Abstract
Critical cases of coronavirus disease 2019 (COVID‐19) are associated with a high risk of mortality. It remains unclear why patients with the same critical condition have different outcomes. We aimed to explore relevant factors that may affect the prognosis of critical COVID‐19 patients. Six critical COVID‐19 inpatients were included in our study. The six patients were divided into two groups based on whether they had a good or poor prognosis. We collected peripheral blood samples at admission and the time point of exacerbation to compare differences in the phenotypes and functions of major populations of immune cells between the groups. On admission, compared to patients with poor prognoses, those with good prognoses had significantly higher counts of monocytes (P < .05), macrophages (P < .05), higher frequency of CD3+CD4+CD45RO+CXCR3+ subsets (P < .05), higher frequency of CD14+CD11C+HLA‐DR+ subset of dendritic cells (P < .05), and a lower count of neutrophils (P < .05). At the time point of exacerbation, the proportions of naïve CD4+ T cells (P < .05), Tregs, and Th2 cells in the poor prognosis group were relatively higher than those in the good prognosis group, and CD4+ memory T cells were relatively lower (P < .05). According to our results, the poor prognosis group showed a worse immune response than the good prognosis group at the time of admission and at exacerbation. Dysregulation of the immune response affects the outcome of critical COVID‐19 patients.
Keywords: COVID‐19, dendritic cells, immune response, lymphocyte subsets, outcome
Highlights
The pathogenesis of COVID‐19 is not fully known.
COVID‐19 patients with the same critical condition had different outcomes.
Immune response plays an important role in defensing against virus infections.
1. INTRODUCTION
Novel coronavirus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is responsible for a large global outbreak and is a major public health issue. 1 The pathogenesis of COVID‐19 is not fully known. Although most COVID‐19 patients have mild symptoms, some become critically ill. It has been reported that some patients with critical COVID‐19 can recover after active treatment but that some critically ill patients continue to deteriorate and even die. 2 It remains unclear why patients with the same critical condition have different outcomes.
A rapid and well‐coordinated immune response is the first line of defense against viral infections. During viral infection, host factors trigger an immune response against the virus. Immune insufficiency or misdirection may promote viral replication and cause tissue damage. 3 A significant immune deficiency during the pathogenesis of SARS and Middle East respiratory syndrome (MERS) has been reported, and restoration of the immune response is key to recovery. 4 An effective host immune response, including innate and adaptive immunity against SARS‐CoV‐2, seems crucial to controlling and resolving viral infection. 5 There is evidence that COVID‐19 is more likely to occur in older men with comorbidities who have weaker immune function. 6 , 7 , 8 However, little is known about the lymphocyte subsets and immune response in patients with COVID‐19. 9
In this study, we aimed to investigate the phenotypes and functions of major populations of immune cells of critical COVID‐19 patients to explore possible factors that may affect the prognosis of the disease.
2. METHODS
2.1. Study design and participants
In this retrospective cohort study, we included six adult inpatients from Beijing YouAn Hospital, Capital Medical University, from 23 January to 8 February 2020. Outcomes were followed up until 8 March 2020. All of the patients were clinically diagnosed with critical infections. The six patients were divided into two groups based on whether they had a good prognosis (three patients) or a poor prognosis (three patients). The criterion for poor prognosis is a hospital stay for over 30 days or death during hospitalization.
We collected peripheral blood samples on admission and at the time point of exacerbation to compare differences in lymphocyte function between the two groups. Next, we analyzed clinical characteristics, expression of infection‐related biomarkers, and lymphocyte subsets between the groups. Epidemiological, demographic, clinical, laboratory test, treatment, and efficacy data were obtained from electronic medical records. Two physicians (LLW and BX) checked all the data carefully.
This study was approved by the ethics committee of Beijing YouAn Hospital, Capital Medical University. All patients signed informed consent forms.
2.2. Criteria
On admission, critical illness was defined according to the “Guidelines of the Diagnosis and Treatment of New Coronavirus Pneumonia” (Revision 7) by the National Health Commission of China, as follows 6 : (a) a breathing rate ≥30 times/minute; (b) a pulse oximeter oxygen saturation (SpO2) ≤93% at rest; and (c) a ratio of the partial pressure of arterial oxygen (PaO2) to the fraction of inspired oxygen (FiO2) ≤300 mm Hg.
