Abstract
Coronavirus disease 2019 (COVID‐19) is a global pandemic that is caused by a novel coronavirus, severe acute respiratory syndrome coronavirus‐2. Data from several countries have shown higher morbidity and mortality among individuals with chronic metabolic diseases, such as diabetes mellitus. In this review, we explore the contributing factors for poorer prognosis in these individuals. As a significant proportion of patients with COVID‐19 also have diabetes mellitus, this adds another layer of complexity to their management. We explore potential interactions between antidiabetic medications and renin–angiotensin–aldosterone system inhibitors with COVID‐19. Suggested recommendations for the use of antidiabetic medications for COVID‐19 patients with diabetes mellitus are provided. We also review pertinent clinical considerations in the management of diabetic ketoacidosis in COVID‐19 patients. In addition, we aim to increase clinicians’ awareness of the metabolic effects of promising drug therapies for COVID‐19. Finally, we highlight the importance of timely vaccinations for patients with diabetes mellitus.
Keywords: COVID‐19, Diabetes, SARS‐CoV‐2
Introduction
Coronavirus disease 2019 (COVID‐19), caused by the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) infection, was first reported in Wuhan, China, in December 2019 1 . Similar to its counterparts, severe acute respiratory syndrome coronavirus (SARS‐CoV) and Middle East respiratory syndrome coronavirus, SARS‐CoV‐2 is highly pathogenic, and can cause severe pneumonia, acute respiratory distress syndrome and multiorgan failure. Furthermore, the rapid transmission of SARS‐CoV‐2 has caused a worldwide pandemic with >6 million cases and 360,000 deaths since June 2020 2 .
Diabetes mellitus affects approximately 463 million people worldwide 3 , while obesity inflicts nearly one‐third of the world’s population 4 . The co‐existence of obesity and diabetes mellitus, also known as “diabesity,” is yet another major pandemic that the world currently faces. Patients with diabesity are at significantly increased risk of developing severe infections and impaired pulmonary function 5 . Furthermore, there are also unique and complex interactions between antidiabetic medications and other commonly used agents for diabetes mellitus‐related comorbidities with COVID‐19 infection. To further complicate this interplay, some of the promising drug therapies are also associated with metabolic effects.
Diabetes mellitus and obesity are risk factors for severity of COVID‐19 infection
Diabetes mellitus is a well‐established risk factor for infections, and the risk increases with poor glycemic control 6 . In general, glycated hemoglobin (HbA1c) >9% has been shown to be associated with 60% increased risk of severe bacterial pneumonia 7 . Although current evidence does not suggest that patients with diabetes mellitus are at higher risk of contracting SARS‐CoV‐2 8 , diabetes mellitus has been listed as the third most prevalent comorbidity, behind cardio‐cerebrovascular disease and hypertension 9 , and is also associated with a two‐ to threefold increase in adverse outcomes 9 . Similarly, obese individuals with body mass index >35 kg/m2 are at nearly sevenfold higher risk of requiring mechanical ventilation 10 . A recent study suggested a lower body mass index threshold of 25 kg/m2 for disease severity stratification in the Asian population 11 . In addition, patients with microvascular and macrovascular complications of diabetes mellitus, as well as obstructive sleep apnea, were found to be at significantly higher risk of severe disease and mortality 12 . Figure 1 summarizes the diverse interactions between these two conditions.
Figure 1.
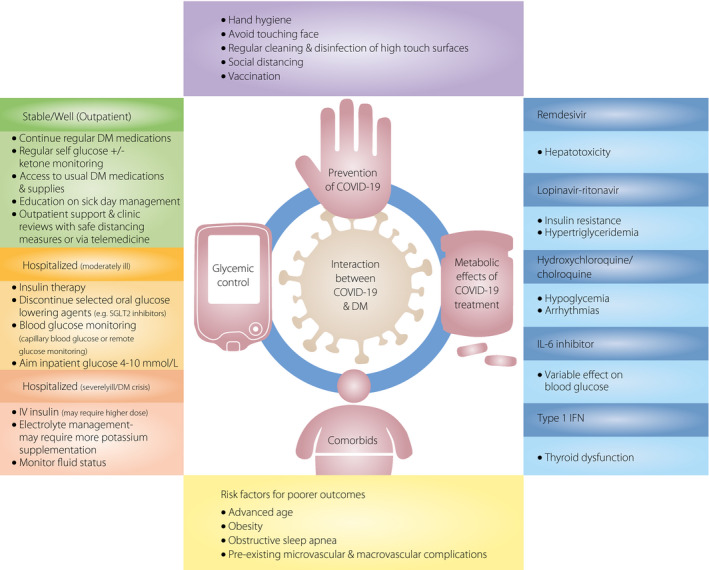
Interaction between coronavirus disease 2019 (COVID‐19) and diabetes mellitus (DM). IFN, interferon; IL‐6, interleukin‐6; SGLT2, sodium–glucose cotransporter 2.
Pathogenic link between diabetes mellitus, obesity and increased severity of COVID‐19
There are several mechanisms that predispose patients with diabetes mellitus to increased disease severity. Diabetes mellitus is associated with immune dysfunction 13 , increased susceptibility to inflammation 14 and reduced viral clearance 15 . Furthermore, a possible association between SARS‐CoV‐2 and the renin–angiotensin–aldosterone system (RAAS) might increase adherence of SARS‐CoV‐2 to target cells and might worsen the severity of COVID‐19 16 , generating controversies about the use of RAAS blockers, which will be discussed further.
Diabetes mellitus is associated with immune dysfunction and increased inflammation
The immune system is dysregulated in hyperglycemia. The humoral system, which mediates immediate defense responses by polymorphs, macrophages and dendritic antigen presenting cells to pathogens, is attenuated in diabetes mellitus 17 . Defects in adaptive immunity are associated with impaired type 1 interferon production 18 . Furthermore, increased generation of advanced glycation end‐products could also inhibit the generation of interferon gamma by T lymphocytes 19 . These could reduce antiviral activity and increase the severity of infection. The low T lymphocyte counts in diabetes mellitus patients might blunt antiviral interferon responses 20 . Furthermore, the co‐existence of diabetes and obesity or “diabesity” is characterized by a pro‐inflammatory state, driven by cytokines, such as interleukin‐6 (IL‐6) and tumor necrosis factor alpha 21 , 22 . These patients are at increased risk of uncontrolled inflammation, which could induce a cytokine storm and contribute to an overall poor prognosis.
