Abstract
Objective
To evaluate the hyperintense signal (HIS) performance on diffusion-weighted imaging (DWI) in diagnosing cerebral venous thrombosis (CVT).
Methods
Seventy-eight patients with CVT hospitalized from January 2004 to January 2015 were retrospectively studied alongside 78 controls without intracranial organic diseases. Diagnostic accuracy indices of HIS on DWI or T2-weighted imaging (T2WI) to diagnose CVT at different sites and states were analyzed.
Results
The overall sensitivity of HIS on DWI for the diagnosis of CVT was significantly lower than that of HIS on T2WI (34.6% vs. 79.5%). HIS on T2WI was more sensitive than HIS on DWI in detecting thrombosis, especially in the superior sagittal sinus and transverse sinus. HIS on DWI was inversely related to the time between disease onset and imaging. Compared with HIS on T2WI, combining HIS on DWI and T2WI did not increase the sensitivity for detecting CVT. HIS on DWI was not detected in the control group, but HIS on T2WI was detected in 26.3% of control individuals. The specificity of HIS on DWI for CVT was higher than that of HIS on T2WI (97.4% vs. 76.9%).
Conclusion
HIS on DWI has a lower sensitivity, but a higher specificity, than HIS on T2WI for diagnosing CVT.
Keywords: Cerebral venous thrombosis, magnetic resonance imaging, diffusion-weighted imaging, T2-weighted imaging, hyperintense signal, diagnostic sensitivity, diagnostic specificity
Introduction
Cerebral venous thrombosis (CVT) is a rare cerebrovascular disease that accounts for approximately 0.5% to 1% of all strokes.1,2 Because specific signs and symptoms of CVT are lacking, a diagnosis of CVT mainly relies on neuroimaging findings. T2-weighted imaging (T2WI) with magnetic resonance venography (MRV) is the first line of imaging modalities.2 The use of T2WI is advantageous because it diminishes the likelihood that hypoplasia of a venous sinus is misdiagnosed as CVT, by producing a relatively hyperintense signal (HIS) as a result of the absence of flow void signal and paramagnetic ingredients. As well as HIS on T2WI, thrombus in a venous sinus can also result in HIS at the corresponding site on diffusion-weighted imaging (DWI).
Studies of the utility of DWI for diagnosing CVT have revealed heterogeneous findings. Both variable and reversible changes in the apparent diffusion coefficient (ADC) at the time of acute events in CVT have been observed.3,4 Using DWI for CVT, it has also been speculated that cytotoxic edema (the movement of water from extracellular space into cells) can be the cause of CVT.5 The sensitivity of HIS on DWI for CVT has been evaluated in a small series of cases. Favrole et al.,6 using DWI, detected recent CVTs in 20 occluded vein(s) or sinus(es) in 41% (12/28) of patients. These authors also suggested that the presence of HIS on DWI in occluded veins at the time of diagnosis might be predictive of a lower rate of vessel recanalization 2 or 3 months later. Furthermore, Yıldız et al.7 evaluated different DWI findings for the diagnosis of CVT, and suggested that DWI can provide an additional clue in the diagnosis of CVT patients, and may be important for diagnosing patients without clinical signs of CVT.
Previous studies have reported variable outcomes with regard to HIS on DWI in patients with CVT. HIS on DWI has been found in as few as 6% (vs. 53% on T2*WI)8 and 21% (vs. 93% on T2*WI)9 of cases to as high as 80% (vs. 81% on T2WI)10 and 83%11 of cases. However, the diagnostic use of HIS on DWI to diagnose CVT has been less studied, and remains relatively obscure. In the present study, we retrospectively analyzed CVT cases who had undergone DWI at our center, with the aim to investigate the diagnostic performance of DWI in diagnosing CVT.
Patients and methods
Patient enrollment
For this retrospective study, data were acquired from the database of the Chinese PLA General Hospital for patients with CVT who had been hospitalized in the Neurology Department between January 2004 and January 2015. The inclusion criteria for patients were: (1) clinical signs and symptoms consistent with CVT; (2) occlusion or severe stenosis of the cerebral venous sinus or internal jugular vein (IJV) confirmed by digital subtraction angiography (DSA) and/or MRV and/or computed tomographic venography;12,13 and (3) high-quality brain transverse section images of DWI and T2WI were available. Patients with other neurological diseases, such as intracranial infection, tumor, inflammation, arterial stroke, or focal short-segment stenosis of the transverse sinus (TS) or sigmoid sinus (SiS) were excluded. The control group comprised age- and sex-matched individuals with no intracranial organic disease or CVT-related signs or symptoms, but who had undergone craniocerebral MRI including DWI as part of their medical examination. Informed consent was not required for this study because the subjects’ details were de-identified during retrospective analyses. The study was approved by the Ethics Committee of the Chinese PLA General Hospital.