The criterion for disease exacerbation refers to cases with rapid deterioration with severe hypoxemic respiratory failure: (a) pulse oximeter oxygen saturation cannot reach 93% on 15 L/minute flow of oxygen via a face mask; (b) chest computed tomography scan revealed diffuse ground‐glass opacities and consolidation in the dependent segments of both lungs consistent with acute respiratory distress syndrome (ARDS); and (c) patients need to be initiated on invasive mechanical ventilation if no signs of improvement were observed under the standard treatments.
2.3. Sample preparation for mass cytometry
Blood cells were collected from the six critical COVID‐19 patients. Peripheral blood mononuclear cells (PBMCs) from healthy donors were used as a control group. All blood cells were cultured with 2 μM cisplatin (195‐Pt, Fluidigm) for 2 minutes before quenching with CSB (Fluidigm) to identify viability during the mass cytometry analysis and fixed using a fix I (Fluidigm) buffer according to a previous publication. 10
2.4. Mass cytometry antibody staining and data acquisition on Helios
A metal‐conjugated antibody cocktail was used to stain the cells. The cells were counted and diluted to 1 × 106 cells/mL in phosphate‐buffered saline (PBS), permeabilized before being washed three times in CSB, cultured with an antibody cocktail in a total of 50 μL of CSB for 30 minutes at room temperature, washed three times in CSB and incubated with a 0.125‐μm intercalator in a fixation and permeabilization buffer (Fluidigm) at 4℃ overnight. The cells were then washed three times with ice‐cold PBS and three times with deionized water. Before data acquisition, the samples were resuspended in deionized water containing 10% EQ 4 Element Beads (Fluidigm), and the concentration of the cells was adjusted to 1 × 106 cells/mL. Data acquisition was performed using a Helios mass cytometer (Fluidigm).
2.5. CyTOF data analysis
All fcs files were uploaded to Cytobank, data cleaning was performed according to a previous paper, 11 and the populations of single living cells were exported as fcs files for further analysis. Arcsinh transform was performed to determine the signal intensities of all channels. A viSNE analysis method was performed as previously described. PhenoGraph analysis was carried out as described. 12
2.6. Statistical analysis
Continuous variables are described as means and standard deviations or medians and interquartile range (IQR) values. We used unpaired t tests to compare differences between the good prognosis group and the poor prognosis group, as appropriate.
3. RESULTS
3.1. Demographic and clinical characteristics of the six critical COVID‐19 patients on admission
In total, the median age of the six patients was 74.5 years (IQR: 63.5‐77.3; range: 59‐78 years), and five patients were men. Five patients had confirmed COVID‐19 patient exposure histories and a clustered disease onset. The median incubation period of the poor prognosis group (14 days) was longer than that of the good prognosis group (4 days). All six patients had fever and shortness of breath. Five patients had a cough. Two patients had symptoms of fatigue (33.3%), and one patient had nausea and vomiting (16.7%). No patient had diarrhea. Of the six patients, 3 (50.0%) had hypertension. None of the patients had diabetes or coronary heart disease (Table 1).
Table 1.
Clinical characteristics of the six patients with COVID‐19
| Good prognosis (n = 3) | Poor prognosis (n = 3) | |||||
|---|---|---|---|---|---|---|
| Clinical characteristics (symptoms) | Case 1 | Case 2 | Case 3 | Case 1 | Case 2 | Case 3 |
| Age, y | 65 | 78 | 75 | 77 | 59 | 74 |
| Sex | M | M | M | M | M | F |
| COVID‐19 type | Critical | Critical | Critical | Critical | Critical | Critical |
| History of travelling in Hubei | + | + | + | − | + | + |
| Cluster onset | + | + | + | − | + | + |
| Incubation period | 2 | 6 | 4 | NA | 11 | 17 |
| Durations from illness onset to exacerbation | 11 | 10 | 9 | 11 | 9 | 8 |
| Days with critical illness | 18 | 16 | 13 | 31 | 28 | 26 |
| Symptoms | ||||||
| Fever | + | + | + | + | + | + |
| Cough | + | + | + | + | + | − |
| Fatigue | − | + | − | + | − | − |
| Shortness of breath | + | + | + | + | + | + |
| Nausea or vomiting | − | − | + | − | − | − |
| Diarrhea | − | − | − | − | − | − |
| Coexisting disorders | ||||||
| Diabetes | − | − | − | − | − | − |
| Hypertension | + | + | − | − | − | + |
| Coronary heart disease | − | − | − | − | − | − |
Note: N is the total number of patients with available data. + = positive, − = negative.
