Abstract
To characterize the expression of steroidogenic enzymes implicated in the development of ovarian steroid cell tumors, not otherwise specified (SCT-NOS). We present 4 ovarian SCT-NOS evaluated by immunohistochemical staining of steroidogenic enzymes as an approach to define this entity pathologically. All 4 ovarian SCT-NOS showed increased expression for cholesterol side-chain cleavage enzyme (CYP11A1), 17α-hydroxylase (CYP17A1), 17β-hydroxysteroid dehydrogenase 1 (HSD17B1), aldo-ketoreductase type 1 C3 (AKR1C3), 3β-hydroxysteroid dehydrogenase 2 (HSD3B2), 5α-reductase type 2 (SRD5A2), steroid sulfatase (SULT2A1), estrogen sulfotransferase (EST), and aromatase (CYP19A1). Expression was negative for 21-hydroxylase (CYP21A2) and 17β-hydroxysteroid dehydrogenase 2 (HSD17B2). 17β-hydroxysteroid dehydrogenase 3 (HSD17B3) and 5α-reductase type 1 (SRD5A1) showed variable expression. Our analysis reveals a novel finding of increased expression of AKR1C3, HSD17B1, SRD5A2, SULT2A1, and EST in ovarian SCT-NOS, which is clinically associated with androgen excess and virilization. Further studies are needed to validate these enzymes as new markers in the evaluation of hyperandrogenic ovarian conditions.
Keywords: ovarian tumor, hyperandrogenism, steroidogenesis
Introduction
Mild hyperandrogenism, such as hirsutism, in postmenopausal women can be part of the normal aging process, but frank virilization can be challenging for the patient.1 A rapid progression of virilization in any woman with marked elevation in testosterone levels strongly suggests the need to rule out adrenal or ovarian tumors.2 Differential diagnoses include hyperandrogenic syndromes like polycystic ovarian syndrome (PCOS), nonclassic congenital adrenal hyperplasia (NCCAH), ovarian hyperthecosis, Cushing syndrome, and iatrogenic hyperandrogenism (Table 1).3
Table 1.
Differential Diagnosis.3
| Symptomatic differential diagnosis (hirsutism) | Pathology differential diagnosis |
|---|---|
| Polycystic ovarian syndrome Ovarian hyperthecosis Cushing syndrome Nonclassic congenital adrenal hyperplasia Adrenocortical carcinoma Ovarian tumors |
Granulosa cell tumors Thecomas Clear cell carcinomas Oxyphilic struma ovarii Ovarian not otherwise specified |
Steroid cell tumors are very rare and represent 0.1% of all ovarian tumors.4 They are divided into 3 subtypes based on the cells of origin. Stromal luteomas arise from ovarian stroma, Leydig cell tumors arise from Leydig cells in the hilum, and steroid cell tumors, not otherwise specified (SCT-NOS) are of unknown lineage (grouped by exclusion from the other 2 subtypes).4 This latter tumor subtype constitutes about 50% to 60% of all steroid cell tumors.4,5 Incidence is highest in the third and fourth decades, and they are clinically androgenic in about 50% of cases.4,6 Ovarian SCT-NOS are mostly benign and unilateral; however, 5% are bilateral. There may be a malignant potential in 25% to 40% of cases.4,6
Conventional diagnosis is based on histopathology, which includes microscopy, cytology, and immunohistochemical (IHC) markers.7 SCT-NOS were histopathologically described in detail more than 30 years ago by Hayes and Scully.4 They observed grossly that ovarian SCT-NOS are well circumscribed, solid, and noncalcified. On microscopic examination, cells are arranged in small nests, cords, or columns.5 Cytologically, they are round, polygonal with abundant granular eosinophilic cytoplasm. The absence of cytoplasmic Reinke crystals differentiates SCT-NOS from Leydig cell tumors. Immunohistochemically, positive staining for steroidogenic factor (SF-1), inhibin, and calretinin serve as important markers for steroid cell tumors.7
In the present study, we analyzed 4 ovarian SCT-NOS in 1 premenopausal and 2 postmenopausal patients who presented with virilization. We performed IHC analysis of steroidogenic enzymes to better define this entity.