Imaging protocols
MRIs were obtained on a 1.5-T scanner (TwinSpeed HDxt, GE Healthcare, Waukesha, WI, USA). Typical parameters were: (1) axial T2WI: fast spin-echo imaging (TR/TE = 5000 ms/114 ms, slice thickness = 5.0 mm, slice gap = 0.5 mm, field of view [FOV] = 24 × 24 cm, matrix = 288 × 244); (2) axial DWI: single-shot spin-echo echoplanar imaging (TR/TE = 6500 ms/110 ms, slice thickness = 5.0 mm, slice gap = 0.5 mm, FOV = 24 × 24 cm, matrix = 128 × 12, b = 1000 s/mm2).
Imaging analysis
Patients with CVT were categorized into three groups according to the length of the interval between disease onset and MRI scan: 1) ≤7 days; 2) 8 to 30 days; and 3) ≥31 days. The diagnosis of CVT and the site of thrombosis on the DSA and/or MRV were made by a neurologist with 10 years of experience in cerebrovascular diseases. HIS on T2WI and HIS on DWI were determined by two neurologists who were blinded to the clinical and angiographic data of patients (Figure 1). Kappa statistics were applied for interobserver agreement. When disagreement arose in the independent analysis, a consensus was reached through discussion. The venous sinuses and veins studied in each patient included the superior sagittal sinus (SSS), left and right TS, left and right SiS, straight sinus (StS), and left and right IJV.
Figure 1.
Hyperintensities on diffusion-weighted imaging (DWI) caused by thrombosis in the superior sagittal sinus (a–d), transverse sinuses (e), and sigmoid sinuses (f). DWI hypointensity in normal sinuses (g).
Statistical analysis
The sites of thrombosis were determined according to the results of the DSA/MRV findings, and these were used as the gold standard to study the sensitivity of HIS on DWI, HIS on T2WI, and their combined use for the diagnosis of CVT at different sites and stages. The result of a combined analysis of HIS on DWI and HIS on T2WI was considered positive when both modalities detected CVT in a case. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of HIS on DWI or T2WI for CVT were calculated from the true positive, false positive, true negative, and false negative values. Comparative analyses of the diagnostic indices were performed using an R × C table Pearson’s χ2 test, a corrected χ2 test, or a Fisher’s exact probability test. All statistical analyses were performed using SPSS for Windows, version 19.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was defined as p < 0.05.
Results
In this study, 78 patients (45 women and 33 men) with CVT and 78 age- and sex-matched controls were included. The mean age of the patients was 36.1 ± 12.7 years. All 78 patients underwent MRV, and 43 patients also underwent DSA. No patients underwent computed tomographic venography. Of the 624 intracranial venous sinuses and 156 IJVs studied, 287 venous sinuses and 29 IJVs had thrombosis. The interval between disease onset and MRI scan in patients with CVT ranged between 1 and 1,825 days (median 34 days).
Thromboses occurred mostly in the TS, followed by the SSS, SiS, IJV, and StS. The right TS, SiS, and IJV more commonly harbored thrombosis compared with the left TS, SiS, and IJV (Table 1, Figure 2). The detection of HIS on DWI according to the interval between the onset of symptoms and MRI scan are summarized in Table 2, as are the between-group comparisons.
Table 1.
The sensitivities of HIS on DWI, HIS on T2*WI, and a combination of both for CVT diagnosis.
