Abstract
Forty-eight Quarter Horse geldings (3 to 8 yr of age) were used to determine the effects of dietary chromium (Cr), in the form of Cr propionate (Cr Prop) on insulin sensitivity. Horses were blocked by age, body condition score, and glucose response to concentrate feeding on day 0 and randomly assigned to treatments. Treatments consisted of 0, 2, 4, or 8 mg Cr/d from Cr Prop. Horses were fed daily a concentrate mix at a rate of 0.2 kg/100 kg body weight (BW) and grass hay at 1.75 to 2.0 kg/100 kg BW. All horses were fed the control diet for 7 d prior to the initiation of the study. After an overnight fast, blood samples from the jugular vein were obtained at 0, 2, and 4 h after concentrate feeding on days 0 and 28 for the determination of glucose, nonesterified fatty acids, and insulin. A glucose tolerance test (GTT) was conducted on day 42. Glucose was infused via jugular vein catheters, and blood samples were collected at various times relative to dosing for glucose and insulin determination. Plasma glucose on day 28 was affected (P < 0.05) by treatment, time, and treatment × time. Horses fed 4 mg Cr/d had lesser (P < 0.05) plasma glucose concentrations than those in the other treatments at 0 h. At 2 h post-feeding glucose concentrations were greater (P < 0.05) in horses fed 0 or 8 mg Cr/d than in those given 4 mg Cr. Horses fed 2 mg Cr/d had lesser (P < 0.05) plasma glucose at 4 h post feeding compared with those fed 0 or 8 mg Cr. Plasma glucose did not differ among horses receiving 2 or 4 mg Cr/d at 2 or 4 h. Serum insulin was affected (P < 0.05) by treatment, time, and treatment × time. Insulin concentrations were greater (P < 0.05) in horses fed 0 or 2 mg Cr/d than in those given 4 or 8 mg Cr at 0 h. At 4 h post-feeding insulin concentrations were greater (P < 0.05) in horses given 0 or 8 mg Cr than in those fed 2 or 4 mg Cr/d. Plasma glucose was affected (P < 0.05) by treatment and time, but not by treatment × time following the GTT. Mean plasma glucose (across sampling times) concentrations were greater (P < 0.05) in controls than in horses fed 2 or 4 mg Cr/d. Glucose concentrations following the GTT did not differ among controls and horses given 8 mg Cr/d. Following glucose infusion, serum insulin concentrations were greater (P < 0.05) in horses fed 2 or 4 mg Cr and tended to be greater in those fed 8 mg Cr/d compared with controls. The results of this study indicate that 2 or 4 mg Cr/d from Cr Prop increased insulin sensitivity in adult horses following oral carbohydrate consumption.
Keywords: chromium propionate, glucose, horses, insulin
Introduction
Chromium (Cr) functions to potentiate the action of insulin in insulin-sensitive tissues. Although the mode of action whereby Cr enhances insulin responsiveness has not been clearly defined, a number of different mechanisms have been proposed (Hua et al., 2012; Hoffman et al., 2014). Considerable research with Cr in human nutrition resulted in the Institute of Medicine establishing an adequate intake of Cr for humans in 2001 (National Academies, 2001).
Aging and obesity have been associated with insulin resistance in horses (Powell et al., 2002; Nielsen et al., 2010). However, controlled studies evaluating the effect of dietary Cr on insulin sensitivity in horses are limited. Yearling horses supplemented with 0.42 mg Cr (from Cr picolinate)/kg concentrate had greater glucose clearance rates following an intravenous (IV) glucose tolerance test (GTT) than controls (Ott and Kivipelto, 1999). Thoroughbred horses supplemented with 5 mg Cr/d, from Cr yeast, tended to have lower plasma glucose and insulin concentrations at 1 h after concentrate feeding than controls (Pagan et al., 1995). Chromium propionate (Cr Prop) has been shown to enhance insulin sensitivity in growing cattle (Spears et al., 2012) and broilers (Brooks et al., 2016). The present study was conducted to determine the effects of dietary Cr, as Cr Prop, on the measures of insulin sensitivity in adult horses. This work was conducted as part of the utility data support for the use of Cr Prop in horses via a food additive petition filed with the U.S. Food and Drug Administration Center for Veterinary Medicine.
Materials and Methods
Forty-eight healthy Quarter Horse geldings (3 to 8 yr of age) were used in this study. Prior to the initiation of the study, all animal care, handling, and procedures described herein were approved by North Carolina State University Animal Care and Use Committee. The study was conducted as four trials, due to a limitation of individual stalls at the testing facility. Horses were procured on four separate occasions from a single horse farm in Vale, NC. Horses were evaluated by a veterinarian on arrival for soundness and other health issues and given a Coggins Test. All horses tested negative for Coggins. Horses were vaccinated for Rabies, Tetanus Toxoid, Eastern and Western Encephalitis, and West Nile Virus and dewormed prior to the initiation of the study. After arrival, horses were housed in a dry lot pen and fed hay and limited oats for 14 to 28 d. Horses were then moved to individual stalls and fed the control diet for 7 d prior to the initiation of the study. Horses were weighed and assigned a body condition score prior to the initiation of the study. On day 0, blood samples were collected from horses at 0, 2, and 4 h following concentrate feeding and analyzed the same day for plasma glucose concentrations. In each trial, 12 horses were blocked by age and body condition score and randomly assigned within a block to treatments. Glucose responses to concentrate feeding on day 0 were also considered and some horses were moved within their block to a different treatment in an attempt to balance treatments for glucose response to feeding. Treatments consisted of 0, 2, 4, or 8 mg of supplemental Cr per head per day from Cr Prop (KemTRACE Chromium; Kemin Agrifoods North America, Des Moines, IA).
Horses were housed and fed individually in pens measuring 3.7 m × 12.2 m. Pens were cleaned daily to remove manure and wasted hay. Horses were observed twice daily for any health-related issues.
Ingredient composition of the concentrate mix is shown in Table 1 and the analyzed composition of concentrate mixes are presented in Table 2 for each trial. The concentrate mix was fed at the rate of 0.2 kg per 100 kg of body weight (BW) per day. Horses were also offered 1.75 to 2.0 kg of grass hay per 100 kg of BW per day. Analyzed compositions of hays fed during the trials are shown in Table 3. Hays fed during the studies were predominately orchardgrass or timothy. Body weights and body condition scores were obtained at 14-d intervals, and hay allotments were adjusted, if necessary, to maintain optimum body condition. If a horse did not consume all hay offered at a feeding, then the amount of hay offered at the next feeding was reduced to minimize orts. The complete ration (hay plus concentrate) was formulated to meet or exceed all nutrient requirements for adult horses, with a mature BW of 500 kg (NRC, 2007). In addition to the concentrate mix and hay, each horse was fed 50 g of ground corn per day that provided 0, 2, 4, or 8 mg Cr from Cr Prop. Analyzed Cr concentrations in the Cr–corn mixes are presented in Table 4. The corn supplement containing the Cr treatments was top-dressed on the morning feeding of concentrate. All horses consumed the entirety of their concentrate mix and corn supplement throughout the study. Hay orts were weighed and recorded as necessary. Samples of hay, concentrate mix, and corn supplements were collected weekly and composited within a trial for the analysis of Cr and other nutrients. Water samples were also collected in each trial for Cr analysis. In trial 1, water samples collected analyzed 0.143 µg Cr/L. Water samples collected in trials 2, 3, and 4 analyzed below our limit of detection (0.100 µg Cr/L).
Table 1.
Ingredient composition of the concentrate mix
| Ingredient | % as fed |
|---|---|
| Oats | 92.40 |
| Molasses | 4.00 |
| Salt | 3.00 |
| Trace mineral mix1 | 0.50 |
| Vitamin mix2 | 0.25 |
1Supplied per kg of concentrate mix: 400 mg Zn (as zinc sulfate); 400 mg Mn (as manganese sulfate); 100 mg Cu (as basic copper chloride); 10 mg Se (as sodium selenite); 0.5 mg I (as calcium iodate); and 0.5 mg Co (as cobalt carbonate).
2Supplied per kg of concentrate mix: vitamin A 16,600 IU; vitamin D3 3,650 IU; vitamin E 555 IU; thiamine, 36.2 mg; and riboflavin, 22.2 mg.
Table 2.