Methods
Immunohistochemical Staining
We performed immunohistochemistry on the representative sections of the ovarian SCT-NOS evaluating the immunoreactivity of steroidogenic enzymes. The IHC analysis was performed with the streptavidin-biotin amplification method using a Histofine Kit (Nichirei, Tokyo, Japan). Primary antibodies used in this study are summarized in Supplemental Table 1 (available online). The antigen-antibody complex was visualized by DAB solution (1 mM 3,3-diaminobenzidine), 50 mM Tris–HCl buffer, pH 7.6, and 0.006% hydrogen peroxidase, and counterstained with hematoxylin.8 We confirmed the specificity of all of the primary antibodies examined in this study, and showed the representative images of positive (with primary antibodies) and negative control (without negative antibodies; Supplemental Figure 1 [available online]).9,10
Scoring of IHC Expression
The IHC expression of the steroidogenic enzymes was assessed using a semiquantitative approach of H-score. Briefly, 100 tumor cells were assessed in each case, and the H-score was generated by adding the percentage of strongly stained nuclei (3×), the percentage of moderately stained nuclei (2×), and the percentage of weakly stained nuclei (1×), giving a possible score range of 0 to 300. The score was independently obtained by 2 of the authors (YY and HS), and the mean of the 2 values was considered as final score. An H-score <10 was considered negative for the expression of the steroidogenic enzyme. A score of >10 was considered as positive of which a score of <100 was considered weakly positive.11
Case 1
A 43-year-old woman was evaluated for signs and symptoms of hyperandrogenism. She was postmenopausal for several years prior to presentation. Her past medical history included systemic lupus erythematosis, kidney transplant with chronic kidney disease stage IV, bilateral complex cystic kidney masses, right adrenal adenoma, hysterectomy with right oophorectomy for uterine fibroids, and right ovarian cysts. Physical examination revealed facial hirsutism, frontotemporal alopecia, pustular acne, coarse terminal body hair, and clitoromegaly. Ferriman-Gallwey score was 28, nearly involving all 9 androgen-sensitive areas.
Hormonal evaluation showed an elevated morning testosterone level of 700 ng/dL, confirmed on repeat testing, and an elevated free testosterone of 120.5 pg/mL. Basal 17-hydroxyprogesterone (17-OHP; 291.0 ng/dL) and androstenedione (2.46 ng/mL) were slightly abnormal (Table 2). However, the cosyntropin stimulation test for 17-OHP ruled out NCCAH. Dehydroepiandrosterone sulfate (DHEAS) level was normal, and gonadotropins were in the menopausal range (Table 2). The 1 mg dexamethasone suppression test (DST) showed mild elevation of cortisol, however, the 24-hour urine-free cortisol excretion was normal. Her plasma metanephrines were within normal range (Table 2).
Table 2.
Hormonal Assaya.