Hyperglycemia and obesity are associated with alterations in pulmonary function and reduced viral clearance
Studies have shown that hyperglycemia can directly increase glucose concentrations in the airways and affect pulmonary function, as well as alter pulmonary vascular permeability and alveolar epithelial function 23 . These factors might contribute to increased severity of respiratory infections. Furthermore, a recent study has also shown delayed clearance of SARS‐CoV‐2 in patients with diabetes mellitus 15 .
With regard to obesity, pulmonary function studies have shown a restrictive pattern and reduced lung volumes in obese individuals 10 . The reduced cardiorespiratory reserve, coupled with difficulty in ventilation, could account for the significant increased disease severity in these patients 5 , 10 , 11 .
Increased adherence of SARS‐CoV‐2 to target cells
SARS‐CoV‐2 has glycoprotein spikes on its surface, which attach to angiotensin‐converting enzyme 2 (ACE2) receptors on target cells. On binding to ACE2, the virus is processed by proteases, such as the transmembrane serine protease 2 and furin, resulting in the internalization of the virion complex 20 . ACE2 and furin expression are increased in diabetes mellitus, which might facilitate viral entry and replication 20 , 24 .
Potential effects of SARS‐CoV‐2 on pancreatic function
The binding of SARS‐CoV to the ACE2 receptors on pancreatic islets could potentially cause acute diabetes 25 . In a study by Yang et al. 25 , just two of 39 patients with SARS‐CoV and labeled to have diabetes mellitus during admission continued to have diabetes mellitus after 3 years. Further characterization showed significant immunostaining for ACE2 in the pancreatic islets, but this was weak in the exocrine tissues. In addition, SARS‐CoV‐2 might be associated with elevated amylase, lipase and focal changes to the pancreas, raising the possibility of pancreatic injury 26 . Other viruses, such as enteroviruses, Coxsackie B virus and cytomegalovirus, had previously been found to be associated with the development of type 1 diabetes mellitus 27 . We recently reported a case of diabetic ketoacidosis (DKA) precipitated by COVID‐19 in a patient with newly diagnosed diabetes mellitus 28 . Similar to other acute illnesses that necessitate hospitalization among patients with diabetes mellitus, inpatient glycemic management and being alert to the potential risk of DKA are crucial. However, the long‐term effects of SARS‐CoV‐2 are unclear, and long‐term follow up will be required to determine the magnitude of its impact on pancreatic function and the consequent risk of developing diabetes mellitus.
Management of diabetes mellitus in a patient with COVID‐19
Glycemic control is important for all patients. Previous experiences with SARS‐CoV and current data with COVID‐19 have shown that hyperglycemia and diabetes mellitus are significant risk factors for complications and mortality 29 .
One of the main challenges in the management of acutely unwell COVID‐19 patients with diabetes mellitus is the reduced oral intake. As such, the dosage of usual oral antidiabetic agents and/or insulin might have to be reduced and adjusted accordingly to avoid hypoglycemia.
In the next section, we review the different classes of oral antidiabetic agents, and their effects on infection and inflammation, and provide recommendations on their use during acute illness.
Metformin
In stable patients with normal oral intake and who do not have nausea and vomiting, metformin can be continued. Interestingly, metformin has gained recent interest given its potential role in immunomodulation. Animal studies have shown reduced expression of pro‐inflammatory cytokines, such as tumor necrosis factor alpha and IL‐6, associated with continued metformin use in sepsis 30 . Metformin has also been shown to improve survival in mice infected with Legionella pneumophila 31 . However, in the critically ill patient with acute renal, hepatic injury or hemodynamic instability, metformin should be avoided due to the risk of lactic acidosis.
Dipeptidyl peptidase‐4 inhibitors
Concerns regarding the slight increased risk of nasopharyngeal 32 and urinary tract infections 33 have arisen from the use of dipeptidyl peptidase‐4 (DPP4) inhibitors. However, a meta‐analysis by Cai et al. 34 did not report significant differences in DPP4 inhibitor use with increased risk of upper respiratory tract infections. Another cohort study also did not show an association between DPP4 inhibitor and risk of pneumonia 35 .
In addition, DPP4 has been shown to be a receptor for cellular entry of Middle East respiratory syndrome coronavirus 36 . Whether this translates into increased susceptibility to certain coronavirus infections or increases the severity of coronavirus infections is currently unclear. At present, the use of DPP4 inhibitors did not show any difference in lymphocyte function or production of inflammatory cytokines in human studies 37 . Further studies are required to elicit potential therapeutic benefits of DPP4 inhibitors in SARS‐CoV‐2 infection. In stable patients with satisfactory oral intake, clinicians might elect to continue DPP4 inhibitors.
Glucagon‐like peptide‐1 receptor agonists
There is emerging evidence of the potential anti‐inflammatory properties arising from glucagon‐like peptide‐1 (GLP‐1) receptor signaling 38 . GLP‐1 receptor agonist treatment in mice infected with respiratory syncytial virus is associated with a significant reduction in inflammatory cytokine production and attenuation of inflammation in the respiratory epithelium 39 . Furthermore, GLP‐1 receptor agonist therapy in the intensive care unit setting is associated with a reduction of hypoglycemia, glucose variability and catabolism by suppressing glucagon 40 , all of which can be protective in these critically ill patients. However, delayed gastric emptying, which is common in the critically ill, might affect the extent of the benefits of glycemic control. Its use is also relatively contraindicated in patients with renal impairment. Currently, there is insufficient evidence to support for or against the use of GLP‐1 receptor agonists in the context of the coronavirus infection.
Thiazolidinedione, sulphonylurea, meglitinide and sodium–glucose cotransporter 2 inhibitors
Studies have suggested increased ACE2 expression associated with thiazolidinedione use, raising concerns about possible increased susceptibility to SARS‐CoV‐2 infection 41 . However, in view of the adverse effects, such as fluid retention, which is commonly associated with thiazolidinedione, it should be discontinued in acutely ill patients. Similarly, sulphonylureas and sodium\x96 glucose cotransporter 2 inhibitors are generally unfavorable in the setting of acute illness. Sulphonylurea and meglitinide increase the risk of hypoglycemia in the presence of poor oral intake.
Sodium–glucose cotransporter 2 inhibitors are associated with increased risks of dehydration and euglycemic DKA, particularly in the setting of an acute illness.
Insulin
Insulin has been the treatment of choice for optimization of glycemic control in acutely ill patients. Several landmark studies have shown mortality and morbidity benefits associated with the use of intensive insulin therapy. Intravenous insulin can be administered as a continuous infusion that allows rapid titration. Furthermore, insulin has been shown to downregulate ACE2 receptors 42 , but more research is required to identify direct clinical benefits of insulin in the context of COVID‐19. In addition, observational studies have reported significantly higher insulin requirements among COVID‐19 patients 43 , supporting the postulation that β‐cell dysfunction might be induced by SARS‐CoV‐2.