Abbreviations: COVID‐19 , coronavirus disease 2019; F, female; M, male; NA, not available.
3.2. Laboratory findings for the six critical COVID‐19 patients
Table 2 presents the laboratory findings for the six patients with COVID‐19. Compared to the patients with good prognoses, those with poor prognoses had higher leukocyte (15.18 vs 5.58 × 109) and neutrophil (13.94 vs 4.40 × 109) counts and higher procalcitonin levels (0.24 vs 0.13 ng/mL) on admission. The platelet (PLT) (90.00 vs 161.00) counts of the poor prognosis group were lower than those of the good prognosis group. Lymphocyte counts and C‐reactive protein levels were similar between the two groups. Compared to the good prognosis group, the poor prognosis group had higher levels of alanine aminotransferase (134.30 vs 34.33 U/L), aspartate aminotransferase (161.70 vs 27.00 U/L), creatinine kinase (CK) (472.70 vs 132.00 U/L), CK‐MB (2.08 vs 0.60 ng/mL), myoglobin (363.70 vs 65.33 ng/mL), and troponin (TNI) (0.41 vs 0.04 ng/mL) on admission. In addition, the level of albumin (32.50 vs 39.43 g/L) and the oxygenation index (PaO2/FiO2) (239.50 vs 281.00 mm Hg) were lower in the poor prognosis group than in the good prognosis group, and the prothrombin time (13.97 vs 11.77 second) of the poor prognosis group was longer than that of the good prognosis group. All the above data were not statistically significant. Compared with the good prognosis group, the poor prognosis group had worse liver injury, myocardial injury, respiratory impairment, and coagulation impairment. Renal function on admission was similar between the two groups.
Table 2.
Laboratory findings for the six patients with COVID‐19
| Good prognosis (n = 3) | Poor prognosis (n = 3) | ||||
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | ||||
| Laboratory findings | Normal range | Admission | Exacerbation | Admission | Exacerbation |
| WBC, ×109/L | 3.5‐9.5 | 5.58 ± 1.91 | 9.51 ± 1.55 | 15.18 ± 4.56 | 10.28 ± 1.33 |
| N, ×109/L | 1.8‐6.3 | 4.40 ± 2.10 | 8.62 ± 1.51 | 13.94 ± 4.26 | 8.46 ± 1.57 |
| L, ×109/L | 1.1‐3.2 | 0.72 ± 0.16 | 0.38 ± 0.04 | 0.70 ± 0.30 | 1.13 ± 0.09 |
| PLT, ×109/L | 125‐350 | 161.00 ± 36.09 | 186.0 ± 42.72 | 90.00 ± 13.45 | 57.67 ± 10.71 |
| PCT, ng/mL | <0.1 | 0.13 ± 0.01 | 0.17 ± 0.04 | 0.24 ± 0.06 | 1.76 ± 0.64 |
| CRP, mg/L | <3 | 53.20 ± 35.83 | 79.97 ± 28.08 | 39.40 ± 15.07 | 68.10 ± 22.14 |
| ALT, U/L | 7‐40 (F); 9‐50 (M) | 34.33 ± 10.35 | 46.67 ± 6.17 | 134.30 ± 51.20 | 29.00 ± 6.03 |
| AST, U/L | 13‐35 (F); 15‐40 (M) | 27.00 ± 14.22 | 54.33 ± 7.67 | 161.70 ± 92.19 | 71.00 ± 33.18 |
| ALB, g/L | 40‐55 | 39.43 ± 1.75 | 29.50 ± 2.51 | 32.50 ± 2.40 | 34.77 ± 3.06 |
| Crea, µmol/L | 41‐81 | 78.00 ± 10.07 | 58.33 ± 9.96 | 58.33 ± 8.09 | 51.67 ± 6.56 |
| eGFR, mL/min | >90 | 82.10 ± 7.11 | 97.93 ± 7.72 | 95.73 ± 3.64 | 101.40 ± 7.83 |
| CK, U/L | 40‐200 (F); 50‐310 (M) | 132.00 ± 22.37 | 166.00 ± 75.18 | 472.7 ± 350.70 | 268.30 ± 158.40 |
| CK‐MB, ng/mL | <3.6 | 0.60 ± 0.19 | 2.25 ± 1.04 | 2.08 ± 1.14 | 5.20 ± 2.04 |
| MYO, ng/mL | 9‐82 (F); 16‐96 (M) | 65.33 ± 15.43 | 76.67 ± 20.50 | 363.70 ± 194.40 | 3058.00 ± 1847.00 |
| TNI, ng/mL | <0.056 | 0.04 ± 0.03 | 0.02 ± 0.02 | 0.41 ± 0.36 | 0.53 ± 0.43 |