Chemical composition of concentrate mixes
| Trial 1 | Trial 2 | Trial 3 | Trial 4 | |
|---|---|---|---|---|
| DM, % | 89.9 | 90.8 | 92.4 | 91.5 |
| CP, % DM | 10.9 | 10.3 | 11.2 | 13.5 |
| ADF, % DM | 14.4 | 16.0 | 11.7 | 13.6 |
| NDF, % DM | 27.0 | 30.5 | 22.0 | 27.0 |
| Calcium, % DM | 0.18 | 0.11 | 0.15 | 0.13 |
| Phosphorus, % DM | 0.42 | 0.38 | 0.37 | 0.42 |
| Magnesium, % DM | 0.14 | 0.13 | 0.13 | 0.15 |
| Potassium, % DM | 0.52 | 0.52 | 0.47 | 0.64 |
| Sodium, % DM | 0.59 | 0.75 | 0.71 | 0.66 |
| Iron, mg/kg DM | 80 | 79 | 81 | 126 |
| Zinc, mg/kg DM | 242 | 420 | 350 | 421 |
| Copper, mg/kg DM | 106 | 105 | 93 | 88 |
| Manganese, mg/kg | 193 | 242 | 245 | 245 |
| Cr, mg/kg | 0.322 | 0.287 | 0.248 | 0.345 |
Table 3.
Chemical composition of hays fed
| Trial 11 | Trial 21 | Trial 31 | Trial 41 | |
|---|---|---|---|---|
| DM, % | 90.5 | 90.5 | 91.0 | 91.5 |
| CP, % DM | 12.8 | 12.9 | 15.9 | 10.1 |
| ADF, % DM | 39.4 | 37.8 | 38.0 | 49.1 |
| NDF, % DM | 58.2 | 58.5 | 64.9 | 64.9 |
| Calcium, % DM | 0.59 | 0.56 | 0.49 | 0.58 |
| Phosphorus, % DM | 0.30 | 0.27 | 0.36 | 0.15 |
| Magnesium, % DM | 0.25 | 0.25 | 0.25 | 0.14 |
| Potassium, % DM | 1.75 | 1.72 | 2.45 | 1.50 |
| Sodium, % DM | 0.04 | 0.02 | 0.04 | 0.01 |
| Iron, mg/kg DM | 99 | 116 | 191 | 138 |
| Zinc, mg/kg DM | 22 | 22 | 24 | 14 |
| Copper, mg/kg DM | 8 | 8 | 8 | 7 |
| Manganese, mg/kg | 79 | 76 | 72 | 29 |
| Cr, mg/kg | 0.117 | 0.161 | 0.208 | 0.164 |
1Average of a.m. and p.m. hay composite.
Table 4.
Analyzed Cr concentrations in Cr–corn mixes
| Trial 1 | Trial 2 | Trial 3 | Trial 4 | |
|---|---|---|---|---|
| ---------------- Cr, mg/kg DM -------------- | ||||
| Control | 0.101 | 0.096 | 0.084 | 0.103 |
| 2 mg Cr/d | 48.3 | 52.4 | 56.1 | 56.9 |
| 4 mg Cr/d | 94.8 | 104.3 | 92.2 | 103.5 |
| 8 mg Cr/d | 174.5 | 198.1 | 172.8 | 162.4 |
Post-feeding sampling
Horses were fasted overnight, and blood samples from the jugular vein were obtained at 0, 2, and 4 h after feeding the concentrate mix on day 0 for the determination of plasma glucose, serum nonesterified fatty acids (NEFA), and serum insulin. On day 28 of each trial, horses were again fasted overnight, and jugular blood samples were taken at 0, 2, and 4 h post feeding for plasma glucose, serum NEFA, and serum insulin determination. On blood sampling days (0 and 28 d), the entire daily allowance (0.2 kg per 100 kg BW) of concentrate mix was given following the 0 h sampling. Blood for plasma glucose determination was collected in evacuated tubes containing sodium fluoride and potassium oxalate and kept on ice until centrifuged. Samples for serum insulin and NEFA were collected in evacuated tubes with no additive and kept on ice until centrifuged. All blood samples were centrifuged at 1,200 × g for 20 min at 10 °C. Plasma samples from day 0 were analyzed for glucose the same day as collected. Serum samples from both collection days and plasma obtained after centrifugation on day 28 were frozen at −20 °C until analyzed.
Glucose tolerance test
A GTT was conducted on day 42 of each trial. On day 42, horses did not receive their morning concentrate mix but were fed limited hay and their 50 g corn–Cr supplement. An indwelling catheter was inserted in the jugular vein on the morning of glucose administration. Horses were then returned to their pen for 1 to 2 h prior to glucose infusion. Each horse was infused via the catheter with a 50% dextrose solution to provide 0.20 g of glucose per kg of BW in trial 1. In subsequent trials (2, 3, and 4), the dose of glucose infused was reduced to 0.10 g/kg BW. Following glucose infusion, the catheter was immediately flushed with saline. Glucose was infused over a course of 1.5 to 2.5 min. Blood samples were collected at −10, 0, 5, 10, 15, 20, 30, 45, 60, 90, 120, 150, and 180 min relative to glucose dosing. Following the blood collection, one aliquot of blood was placed in an evacuated tube containing sodium fluoride and potassium oxalate for plasma glucose analysis while a second aliquot was placed in an evacuated tube with no additive for serum insulin determination. Blood was kept on ice until being centrifuged on the day of collection at 1,200 × g. Samples were then stored at −20 °C until analyzed.
Glucose clearance rate and half-life following the GTT were calculated from 15- to 45-min following glucose infusion as described by Kaneko (1989). Areas under the curves (AUC) for glucose and insulin relative to basal concentrations were calculated using trapezoidal geometry.
Laboratory analysis
Hays and concentrate mixes fed in each trial were analyzed for chemical components at a commercial laboratory (Dairy One Cooperative Inc., Ithaca, NY). Corn–Cr supplements, concentrate mixes, and hays were prepared for Cr analysis by wet ashing with trace mineral grade nitric acid (Trace Metal Grade, Fisher Scientific, Raleigh, NC) using a microwave digestion procedure. Chromium was measured by electrothermal atomic absorption spectrophotometry as described previously (Lloyd et al., 2010). Bovine muscle obtained from the National Institute of Standards and Technology and certified to contain 71 ± 38 ng Cr/g was used as a reference standard.
Plasma glucose was measured using the glucose hexokinase assay kit from Sigma-Aldrich (kit no. GAHK-20; St. Louis, MO). Serum NEFA concentrations were determined enzymatically using the NEFA-HR(2) kit (FujiFilm Wako Diagnostics, Richmond, VA). Serum insulin concentrations were determined using a radioimmunoassay kit (kit no. HI-14K; EMD Millipore Corporation, Billerica, MA). The inter- and intra-assay coefficient of variation for glucose, insulin, and NEFA were 4.9% and 2.5%, 7.5% and 5.8%, and 7.2% and 4.1%, respectively.
Statistical analysis
All data were analyzed using the MIXED procedure of SAS Institute (2008) for a randomized complete block design. Glucose, insulin, and NEFA concentrations after feeding on day 0 were analyzed by repeated measures with the model, including treatment, trial, block, time, and all two and three-way interactions. The model for glucose and insulin AUC on day 0 included treatment, trial, block, treatment × trial, treatment × block, and trial × block. The statistical models for glucose, insulin, and NEFA concentrations after the feeding of day 28 were the same as those above except that day 0 values were used as a covariant if found to be significant. For repeated measures, day 0 values at each sampling time were used as a covariant for the same sampling times on day 28. Day 0 AUC were used as a covariant for glucose and insulin AUC on d 28. When the overall effect of treatment was significant, differences among treatments were determined using the PDiff option of SAS.
The average of the -10 and 0 minute time samples were used as the basal or 0 time value in the statistical analysis of the GTT data. The model for glucose AUC, half-life, and clearance rate, and basal glucose for the GTT test included treatment, trial, block, treatment × trial, treatment × block, and trial × block. The model for glucose and insulin repeated measures included treatment, trial, block, time, and all two and three-way interactions. Day 0 glucose or insulin values were not used as a covariant for the GTT data because glucose was given by a different route (IV vs. orally).
Results and Discussion
Initial and final BW and BW gain during the 42-d study were not affected by treatment (Table 5). In previous studies, Cr supplementation has not affected BW gain in yearlings (Ott and Kivipelto, 1999) or adult horses (Uyanik et al., 2008). Feed intake and body condition scores were also similar across treatments (Table 5). Based on feed intake over the entire study, the 2, 4, and 8 mg Cr/d treatments would have supplied 0.18, 0.36, and 0.74 mg Cr/kg of diet, respectively.