The classes of antidiabetic medications, their effects in the context of COVID‐19 and the recommendations during acute illness are summarized in Table 1.
Table 1.
Summary of antidiabetic medications, effects on coronavirus disease 2019 and recommendations on their use during acute illness
| Antidiabetic medication | Effects on infection | Recommendations during acute illness |
|---|---|---|
| Metformin |
Reduces inflammatory cytokines May reduce viral replication |
Avoid in the setting of renal, hepatic failure or critically ill due to risk of lactic acidosis |
| DPP4‐inhibitor | May be associated with disease severity in MERS‐CoV, but effect on SARS‐CoV‐2 not defined | More data needed for the acutely ill patient. May consider continuing in patients who are well with satisfactory oral intake |
| GLP‐1 receptor agonist | Significant reduction in inflammatory responses in animal models | More data needed for the acutely ill patient. May consider continuing in patients who are well with satisfactory oral intake |
| Thiazolidinediones | May be associated with increased ACE2 expression, but clinical implication is unclear | Discontinue in acutely ill patients due to risk of fluid retention |
| Sulphonylurea/meglitinides | No apparent direct effect in SARS‐CoV‐2 | Discontinue in patients with poor oral intake due to hypoglycemia risk |
| SGLT‐2 inhibitors | No apparent direct effect in SARS‐CoV‐2 | Discontinue in acute illness due to risk of euglycemic DKA and further dehydration |
| Insulin | May downregulate ACE2 receptors | Treatment of choice in acutely ill patients to achieve glycemic targets with dose titration based on glucose levels |
ACE2, angiotensin‐converting enzyme 2; DKA, diabetic ketoacidosis; DPP4, dipeptidyl peptidase‐4; GLP‐1, glucagon‐like peptide‐1; MERS‐CoV, Middle East respiratory syndrome coronavirus; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus‐2; SGLT‐2, sodium–glucose cotransporter 2.
Practical considerations for inpatient glycemic control in patients with COVID‐19
Maintaining good glycemic control is important for these patients. In a retrospective study by Bode et al. 44 examining the outcomes of inpatient glycemic control of 1,122 patients with COVID‐19, uncontrolled hyperglycemia (defined as ≥2 episodes of blood glucose >10 mmol/L) was associated with a nearly fivefold increase in mortality and increased length of hospitalization. Zhu et al. 45 showed that inpatients whose blood glucose was maintained between 3.9 and 10 mmol/L had significantly lower rates of complications and all‐cause mortality. Frequent monitoring of blood glucose is essential with the aim of maintaining blood glucose levels within the recommended target of 4–10 mmol/L 43 . Furthermore, it is important to emphasize that inpatient diabetes management is highly dynamic. The titration of antidiabetic medications needs to be guided by ongoing glucose measurements and trends, illness severity, route of nutrition and concomitant medications that might affect glucose levels, such as glucocorticoids. The need for frequent inpatient blood glucose monitoring increases exposure of healthcare workers to SARS‐CoV‐2. Besides donning personal protective equipment and strict adherence to recommendations from the Centers for Disease Control and Prevention in preventing transmission of pathogens during glucose monitoring 46 , additional care needs to be taken to reduce the spread of COVID‐19 during capillary glucose monitoring, as viable SARS‐CoV‐2 might be present on contact surfaces. It is therefore important to clean and disinfect the glucometer after each and every use, and to use disposable safety lancets. Where possible, the responsibility for routine cleaning and disinfection should be assigned to appropriately‐trained personnel 47 .
If resources permit, the use of remote glucose monitoring might prove beneficial. Real‐time continuous glucose monitoring has been reported as a useful alternative for COVID‐19 patients with diabetes mellitus, allowing rapid titration of insulin doses, while minimizing the risk of staff exposure.
Management of diabetic emergencies in COVID‐19
Patients who are acutely ill are at risk of diabetic emergencies. In a study involving a cohort of 174 Chinese patients with COVID‐19, 37 had diabetes mellitus, of which two developed DKA 49 .
DKA occurs as a result of insulin deficiency and increased counterregulatory responses, which favor the production of ketones. The unique interactions between SARS‐CoV‐2 and the RAAS might provide yet another mechanism in the pathophysiology of DKA. First, as alluded to earlier, direct entry of SARS‐CoV‐2 into pancreatic islet cells might worsen β‐cell injury 25 . Second, downregulation of ACE2 after viral entry can lead to unopposed angiotensin II, which might impede insulin secretion 50 .
In addition, the relationship between SARS‐CoV‐2 and the RAAS can complicate DKA management. As angiotensin II increases pulmonary vascular permeability and worsens damage to lung parenchyma 16 , fluid replacement needs to be administered judiciously to avoid aggravating pulmonary injury. This also raises the importance of careful assessment of fluid status through objective hemodynamic parameters to determine the amount of fluid replacement.
Another important aspect in DKA management is that of monitoring and correcting electrolyte abnormalities. As angiotensin II stimulates aldosterone secretion and increases renal potassium loss, this can potentiate the risk of hypokalemia, which might necessitate additional potassium supplementation in order to continue intravenous insulin to suppress ketogenesis 28 .
Use of RAAS inhibitors in COVID‐19
Many patients with diabetes mellitus have other comorbidities and are taking RAAS inhibitors. The complex relationship between the RAAS and SARS‐CoV‐2 has led to controversies surrounding the use of these agents.
As ACE2 is the key receptor that facilitates entry of SARS‐CoV‐2, it is postulated that ACE inhibition could lead to a compensatory increase in the ACE2 expression with concerns that this might provide increased binding sites for viral entry into pneumocytes 16 .
However, after SARS‐CoV‐2 entry, the expression of ACE2 was found to be significantly downregulated, which could be associated with significant lung injury 20 . This can be attributable to the physiological action of ACE2, which catalyzes the breakdown of angiotensin II to angiotensin (1‐7), the latter having anti‐inflammatory and anti‐oxidant properties that protects the lungs against acute respiratory distress syndrome 51 . With regard to the effects of RAAS inhibitors on ACE2 expression in humans, studies have shown conflicting results. Although Ferrario et al. 52 previously reported that lisinopril and losartan are associated with a significant increase in ACE2 levels, others did not report an effect on ACE2 among patients treated with RAAS inhibitors 16 , 53 . At this point, there is insufficient evidence to conclude whether RAAS inhibition is beneficial or harmful in COVID‐19.