| LA, mmol/L | 0.4‐2.0 | 2.23 ± 0.32 | 1.96 ± 0.37 | 2.20 ± 0.12 | 4.29 ± 2.41 |
| PaO2/FiO2, mm Hg | 400‐500 | 281.00 ± 11.15 | 170.10 ± 34.22 | 239.50 ± 28.13 | 168.30 ± 66.88 |
| PT, s | 9.9‐12.8 | 11.77 ± 0.38 | 12.10 ± 0.21 | 13.97 ± 0.68 | 13.63 ± 0.52 |
Note: N is the total number of patients with available data.
Abbreviations: ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatinine kinase; COVID‐19, coronavirus disease 2019; F, female; M, male; MYO, myoglobin; PT, prothrombin time; SD, standard deviation; TNI, troponin.
3.3. Complications, treatment, and outcomes of the six critical COVID‐19 patients
All six patients had pneumonia, ARDS, acute liver injury, and acute myocardial injury. Moreover, three poor prognosis patients had acute kidney injury. Only one patient in the poor prognosis group had septic shock. All six patients were treated with oxygen therapy and systemic corticosteroids. Intravenous antibiotics were given to the three patients with a poor prognosis. One patient in the good prognosis group and the three patients in the poor prognosis group were admitted to the intensive care unit (ICU). Three poor prognosis patients received invasive mechanical ventilation, extracorporeal membrane oxygenation, and continuous renal replacement therapy. Overall, the median durations from illness onset to exacerbation (10 days vs 9 days) of the two groups were similar, but the median number of days with critical illness (28 days vs 16 days) in the poor prognosis group was longer than that in the good prognosis group. Within 30 days after admission, all three patients in the good prognosis group discharged; one patient in the poor prognosis group died, and the other two patients were still under treatment in the hospital (Table 3).
Table 3.
Complications, treatment, and outcomes of the six patients with COVID‐19
| Good prognosis (n = 3) | Poor prognosis (n = 3) | |||||
|---|---|---|---|---|---|---|
| Complications, treatment, and outcomes | Case 1 | Case 2 | Case 3 | Case 1 | Case 2 | Case 3 |
| Complications | ||||||
| Septic shock | − | − | − | + | − | − |
| Acute respiratory distress syndrome | + | + | + | + | + | + |
| Acute kidney injury | − | − | − | + | + | + |
| Acute liver injury | + | + | + | + | + | + |
| Acute myocardial injury | + | + | + | + | + | + |
| Pneumonia | + | + | + | + | + | + |
| Supportive treatment | ||||||
| Administration of intravenous antibiotics | − | − | − | + | + | + |
| Administration of systemic corticosteroids | + | + | + | + | + | + |
| Oxygen therapy | + | + | + | + | + | + |
| Mechanical ventilation | ||||||
| Invasive | − | − | − | + | + | + |
| Noninvasive | − | − | − | − | − | − |
| Use of extracorporeal membrane oxygenation | − | − | − | + | + | + |
| Use of continuous renal replacement therapy | − | − | − | + | + | + |
| Intensive care unit admission | + | − | − | + | + | + |
| Clinical outcomes | ||||||
| Discharge from hospital | + | + | + | − | − | − |
| Death | − | − | − | + | − | − |
| Staying in hospital | − | − | − | − | + | + |
Note: N is the total number of patients with available data. + = positive, − = negative.