| Results: case 1 | Results: case 2 | Results: case 3 | |
|---|---|---|---|
| Testosterone total | 700, 608 (9-55 ng/dL)b | 459, 402 (3-41 ng/dL) | 421 (2-45 ng/dL) |
| Testosterone free | 120.5 (1.1-5.8 pg/mL) | 8.6, 6.3 (0.10-0.85 ng/dL) | 49.3 (0.1-6.4 pg/dL) |
| Estradiol | 59 (0-55 pg/mL) | 37.1 (<6.0-54 pg/mL) | 55 (19-357 pg/mL) |
| FSH | 29.8 (25.8-134.8 mIU/mL) | 36.9 (25.8-134.8 mIU/mL) | 7.4 (1.9-16.9 mIU/mL) |
| LH | 38.5 (7.7-58.5 mIU/mL) | 24.8 (7.7-58.5 mIU/mL) | 5.4 (1.9-16.9 mIU/mL) |
| Androstenedione | 2.46 (0.130-0.820 ng/dL) | 601 (17-99 ng/dL) | 114 (30-235 ng/dL) |
| 17-OH progesterone | 291.0 (<206 ng/dL) | 154 (<206 ng/dL) | 43 (<285 ng/dL) |
| AM cortisol | 21.6 (7.0-22.0 µg/dL) | 21.5 (6.2-19.4 µg/dL) | 3.8 (4.0-22.0 µg/dL) |
| 1-mg-DST | 3.8 (<1.8 µg/dL) | 1.8 (<1.8 µg/dL) | <0.5 (<1.8 µg/dL) |
| Urine-free cortisol 24 hour | 3.0 (<45 µg/g/d) | — | — |
| DHEA-S | 132.1 (57.3-279.2 µg/dL) | 207.4 (29.4-220.5 µg/dL) | 62 (23-266 µg/dL) |
| Plasma metanephrines | 0.19 (0.00-0.49 nmol/L) | 14 (0-62 pg/mL) | — |
| Plasma normetanephrines | 0.34 (0.00-0.89 nmol/L) | 190 (0-145 pg/mL) | — |
Abbreviations: FSH, follicle stimulating hormone; LH, luteinizing hormone; 17-OH, 17-hydroxyprogesterone; DST, dexamethasone suppression test; DHEA-S, dehydroepiandrosterone sulfate.
Diversity in laboratory assays has resulted in different values and units in test results.
Reference ranges are in parentheses.
Transvaginal ultrasound demonstrated a normal sonographic appearing left ovary measuring 2.3 × 1.9 × 1.5 cm with no masses, and absent right ovary and uterus. Magnetic resonance imaging of the abdomen revealed stable right adrenal adenoma and increase in size of left kidney mass measuring 2.2 × 2.1 cm (previously 1.7 cm) raising concern for renal cell carcinoma (Figure 1). This prompted resection of both lesions with a subsequent diagnosis of benign nodular adrenal cortical hyperplasia and left renal hematoma, respectively.
Figure 1.
(A) Magnetic resonance imaging (MRI) abdomen T2 coronal image showing left upper pole renal mass. (B) MRI abdomen T1 transverse image showing right adrenal adenoma. (C) Transvaginal ultrasound showing left ovarian simple cyst.
Although no apparent mass was noted on ovarian imaging, the patient later underwent a laparoscopic left salpingo-oophorectomy for presumed tumor causing high testosterone, which demonstrated a 1.9-cm ovarian tumor. On histopathological examination, the lesion was composed of polygonal cells showing distinct cell borders with abundant granular eosinophilic cytoplasm, centrally located round nuclei with prominent nucleoli, and no Reinke crystals. Mitoses, tumor necrosis, or lymphovascular space invasion was not identified. The Ki67 proliferation index was approximately 3%. The tumor cells showed diffuse and strong expression by SF-1, inhibin, and calretinin by immunohistochemistry. Overall, the morphologic and immunophenotypic findings were consistent with ovarian SCT-NOS. Three weeks postsurgery, testosterone level normalized to 11 ng/dL, and estrogen replacement therapy was started to help with hot flashes.
Case 2
A 64-year-old postmenopausal woman with a history of type 2 diabetes mellitus, hypertension, coronary artery disease, and prior stroke, was evaluated for signs and symptoms of hyperandrogenism. Physical examination revealed an obese female with mild facial hirsutism, oily skin, and coarse terminal hair on her abdomen.
Biochemical evaluation revealed high total testosterone levels of 459 ng/dL, elevated free testosterone levels of 8.6 pg/mL, and high androstenedione levels of 601 ng/dL. 17-OHP and DHEAS levels were within normal range, while gonadotropins were in the menopausal range. The serum cortisol after a DST, and plasma metanephrines were within normal range (Table 2). Thus, based on the biochemical findings, her initial presumptive diagnosis was ovarian hyperthecosis.