Despite these uncertainties, abrupt cessation of RAAS inhibitors might be associated with more harm. Many diabetes mellitus patients have concomitant cardiovascular diseases and are at risk of decompensation if these medications are stopped. At present, professional societies worldwide have therefore recommended continuation of RAAS inhibitors 54 .
Metabolic complications of treatments of COVID‐19
Moving forward, there are currently numerous trials underway in search of effective treatments for this infection. We aim to provide a summary of the current treatments, mechanisms of actions, and highlight some of these agents that are associated with the effects on glucose and/or lipid metabolism.
In brief, the SARS‐CoV‐2 replication cycle starts by gaining host entry through the S protein, facilitated by host transmembrane serine protease 2 20 . Viral polyproteins are synthesized by ribonucleic acid polymerase, followed by assembly of structural proteins and release of new viral particles. There are several drug targets that might interfere with the replication cycle of SARS‐CoV‐2, including chloroquine and hydroxychloroquine, which reduce viral entry; lopinavir–ritonavir, which inhibit proteolysis; and tocilizumab, which disrupts IL‐6 signaling. Other potential candidates include the transmembrane serine protease 2 inhibitor, camostat mesylate, which has been shown to prevent viral cell entry, as well as remdesivir, which inhibits ribonucleic acid polymerase 55 . Type 1 interferon might interfere with viral replication and minimize systemic inflammation 56 . As the use of these medications is likely to increase with the growing number of COVID‐19 cases, we highlight three agents with accompanying metabolic effects, which might be beneficial or detrimental by exacerbating the underlying metabolic comorbidities already established in some of these patients.
Chloroquine and hydroxychloroquine
These two agents inhibit SARS‐CoV‐2 entry, proteolytic processing and might also have immunomodulatory effects by reducing cytokine production 55 .
Hydroxychloroquine has been shown to improve insulin and glucose metabolism. Studies have shown a significant reduction in HbA1c and reduction in insulin doses 57 . Although the exact mechanisms remain to be elucidated, the improvements in glycemic control are likely associated with reduced insulin degradation 58 , increased insulin binding to its receptor with an increase in the half‐life of the insulin receptor complex 59 , 60 and increases insulin secretion 61 . Given the potential benefits of hydroxychloroquine/chloroquine on glucose metabolism, close glycemic monitoring in diabetes mellitus patients, timely reduction of the dosages of antidiabetic medications and insulin in patients who receive these treatments is essential to avoid hypoglycemia.
Although in vitro studies have shown antiviral activity of hydroxychloroquine/chloroquine against SARS‐CoV‐2 62 , 63 , observational studies did not show a significant reduction in the need for intubation or mortality 64 , 65 . Furthermore, there is concern about the cardiovascular safety associated with this class of medication 66 . Electrophysiological studies suggest that hydroxychloroquine/chloroquine use might interfere with cardiac channels, lead to prolongation of action potential and cause life‐threatening arrhythmias 67 . Thus, the efficacy and safety of hydroxychloroquine/chloroquine in the treatment of COVID‐19 are currently inconclusive and await further confirmation with randomized controlled trials.
Lopinavir–ritonavir
Lopinavir–ritonavir are protease inhibitors used in the treatment of human immunodeficiency virus. The mechanism of action is thought to inhibit 3‐chymotrypsin‐like protease in viral ribonucleic acid processing 55 .
Protease inhibitors have been shown to inhibit glucose uptake 68 . Euglycemic, hyperinsulinemia clamp studies showed a reduction in glucose disposal with lopinavir–ritonavir use 69 . The increase in peripheral insulin resistance might be secondary to dysregulation in insulin signaling, by causing inhibition of glucose uptake 70 and phosphorylation of the insulin receptor 71 .
With regard to lipid metabolism, among HIV patients taking lopinavir–ritonavir, triglyceride levels nearly doubled within 3 months of initiation 72 . Another study showed that hypertriglyceridemia can occur within 2 weeks of therapy 73 .
Protease inhibitors stimulate hepatic triglyceride synthesis 74 , and inhibit chylomicron uptake and triglyceride clearance 75 . As severe hypertriglyceridemia is a risk factor for acute pancreatitis, it is important to monitor the lipid levels of patients initiated on this treatment, especially for diabetes mellitus patients, who are at higher risk of developing severe hypertriglyceridemia.
The efficacy of lopinavir–ritonavir is currently inconclusive. Its use was previously reported to be associated with reduced mortality at 28 days, and shortened intensive care unit admissions and duration of viral shedding 76 . However, a more recent randomized controlled trial involving 199 patients with COVID‐19 infection treated with lopinavir–ritonavir did not show a mortality benefit 77 .
IL‐6 receptor antagonist
Tocilizumab is a biological agent that binds to the IL‐6 receptor, interferes with IL‐6 signaling and attenuates the “cytokine storm” in severe COVID‐19 infection 55 . More commonly used in rheumatic conditions, tocilizumab has been shown to be associated with contrasting effects on glucose metabolism in different tissues. IL‐6 has been shown to have an unfavorable effect on glucose metabolism by increasing hepatic insulin resistance 78 , 79 . The use of tocilizumab is associated with a small, but significant, improvement in HbA1c at 1 and 3 months of initiation of tocilizumab in patients with rheumatoid arthritis, reflecting improved insulin sensitivity from IL‐6 inhibition 80 . However, IL‐6 has a complex role in modulating insulin sensitivity, being both an enhancer and inhibitor of insulin action on different tissues, and having differential roles in regulating metabolism in individuals with diabetes, as compared with individuals with normal glucose tolerance. It has been postulated that the higher circulating levels of IL‐6 in patients with diabetes mellitus serves as a compensatory mechanism to promote glucose uptake in skeletal muscle, and thus, the use of IL‐6 inhibitors might adversely impact glucose homeostasis in skeletal muscles 81 . Nevertheless, the impact of short‐term use of IL‐6 receptor antagonist for treatment of COVID‐19 on glucose metabolism is currently unclear and needs to be corroborated by further research.
Type 1 interferon
Thyroid dysfunction is a common side‐effect of interferon therapy, and its prevalence has been reported to be up to 35% 82 . The development of thyroid dysfunction does not appear to be related to the dose of interferon therapy 83 . However, among those who develop thyroid dysfunction and with positive thyroid autoantibodies, 50% continue to carry the antibodies after interferon therapy is stopped, necessitating the need for long‐term follow up 84 .