| Site of thrombosis | Number of cases | HIS on DWI |
HIS on T2*WI |
HIS on DWI and T2*WI |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| + | − | Sensitivity (%) | + | − | Sensitivity (%) | + | − | Sensitivity (%) | ||
| SSS | 53 | 28 | 25 | 52.8 | 45 | 8 | 84.9 | 45 | 8 | 84.9 |
| STS | 20 | 1 | 19 | 5.0 | 3 | 17 | 15.0 | 3 | 17 | 15.0 |
| Left TS | 55 | 7 | 48 | 12.7 | 37 | 18 | 67.3 | 38 | 17 | 69.1 |
| Left SIS | 40 | 1 | 39 | 2.5 | 11 | 29 | 27.5 | 11 | 29 | 27.5 |
| Left IJV | 10 | 0 | 10 | 0 | 1 | 9 | 10.0 | 1 | 9 | 10.0 |
| Right TS | 64 | 17 | 47 | 26.6 | 43 | 21 | 67.2 | 43 | 21 | 67.2 |
| Right SIS | 51 | 4 | 47 | 7.8 | 19 | 32 | 37.3 | 19 | 32 | 37.3 |
| Right IJV | 19 | 3 | 16 | 15.8 | 8 | 11 | 42.1 | 8 | 11 | 42.1 |
Abbreviations: CVT: cerebral venous thrombosis; DWI: diffusion-weighted imaging; HIS: hyperintense signal; IJV: internal jugular vein; SIS: sigmoid sinus; SSS: superior sagittal sinus; STS: straight sinus; T2*WI: T2*-weighted imaging.
Figure 2.
Normal superior sagittal sinus (A), transverse sinus (B), and sigmoid sinus (C) on diffusion-weighted imaging (DWI), T1-weighted imaging (T1WI), and T2-weighted imaging (T2WI). Thromboses in the superior sagittal sinus (A′), transverse sinus (B′), and sigmoid sinus (C′) on DWI, T1WI, and T2WI. The arrows indicate the sites of the venous sinuses. Normal venous sinuses manifest as “flow-void” hypointense signals. Venous sinuses with thrombosis manifest as hyperintense signals.
Table 2.
Different stages of HIS on DWI for CVT diagnosis.
| Interval between onset of symptoms and MRI | Number of cases | HIS in any sinus or IJV on DWI |
Intergroup comparison |
χ 2 | P | ||
|---|---|---|---|---|---|---|---|
| + | − | χ 2 | P | ||||
| ≤7 days | 10 | 7 (70.0%) | 3 (30.0%) | 0.007a | 0.931 | 16.492 | 0.000 |
| 8–30 days | 26 | 16 (61.5%) | 10 (38.5%) | 12.696b | 0.000 | ||
| ≥31 days | 42 | 8 (19.0%) | 34 (43.6%) | 7.885c | 0.005 | ||
Abbreviations: CVT, cerebral venous thrombosis; DWI, diffusion-weighted imaging; HIS, hyperintense signal; IJV, internal jugular vein; MRI: magnetic resonance imaging. a≤7 days group vs. 8 to 30 days group, b8 to 30 days group vs. ≥31 days group, c≤7 days group vs. ≥31 days group.
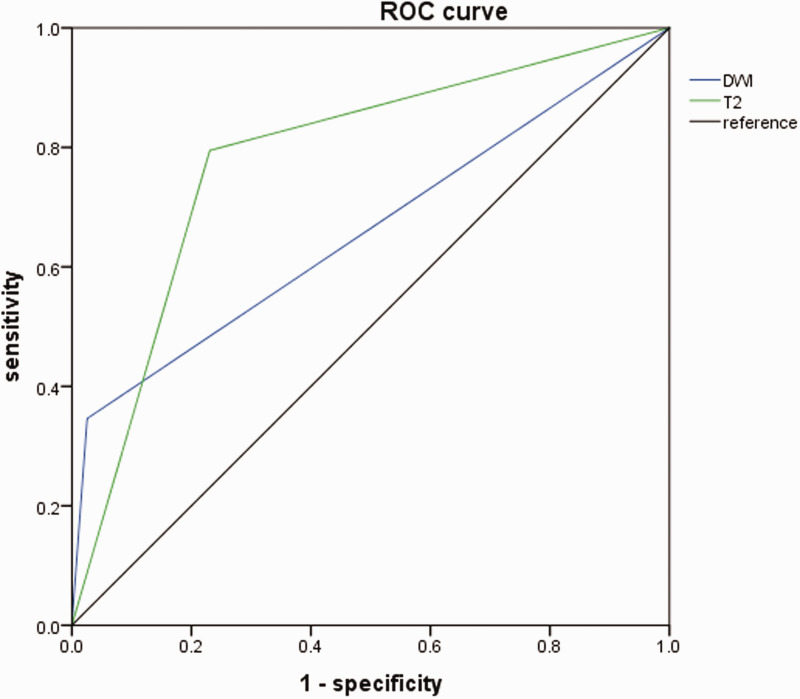
The overall sensitivity of HIS on DWI for CVT was 34.6%, which was significantly lower than the sensitivity of HIS on T2WI (79.5%; Table 3; Figure 3). The overall specificity of HIS on DWI for CVT was significantly higher than the specificity of HIS on T2WI (97.4% vs. 76.9%). The sensitivity, specificity, PPV, and NPV of DWI, T2WI, and DWI plus T2WI are shown in Table 3.