Transvaginal ultrasound showed bilateral ovarian masses measuring 2.3 × 1.9 × 2.2 cm in the right ovary and 1.2 × 1.1 × 0.9 cm in the left ovary. Imaging also revealed an incidental 2-cm lipid-rich right adrenal adenoma, which was nonsecreting based on hormonal evaluation.
The patient underwent bilateral salpingo-oophorectomy, which on gross examination demonstrated a 2.5-cm lesion in the right ovary and 1.0-cm lesion in the left ovary. These bilateral lesions were histologically similar to the tumor in case 1 and displayed diffuse expression for SF-1, calretinin, and inhibin by immunohistochemistry, thereby consistent with bilateral ovarian SCT-NOS. Both tumors showed a low Ki67 proliferation index: 1% to 2% in the right ovarian tumor and 2% to 3% in the left ovarian tumor. Ten days postoperatively, total testosterone decreased to a normal value (39.0 ng/dL).
Case 3
A 34-year-old premenopausal woman with a history of gout, depression, and allergic rhinitis was evaluated for signs and symptoms of hyperandrogenism and secondary amenorrhea for 1.5 years. She was previously evaluated by gynecology and briefly treated with oral contraceptives without any improvement. Physical examination revealed morbid obesity, hypopigmented abdominal striae, facial hirsutism, acne, and coarse terminal body hair without clitoromegaly. Ferriman-Gallwey score was 20, involving nearly all 9 androgen-sensitive areas.
Biochemical evaluation showed elevated testosterone of 351 ng/dL, confirmed with repeat testing, and an elevated free testosterone of 49.3 pg/mL (see Table 2). Luteinizing hormone, follicle stimulating hormone, prolactin, insulin-like growth factor 1, cortisol, 17-OHP, androstenedione, estradiol, thyroid stimulating hormone, and a DST were all normal. Hemoglobin A1c was 5.9%, consistent with prediabetes. Imaging with transvaginal ultrasound and computed tomography of the abdomen and pelvis did not reveal any ovarian or adrenal masses.
Given a family history of ovarian and uterine cancer in her mother and presumed ovarian source of excessive testosterone, it was decided to proceed with hysterectomy and bilateral salpingo-oophorectomy. The right ovary demonstrated a 1.5-cm tumor that was histologically similar to those in cases 1 and 2 and showed diffuse expression for SF-1 by immunohistochemistry, consistent with ovarian SCT-NOS. The tumor showed scattered mitotic figures with a count of 2/10 high-power fields. The Ki67 proliferation index was approximately 3%. Postsurgical evaluation revealed normalization of her testosterone to 7.1 ng/dL.
Results
In order to understand the specific steroidogenic enzymes expressed in ovarian SCT-NOS, we performed IHC staining for the enzymes involved in the ovarian steroidogenic pathway.
The tumor cells from all 4 specimens showed diffuse moderate-to-strong expression for cholesterol side-chain cleavage enzyme (CYP11A1), 17α-hydroxylase (CYP17A1), 17β-hydroxysteroid dehydrogenase 1 (HSD17B1), aldo-ketoreductase type 1 C3 (AKR1C3), 3β-hydroxysteroid dehydrogenase 2 (HSD3B2), 5α-reductase type 2 (SRD5A2), steroid sulfatase (SULT2A1), estrogen sulfotransferase (EST), and aromatase (CYP19A1). Expression was negative for 21-hydroxylase (CYP21A2), and 17β-hydroxysteroid dehydrogenase 2 (HSD17B2). 5α-reductase type 1 (SRD5A1) and 17β-hydroxysteroid dehydrogenase 3 (HSD17B3) showed variable weak expression (see Table 3 and Figure 2).
Table 3.