Table 5.
Effects of dietary Cr on horse body weights, body condition scores, and feed intake
| Supplemental Cr, mg/d | |||||
|---|---|---|---|---|---|
| 0 | 2 | 4 | 8 | SE | |
| BW, kg | |||||
| Initial | 458.8 | 455.6 | 473.6 | 458.6 | 14.3 |
| Final | 479.0 | 476.5 | 489.4 | 475.4 | 14.7 |
| BW gain, kg | 20.3 | 16.0 | 15.9 | 16.8 | 2.3 |
| Body condition score1 | |||||
| Initial | 4.08 | 4.21 | 4.29 | 4.54 | 0.18 |
| Final | 4.67 | 4.76 | 4.68 | 4.75 | 0.18 |
| Feed intake, kg/d | |||||
| Hay | 9.90 | 10.07 | 9.98 | 9.87 | 0.33 |
| Concentrate mix | 0.97 | 0.98 | 1.00 | 0.98 | 0.003 |
| Total intake | 10.87 | 11.05 | 10.98 | 10.85 | 0.03 |
11 = poor; 2 = very thin; 3 = thin; 4 = moderately thin; 5 = moderate; 6 = moderately fleshy; 7 = fleshy; 8 = fat.
Post-feeding blood metabolites
Three horses were being treated on or around day 28 in trial 3, which may have affected their blood metabolite data after feeding. One horse in the 2 mg Cr treatment was being treated with phenylbutazone for an infection in his right front foot. A control horse had a laceration in his upper eyelid that required four stitches and was being treated with an antibiotic ointment and Banamine. One horse receiving 8 mg Cr/d was treated with oral antibiotics and Banamine for swelling in his lymph node area. These three horses were not included in the statistical analysis of blood metabolite data, because their illness or drug treatment may have affected their glucose and insulin response to concentrate feeding.
In trial 3, the hay was inadvertently not removed from horses the night before the day 28 blood sampling. Therefore, the horses were probably not in a truly fasted state prior to concentrate feeding. This resulted in plasma NEFA concentrations being lower prior to feeding in trial 3 (X = 33.1 µEq/L) compared with the other trials (X = 112, 64, and 113 µEq/L for trials 1, 2, and 4, respectively). In horses fed only mixed grass hay, withholding hay resulted in much greater plasma NEFA concentrations than those observed in horses that continued to receive hay (Frank et al., 2002). Plasma NEFA data statistically analyzed without trial 3 were included in the analysis. Failure to remove hay did not appear to affect glucose or insulin concentrations at 0 h prior to feeding in trial 3. Bertin et al. (2016) reported that fasting did not affect baseline glucose or insulin concentrations in horses fed hay. Data from trial 3 were included in the statistical analysis of glucose and insulin responses to concentrate feeding on day 28.
Plasma glucose concentrations on day 0 were not affected by treatment. The day 0 glucose covariant was highly significant (P < 0.01) for basal glucose, glucose repeated measures, and glucose AUC on day 28. Plasma glucose on day 28 was not affected (P > 0.10) by a treatment × trial or treatment × block interaction. Basal glucose concentrations were affected by treatment (P = 0.02) and trial (P = 0.01; Tables 6). Horses receiving 4 mg Cr/d had lesser (P < 0.05) basal glucose concentrations than those fed 0, 2, or 8 mg Cr/d. Plasma glucose on day 28 was affected by treatment (P = 0.01), time (P < 0.01), and by a treatment × time interaction (P = 0.05) when analyzed by repeated measures (Table 6). Mean plasma glucose concentrations (across times) in control horses were greater (P = 0.01) than in those fed 4 mg Cr/d and tended (P = 0.12) to be greater than in horses given 2 mg Cr/d. Horses receiving 4 mg Cr/d had lesser (P < 0.05) plasma glucose concentrations than those in the other treatment groups at 0 h. At 2 h post feeding, plasma glucose concentrations were greater (P < 0.05) in horses fed 0 or 8 mg Cr/d than in horses fed 4 mg Cr/d. Horses fed 2 mg Cr/d had lesser (P < 0.05) plasma glucose concentrations at 4 h post feeding compared with those fed 0 or 8 mg Cr/d. Plasma glucose concentrations did not differ among horses receiving 2 or 4 mg Cr/d at 2 or 4 h post feeding. Glucose AUC was also affected by treatment (P = 0.02) with controls having a greater (P = 0.02) AUC than horses fed 4 mg Cr/d. Horses receiving 2 or 4 mg Cr/d also had a greater (P < 0.05) glucose AUC than those fed 8 mg Cr/d.
Table 6.
Effects of dietary Cr on plasma glucose concentrations and AUC following concentrate feeding on day 28
| Supplemental Cr, mg/d | P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 8 | SE | Day 0 glucose | Trt | Time | Trt × time | |
| Glucose | |||||||||
| Basal concentration, mg/dL | 81.4a | 82.1a | 77.5b | 81.3a | 1.1 | 0.001 | 0.02 | ||
| AUC, mg/dL * min | 23,181a,c | 22,183a,b | 21,425b | 23,769c | 524 | 0.001 | 0.02 | ||
| Repeated measures, mg/dL | |||||||||
| Mean | 93.3a,c | 89.3a | 86.9b | 94.8c | 1.8 | 0.001 | 0.01 | 0.001 | 0.05 |
| 0 h | 88.0a | 88.2a | 83.6b | 87.4a | 1.6 | ||||
| 2 h | 100.2a,c | 95.0a,b | 90.7b | 104.3c | 3.3 | ||||
| 4 h | 91.7a,c | 84.6b | 86.4a,b,c | 92.7c | 2.4 | ||||
a–cMeans in a row not bearing a common superscript letter differ (P < 0.05).
Table 7.
Effects of dietary Cr on serum insulin concentrations and AUC following concentrate feeding on day 28
| Supplemental Cr, mg/d | P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 8 | SE | Day 0 insulin | Trt | Time | Trt × time | |
| Insulin | |||||||||
| Basal concentration, µU/mL | 7.1a | 6.6a | 5.4b | 5.3b | 0.4 | 0.001 | 0.01 | ||
| AUC, µU/mL * min | 4,564a,b | 4,022a,b | 3,848a | 4,679b | 292 | 0.006 | 0.14 | ||
| Repeated measures, µU/mL | |||||||||
| Mean | 16.7a,b | 14.6a | 14.2a | 17.6b | 1.0 | 0.004 | 0.05 | 0.001 | 0.03 |
| 0 h | 10.8a | 10.6a | 9.2b | 9.2b | 1.3 | ||||
| 2 h | 21.3 | 20.0 | 19.4 | 24.5 | 2.2 | ||||
| 4 h | 18.0a | 13.1b | 13.8b | 19.0a | 1.5 |
a,bMeans in a row not bearing a common superscript letter differ (P < 0.05).
Serum insulin on day 0 was not affected by treatment. The day 0 covariant was significant for insulin repeated measures (P = 0.004), basal insulin (P = 0.001), and insulin AUC (P = 0.006). Serum insulin concentrations on day 28 were not affected (P > 0.10) by a treatment × trial or a treatment × block interaction. Basal insulin concentrations on day 28 were affected by treatment (P = 0.01) with control horses having greater (P < 0.05) basal serum insulin concentrations than horses receiving 4 or 8 mg Cr/d but not those fed 2 mg Cr/d. When analyzed by repeated measures, serum insulin was affected by treatment (P = 0.05), time (P = 0.001), and treatment × time (P = 0.03). Prior to feeding concentrate (0 h), serum insulin concentrations were greater (P < 0.05) in horses fed 0 or 2 mg Cr/d compared with those receiving 4 or 8 mg Cr/d. Serum insulin concentrations did not differ among treatments at 2 h post feeding. However, at 4 h post feeding, insulin concentrations were greater (P < 0.05) in horses given 0 or 8 mg Cr/d than in horses fed 2 or 4 mg Cr/d. Insulin AUC was not affected (P = 0.14) by treatment.