Interferon‐induced type 1 diabetes mellitus has been reported, but its occurrence is rare. Most of these cases occur in patients who test positive for the glutamic acid decarboxylase antibody 85 , 86 . Checking for glutamic acid decarboxylase positivity might be worthwhile before initiation of interferon therapy.
Remdesivir
Remdesivir use is associated with clinical improvement in >50% of patients with COVID‐19 and shortens the time to recovery 87 , 88 . Remdesivir attenuates hepatic lipid deposition and insulin resistance in mice 89 . Paradoxically, hepatotoxicity is one of its major adverse effects in humans, and needs to be used with caution in patients with underlying liver disease or receiving statin therapy 87 .
The mechanism of action and metabolic effects of the medications used to treat COVID‐19 are summarized in Table 2.
Table 2.
Mechanism of action and metabolic effects of medications used to treat coronavirus disease 2019
| Name of medication | Mechanism of action | Metabolic effects |
|---|---|---|
| Chloroquine/hydroxychloroquine | Inhibit SARS‐CoV‐2 entry and viral replication |
Improves glycemic control and may even cause hypoglycemia May be associated with increased risk of arrhythmias |
| Lopinavir–ritonavir | Inhibit 3‐chymotrypsin‐like protease in viral RNA processing with antiviral activity against SARS‐CoV‐2 |
Increases triglyceride synthesis leading to hypertriglyceridemia Inhibits glucose uptake, which may result in hyperglycemia |
| IL‐6 receptor antagonist | Interferes with IL‐6 signaling and attenuates “cytokine storm” |
Improves hepatic insulin sensitivity May worsen skeletal muscle insulin resistance |
| Type 1 interferon |
Interferes with viral replication Minimizes inflammation |
Thyroid dysfunction Rarely associated with type 1 diabetes mellitus |
| Remdesivir | Inhibits viral RNA polymerase | May cause hepatotoxicity |
IL‐6, interleukin‐6; RNA, ribonucleic acid; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus‐2.
Prevention of COVID‐19 in diabetes mellitus patients
General recommendations
The prevention of COVID‐19 includes maintaining good hand hygiene, abiding by social distancing measures and avoiding close contact with people who are unwell. Diabetes mellitus patients should be encouraged to continue regular self‐monitoring of blood glucose, maintain healthy nutrition, keep physically active with home‐based exercises, and have an adequate supply of and access to diabetes mellitus medications and supplies. Diabetes mellitus patients should also be educated on sick day rules by their healthcare team.
The use of remote consultation has also enabled care to be delivered to diabetes mellitus patients while minimizing their exposure to SARS‐CoV‐2. The use of telemedicine has recently been shown to be effective in the management of even high‐risk diabetes mellitus patients, such as those with newly diagnosed type 1 diabetes mellitus 90 . Before the COVID‐19 pandemic, a Cochrane review by Flodgren et al. 91 showed that interactive telemedicine can effectively assist physicians in the management of diabetes mellitus. Patients allocated to telemedicine consultations had a lower HbA1c compared with usual care at a median of 9 months’ follow up. The COVID‐19 pandemic is expected to accelerate the transformation of healthcare delivery and increase the use of telemedicine in the management of chronic diseases.
Vaccinations in diabetes mellitus patients
Vaccinations have major public health benefits by providing direct protection to those who are vaccinated, as well as indirect protection to the unvaccinated, but susceptible, individuals 92 .
In patients with diabetes mellitus, their innate cellular immune response is decreased, which increases their risk of developing infections. Despite this, influenza vaccination is effective in diabetes patients and it is important to vaccinate this group of vulnerable individuals 93 . McElhaney et al. 94 showed that antibody titers did not differ between elderly patients with and without diabetes mellitus who were vaccinated against influenza. Long‐term antibody titers and antibody persistence were also similar in patients with and without diabetes mellitus for at least up to 6 months 95 . In clinical practice, Wang et al. 96 showed reductions in hospitalizations, respiratory failure and mortality among elderly patients with diabetes mellitus who were vaccinated against influenza. Similar results were also shown in a meta‐analysis involving 170,924 participants, although there were multiple confounders that weakened the evidence 97 . With regard to pneumococcal infections, vaccinations have also shown mortality benefit in bacteremic pneumococcal infection 98 .
The efficacy of vaccinations might be of concern among patients with type 1 diabetes mellitus, as it has been speculated that they might not be able to mount sufficient immunological response 99 . Nevertheless, the overall response to vaccination exceeds 70% among patients with diabetes mellitus, with type 2 diabetes mellitus patients showing similar immune responses to controls 98 . As of 30 May 2020, there were 10 candidate vaccines for COVID‐19 under investigation 100 .
Conclusions
With the exponential increase in the number of new COVID‐19 cases, it has been postulated that this pandemic might persist for the next few months and could even recur seasonally. The coexistence of two global pandemics – COVID‐19 and diabetes mellitus – has significant clinical implications, and impacts on morbidity and mortality. It is therefore crucial for clinicians caring for people with diabetes mellitus and COVID‐19 to be aware of the metabolic risk factors associated with disease severity, and keep abreast of the latest developments emerging on the metabolic interactions between antidiabetic agents, RAAS inhibitors and potential drug treatments for COVID‐19 (Figure 1). Last, but not least, we highlight the importance of timely vaccinations for individuals with diabetes mellitus, with a view of including the COVID‐19 vaccine, when available, so as to offer early protection against this life‐threatening infection. The evidence on COVID‐19 is evolving rapidly, and further metabolic interactions, both acute and long‐term outcomes, will surface with increasing data made available.
Disclosure
The authors declare no conflict of interest.
J Diabetes Investig 2020; 11: 1104–1114
References
- 1. Zhu N, Zhang D, Wang W, et al A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382: 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. COVID‐19 Dashboard by the Center for Systems Science and Engineering (CSSR) at Johns Hopkins University (JHU). https://coronavirus.jhu.edu/map.html. Accessed April 26, 2020.