Steroidogenic Enzyme Expression in Ovarian SCT-NOS.
| Enzyme | Gene | Case 1 |
Case 2 |
Case 2 |
Case 3 |
||||
|---|---|---|---|---|---|---|---|---|---|
| LT ovary SCT-NOS | H-score | LT ovary SCT-NOS | H-score | RT ovary SCT-NOS | H-score | RT ovary SCT-NOS | H-score | ||
| CYP11A1 | CYP11A1 | + | 199.492 | + (weak) | 22.886 | + | 168.293 | + | 175.099 |
| CYP17A1 | CYP17A1 | + | 173.367 | + | 147.419 | + | 184.529 | + | 180.549 |
| CYP19A1 | CYP19A1 | + | 180.944 | + | 187.654 | + (weak) | 96.132 | + | 287.459 |
| CYP21 | CYP21A2 | − | 0.495 | − | 1.938 | − | 0.648 | + (weak) | 77.336 |
| HSD3B2 | HSD3B2 | + | 161.673 | + | 114.65 | + (weak) | 89.88 | + | 114.175 |
| HSD17B1 | HSD17B1 | + | 181.624 | + | 165.089 | + | 103.299 | + | 190.157 |
| HSD17B2 | HSD17B2 | − | 2.111 | + | 192.386 | + | 194.015 | + (weak) | 64.426 |
| HSD17B3 | HSD17B3 | − | 9.091 | + (weak) | 83.242 | + (weak) | 74.829 | + | 173.114 |
| AKR1C3 | AKR1C3 | + | 155.309 | + | 171.306 | + | 177.76 | + | 168.952 |
| SRD5A1 | SRD5A1 | + (weak) | 20 | + (weak) | 18.039 | - | 0.231 | + | 187.359 |
| SRD5A2 | SRD5A2 | + | 197.821 | + | 185.714 | + | 195.641 | + | 131.992 |
| SULT2A1 | SULT2A1 | + | 148.196 | + | 153.15 | + (weak) | 95.77 | + | 183.893 |
| EST | SULT1E1 | + | 176.679 | + | 179.513 | + | 137.408 | + (weak) | 94.155 |
Abbreviations: SCT-NOS, steroid cell tumors, not otherwise specified; LT, left; RT, right; CYP11A1, cholesterol side-chain cleavage enzyme; CYP17A, 17α-hydroxylase; CYP19A1, aromatase; CYP21, 21α-hydroxylase; HSD3B2, 3β-hydroxysteroid dehydrogenase-2; HSD17B1, 17β-hydroxysteroid dehydrogenase-1; HSD17B2, 17β-hydroxysteroid dehydrogenase-2; HSD17B3, 17β-hydroxysteroid dehydrogenase-3; 17-βHSD5/AKR1C3, 17β-hydroxysteroid dehydrogenase-5/aldo-keto reductase 1C3; SRD5A1, 5α-reductase type 1; SRD5A2, 5α-reductase type 2; SULT2A1, steroid sulfatase; EST, estrogen sulfotransferase.
Figure 2.
Immunohistochemical evaluation.
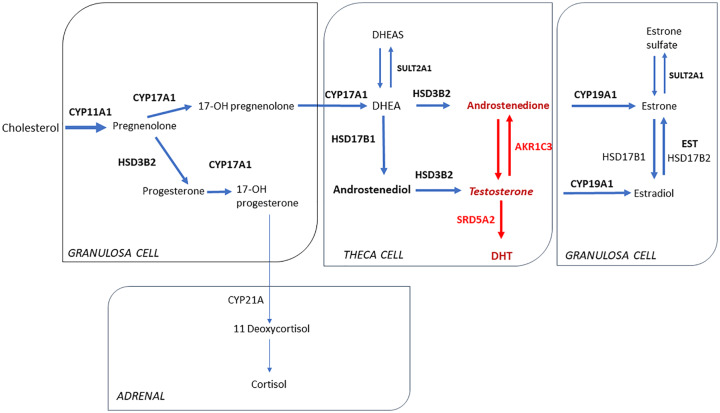
This pattern of enzyme expression is novel in that AKR1C3 and SRD5A2 could be driving androgen synthesis in the ovary (Figure 3).