Abbreviation: COVID‐19 , coronavirus disease 2019.
3.4. Phenotypical and functional analyses of peripheral lymphocyte changes in the six critical COVID‐19 patients
Mass cytometry analyses including a 33 CyTOF marker panel were performed (Table 4). In brief, PBMCs from the six COVID‐19 patients were collected at the time of admission and of exacerbation. Samples from seven healthy donors were collected as controls.
Table 4.
CyTOF marker panel design
| Antigen | Symbol and mass | Antibody clone | Source |
|---|---|---|---|
| CD45 | 89Y | HI30 | Fluidigm |
| CCR6 | 141Pr | G034E3 | Fluidigm |
| CD19 | 142Nd | HIB19 | Fluidigm |
| CD5 | 143Nd | UCHT2 | Fluidigm |
| CD16 | 145Nd | 3G8 | Biolegend |
| IgD | 146Nd | IA6‐2 | Fluidigm |
| CD20 | 147Sm | H1 | Fluidigm |
| CD14 | 148Nd | 134620 | R&D |
| CD25 | 149Sm | 2A3 | Fluidigm |
| CD8a | 150Nd | RPA‐T8 | Fluidigm |
| CD45 | 151Eu | HI30 | Biolegend |
| CD11c | 152Sm | Bu15 | Biolegend |
| CD7 | 153Eu | CD7‐6B7 | Fluidigm |
| CD49d | 154Sm | F10 | Biolegend |
| CD27 | 155Gd | L128 | Fluidigm |
| CXCR3 | 156Gd | G025H7 | Fluidigm |
| CCR4 | 158Gd | 205410 | Fluidigm |
| CD161 | 159Tb | HP‐3G10 | Fluidigm |
| CD28 | 160Gd | CD28.2 | Fluidigm |
| CD45RA | 162Dy | HI100 | Biolegend |
| CD103 | 163Dy | Ber‐ACT8 | Biolegend |
| CXCR5 | 164Dy | 51505 | Fluidigm |
| CD45RO | 166Er | UCHL1 | Biolegend |
| IgA | 167Er | HP6123 | Biolegend |
| CD22 | 168Er | HIB22 | Biolegend |
| CD24 | 169Tm | ML5 | Fluidigm |
| CD3 | 170Er | UCHT1 | Fluidigm |
| CD9 | 171Yb | SN4 C3‐3A2 | Fluidigm |
| IgM | 172Yb | MHM‐88 | Fluidigm |
| HLA‐DR | 173Yb | L243 | Fluidigm |
| CD38 | 174Yb | HIT2 | Biolegend |
| PD‐1 | 175Ho | EH12.2H7 | Fluidigm |
| CD4 | 176Yb | RPA‐T4 | Fluidigm |
| CD11b | 209Bi | ICRF44 | Fluidigm |
We first analyzed the frequencies of the major immune components of PBMCs, and viSNE plots were employed to visualize high‐dimensional CyTOF data and the distribution of the markers used to identify subsets in two dimensions (Figure 1A). The mean value of each population and comparisons among groups represented as minus log10 P values are shown in the heatmap in Figure 1B. The major immune cell components in the COVID‐19 patients were significantly different from those in the healthy donors in terms of neutrophils, B cells, and T cells. However, when comparing the results on admission among the COVID‐19 patients with a good or poor prognosis, we found that the good prognosis group had significantly higher counts of monocytes and macrophages (P < .05, P < .05) and a lower count of neutrophils (P < .05). At time point exacerbation, the proportions of naïve CD4+ T cells (P < .05), Tregs, and Th2 cells were relatively higher in the poor prognosis group than in the good prognosis group, whereas CD4+ memory T cells were relatively lower (P < .05) (Figure 1B).
Figure 1.