The tendency for lower serum insulin coupled with lower plasma glucose concentrations after concentrate feeding in horses given 2 or 4 mg Cr/d is consistent with increased insulin sensitivity. Thoroughbred horses supplemented with 5 mg Cr/d, from Cr yeast, tended to have lower plasma glucose (P = 0.18) and insulin (P < 0.10) concentrations at 1 h after feeding 1.81 kg of concentrate (Pagan et al., 1995). Chromium supplementation (0.30 mg/kg diet) also reduced serum glucose and insulin concentrations at 2 h post feeding in pigs (Evock-Clover et al., 1993).
Mean basal glucose and insulin concentrations prior to feeding were in the normal range (Ralston, 2002) for all treatment groups, suggesting that none of the horses were insulin-resistant. Glucose and insulin responses to concentrate feeding in the present study were similar to those reported previously (Jose-Cunilleras et al., 2004) in adult horses fed 0.20 kg/100 kg BW of steamed oat groats. Horses used in the present study were considered to be mature adults (3 to 8 yr of age), healthy, and nonobese. However, considerable variation in glucose and insulin responses to concentrate feeding were observed among horses on day 0 and within a treatment group on day 28. A number of factors may account for the variation in glucose and insulin responses to concentrate feeding among horses, including differences in 1) rate of concentrate consumption, 2) rate and extent of glucose absorption, and 3) insulin sensitivity. The day 0 covariate for both glucose and insulin were highly significant when used in the day 28 analysis of variance for glucose and insulin. This indicates that using the day 0 covariate for the 28-d analysis corrected for some of the initial variations observed among horses.
In evaluating the plasma NEFA data, it was noted that one horse in trial 1 receiving 8 mg Cr/d was an outlier at the 0 h bleed on both days 0 and 28. This horse had plasma NEFA concentrations at 0 h of 525.5 and 320.6 µEq/L on days 0 and 28, respectively. An outlier test confirmed that this horse was indeed an outlier. This horse was observed to be nervous every time when being bled. This likely resulted in the release of hormones (epinephrine) that cause a rapid release of fatty acids into the bloodstream. Removing the outlier did not greatly affect the overall treatment P-values but reduced standard errors and P-values for individual comparisons to the 8 mg Cr/d treatment. Plasma NEFA concentrations on day 0 were not affected by treatment (P = 0.40). The day 0 covariant for plasma NEFA was not significant and, therefore, was not included in the day 28 analysis of variance. Plasma NEFA concentrations were not affected (P > 0.10) by a treatment × trial or a treatment × block interaction. Plasma NEFA concentrations on day 28 were affected by treatment (P = 0.01), time (P = 0.001), and a treatment × time interaction (P = 0.02; Table 8). Prior to feeding (0 h), horses fed 2 mg Cr/d had lesser (P < 0.05) and those fed 4 mg Cr/d tended (P = 0.08) to have lower plasma NEFA concentrations than controls. Horses receiving 8 mg Cr/d had greater (P < 0.05) NEFA concentrations at 0 h than those fed 2 or 4 mg Cr/d. At 2 h post feeding, NEFA concentrations were lesser (P < 0.05) in horses fed 2 mg Cr/d compared with the other treatment groups. Treatment did not affect NEFA concentrations at 4 h post feeding. Mares supplemented with 5 mg Cr/d (from Cr picolinate) had lower NEFA concentrations around feeding than control mares (Gentry et al., 1999). As expected, plasma NEFA concentrations were greater prior to feeding compared with 2 and 4 h post feeding. Area under the plasma NEFA response curve calculated from 0 to 4 h post feeding was greater (P < 0.05) for controls and horses receiving 8 mg Cr/d compared with those fed 2 mg Cr/d. Plasma NEFA AUC for horses supplemented with 4 mg Cr/d did not differ from the other treatment groups.
Table 8.
Effects of dietary Cr on plasma NEFA concentrations and AUC following concentrate feeding on day 28
| Supplemental Cr, mg/d | P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 8 | SE | Day 0 NEFA | Trt | Time | Trt × time | |
| NEFA | |||||||||
| Basal concentration, µEq/L | 104.7a,c | 64.4b | 79.4a,b | 113.0a | 11.1 | 0.99 | 0.02 | ||
| AUC, µEq/L * min | 11,809a,c | 8,423b | 10,243a,b | 12,576c | 761 | 0.90 | 0.01 | ||
| Repeated measures, µEq/L | |||||||||
| Mean | 55.4a,c | 38.5b | 46.6a,b | 59.4c | 3.9 | 0.90 | 0.01 | 0.001 | 0.02 |
| 0 h | 104.7a,c | 64.4b | 79.4a,b | 118.1c | 10.2 | ||||
| 2 h | 30.7a | 24.7b | 31.0a | 31.4a | 1.8 | ||||
| 4 h | 30.6 | 26.5 | 29.4 | 28.7 | 2.0 | ||||
a–cMeans in a row not bearing a common superscript letter differ (P < 0.05).
Glucose tolerance test
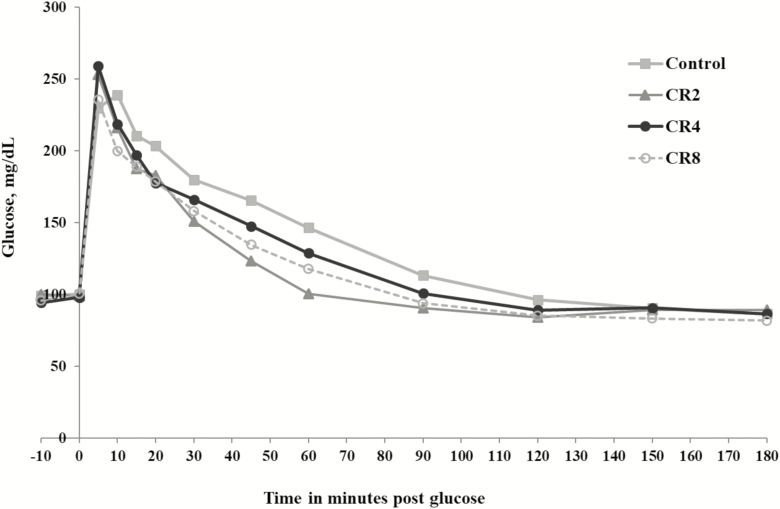
In trial 1, dextrose solution was infused to provide 0.20 g of glucose per kg BW. This dose of glucose resulted in plasma glucose concentrations peaking at approximately 250 mg/dL and staying above 200 mg/dL for at least 20 min in some groups (Figure 1). When plasma glucose exceeds a certain concentration, glucose spillage in urine occurs. This can result in major errors in the measurement of glucose kinetics following IV glucose administration. The renal threshold for glucose in horses has been estimated at 160 to 180 mg/dL (Toth et al., 2009). The amount of glucose infused in trial 1 was below the glucose dose (0.30 g glucose/kg BW) frequently used in previous studies (Hoffman et al., 2003; Toth et al., 2009) to measure glucose tolerance in horses. However, Toth et al. (2009) showed that a considerable loss of glucose in the urine occurs when horses are given 0.30 g glucose/kg BW. Urinary loss of glucose in horses was minimized when 0.10 g glucose/kg BW was used in the GTT (Toth et al., 2009). Because of the high glucose concentrations observed after glucose infusion, results from trial 1 were not included in the statistical analysis of glucose and insulin data following the GTT. Data from horses in trial 1 were included in the statistical analysis of post-feeding blood metabolite data.
Figure 1.
Effect of dietary Cr on plasma glucose concentrations in horses following IV infusion of 0.20 g glucose per kg BW in trial 1 (n = 3 per treatment).