Table 3.
The sensitivity and specificity of HIS on DWI for CVT diagnosis.
| MRI method | DSA/MRV |
Sensitivity (%) | Specificity (%) | AUC | PPV (%) | NPV (%) | kappa | P | ||
|---|---|---|---|---|---|---|---|---|---|---|
| + | − | |||||||||
| DWI | + | 27 | 2 | |||||||
| − | 51 | 76 | 34.6 | 97.4 | 0.660 | 93.1 | 59.8 | 0.321 | 0.000 | |
| T2*WI | + | 62 | 18 | |||||||
| − | 16 | 60 | 79.5 | 76.9 | 0.782 | 77.5 | 78.9 | 0.564 | 0.000 | |
| T1*WI | + | 64 | 54 | |||||||
| − | 14 | 24 | 82.1 | 30.8 | 0.564 | 54.2 | 63.2 | 0.128 | 0.062 | |
| DWI+T2*WI | + | 62 | 20 | |||||||
| − | 16 | 58 | 79.5 | 74.4 | 0.545 | 75.6 | 78.4 | 0.538 | 0.000 | |
Abbreviations: AUC: area under the curve; CVT: cerebral venous thrombosis; DSA: digital subtraction angiography; DWI: diffusion-weighted imaging; HIS: hyperintense signal; NPV: negative predictive value; PPV: positive predictive value; T1*WI: T1*-weighted imaging; T2*WI: T2*-weighted imaging.
Figure 3.
Receiver operator characteristic (ROC) curves showing the differences in diagnostic performances of hyperintense signal on diffusion-weighted imaging (DWI) and T2-weighted imaging (T2).
The sensitivity of T2WI and T2WI plus DWI in diagnosing CVT was the same (Table 1). The correlation coefficient between the sensitivity of DWI and the sensitivity of T2WI in detecting HIS in different sinuses and veins was 0.862. HIS on DWI was not detected in the control group, but HIS on T2WI was detected in 23.1% of control individuals.
Discussion
In the present study, the sensitivity of HIS on DWI for the diagnosis of CVT was low (34.6%), which is consistent with the results obtained previously by Lovblad et al.14 and Favrole et al.6 However, in our study, the specificity of HIS on DWI was higher than that of T2WI. In addition, the combination of HIS on DWI and T2WI was not any more sensitive than that of T2WI alone in detecting CVT. Moreover, all HIS on DWI were observed in patients who also had HIS on T2WI. These results suggest that HIS on DWI might have been influenced by the signal intensity on T2WI, via the T2-shine-through effect. It is therefore not necessary to perform DWI to increase the sensitivity of CVT detection when T2WI is performed.
In our patients, HIS on DWI was detected in 70% of the patients in the ≤7 days (from disease onset to MRI) group, and in 61.5% of the 8 to 30 days group. A high incidence of HIS in the 8 to 30 days group most likely resulted from a late subacute thrombus, which is similar to a late subacute hematoma in terms of the biophysical characteristics that are related to its manifestation on DWI. An intravascular thrombus differs from an intracerebral hematoma in that a thrombus does not contain extracellular fluid; this may result in more severely restricted diffusion and, consequently, a stronger HIS on DWI.
In the present study, the incidence of HIS on DWI for CVT was markedly lower by 31 days after the onset of symptoms. This outcome is consistent with a previous report in which HIS in sinuses were detected in the first DWI of 12 of 28 patients, and 27 patients had disease onset within 30 days. Repeated DWI after 2 to 3 months indicated the complete disappearance of HIS in that study.6 A possible explanation for HIS on DWI being detected 31 days after the onset of symptoms in our patients is that venous thrombosis is a dynamic process; thus, a newly formed thrombus can occur long after the onset of symptoms.
Although HIS on DWI did not increase the sensitivity of MRI to diagnose CVT compared with the conventional T2WI, our results indicated that the value of HIS on DWI may lie in its high specificity. In our study, HIS on T2WI was observed in 23% of individuals without CVT, but no HIS on DWI was observed. The anatomical basis of this observation may be the presence of hypoplasia of the venous sinus, giant arachnoid granulations, or brain herniation into the venous sinus.15–21 These anatomical variations may result in both a stenosis-like appearance on venography imaging and HIS on T2WI.