CyTOF‐based analysis revealed immune cell signatures in the peripheral blood of COVID‐19 patients. A, Subsets of blood cells revealed by CyTOF are indicated, and relative marker expression is also displayed. B, The proportion of immune cell subpopulations is displayed in a heat map using the mean value of each group in min/max form (left). Comparisons of peripheral blood immune cell subsets among groups are displayed in a heat map using Minus log 10 (P value) (right). COVID‐19, coronavirus disease 2019
3.5. Percentage and status of DCs in peripheral blood cells represent differences between the good and poor prognosis groups of critical COVID‐19 patients
We then used PhenoGraph to analyze 19 subjects (seven healthy donors and the six patients with critical COVID‐19 at two‐time points) with 33 markers (Figure 2A). A total of 190 000 cells were analyzed, and 32 clusters were identified according to marker expression (Figure 2B). On admission, the patients with a good prognosis had higher frequencies of CD3+CD4+CD45RO+CXCR3+ subsets (cluster 10, P < .05) and the CD14+CD11C+HLA‐DR+ subset of dendritic cells (DCs) (cluster 25, P < .05) than the poor prognosis patients. To further explore functional differences in immune subpopulations, we also analyzed marker expression patterns in all 32 PhenoGraph metaclusters among all five groups; cluster 2 (CD14−CD11c+HLA‐DR+) and cluster 25 (CD14+CD11b+CD11c+HLA‐DR+) are displayed as examples in Figure 2D. We observed that the good prognosis group patients displayed relatively higher levels of markers such as CD38, CXCR3, CCR5, HLA‐DR, and CD49d.
Figure 2.
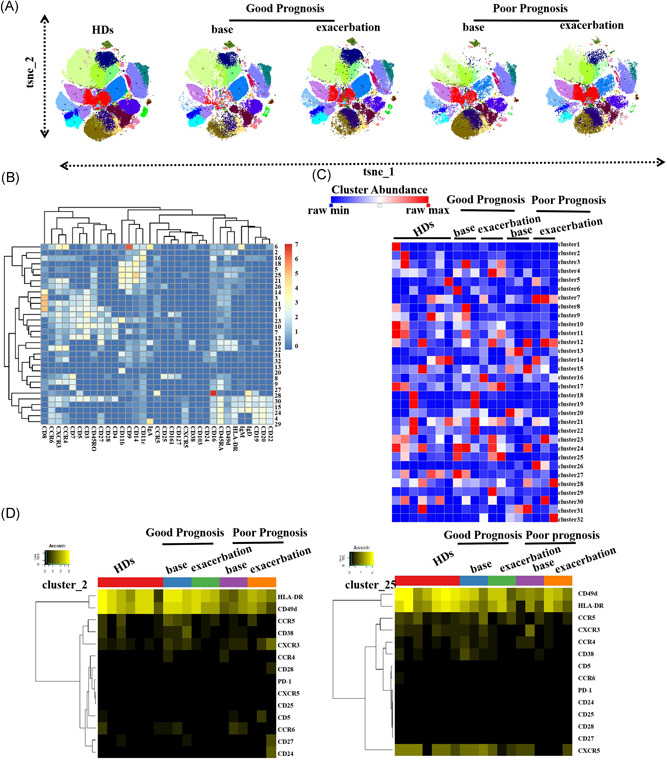
In‐depth phenotyping of immune cells by PhenoGraph. A, The viSNE plot of each group is shown as colored by cluster. B, Normalized marker expression of identified clusters in the heatmap. C, Heatmap of all cluster abundances among groups. D, Protein (active markers, chemokine receptors) expression of metacluster2 (DCs) and 25 (monocytes) are displayed
4. DISCUSSION
Critical cases of COVID‐19 are associated with a high risk of mortality. According to reports, the mortality rates of ICU patients in Jin Yin‐Tan Hospital were between 38% and 62%, and more than 10% required ECMO. 13 , 14 , 15 Thus, clarifying the pathogenesis of critical COVID‐19 to facilitate improvement in treatment is important. 16 , 17 Several studies have described the immune response of COVID‐19 patients. SARS‐CoV‐2 may break down antiviral immunity at an early stage. Elevated T cell exhaustion levels and reduced functional diversity of these cells in the peripheral blood may predict severe progression in COVID‐19 patients. 18 , 19 Our research focuses on the immune response in critical COVID‐19 patients with different outcomes, which is quite different from previous studies.
In our study, the median durations from illness onset to exacerbation were similar in the two groups. The median number of days with critical illness (28 days vs 16 days) in the poor prognosis group was longer than that in the good prognosis group. Furthermore, laboratory investigations on admission revealed more prominent laboratory abnormalities in the poor prognosis group than in the good prognosis group, such as leukocyte counts, neutrophil counts, PLT counts, and procalcitonin levels. It seems that when patients were hospitalized, those with a poor prognosis had already exhibited a more pronounced inflammatory response and more serious multiple organ functional impairment than those with a good prognosis at the early stage of disease.