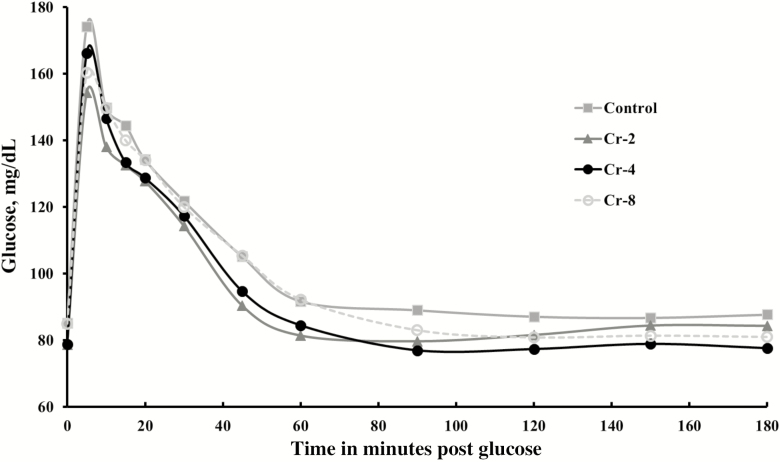
In trials 2, 3, and 4, dextrose was reduced to only provide 0.10 g of glucose per kg BW. Plasma glucose concentrations at 5 min post administration were less than 180 mg/dL when this dose of glucose was infused (Figure 2). Glucose data following the GTT are shown in Table 9. Basal glucose concentrations were affected by treatment (P = 0.01), trial (P = 0.001), and treatment × trial (P = 0.05). Control horses and those fed 8 mg Cr/d had greater (P < 0.05) basal glucose concentrations than horses receiving 2 or 4 mg Cr/d. The treatment × trial interaction was due to Cr supplementation at 2 or 4 mg/d reducing or tending to reduce basal glucose concentrations in trials 3 and 4, but not in trial 2. Other glucose measurements were not affected by a treatment × trial or a treatment × block interaction. Glucose repeated measures were affected by treatment (P = 0.01) and time (P = 0.001), but not by treatment × time (P = 0.14). Mean plasma glucose concentrations (across sampling samples) were greater (P = 0.01) in controls than in horses fed 2 or 4 mg Cr/d. Glucose concentrations following the GTT did not differ (P = 0.10) among controls and horses receiving 8 mg Cr/d. Mean plasma glucose concentrations in horses given 8 mg Cr/d were greater (P = 0.02) than in those fed 2 mg Cr/d and tended (P = 0.06) to be greater compared with horses fed 4 mg Cr/d. Peak glucose concentrations following IV glucose infusion were not affected (P = 0.15) by treatment.
Figure 2.
Effect of dietary Cr on plasma glucose concentrations in horses following IV infusion of 0.10 g glucose per kg BW in trials 2, 3, and 4 (n = 9 per treatment; treatment P = 0.01; time P = 0.001).
Table 9.
Effects of dietary Cr on plasma glucose concentrations, AUC, and kinetics following a GTT on day 42
| Supplemental Cr, mg/d | P-value | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 8 | SE | Trt | Time | Trt × time | |
| Glucose | ||||||||
| Basal concentration, mg/dL | 85.1a | 78.3b | 78.8b | 85.0a,b | 1.6 | 0.01 | ||
| Peak concentration, mg/dL | 174.6 | 155.6 | 166.2 | 160.3 | 5.9 | 0.15 | ||
| AUC, mg/dL * min | ||||||||
| 0 to 45 min | 5,874 | 5,378 | 5,569 | 5,758 | 128 | 0.06 | ||
| 0 to 60 min | 7,350a,c | 6,702b | 6,913a,b | 7,241a,c | 168 | 0.05 | ||
| 0 to 180 min | 17,923 | 16,805 | 16,372 | 17,201 | 437 | 0.10 | ||
| Repeated measures mean, mg/dL | 113.1a,c | 103.7b | 105.1b,c | 109.4a,c | 1.4 | 0.01 | 0.001 | 0.14 |
| Clearance rate, %/min | 1.30 | 1.46 | 1.38 | 1.18 | 0.12 | 0.45 | ||
| Half-life, min | 81.4a | 52.5b | 68.6a,b | 80.3a | 7.3 | 0.04 | ||
a–cMeans in a row not bearing a common superscript letter differ (P < 0.05).
Area under the glucose response curve, calculated from 0 to 60 min following glucose administration, was less (P < 0.05) in horses receiving 2 mg Cr/d compared with controls and horses receiving 8 mg Cr/d (Table 9). Controls also tended (P = 0.08) to have greater glucose AUC from 0 to 60 min than horses given 4 mg Cr/d. Glucose AUC calculated from 0 to 45 (P = 0.06) or 0 to 180 (P = 0.10) min tended to be affected by treatment. Glucose half-life was affected by treatment (P = 0.04). Following glucose administration, glucose half-life was greater (P < 0.05) in the control and 8 mg Cr treatments compared with those fed 2 mg Cr/d. Glucose half-life did not differ among horses fed 2 and 4 mg Cr/d. Glucose clearance rate was not affected by treatment. Yearling horses supplemented with Cr picolinate to provide 0.42 mg Cr/kg of concentrate (approximately 2.9 mg Cr/d) had greater glucose clearance rates than control horses following an IV GTT (Ott and Kivipelto, 1999). The results of this study (Ott and Kivipelto, 1999) may have been confounded by urinary excretion of glucose as glucose was infused at 0.20 g/kg BW, and plasma glucose concentrations exceeded 200 mg/dL following glucose administration. In this study, lower supplemental concentrations of chromium (0.105 or 0.210 mg/kg concentrate) did not significantly enhance glucose clearance rate. Pigs supplemented with 0.20 mg Cr/kg diet, from Cr picolinate, also had greater glucose clearance than control pigs following an IV GTT (Amoikon et al., 1995).
Insulin measurements following IV glucose infusion were not affected (P > 0.07) by treatment × trial or treatment × block interactions. Basal insulin concentrations prior to glucose infusion were affected by trial (P = 0.001), but not by treatment (P = 0.30; Table 10). Serum insulin concentrations were affected by treatment (P = 0.04) and time (P = 0.001) when analyzed by repeated measures (Table 10). Following glucose infusion, serum insulin concentrations were greater (P < 0.05) in horses fed 2 or 4 mg Cr/d compared with controls. Horses receiving 8 mg Cr/d also tended (P = 0.07) to have greater serum insulin concentrations than controls. Peak insulin concentrations following glucose infusion were not affected (P = 0.32) by treatment (Table 10). Area under the insulin response curve calculated from 0 to 45 or 0 to 60 min after glucose infusion was greater (P < 0.05) in horses fed 2 or 4 mg Cr/d compared with controls (Table 10). AUC for insulin from 0 to 180 min was not affected (P = 0.36) by treatment.
Table 10.
Effects of dietary Cr on serum insulin concentrations and AUC following a GTT on day 42
| Supplemental Cr, mg/d | P-value | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 8 | SE | Trt | Time | Trt × time | |
| Insulin | ||||||||
| Basal concentration, µU/mL | 9.8 | 10.5 | 11.8 | 11.4 | 0.8 | 0.30 | ||
| Peak concentration, µU/mL | 22.7a | 32.9b | 28.1a,b | 28.4a,b | 3.1 | 0.32 | ||
| AUC, µU/mL * min | ||||||||
| 0 to 45 min | 763a | 1,150b | 1,022b | 941a,b | 91 | 0.05 | ||
| 0 to 60 min | 984a | 1,437b | 1,312b | 1,227a,b | 96 | 0.04 | ||
| 0 to 180 min | 2,474 | 3,020 | 2,837 | 2,776 | 211 | 0.36 | ||
| Repeated measures mean, µU/mL | 14.8a | 19.1b | 17.8b | 17.4a,b | 1.0 | 0.04 | 0.001 | 0.47 |
a,bMeans in a row not bearing a common superscript letter differ (P < 0.05).
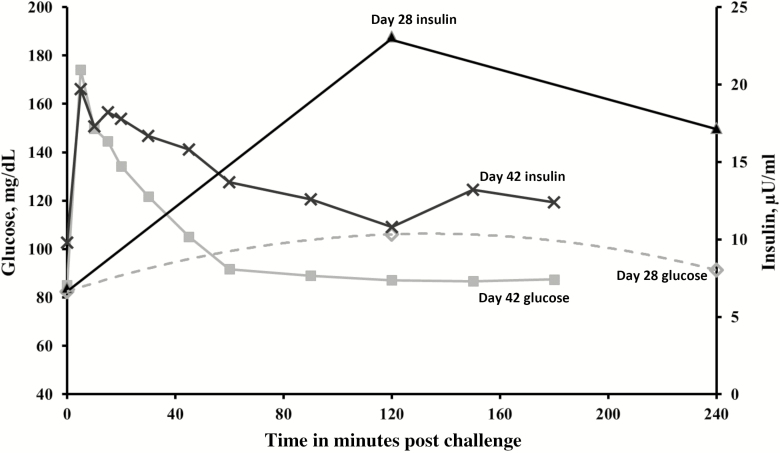
Consistent with previous studies (Duhlmeier et al., 2001; de Laat et al., 2016), insulin release following oral concentrate consumption was greater than following IV glucose administration, despite greater peak plasma glucose concentrations following IV glucose. A comparison of glucose and insulin concentrations following oral concentrate and IV glucose infusion in the control horse is shown in Figure 3. Oral carbohydrate consumption in horses causes secretion of gastrointestinal hormones, referred to as incretins, that promote insulin release under hyperglycemic conditions (de Graaf-Roelfsema, 2014). Glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide (GLP) are incretins that have been shown to affect insulin secretion in horses (Duhlmeier et al., 2001; Bamford et al., 2015). Duhlmeier et al. (2001) found that GIP concentrations did not change after IV glucose administration but increased greatly following an oral GTT. The greater GIP concentrations after oral glucose were associated with greater plasma insulin concentrations compared with IV glucose administration. More recently, a strong positive association between circulating GLP and insulin concentrations was also observed in horses following a concentrate meal (Bamford et al., 2015) and, in ponies, considered insulin resistant, after an oral nonstructural carbohydrate challenge (de Laat et al., 2016).