- 3. International Diabetes Federation . IDF Diabetes Atlas,9th: edn. Belgium: Brussels, 2019. https://www.diabetesatlas.org/en/. Accessed April 26, 2020. [Google Scholar]
- 4. Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism 2019; 92: 6–10. [DOI] [PubMed] [Google Scholar]
- 5. Sattar Naveed, McInnes Iain B., McMurray John J.V. Obesity Is a Risk Factor for Severe COVID‐19 Infection. Circulation 2020; 142: 4–6. [DOI] [PubMed] [Google Scholar]
- 6. Critchley JA, Carey IM, Harris T, et al Glycemic control and risk of infections among people with type 1 or type 2 diabetes in a large primary care cohort study. Diabetes Care 2018; 41: 2127–2135. [DOI] [PubMed] [Google Scholar]
- 7. Akbar DH. Bacterial pneumonia: comparison between diabetics and non‐diabetics. Acta Diabetol 2001; 38: 77–82. [DOI] [PubMed] [Google Scholar]
- 8. Fadini GP, Morieri ML, Longato E, et al Prevalence and impact of diabetes among people infected with SARS‐CoV‐2. J Endocrinol Invest 2020; 43: 867–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li B, Yang J, Zhao F, et al Prevalence and impact of cardiovascular metabolic diseases on COVID‐19 in China. Clin Res Cardiol 2020; 109: 531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simonnet A, Chetboun M, Poissy J, et al High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus‐2 (SARS‐CoV‐2) Requiring Invasive Mechanical Ventilation. Obesity 2020;28: 1195–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ong SWX, Young BE, Leo YS, et al Association of higher body mass index (BMI) with severe coronavirus disease 2019 (COVID‐19) in younger patients. Clin Infect Dis 2020; ciaa548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cariou B, Hadjadj S, Wargny M, et al Phenotypic characteristics and prognosis of inpatients with COVID‐19 and diabetes: the CORONADO study. Diabetologia 2020; 63: 1500–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol Med Microbiol 1999; 26: 259–65. [DOI] [PubMed] [Google Scholar]
- 14. Tsalamandris S, Antonopoulos AS, Oikonomou E, et al The role of inflammation in diabetes: current concepts and future perspectives. Eur Cardiol 2019; 14: 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen X, Wenjia H, Ling J, et al Hypertension and diabetes delay the viral clearance in COVID‐19 patients. medRxiv 2020. 10.1101/2020.03.22.20040774. [DOI] [Google Scholar]
- 16. Vaduganathan M, Vardeny O, Michel T, et al Renin‐angiotensin‐aldosterone system inhibitors in patients with Covid‐19. N Engl J Med 2020; 382: 1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol 2002; 20: 197–216. [DOI] [PubMed] [Google Scholar]
- 18. Kreuzer D, Nikoopour E, Au BC, et al Reduced interferon‐α production by dendritic cells in type 1 diabetes does not impair immunity to influenza virus. Clin Exp Immunol 2015; 179: 245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Akirav EM, Henegariu O, Preston‐Hurlburt P, et al The receptor for advanced glycation end products (RAGE) affects T cell differentiation in OVA induced asthma. PLoS One 2014; 23: e95678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muniyappa R, Gubbi S. COVID‐19 pandemic, corona viruses, and diabetes mellitus. Am J Physiol Endocrinol Metab 2020; 318: E736–E741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pal R, Bhansali A. COVID‐19, diabetes mellitus and ACE2: the conundrum. Diabetes Res Clin Pract 2020; 29: 108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rodríguez‐Hernández H, Simental‐Mendía LE, Rodríguez‐Ramírez G, et al Obesity and inflammation: epidemiology, risk factors, and markers of inflammation. Int J Endocrinol 2013; 2013: 678159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Philips BJ, Meguer JX, Redman J, et al Factors determining the appearance of glucose in upper and lower respiratory tract secretions. Intensive Care Med 2003; 29: 2204–2210. [DOI] [PubMed] [Google Scholar]
- 24. Fernandez C, Rysä J, Almgren P, et al Plasma levels of the proprotein convertase furin and incidence of diabetes and mortality. J Intern Med 2018; 284: 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang JK, Lin SS, Ji XJ, et al Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol 2010; 47: 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang F, Wang H, Fan J, et al Pancreatic injury patterns in patients with coronavirus disease pneumonia. Gastroenterology 2020. 10.1053/j.gastro.2020.03.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Filippi CM, von Herrath MG. Viral trigger for type 1 diabetes: pros and cons. Diabetes 2008; 57: 2863–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chee YJ, Ng SJH, Yeoh E. Diabetic ketoacidosis precipitated by Covid‐19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract 2020; 24: 108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang JK, Feng Y, Yuan MY, et al Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med 2006; 23: 623–628. [DOI] [PubMed] [Google Scholar]
- 30. Kim J, Kwak HJ, Cha JY, et al Metformin suppresses lipopolysaccharide (LPS)‐induced inflammatory response in murine macrophages via activating transcription factor‐3 (ATF‐3) induction. J Biol Chem 2014; 289: 23246–23255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kajiwara C, Kusaka Y, Kimura S, et al Metformin mediates protection against legionella pneumonia through activation of AMPK and mitochondrial reactive oxygen species. J Immunol 2018; 200: 623–631. [DOI] [PubMed] [Google Scholar]
- 32. Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta‐analysis. JAMA 2007; 298: 194–206. [DOI] [PubMed] [Google Scholar]
- 33. Richter B, Bandeira‐Echtler E, Bergerhoff K, et al Dipeptidyl peptidase‐4 (DPP‐4) inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev 2008: CD006739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cai L, Cai Y, Lu ZJ, et al The efficacy and safety of vildagliptin in patients with type 2 diabetes: a meta‐analysis of randomized clinical trials. J Clin Pharm Ther 2012; 37: 386–398. [DOI] [PubMed] [Google Scholar]
- 35. Wvan der Zanden R, de Vries F, Lalmohamed A, et al Use of dipeptidyl‐peptidase‐4 inhibitors and the risk of pneumonia: a population‐based cohort study. PLoS One 2015; 10: e0139367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Song W, Wang Y, Wang N, et al Identification of residues on human receptor DPP4 critical for MERS‐CoV binding and entry. Virology 2014; 471–473: 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Poppel PC, Gresnigt MS, Smits P, et al The dipeptidyl peptidase‐4 inhibitor vildagliptin does not affect ex vivo cytokine response and lymphocyte function in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 2014; 103: 395–401. [DOI] [PubMed] [Google Scholar]