Because MRI is currently a routine neuroimaging modality employed in patients with suspected CVT, knowledge about the different features of CVT on different MRI sequences is crucial for establishing a diagnosis. Thrombus in a venous sinus or vein is composed of a fibrin network and trapped red blood cells. The imaging features of an evolving intracerebral hematoma and its underlying biophysical mechanisms might also be applicable to an intravascular clot, at least to some extent. On DWI, the hematoma core is reported to be hyperintense at the hyperacute and late subacute stages, but hypointense at the acute, early subacute, and chronic stages.22–24 Compared with normal white matter, stable-but-reduced diffusion is observed in hyperacute, acute, and early subacute hematomas containing hemoglobin within intact red blood cells.22,23,25
With simultaneous quantitative analysis of ADCs and the signal intensity ratio of intracerebral hematoma on DWI and T2WI, there was a positive correlation of the signal intensity ratio on DWI with that on T2WI, but not with the ADC.23 The signal intensities of hematoma on DWI may be dominated by those on T2WI via T2-shine-through or T2-blackout effects. A previous study provided further evidence that signal intensities on T2WI, rather than diffusion status, determine the signal intensity on DWI; they demonstrated that there was no significant difference between the diffusion status in hematomas containing intact red blood cells and normal white matter.26
Our study had some limitations. First, this was a retrospective study with a relatively small sample size, and we included patients who were admitted over a long period of time. In addition, MRI was not repeated at scheduled times; thus, it was not possible to precisely describe the evolution of signal intensity on DWI at different stages of CVT. Second, the ADC data were not available for most patients, and so the exact mechanisms underlying HIS on DWI were not able to be ascertained. Finally, the proportion of thrombosis in TS and SiS might have been overestimated because coexisting hypoplasia can be misdiagnosed as thrombosis using the criteria that we employed.
In summary, our results verified that HIS can be found at sites of thrombosis on DWI in some patients with CVT. The diagnostic value of HIS on DWI for CVT may depend on its high specificity. HIS on T2WI had a higher sensitivity than HIS on DWI for diagnosing CVT, especially in the SSS and TS. However, HIS on T2WI had a low specificity compared with HIS on DWI. DWI can be useful to differentiate CVT from intravenous non-thrombotic tissue, which can result in the manifestation of stenosis on venography imaging and HIS on T2WI.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Cheng-lin Tian https://orcid.org/0000-0002-2455-6930
References
- 1.Stam J. Thrombosis of the cerebral veins and sinuses. N Eng J Med 2005; 353: 314–315. [PubMed] [Google Scholar]
- 2.Saposnik G, Barinagarrementeria F, Brown RD, et al. Diagnosis and management of cerebral venous thrombosis a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42: 1158. [DOI] [PubMed] [Google Scholar]
- 3.Corvol JC, Oppenheim C, Manai R, et al. Diffusion-weighted magnetic resonance imaging in a case of cerebral venous thrombosis. Stroke 1998; 29: 2649–2652. [DOI] [PubMed] [Google Scholar]
- 4.Ducreux D, Oppenheim C, Vandamme X, et al. Diffusion-weighted imaging patterns of brain damage associated with cerebral venous thrombosis. AJNR Am J Neuroradiol 2001; 22: 261–268. [PMC free article] [PubMed] [Google Scholar]
- 5.Forbes KP, Pipe JG, Heiserman JE. Evidence for cytotoxic edema in the pathogenesis of cerebral venous infarction. AJNR Am J Neuroradiol 2001; 22: 450–455. [PMC free article] [PubMed] [Google Scholar]
- 6.Favrole P, Guichard JP, Crassard I, et al. Diffusion-weighted imaging of intravascular clots in cerebral venous thrombosis. Stroke 2004; 35: 99–103. [DOI] [PubMed] [Google Scholar]