Figure 3.
Major pathways of ovarian steroidogenesis in solid lines. Red arrows show increased enzyme expression in ovarian NOS.
Discussion
In this study, our index cases presented with hyperandrogenism from an ovarian tumor, which on histopathological examination was consistent with the diagnosis of ovarian SCT-NOS. Subsequently, we further characterized the tumors with IHC analysis and compared the expression of the steroidogenic enzymes involved with other ovarian hyperandrogenic conditions published in the literature.
During our evaluation for hyperandrogenism, we ruled out the diagnosis of NCCAH and iatrogenic hyperandrogenism. More so, it was clear that SCT-NOS was the diagnosis after the laboratory workup and improvement of symptoms after surgical resection.
Our results revealed diffuse expression for the following steroidogenic enzymes by IHC analysis: CYP11A1, CYP17A1, CYP19A1, AKR1C3, HSD17B1, HSD3B2, SRD5A2, SULT2A1, and EST (Figure 2). Similar to this study, previous studies of ovarian steroid cell tumors have shown intense IHC expression for enzymes CYP11A1, CYP17A1, and CYP19A1; however, expression for AKR1C3 was not assessed.12 Our analysis of AKR1C3 showed increased ovarian expression for this enzyme. Previous studies have also shown positive CYP11A1, CYP17A1, and HSD3B2 as part of the steroidogenic pathway in patients with PCOS. More recently, evidence has emerged for the role of AKR1C3 in androgen-activation in subcutaneous adipose tissue of PCOS patients,13 and in the ovarian stroma adjacent to ovarian epithelial tumors.14 In addition to PCOS, the expression of CYP17A1 is also present in ovarian hyperthecosis and normal ovarian tissue.
Expression of AKR1C3 is critical in the conversion of androstenedione to testosterone in ovarian theca cells.15,16 In addition to reducing the steroid precursor 4-androstene-3,17-dione to testosterone, AKR1C3 is also involved in the reduction of estrone to 17β-estradiol in target tissues.17 AKR1C3 is overexpressed in a wide variety of cancers, and its increased activity is believed to promote cellular proliferation in hormone-dependent cancers.18 Given the expression of AKR1C3 in these tumors, it could be hypothesized that they might originate from theca cells.19 To the best of our knowledge, the cases described in this study are the first to demonstrate the presence of AKR1C3 in ovarian SCT-NOS.
The other novel result from this analysis showed increased expression of SRD5A2 in SCT-NOS. Testosterone is converted to dihydrotestosterone (DHT) by the SRD5A2 enzyme that is expressed in target tissues.20 DHT is a more potent androgen than testosterone, and it is responsible for secondary masculine characteristics including body hair and voice deepening.15 The SRD5A enzyme has 2 isoforms–type 1 is found in scalp and other peripheral tissues, and type 2 is the predominant form of the enzyme found in male reproductive tissues.21,22
HSD17B1 was also found to be expressed in SCT-NOS, which is fundamental in the conversion of DHEA into androstenediol.23 However, HSD17B3 expression was either weak or negative in most of our SCT-NOS likely because it is predominantly found in Leydig cells where it converts androstenedione to testosterone.14
Based on the observations from the present series of SCT-NOS, it appears that the main pathway driving testosterone production is from androstenedione to testosterone via AKR1C3.
Interestingly, 2 of the 3 cases had nonfunctional adrenal adenomas. There have been other reports of an association of ovarian tumors with adrenal masses.1,24 Further studies are needed to assess if these concurrences represent a significant pathological pattern.
Given the clinical challenges posed by these cases, IHC analysis of the steroidogenic pathway may serve as a useful tool in localization of specific enzyme sites involved in ovarian steroidogenesis.
