et al. Guillain‐Barré syndrome associated with SARS‐CoV‐2. N Engl J Med 2020; 382: 2574–2576. 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gutierrez‐Ortiz C, Mendez A, Rodrigo‐Rey S, et al. Miller–Fisher syndrome and polyneuritis cranialis in COVID‐19. Neurology 2020. 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 41. Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain–Barré syndrome associated with SARS‐CoV‐2 infection: causality or coincidence? Lancet Neurol 2020; 19: 383–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Scheidl E, Canseco DD, Hadji‐Naumov A, Bereznai B. Guillain–Barré syndrome during SARS‐CoV‐2 pandemic: a case report and review of recent literature. J Peripher Nerv Syst 2020; 25: 204–7. 10.1111/jns.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lu L, Xiong W, Liu D, et al. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: a retrospective multicenter study. Epilepsia 2020; 61: e49–53. 10.1111/epi.16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Neurological manifestations distribution by continent.
Table S2. Neurological manifestations distribution by general COVID‐19 symptoms.
Table S3. Neurological manifestations distribution by time of occurrence.
Table S4. Neurological manifestations severity distribution by place of observation.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