Figure 3.
Plasma glucose and serum insulin concentrations in control horses following oral concentrate feeding (day 28) or IV glucose infusion (day 42) (n = 9).
Because oral concentrate consumption has such a large effect on insulin release in horses, the results obtained in the present study following oral concentrate consumption would appear to be more physiological than the results obtained after IV glucose infusion. Following oral concentrate feeding, 4 mg Cr/d was the minimum dose of Cr that resulted in a significant improvement in insulin sensitivity, based on reduced plasma glucose concentrations and glucose AUC after concentrate feeding. Glucose AUC is considered the best measure of glucose tolerance in horses (Frank et al., 2010). The lower level of Cr supplementation (2 mg Cr/d) tended to reduce glucose concentrations and glucose AUC following concentrate feeding relative to the control treatment. When glucose was administered IV, horses fed 2 and 4 mg Cr/d had lower plasma glucose concentrations across all pre- and post-glucose infusion sampling times than in control horses.
The present study indicates that supplementing 4 mg Cr/d, from Cr Prop, enhances insulin sensitivity following concentrate feeding in healthy adult horses. Supplementing Cr to adult horses may help prevent horses from becoming insulin resistant later in life. It has been demonstrated that old or geriatric horses need more insulin to manage an oral concentrate dose. Old horses (27.0 ± 0.2 yr) had greater insulin concentrations and insulin:glucose ratios after oral glucose administration than young (6.8 ± 0.4 yr) or middle (15.2 ± 0.4 yr) age horses (Malinowski et al., 2002). Adult horses (5 to 13 yr) also had lesser fasting and peak insulin concentrations than aged horses (18 to 24 yr) after receiving a hay-concentrate meal (Jacob et al., 2018). Mature horses (14.2 ± 0.5 yr) had much greater peak insulin concentrations but slightly lower peak glucose concentrations than 2-yr-old horses following the consumption of 1.5 g/kg BW of concentrate (Nielsen et al., 2010). Hyperinsulinemia in insulin-resistant horses is believed to be due to insulin secretion increasing to compensate for reduced insulin responsiveness by tissues (de Graaf-Roelfsema, 2014).
Studies have indicated that humans with non-insulin-dependent diabetes are more likely to respond to Cr supplementation than humans with normal glucose tolerance (Balk et al., 2007). Research examining the effect of Cr supplementation on insulin sensitivity in insulin-resistant horses is limited. Vervuert et al. (2010) supplemented 27 insulin-resistant ponies and horses with 0 or 25 µg Cr (Cr yeast)/kg BW for 4 wk. An oral starch tolerance test was performed at the beginning and end of the study following a 12-h fast. Compared with the pre-experimental starch tolerance test, plasma insulin concentrations increased to a lesser degree after 4 wk in Cr-supplemented horses. In horses receiving control yeast, insulin responses to the starch challenge were similar in the pre- and post-experimental period. The decrease in insulin response after 4 wk of Cr supplementation was not associated with a lower plasma glucose response following the oral starch challenge.
Preventing insulin resistance or improving insulin sensitivity in insulin-resistant horses has a number of possible health implications. Obesity in mares is associated with elevated insulin concentrations and reduced insulin sensitivity (Powell et al., 2002; Vick et al., 2007). It is unclear if obesity induces insulin resistance or if insulin-resistant horses are more predisposed to obesity (Frank et al., 2010). Hyperinsulinemia observed during obesity may occur to compensate for systemic insulin resistance (de Graef-Roelfsema, 2014). However, there is some evidence in mice that hyperinsulinemia may cause obesity and insulin resistance (Mehran et al., 2012). Insulin resistance in horses also has been associated with laminitis and infusing large amounts of insulin to cause hyperinsulinemia-induced laminitis in horses (de Laat et al., 2016). Collectively obesity, hyperinsulinemia, insulin resistance, and predisposition toward laminitis are principle components of the equine metabolic syndrome (Frank et al., 2010).
The improved insulin sensitivity in horses fed 4 mg Cr/d after concentrate feeding suggests that the control diet was inadequate in Cr for maximizing insulin sensitivity. Hays fed in the present study contained 0.10 to 0.22 mg Cr/kg DM, and the concentrate mix analyzed 0.30 mg Cr/kg DM across all trials. A recent study indicated that unprocessed grains contain less than 0.05 mg Cr/kg DM (Spears et al., 2017). Harvested forages contained greater Cr concentrations than cereal grains, but some of the Cr in forages was probably due to Cr contamination from soil and metal contact during harvesting and processing for the analysis (Spears et al., 2017). Little is known regarding the bioavailability of Cr from animal feedstuffs. In humans, the bioavailability of Cr naturally present in foods has been shown to be low (Anderson and Kozlovsky, 1985). Chromium Prop has been shown to provide bioavailable Cr, based on its ability to enhance insulin sensitivity in cattle fed a basal diet containing 0.20 mg Cr/kg DM (Spears et al., 2012) and in broilers fed basal diets analyzing 0.43 to 0.45 mg Cr/kg diet (Brooks et al., 2016).
Route of carbohydrate administration affected the response of insulin to Cr supplementation. In contrast to results obtained after concentrate feeding, serum insulin concentrations following IV glucose infusion were greater in horses receiving 2 or 4 mg Cr/d than in control horses. The increase in insulin following IV glucose infusion was much smaller in horses in the present study compared with those previously observed in pigs (Guan et al., 2000) and cattle (Hayirli et al., 2001; Stahlhut et al., 2005; Spears et al., 2012). When compared with baseline concentrations, serum insulin increased 2.3-fold following IV glucose infusion in control horses. In control pigs, serum insulin concentrations increased approximately 9-fold after IV glucose administration (Guan et al., 2000). Serum insulin concentrations increased 11.3-fold in control prepartum dairy cows and 16.5-fold in postpartum dairy cows following IV glucose infusion (Hayirli et al., 2001). Following IV glucose infusion, serum insulin concentrations increased 6.6-fold in control beef heifers (Spears et al., 2012) and approximately 6.5-fold in pre- and postpartum beef cows (Stahlhut et al., 2005). This suggests that gastrointestinal-released incretins may be more important in promoting insulin release under hyperglycemic conditions in horses than in pigs and cattle.
The timing of peak serum insulin concentrations and the rate of decline in serum insulin following IV glucose administration also differed greatly from what has been observed in pigs and cattle. In pigs and cattle, serum insulin concentrations peak by 5 to 10 min following IV glucose infusion and then decrease rapidly reaching baseline concentrations by 30 (Guan et al., 2000) to 60 min (Stahlhut et al., 2005; Spears et al., 2012). In the present study, serum insulin concentrations did not return to baseline values until 120 min following glucose administration. Serum insulin concentrations at 5 min post glucose infusion were higher than baseline insulin concentrations in all but 1 of the 36 horses. Compared to 5 min, serum insulin was slightly lower in samples collected at 10 and 15 min. However, serum insulin concentrations, across all treatments, at 5 min did not differ from those in samples collected at 20 (P = 0.80) or 30 min (P = 0.76) post glucose administration. For several horses, peak insulin concentrations occurred between 20 and 60 min after IV glucose infusion. This suggests that the initial release of insulin from the pancreas was inadequate to handle the glucose load and a second release of insulin occurred.