Conclusion
In the present study, we characterized ovarian SCT-NOS by IHC. Ovarian SCT-NOS have an unknown prevalence and is currently underdiagnosed. This entity should be considered in patients who present with rapid virilization and elevated testosterone levels. Clinically, the diagnosis of SCT-NOS is challenging as imaging does not always reveal any ovarian lesions. Histologically, specific enzymes involved in ovarian steroidogenesis may help to define the site of origin for androgen excess in ovarian tumors. Further studies are needed to validate our novel findings of increased expression of AKR1C3, HSD17B1, SRD5A2, SULT2A1, and EST in ovarian SCT-NOS.
Supplemental Material
Supplemental material, Supplemental_figure_1 for Expression of Key Androgen-Activating Enzymes in Ovarian Steroid Cell Tumor, Not Otherwise Specified by Evana Valenzuela Scheker, Amita Kathuria, Ashwini Esnakula, Hironobu Sasano, Yuto Yamazaki, Sergei Tevosian, Richard J. Auchus, Hans K. Ghayee and Gauri Dhir in Journal of Investigative Medicine High Impact Case Reports
Supplemental material, Supplemental_material for Expression of Key Androgen-Activating Enzymes in Ovarian Steroid Cell Tumor, Not Otherwise Specified by Evana Valenzuela Scheker, Amita Kathuria, Ashwini Esnakula, Hironobu Sasano, Yuto Yamazaki, Sergei Tevosian, Richard J. Auchus, Hans K. Ghayee and Gauri Dhir in Journal of Investigative Medicine High Impact Case Reports
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Verbal informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Tutzer M, Winnykamien I, Davila Guardia JD, Castelo-Branco C. Hyperandrogenism in post-menopausal women: a diagnosis challenge. Gynecol Endocrinol. 2014;30:23-25. [DOI] [PubMed] [Google Scholar]
- 2. Alpanes M, Gonzalez-Casbas JM, Sanchez J, Pián H, Escobar-Morreale HF. Management of postmenopausal virilization. J Clin Endocrinol Metab. 2012;97:2584-2588. [DOI] [PubMed] [Google Scholar]
- 3. Young R, Scully RE. Sex cord-stromal, steorid cell, and other ovarian tumors with endocrine, paraendocrine, and paraneoplastic manifestations. In: Kurman RJ, Ellenson LH, Ronnett BM. eds. Blaustein’s Pathology of the Female Genital Tract. Springer; 1987;826-832. [Google Scholar]
- 4. Hayes MC, Scully RE. Ovarian steroid cell tumors (not otherwise specified). A clinicopathological analysis of 63 cases. Am J Surg Pathol. 1987;11:835-845. [DOI] [PubMed] [Google Scholar]
- 5. Sood N, Desai K, Chindris AM, Lewis J, Dinh TA. Symptomatic ovarian steroid cell tumor not otherwise specified in a post-menopausal woman. Rare Tumors. 2016;8:6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taylor HB, Norris HJ. Lipid cell tumors of the ovary. Cancer. 1967;20:1953-1962. [DOI] [PubMed] [Google Scholar]
- 7. Zhao C, Vinh TN, McManus K, Dabbs D, Barner R, Vang R. Identification of the most sensitive and robust immunohistochemical markers in different categories of ovarian sex cord-stromal tumors. Am J Surg Pathol. 2009;33:354-366. [DOI] [PubMed] [Google Scholar]
- 8. Sasano H, Edwards DP, Anderson TJ, et al. Validation of new aromatase monoclonal antibodies for immunohistochemistry: progress report. J Steroid Biochem Mol Biol. 2003;86:239-244. [DOI] [PubMed] [Google Scholar]
- 9. Kubota-Nakayama F, Nakamura Y, Konosu-Fukaya S, et al. Expression of steroidogenic enzymes and their transcription factors in cortisol-producing adrenocortical adenomas: immunohistochemical analysis and quantitative real-time polymerase chain reaction studies. Hum Pathol. 2016;54:165-173. doi: 10.1016/j.humpath.2016.03.016 [DOI] [PubMed] [Google Scholar]