To map the peripheral lymphocyte signature of COVID‐19 patients diagnosed with critical disease and further identify the immune subpopulations that might be associated with different outcomes, the major immune cell components in COVID‐19 patients were analyzed. At the time of admission, the good prognosis patients had higher frequencies of CD3+CD4+CD45RO+CXCR3+ subsets compared to the poor prognosis patients. This subset can be recognized as memory helper T‐cell type 1 (Th1). Evidence has indicated that the Th1‐type response plays a key role in the successful control of SARS‐CoV, MERS‐CoV, and SARS‐CoV‐2. 18 Moreover, expansion of antigen‐specific CD8+ T cells depending on Th1 cells has a significant effect on virus clearance. 19 It has been reported that the total number of natural killer cells and CD8+ T cells is markedly decreased in patients with SARS‐CoV‐2 infection. 20 , 21 Our results indicate that memory Th1 cells may play an important protective role in defense against fatal cases of coronavirus infection, which is consistent with previous research results.
DCs are the most powerful full‐time antigen‐presenting cells (APCs), and they can efficiently ingest, process, and present antigens. 22 According to our results, good prognosis patients had higher frequencies of the CD14+CD11C+HLA‐DR+ subset of DCs than did poor prognosis patients on admission. Previous studies have shown that CD11C+ DCs coexpressing the monocyte marker CD14 may be directly involved in the immunopathology of some diseases. These cells can exhibit an efficient antigen‐presentation capacity and constitutive secretion of tumor necrosis factor‐alpha, which suggests an active immune response. 23 , 24 However, some viruses can hinder the body's antiviral response, resulting in more serious infection. Similar to our observations, Wilk AJ's 25 study found that plasmacytoid DCs and conventional DCs were depleted in critical COVID‐19 patients. Critical COVID‐19 patients with a poor immune response at disease onset will fail to defend themselves against the coronavirus infection.
At the time of admission, further profiling of immune cell subpopulations revealed that neutrophils were significantly increased in the poor prognosis group. Increased neutrophil counts have been reported in patients with critical COVID‐19. 9 At the time point of exacerbation, the poor prognosis group had relatively higher levels of naïve CD4+ T cells, Tregs, and Th2 cells than the good prognosis group. These results indicate immunosuppression and an inflammatory response in those with a poor prognosis, consistent with our laboratory investigations. 26
Our study has some limitations. It is necessary to mention that our findings were based on a limited number of cases and CyTOF antibodies. In addition, our study lacks immune functional analyses, such as cell type‐specific cytokine production. Validation in other patients should be performed in the future.
In conclusion, dysregulation of the immune response affects outcome in critical COVID‐19 patients. Our data highlight the immunological features in critical COVID‐19 patients and can provide therapeutic strategies through an improved understanding of the mechanisms of immune dysregulation. Future studies should be carried out to explore links between disease outcome and immune cell‐type abundances in patients with SARS‐COV‐2 infections.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
LW was responsible for the clinical data collection, data analysis, and drafting of the manuscript. WW contributed to the mass cytometry analysis, data analysis, and drafting of the manuscript. BX and DC contributed to the study design and manuscript revision.
ACKNOWLEDGMENTS
The authors would like to thank the SARS‐CoV‐2 medical and nursing team of Beijing YouAn Hospital, Capital Medical University, who are fighting against the illness, and Professor Ronghua Jin for advice on the medical treatment of critically ill patients.
Wei L‐l, Wang W‐j, Chen D‐x, Xu B. Dysregulation of the immune response affects the outcome of critical COVID‐19 patients. J Med Virol. 2020;92:2768–2776. 10.1002/jmv.26181
Lin‐lin Wei and Wen‐jing Wang contributed equally.
Contributor Information
De‐xi Chen, Email: dexichen@ccmu.edu.cn.
Bin Xu, Email: xubin1016@126.com.