The low release of insulin in horses following IV glucose may explain why Cr supplementation affected serum insulin concentrations differently from that observed following concentrate feeding. The greater serum insulin concentrations in horses supplemented with Cr following IV glucose administration indicate that Cr affected either the pancreatic release or turnover of insulin. In cattle, Cr supplementation either decreased (Stahlhut et al., 2005; Spears et al., 2012) or did not affect (Hayirli et al., 2001) insulin concentrations following IV glucose infusion. However, pigs supplemented with 0.20 mg Cr/kg diet, from a high Cr yeast, had a greater release of insulin than controls after glucose administration (Guan et al., 2000). We were not able to estimate insulin clearance or half-life in the present study because peak insulin concentrations varied greatly among horses in all treatment groups. Chromium has been reported to stabilize insulin against chymotrypsin-mediated hydrolysis in vitro (Govindaraju et al., 1989). Insulin clearance primarily depends on degradation in the liver via insulin-degrading enzyme. Insulin conjugated with Cr instead of zinc reduced plasma insulin clearance when injected into diabetic mice by inhibiting hepatic insulin-degrading enzyme (Wang et al., 2014).
It is unclear why horses receiving 8 mg Cr/d responded differently to oral concentrate feeding than those fed 2 or 4 mg Cr/d. Horses fed 8 mg/d of Cr had lower fasting insulin concentrations than controls on day 28, but glucose and insulin responses following concentrate feeding were similar to controls. Insulin responses following IV glucose infusion in horses fed 8 mg Cr/d tended to go in the same direction as the 2 and 4 mg Cr treatments. Glucose repeated measures, across all sampling times, tended (P = 0.10) to be lesser in horses receiving 8 mg Cr/d than in controls. However, glucose concentrations (across sampling times) after IV glucose infusion were greater in horses fed 8 mg Cr/d compared with those receiving 2 or 4 mg Cr/d. In lactating dairy cows, glucose measurements following an IV glucose challenge were similar in controls and cows given 15.7 mg Cr (from Cr methionine)/d (Hayirli et al., 2001). However, cows receiving 3.7 or 7.7 mg Cr/d had lower peak glucose concentrations and greater glucose clearance rates than control cows following IV glucose administration. In growing heifers, Cr supplementation reduced insulin release and glucose AUC after IV glucose, and no differences were observed among heifers supplemented with 3, 6, or 9 mg Cr/d (Spears et al., 2012).
Conclusions
Horses supplemented with 4 mg Cr/d had lower plasma glucose concentrations and glucose AUC than control horses following concentrate feeding. Serum insulin concentrations were lower in horses fed 2 or 4 mg Cr/d compared with controls at 4 h after concentrate feeding. The lower plasma glucose response, coupled with reduced serum insulin concentrations following concentrate feeding in horses supplemented with 4 mg Cr/d, is consistent with Cr enhancing insulin sensitivity. The 4 mg Cr/d treatment would be equivalent to 0.36 mg Cr/kg of diet.
Acknowledgments
The use of trade names in this publication does not imply endorsement by North Carolina State University or criticism of similar products not mentioned. We acknowledge Matt Newhouse (Kemin Agrifoods North America, Inc., Des Moines, IA) for support in monitoring this study and associated data with the use of good laboratory-like practices. Appreciation is expressed to Lawson Walston, Tabatha Wilson, Caitlin Pritchett, Daniel Adams, Morghan Bowman, Caroline Chang, and Jennifer Gill at North Carolina State University for assistance with animal care and sampling. This research was partially supported by a grant from Kemin Agifoods North America, Inc.
Glossary
Abbreviations
- AUC
area under the curve
- BW
body weight
- Cr Prop
chromium propionate
- GIP
glucose-dependent insulinotropic polypeptide
- GLP
glucagon-like peptide
- GTT
glucose tolerance test
- IV
intravenous
- NEFA
nonesterified fatty acids
Conflict of interest statement
K.K., J.H., and W.R. are employed by Kemin AgriFoods North America, Inc. J.S. is a consultant for Kemin AgriFoods North America, Inc.
Literature Cited
- Amoikon E. K., Fernandez J. M., Southern L. L., Thompson D. L. Jr, Ward T. L., and Olcott B. M.. 1995. Effect of chromium tripicolinate on growth, glucose tolerance, insulin sensitivity, plasma metabolites, and growth hormone in pigs. J. Anim. Sci. 73:1123–1130. doi: 10.2527/1995.7341123x [DOI] [PubMed] [Google Scholar]
- Anderson R. A., and Kozlovsky A. S.. 1985. Chromium intake, absorption and excretion of subjects consuming self-selected diets. Am. J. Clin. Nutr. 41:1177–1183. doi: 10.1093/ajcn/41.6.1177 [DOI] [PubMed] [Google Scholar]
- Balk E. M., Tatsioni A., Lichtenstein A. H., Lau J., and Pittas A. G.. 2007. Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized controlled trials. Diabetes Care 30:2154–2163. doi: 10.2337/dc06-0996 [DOI] [PubMed] [Google Scholar]
- Bamford N. J., Baskerville C. L., Harris P. A., and Bailey S. R.. 2015. Postprandial glucose, insulin, and glucagon-like peptide-1 responses of different equine breeds adapted to meals containing micronized maize. J. Anim. Sci. 93:3377–3383. doi: 10.2527/jas.2014-8736 [DOI] [PubMed] [Google Scholar]
- Bertin F. R., Taylor S. D., Bianco A. W., and Sojka-Kritchevsky J. E.. 2016. The effect of fasting duration on baseline blood glucose concentration, blood insulin concentration, glucose/insulin ratio, oral sugar test, and insulin response test results in horses. J. Vet. Intern. Med. 30:1726–1731. doi: 10.1111/jvim.14529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks M. A., Grimes J. L., Lloyd K. E., Krafka K., Lamptey A., and Spears J. W.. 2016. Chromium propionate in broilers: effect on insulin sensitivity. Poult. Sci. 95:1096–1104. doi: 10.3382/ps/pew018 [DOI] [PubMed] [Google Scholar]
- de Graaf-Roelfsema E. 2014. Glucose homeostasis and the enteroinsular axis in the horse: a possible role in equine metabolic syndrome. Vet. J. 199:11–18. doi: 10.1016/j.tvjl.2013.09.064 [DOI] [PubMed] [Google Scholar]
- de Laat M. A., McGree J. M., and Sillence M. N.. 2016. Equine hyperinsulinemia: investigation of the enteroinsular axis during insulin dysregulation. Am. J. Physiol. Endocrinol. Metab. 310:E61–E72. doi: 10.1152/ajpendo.00362.2015 [DOI] [PubMed] [Google Scholar]
- Duhlmeier R., Deegen E., Fuhrmann H., Widdel A., and Sallmann H. P.. 2001. Glucose dependent insulinotropic polypeptide (GIP) and the enteroinsular axis in equines (Equus caballus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 129:563–575. doi: 10.1016/S1095-6433(01)00295-1 [DOI] [PubMed] [Google Scholar]
- Evock-Clover C. M., Polansky M. M., Anderson R. A., and Steele N. C.. 1993. Dietary chromium supplementation with or without somatotropin treatment alters serum hormones and metabolites in growing pigs without affecting growth performance. J. Nutr. 123:1504–1512. doi: 10.1093/jn/123.9.1504 [DOI] [PubMed] [Google Scholar]
- Frank N., Geor R. J., Bailey S. R., Durham A. E., and Johnson P. J.; American College of Veterinary Internal Medicine 2010. Equine metabolic syndrome. J. Vet. Intern. Med. 24:467–475. doi: 10.1111/j.1939-1676.2010.0503.x [DOI] [PubMed] [Google Scholar]
- Frank N., Sojka J. E., and Latour M. A.. 2002. Effect of withholding feed on concentration and composition of plasma very low density lipoprotein and serum nonesterified fatty acids in horses. Am. J. Vet. Res. 63:1018–1021. doi: 10.2460/ajvr.2002.63.1018 [DOI] [PubMed] [Google Scholar]