- 38. Lee YS, Jun HS. Anti‐inflammatory effects of GLP‐1‐based therapies beyond glucose control. Mediators Inflamm 2016; 2016: 3094642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bloodworth MH, Rusznak M, Pfister CC, et al Glucagon‐like peptide 1 receptor signaling attenuates respiratory syncytial virus‐induced type 2 responses and immunopathology. J Allergy Clin Immunol 2018; 142: 683–687.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mustafa OG, Whyte MB. The use of GLP‐1 receptor agonists in hospitalised patients: an untapped potential. Diabetes Metab Res Rev 2019; 35: e3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID‐19 infection? Lancet Respir Med 2020; 8: e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roca‐Ho H, Riera M, Palau V, et al Characterization of ACE and ACE2 expression within different organs of the NOD mouse. Int J Mol Sci 2017; 18: 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bornstein SR, Rubino F, Khunti K, et al Practical Recommendations for the management of diabetes in patients with COVID‐19. Lancet Diabetes Endocrinol 2020; 8: 546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bode B, Garett V, Messler J, et al Glycemic characteristics and clinical outcomes of COVID‐19 patients hospitalized in the United States. J Diabetes Sci Technol 2020; 14: 813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhu L, She Z, Cheng X, et al Association of blood glucose control and outcomes in patients with COVID‐19 and pre‐existing type 2 diabetes. Cell Metab 2020; 31: 1068–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Centers for Disease Control and Prevention . Infection Prevention during Blood Glucose Monitoring and Insulin Administration, 2011. https://www.cdc.gov/injectionsafety/blood‐glucose‐monitoring.html Accessed June 1, 2020
- 47. Geaghan SM. Infection transmission associated with point of care testing and the laboratory's role in risk reduction. EJIFCC 2014; 25: 188–194. [PMC free article] [PubMed] [Google Scholar]
- 48. Shehav‐Zaltzman G, Segal G, Konvalina N, et al Remote Glucose monitoring of hospitalized, quarantined patients with diabetes and COVID‐19. Diabetes Care 2020;43: e75–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guo W, Li M, Dong Y, et al Diabetes is a risk factor for the progression and prognosis of COVID‐19. Diabetes Metab Res Rev 2020; 31: e3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Carlsson PO, Berne C, Jansson L. Angiotensin II and the endocrine pancreas: effects on islet blood flow and insulin secretion in rats. Diabetologia 1998; 41: 127–133. [DOI] [PubMed] [Google Scholar]
- 51. Vrigkou E, Tsangaris I, Bonovas S, et al The evolving role of the renin‐angiotensin system in ARDS. Crit Care 2017; 21: 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ferrario CM, Jessup J, Chappell MC, et al Effect of angiotensin‐converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin‐converting enzyme 2. Circulation 2005; 111: 2605–2610. [DOI] [PubMed] [Google Scholar]
- 53. Campbell DJ, Zeitz CJ, Esler MD, et al Evidence against a major role for angiotensin converting enzyme‐related carboxypeptidase (ACE2) in angiotensin peptide metabolism in the human coronary circulation. J Hypertens 2004; 22: 1971–1976. [DOI] [PubMed] [Google Scholar]
- 54. De Simone G. Position statement of the ESC Council on Hypertension on ACE‐inhibitors and angiotensin receptor blockers, 2020. https://www.escardio.org/Councils/Council‐on‐Hypertension‐(CHT)/News/position‐statement‐of‐the‐esc‐council‐on‐hypertension‐on‐ace‐inhibitors‐and‐ang. Accessed 22 April, 2020.
- 55. Sanders JM, Monogue ML, Jodlowski TZ, et al Pharmacologic treatments for coronavirus disease 2019 (COVID‐19). JAMA 2020; 323:1824–1836. [DOI] [PubMed] [Google Scholar]
- 56. Prokunina‐Olsson L, Alphonse N, Dickenson RE, et al COVID‐19 and emerging viral infections: the case for interferon lambda. J Exp Med 2020; 217: e20200653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Quatraro A, Consoli G, Magno M, et al Hydroxychloroquine in decompensated, treatment‐refractory noninsulin‐dependent diabetes mellitus. A new job for an old drug? Ann Intern Med 1990; 112: 678–681. [DOI] [PubMed] [Google Scholar]
- 58. Blazar BR, Whitley CB, Kitabchi AE, et al In vivo chloroquine‐induced inhibition of insulin degradation in a diabetic patient with severe insulin resistance. Diabetes 1984; 33: 1133–1137. [DOI] [PubMed] [Google Scholar]
- 59. Bevan AP, Christensen JR, Tikerpae J, et al Chloroquine augments the binding of insulin to its receptor. Biochem J 1995; 311(Pt 3): 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Paul H. Managing uncontrolled type 2 diabetes: role of hydroxychloroquine in therapy as AD on antidiabetic agent: a case study. EC Endocrinology and Metabolic Research 2018. https://www.ecronicon.com/ecemr/pdf/ECEMR‐03‐00042.pdf. Accessed on 25 April, 2020.
- 61. Powrie JK, Smith GD, Shojaee‐Moradie F, et al Mode of action of chloroquine in patients with non‐insulin‐dependent diabetes mellitus. Am J Physiol 1991; 260(6 Pt 1): E897–E904. [DOI] [PubMed] [Google Scholar]
- 62. Wang M, Cao R, Zhang L, et al Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res 2020; 30: 269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yao X, Ye F, Zhang M, et al In Vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clin Infect Dis 2020. 10.1093/cid/ciaa237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mahévas M, Tran VT, Roumier M, et al Clinical efficacy of hydroxychloroquine in patients with covid‐19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020; 369: m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Geleris J, Sun Y, Platt J, et al Observational study of hydroxychloroquine in hospitalized patients with Covid‐19. N Engl J Med 2020; 382: 2411–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Oren O, Yang EH, Gluckman TJ, et al The use of chloroquine and hydroxychloroquine in COVID‐19 and cardiovascular implications: understanding safety discrepancies to improve interpretation and design of clinical trials. Circ Arrhythm Electrophysiol 2020; 13 10.1161/CIRCEP.120.008688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Roden DM, Harrington RA, Poppas A, et al Considerations for drug interactions on QTc in exploratory COVID‐19 (Coronavirus Disease 2019) treatment. Circulation 2020; 141: e906–e907. [DOI] [PubMed] [Google Scholar]