REFERENCES
- 1. Sohrabi C, Alsafi Z, O'Neill N, et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID‐19). Int J Surg. 2020;76:71‐76. 10.1016/j.ijsu.2020.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang B, Wang L, Kong X, et al. Long‐term coexistence of SARS‐CoV‐2 with antibody response in COVID‐19 patients. J Med Virol. 2020. 10.1002/jmv.25946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li G, Fan Y, Lai Y, et al. Coronavirus infections and immune responses. J Med Virol. 2020;92(4):424‐432. 10.1002/jmv.25685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mahallawi WH, Khabour OF, Zhang Q, Makhdoum HM, Suliman BA. MERS‐CoV infection in humans is associated with a pro‐inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8‐13. 10.1016/j.cyto.2018.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tufan A, Avanoğlu Güler A, Matucci‐Cerinic M. COVID‐19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk J Med Sci. 2020;50(SI‐1):620‐632. 10.3906/sag-2004-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Diagnosis and Treatment Protocol for Novel Coronavirus Infection‐Induced Pneumonia version 7 (trial). National Health and Health Commission of China . http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf. Accessed May 22, 2020.
- 7. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang Y, Lu Q, Liu M, et al. Epidemiological and clinical features of the 2019 novel coronavirus outbreak in China. medRxiv. 2020. 10.1101/2020.02.10.20021675v2 [DOI] [Google Scholar]
- 9. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis. 2020:ciaa248. 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2‐mesenchymal stem cells improves the outcome of patients with COVID‐19 pneumonia. Aging Dis. 2020;11(2):216‐228. 10.14336/AD.2020.0228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van Unen V, Höllt T, Pezzotti N, et al. Visual analysis of mass cytometry data by hierarchical stochastic neighbour embedding reveals rare cell types. Nat Commun. 2017;8(1):1740. 10.1038/s41467-017-01689-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levine JH, Simonds EF, Bendall SC, et al. Data‐driven phenotypic dissection of AML reveals progenitor‐like cells that correlate with prognosis. Cell. 2015;162(1):184‐197. 10.1016/j.cell.2015.05.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goh KJ, Choong MC, Cheong EH, et al. Rapid progression to acute respiratory distress syndrome: review of current understanding of critical illness from COVID‐19 infection. Ann Acad Med Singapore. 2020;49(3):108‐118. [PubMed] [Google Scholar]
- 14. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8:475‐481. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rokni M, Ghasemi V, Tavakoli Z. Immune responses and pathogenesis of SARS‐CoV‐2 during an outbreak in Iran: comparison with SARS and MERS. Rev Med Virol. 2020;30(3):e2107. 10.1002/rmv.2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Giamarellos‐Bourboulis EJ, Netea MG, Rovina N, et al. Complex immune dysregulation in COVID‐19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992‐1000. 10.1016/j.chom.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID‐19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 19. Singh M, Thakral D, Rishi N, Kar HK, Mitra DK. Functional characterization of CD4 and CD8 T cell responses among human papillomavirus infected patients with ano‐genital warts. Virusdisease. 2017;28(2):133‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zheng HY, Zhang M, Yang CX, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID‐19 patients. Cell Mol Immunol. 2020;17(5):541‐543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID‐19 patients. Cell Mol Immunol. 2020;17(5):533‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goyvaerts C, Breckpot K. The journey of in vivo virus engineered dendritic cells from bench to bedside: a bumpy road. Front Immunol. 2018;9:2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Beitnes AC, Ráki M, Brottveit M, Lundin KE, Jahnsen FL, Sollid LM. Rapid accumulation of CD14+CD11c+ dendritic cells in gut mucosa of celiac disease after in vivo gluten challenge. PLoS One. 2012;7(3):e33556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Micheletti A, Finotti G, Calzetti F, et al. slanDCs/M‐DC8+ cells constitute a distinct subset of dendritic cells in human tonsils. Oncotarget. 2016;7(1):161‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilk AJ, Rustagi A, Zhao NQ, et al. A single‐cell atlas of the peripheral immune response to severe COVID‐19 [published online ahead of print Jun 8, 2020]. medRxiv. 10.1038/s41591-020-0944-y [DOI] [Google Scholar]
- 26. Levine AG, Mendoza A, Hemmers S, et al. Stability and function of regulatory T cells expressing the transcription factor T‐bet. Nature. 2017;546(7658):421‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]