- 7.Yıldız ME, Ozcan UA, Turk A, et al. Diffusion-weighted MR imaging findings of cortical vein thrombosis at 3 T. Clin Neuroradiol 2015; 25: 249–256. [DOI] [PubMed] [Google Scholar]
- 8.Bidar F, Faeghi F, Ghorbani A. Assessment of cerebral venous sinus thrombosis using T2 (*)-weighted gradient echo magnetic resonance imaging sequences. Iran J Neurol 2016; 15: 96–99. [PMC free article] [PubMed] [Google Scholar]
- 9.Idbaih A, Boukobza M, Crassard I, et al. MRI of clot in cerebral venous thrombosis: high diagnostic value of susceptibility-weighted images. Stroke 2006; 37: 991–995. [DOI] [PubMed] [Google Scholar]
- 10.Mullins ME, Grant PE, Wang B, et al. Parenchymal abnormalities associated with cerebral venous sinus thrombosis: assessment with diffusion-weighted MR imaging. AJNR Am J Neuroradiol 2004; 25: 1666–1675. [PMC free article] [PubMed] [Google Scholar]
- 11.Lovblad K, Schneider J, El-Koussy M, et al. Diffusion-weighted MRI in human cerebral venous thrombosis. Proc Intl Sot Mag Reson Med 2000; 8: 1225. [Google Scholar]
- 12.Ameri A, Bousser MG. Cerebral venous thrombosis. Neurol Clin 1992; 10: 87–111. [PubMed] [Google Scholar]
- 13.Dormont D, Anxionnat R, Evrad S. MRI in cerebral venous thrombosis. J Neuroradiol 1994; 21: 81–99. [PubMed] [Google Scholar]
- 14.Lövblad KO, Bassetti C, Schneider J, et al. Diffusion-weighted MRI in cerebral venous thrombosis. Cerebrovasc Dis 2001; 11: 169. [DOI] [PubMed] [Google Scholar]
- 15.Durst CR, Ornan DA, Reardon MA, et al. Prevalence of dural venous sinus stenosis and hypoplasia in a generalized population. J Neurointerv Surg 2016; 8: 1173. [DOI] [PubMed] [Google Scholar]
- 16.Leach JL, Meyer K, Jones BV, et al. Large arachnoid granulations involving the dorsal superior sagittal sinus: findings on MR imaging and MR venography. Ajnr Am J Neuroradiol 2008; 29: 1335–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang L, Korogi Y, Sugahara T, et al. Normal structures in the intracranial dural sinuses: delineation with 3D contrast-enhanced magnetization prepared rapid acquisition gradient-echo imaging sequence. Am J Neuroradiol 2002; 23: 1739–1746. [PMC free article] [PubMed] [Google Scholar]
- 18.Battal B, Hamcan S, Akgun V, et al. Brain herniations into the dural venous sinus or calvarium: MRI findings, possible causes and clinical significance. Eur Radiol 2016; 26: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 19.Malekzadehlashkariani S, Wanke I, Rüfenacht DA, et al. Brain herniations into arachnoid granulations: about 68 cases in 38 patients and review of the literature. Neuroradiology 2016; 58: 443–457. [DOI] [PubMed] [Google Scholar]
- 20.Keyzer BD, Bamps S, Calenbergh FV, et al. Giant arachnoid granulations mimicking pathology: a report of three cases. Neuroradiol J 2014; 27: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Battal B, Castillo M. Brain herniations into the dural venous sinuses or calvarium: MRI of a recently recognized entity. Neuroradiol J 2014; 27: 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang BK, Na DG, Ryoo JW, et al. Diffusion-weighted MR imaging of intracerebral hemorrhage. Chinese J Med Imaging Technol 2008; 2: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silvera S, Oppenheim C, Touzé E, et al. Spontaneous intracerebral hematoma on diffusion-weighted images: influence of T2-shine-through and T2-blackout effects. Ajnr Am J Neuroradiol 2005; 26: 236. [PMC free article] [PubMed] [Google Scholar]
- 24.Ebisu T, Tanaka C, Umeda M, et al. Hemorrhagic and nonhemorrhagic stroke: diagnosis with diffusion-weighted and T2-weighted echo-planar MR imaging. Radiology 1997; 203: 823–828. [DOI] [PubMed] [Google Scholar]
- 25.Atlas SW, Dubois P, Singer MB, et al. Diffusion measurements in intracranial hematomas: implications for MR imaging of acute stroke. Ajnr Am J Neuroradiol 2000; 21: 1190–1194. [PMC free article] [PubMed] [Google Scholar]
- 26.Maldjian JA, Listerud J, Moonis G, et al. Computing diffusion rates in T2-dark hematomas and areas of low T2 signal. Ajnr Am J Neuroradiol 2001; 22: 112–118. [PMC free article] [PubMed] [Google Scholar]