- 68. Koster JC, Remedi MS, Qiu H, et al HIV protease inhibitors acutely impair glucose‐stimulated insulin release. Diabetes 2003; 52: 1695–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lee GA, Lo JC, Aweeka F, et al Single‐dose lopinavir‐ritonavir acutely inhibits insulin‐mediated glucose disposal in healthy volunteers. Clin Infect Dis 2006; 43: 658–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vyas AK, Koster JC, Tzekov A, et al Effects of the HIV protease inhibitor ritonavir on GLUT4 knock‐out mice. J Biol Chem 2010; 285: 36395–36400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Djedaini M, Peraldi P, Drici MD, et al Lopinavir co‐induces insulin resistance and ER stress in human adipocytes. Biochem Biophys Res Commun 2009; 386: 96–100. [DOI] [PubMed] [Google Scholar]
- 72. Lee GA, Seneviratne T, Noor MA, et al The metabolic effects of lopinavir/ritonavir in HIV‐negative men. AIDS 2004; 18: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Purnell JQ, Zambon A, Knopp RH, et al Effect of ritonavir on lipids and post‐heparin lipase activities in normal subjects. AIDS 2000; 14: 51–57. [DOI] [PubMed] [Google Scholar]
- 74. Lenhard JM, Croom DK, Weiel JE, et al HIV protease inhibitors stimulate hepatic triglyceride synthesis. Arterioscler Thromb Vasc Biol 2000; 20: 2625–2629. [DOI] [PubMed] [Google Scholar]
- 75. Carr A, Samaras K, Chisholm DJ, et al Pathogenesis of HIV‐1‐protease inhibitor‐associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet 1998; 351: 1881–1883. [DOI] [PubMed] [Google Scholar]
- 76. Yan D, Liu X‐Y, Zhu Y‐N, et al Factors associated with prolonged viral shedding and impact of Lopinavir/Ritonavir treatment in patients with SARS‐CoV‐2 infection. medRxiv 2020. 10.1101/2020.03.22.20040832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kunz KM. A trial of lopinavir‐ritonavir in Covid‐19. N Engl J Med 2020; 382: e68. [DOI] [PubMed] [Google Scholar]
- 78. Schultz O, Oberhauser F, Saech J, et al Effects of inhibition of interleukin‐6 signalling on insulin sensitivity and lipoprotein (a) levels in human subjects with rheumatoid diseases. PLoS One 2010; 5: e14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Klover PJ, Clementi AH, Mooney RA. Interleukin‐6 depletion selectively improves hepatic insulin action in obesity. Endocrinology 2005; 146: 3417–3427. [DOI] [PubMed] [Google Scholar]
- 80. Otsuka Y, Kiyohara C, Kashiwado Y, et al Effects of tumor necrosis factor inhibitors and tocilizumab on the glycosylated hemoglobin levels in patients with rheumatoid arthritis; an observational study. PLoS One 2018; 13: e0196368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jiang LQ, Duque‐Guimaraes DE, Machado UF, et al Altered response of skeletal muscle to IL‐6 in type 2 diabetic patients. Diabetes 2013; 62: 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Koh LK, Greenspan FS, Yeo PP. Interferon‐alpha induced thyroid dysfunction: three clinical presentations and a review of the literature. Thyroid 1997; 7: 891–896. [DOI] [PubMed] [Google Scholar]
- 83. Dalgard O, Bjøro K, Hellum K, et al Thyroid dysfunction during treatment of chronic hepatitis C with interferon alpha: no association with either interferon dosage or efficacy of therapy. J Intern Med 2002; 251: 400–406. [DOI] [PubMed] [Google Scholar]
- 84. Carella C, Mazziotti G, Amato G, et al Clinical review 169: Interferon‐alpha‐related thyroid disease: pathophysiological, epidemiological, and clinical aspects. J Clin Endocrinol Metab 2004; 89: 3656–3661. [DOI] [PubMed] [Google Scholar]
- 85. Fabris P, Betterle C, Floreani A, et al Development of type 1 diabetes mellitus during interferon alfa therapy for chronic HCV hepatitis. Lancet 1992; 340: 548. [DOI] [PubMed] [Google Scholar]
- 86. Fabris P, Betterle C, Greggio NA, et al Insulin‐dependent diabetes mellitus during alpha‐interferon therapy for chronic viral hepatitis. J Hepatol 1998; 28: 514–517. [DOI] [PubMed] [Google Scholar]
- 87. Grein J, Ohmagari N, Shin D, et al Compassionate use of Remdesivir for patients with severe Covid‐19. N Engl J Med 2020; 382: 2327–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Beigel JH, Tomashek KM, Dodd LE, et al Remdesivir for the treatment of Covid‐19 – preliminary report 2020. N Engl J Med 2020. 10.1056/nejmoa2007764 [DOI] [PubMed] [Google Scholar]
- 89. Li YN, Su Y. Remdesivir attenuates high fat diet (HFD)‐induced NAFLD by regulating hepatocyte dyslipidemia and inflammation via the suppression of STING. Biochem Biophys Res Commun 2020; 526: 381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Garg SK, Rodbard D, Hirsch IB, et al Managing new‐onset type 1 diabetes during the COVID‐19 pandemic: challenges and opportunities. Diabetes Technol Ther 2020; 22: 431–439. [DOI] [PubMed] [Google Scholar]
- 91. Flodgren G, Rachas A, Farmer AJ, et al Interactive telemedicine: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2015; 9: CD002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Anderson EJ, Daugherty MA, Pickering LK, et al Protecting the community through child vaccination. Clin Infect Dis 2018; 67: 464–471. [DOI] [PubMed] [Google Scholar]
- 93. Goeijenbier M, van Sloten TT, Slobbe L, et al Benefits of flu vaccination for persons with diabetes mellitus: a review. Vaccine 2017; 12: 5095–5101. [DOI] [PubMed] [Google Scholar]
- 94. McElhaney JE, Garneau H, Camous X, et al Predictors of the antibody response to influenza vaccination in older adults with type 2 diabetes. BMJ Open Diabetes Res Care 2015; 3: e000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Seo YB, Baek JH, Lee J, et al Long‐term immunogenicity and safety of a conventional influenza vaccine in patients with type 2 diabetes. Clin Vaccine Immunol 2015; 22: 1160–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wang IK, Lin CL, Chang YC, et al Effectiveness of influenza vaccination in elderly diabetic patients: a retrospective cohort study. Vaccine 2013; 31: 718–724. [DOI] [PubMed] [Google Scholar]
- 97. Remschmidt C, Wichmann O, Harder T. Vaccines for the prevention of seasonal influenza in patients with diabetes: systematic review and meta‐analysis. BMC Med 2015; 13: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Smith SA, Poland GA. Use of influenza and pneumococcal vaccines in people with diabetes. Diabetes Care 2000; 23: 95–108. [DOI] [PubMed] [Google Scholar]
- 99. Ruben FL, Fireman P, LaPorte RE, et al Immune responses to killed influenza vaccine in patients with type 1 diabetes: altered responses associated with HLA‐DR 3 and DR 4. J Lab Clin Med 1988; 112: 595–602. [PubMed] [Google Scholar]
- 100. World Health Organization . Draft landscape of COVID‐19 candidate vaccines, 2020. https://www.who.int/who‐documents‐detail/draft‐landscape‐of‐covid‐19‐candidate‐vaccines Accessed May 31, 2020.