- Gentry L. R., Thompson D. L., Fernandez J. M., Smith L. A., Horohov D. W., and Leise B. S.. 1999. Effects of chromium tripicolinate supplementation on plasma hormone and metabolite concentrations and immune function in adult mares. J. Equine Vet. Sci. 19:259–265. doi: 10.1016/S0737-0806(99)80330-X [DOI] [Google Scholar]
- Govindaraju K., Ramasami T., and Ramaswamy D.. 1989. Chymotrypsin-catalyzed hydrolysis of chromium (III) derivatives of insulin: evidence for stabilization of the protein through interactions with metal ions. J. Inorg. Biochem. 35:127–135. doi: 10.1016/0162-0134(89)80005-4 [DOI] [PubMed] [Google Scholar]
- Guan X., Matte J. J., Ku P. K., Snow J. L., Burton J. L., and Trottier N. L.. 2000. High chromium yeast supplementation improves glucose tolerance in pigs by decreasing hepatic extraction of insulin. J. Nutr. 130:1274–1279. doi: 10.1093/jn/130.5.1274 [DOI] [PubMed] [Google Scholar]
- Hayirli A., Bremmer D. R., Bertics S. J., Socha M. T., and Grummer R. R.. 2001. Effect of chromium supplementation on production and metabolic parameters in periparturient dairy cows. J. Dairy Sci. 84:1218–1230. doi: 10.3168/jds.S0022-0302(01)74583-3 [DOI] [PubMed] [Google Scholar]
- Hoffman R. M., Boston R. C., Stefanovski D., Kronfeld D. S., and Harris P. A.. 2003. Obesity and diet affect glucose dynamics and insulin sensitivity in Thoroughbred geldings. J. Anim. Sci. 81:2333–2342. doi: 10.2527/2003.8192333x [DOI] [PubMed] [Google Scholar]
- Hoffman N. J., Penque B. A., Habegger K. M., Sealls W., Tackett L., and Elmendorf J. S.. 2014. Chromium enhances insulin responsiveness via AMPK. J. Nutr. Biochem. 25:565–572. doi: 10.1016/j.jnutbio.2014.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Y., Clark S., Ren J., and Sreejayan N.. 2012. Molecular mechanisms of chromium in alleviating insulin resistance. J. Nutr. Biochem. 23:313–319. doi: 10.1016/j.jnutbio.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S. I., Geor R. J., Weber P. S. D., Harris P. A., and McCue M. E.. 2018. Effect of age and dietary carbohydrate profiles on glucose and insulin dynamics in horses. Equine Vet. J. 50:249–254. doi: 10.1111/evj.12745 [DOI] [PubMed] [Google Scholar]
- Jose-Cunilleras E., Taylor L. E., and Hinchcliff K. W.. 2004. Glycemic index of cracked corn, oat groats and rolled barley in horses. J. Anim. Sci. 82:2623–2629. doi: 10.2527/2004.8292623x [DOI] [PubMed] [Google Scholar]
- Kaneko J. J. 1989. Carbohydrate digestion and its diseases. In: Kaneko J. J., editor. Clinical biochemistry of domestic animals. 4th ed. San Diego (CA): Academic Press; p. 44–85. [Google Scholar]
- Lloyd K. E., Fellner V., McLeod S. J., Fry R. S., Krafka K., Lamptey A., and Spears J. W.. 2010. Effects of supplementing dairy cows with chromium propionate on milk and tissue chromium concentrations. J. Dairy Sci. 93:4774–4780. doi: 10.3168/jds.2010-3198 [DOI] [PubMed] [Google Scholar]
- Malinowski K., Betros C. L., Flora L., Kearns C. F., and McKeever K. H.. 2002. Effect of training on age-related changes in plasma insulin and glucose. Equine Vet. J. Suppl. 34:147–153. doi: 10.1111/j.2042-3306.2002.tb05408x [DOI] [PubMed] [Google Scholar]
- Mehran A. E., Templeman N. M., Brigidi G. S., Lim G. E., Chu K. Y., Hu X., Botezelli J. D., Asadi A., Hoffman B. G., Kieffer T. J., . et al. 2012. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab. 16:723–737. doi: 10.1016/j.cmet.2012.10.019 [DOI] [PubMed] [Google Scholar]
- National Academies 2001. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington (DC):National Academies Press. [PubMed] [Google Scholar]
- Nielsen B. D., O’Connor-Robison C. I., Spooner H. S., and Shelton J.. 2010. Glycemic and insulinemic responses are affected by age of horse and method of feed processing. J. Equine Vet. Sci. 30:249–258. doi: 10.1016/j.jevs.2010.03.008 [DOI] [Google Scholar]
- NRC 2007. Nutrient requirements of horses. 7th rev. ed. Washington (DC): National Academies Press. [Google Scholar]
- Ott E. A., and Kivipelto J.. 1999. Influence of chromium tripicolinate on growth and glucose metabolism in yearling horses. J. Anim. Sci. 77:3022–3030. doi: 10.2527/1999.77113022x [DOI] [PubMed] [Google Scholar]
- Pagan J. D., Jackson S. G., and Duren S. E.. 1995. The effect of chromium supplementation on metabolic response to exercise in Thoroughbred horses. In: Lyons, T. P., and K. A. Jacques, editors. Biotechnology in the feed industry. Proceedings of Alltech 11th Annual Symposium. Nottingham (UK): Nottingham University Press; p. 263–270. [Google Scholar]
- Powell D. M., Reedy S. E., Sessions D. R., and Fitzgerald B. P.. 2002. Effect of short-term exercise training on insulin sensitivity in obese and lean mares. Equine Vet J. Suppl. 34:81–84. doi: 10.1111/j.2042-3306.2002.tb05396.x [DOI] [PubMed] [Google Scholar]
- Ralston S. L. 2002. Insulin and glucose regulation. Vet. Clin. North Am. Equine Pract. 18:295–304, vii. doi: 10.1016/s0749-0739(02)00014-7 [DOI] [PubMed] [Google Scholar]
- SAS Institute 2008. User’s guide: statistics. Version 9.2 ed. Cary (NC): SAS Institute, Inc. [Google Scholar]
- Spears J. W., Lloyd K. E., and Krafka K.. 2017. Chromium concentrations in ruminant feed ingredients. J. Dairy Sci. 100:3584–3590. doi: 10.3168/jds.2016-12153 [DOI] [PubMed] [Google Scholar]
- Spears J. W., Whisnant C. S., Huntington G. B., Lloyd K. E., Fry R. S., Krafka K., Lamptey A., and Hyda J.. 2012. Chromium propionate enhances insulin sensitivity in growing cattle. J. Dairy Sci. 95:2037–2045. doi: 10.3168/jds.2011-4845 [DOI] [PubMed] [Google Scholar]
- Stahlhut H. S., Whisnant C. S., Lloyd K. E., Baird E. J., Legleiter L. R., Hansen S. L., and Spears J. W.. 2005. Effect of chromium supplementation and copper status on glucose and lipid metabolism in Angus and Simmental beef cows. Anim. Feed Sci. Technol. 128:253–265. doi: 10.1016/j.anifeedsci.2005.11.002 [DOI] [Google Scholar]
- Toth F., Frank N., Elliott S. B., Perdue K., Geor R. J., and Boston R. C.. 2009. Optimisation of the frequently sampled intravenous glucose tolerance test to reduce urinary glucose spilling in horses. Equine Vet. J. 41:844–851. doi: 10.2746/042516409X439661 [DOI] [PubMed] [Google Scholar]
- Uyanik F., Guclu B. K., Bekyurek T., Mustafa M. A. U., Canoo lu E., Dem ral O., Erdem O., Guvnec K., Gurbulak K., and Sayal A.. 2008. The effect of chromium supplementation on body weight, serum glucose, proteins, lipids, minerals and ovarian follicular activity in working horses. J. Anim. Vet. Adv. 7:771–776. doi:https://medwelljournals.com/abstract/?doi=javaa.2008.771.776 [Google Scholar]
- Vervuert I., Oβwald B., Cuddeford D., and Coenen M.. 2010. Effect of chromium yeast supplementation on postprandial glycaemic and insulinaemic responses in insulin-resistant ponies and horses. Pferdeheilkunde. 26:245–250. doi: 10.21836/PEM20100221 [DOI] [Google Scholar]
- Vick M. M., Adams A. A., Murphy B. A., Sessions D. R., Horohov D. W., Cook R. F., Shelton B. J., and Fitzgerald B. P.. 2007. Relationships among inflammatory cytokines, obesity, and insulin sensitivity in the horse. J. Anim. Sci. 85:1144–1155. doi: 10.2527/jas.2006-673 [DOI] [PubMed] [Google Scholar]
- Wang Z. Q., Yu Y., Zhang X. H., and Komorowski J.. 2014. Chromium-insulin reduces insulin clearance and enhances insulin signaling by suppressing hepatic insulin-degrading enzyme and proteasome protein expression in KKAy mice. Front. Endocrinol. (Lausanne). 5:99. doi: 10.3389/fendo.2014.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]