- 10. Tezuka Y, Yamazaki Y, Ono Y, et al. Unique sex steroid profiles in estrogen-producing adrenocortical adenoma associated with bilateral hyperaldosteronism. J Endocr Soc. 2020;4:bvaa004. doi: 10.1210/jendso/bvaa004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ishibashi H, Suzuki T, Suzuki S, et al. Sex steroid hormone receptors in human thymoma. J Clin Endocrinol Metab. 2003;88:2309-2317. [DOI] [PubMed] [Google Scholar]
- 12. Sasano H, Okamoto M, Mason JI, et al. Immunohistochemical studies of steroidogenic enzymes (aromatase, 17 alpha-hydroxylase and cholesterol side-chain cleavage cytochromes P-450) in sex cord-stromal tumors of the ovary. Hum Pathol. 1989;20:452-457. [DOI] [PubMed] [Google Scholar]
- 13. O’Reilly MW, Kempegowda P, Walsh M, et al. AKR1C3-mediated adipose androgen generation drives lipotoxicity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102:3327-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blanco LZ, Jr, Kuhn E, Morrison JC, Bahadirli-Talbott A, Smith-Sehdev A, Kurman RJ. Steroid hormone synthesis by the ovarian stroma surrounding epithelial ovarian tumors: a potential mechanism in ovarian tumorigenesis. Mod Pathol. 2017;30:563-576. [DOI] [PubMed] [Google Scholar]
- 15. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32:81-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qin KN, Rosenfield RL. Expression of 17 beta-hydroxysteroid dehydrogenase type 5 in human ovary: a pilot study. J Soc Gynecol Investig. 2000;7:61-64. [DOI] [PubMed] [Google Scholar]
- 17. Rizner TL, Penning TM. Role of aldo-keto reductase family 1 (AKR1) enzymes in human steroid metabolism. Steroids. 2014;79:49-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447-4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nelson VL, Qin KN, Rosenfield RL, et al. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86:5925-5933. [DOI] [PubMed] [Google Scholar]
- 20. Bruchovsky N, Wilson JD. The intranuclear binding of testosterone and 5-alpha-androstan-17-beta-ol-3-one by rat prostate. J Biol Chem. 1968;243:5953-5960. [PubMed] [Google Scholar]
- 21. Jenkins EP, Andersson S, Imperato-McGinley J, Wilson JD, Russell DW. Genetic and pharmacological evidence for more than one human steroid 5 alpha-reductase. J Clin Invest. 1992;89:293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Andersson S, Berman DM, Jenkins EP, Russell DW. Deletion of steroid 5 alpha-reductase 2 gene in male pseudohermaphroditism. Nature. 1991;354:159-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Labrie F, Luu-The V, Lin SX, et al. The key role of 17 beta-hydroxysteroid dehydrogenases in sex steroid biology. Steroids. 1997;62:148-158. [DOI] [PubMed] [Google Scholar]
- 24. Sullivan H, Dennis BA. Visual vignette. Endocr Pract. 2016;22:1137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_figure_1 for Expression of Key Androgen-Activating Enzymes in Ovarian Steroid Cell Tumor, Not Otherwise Specified by Evana Valenzuela Scheker, Amita Kathuria, Ashwini Esnakula, Hironobu Sasano, Yuto Yamazaki, Sergei Tevosian, Richard J. Auchus, Hans K. Ghayee and Gauri Dhir in Journal of Investigative Medicine High Impact Case Reports
Supplemental material, Supplemental_material for Expression of Key Androgen-Activating Enzymes in Ovarian Steroid Cell Tumor, Not Otherwise Specified by Evana Valenzuela Scheker, Amita Kathuria, Ashwini Esnakula, Hironobu Sasano, Yuto Yamazaki, Sergei Tevosian, Richard J. Auchus, Hans K. Ghayee and Gauri Dhir in Journal of Investigative Medicine High Impact Case Reports