Abstract
Background:
In recent years, more and more studies have shown that various inflammatory factors have predictive effects on the prognosis of various human tumors. However, the prognostic role of interleukin 18 (IL-18) remains controversial. Furthermore, its role in radiation-induced injuries relating to radiotherapy (RT) is also unclear. In this study, we conducted the meta-analysis to clarify its roles in prognosis of human tumors and radiation-induced injuries relating to RT.
Methods:
We comprehensively searched PubMed, Embase, and Cochrane Library to identify studies published before November 2019 involving patients with cancer expressing IL-18 and which reported overall survival (OS) during the follow-up period.
Results:
A total of 1376 samples from 16 studies showed that high expression of IL-18 is closely related to prognosis and OS for patients with carcinoma (hazard ratio [HR]: 2.12; 95% CI: 1.81-2.49; P = .04; random-effect model). In addition, subgroup analysis proved that high expression of IL-18 was related to poor OS of hematologic tumor (HR: 2.03, 95% CI: 1.44-2.86, P < .00001), hepatocellular carcinoma (HR: 1.99, 95% CI: 1.38-2.86, P = .0002), and gastric cancer (HR: 2.00, 95% CI: 1.12-3.57, P = .02).
Conclusions:
High expression of IL-18 is related with poor prognosis of carcinoma.
Keywords: IL-18, cancer, meta-analysis, biomarker, radiation injuries
Introduction
The incidence and mortality of carcinoma are rapidly growing all over the world. In 2018, it was estimated that there were 17.0 million new cancer cases (excluding 1.1 million nonmelanoma skin cancer) and 9.5 million cancer deaths (excluding 0.1 million nonmelanoma skin cancer).1 It was acknowledged that the early diagnosis plays an important role in treatment of cancer. Therefore, it is necessary to search new biomarkers for early diagnosis of cancers.
Interleukin (IL)-18, previously known as “Interferon gamma-inducing factor,” was initially purified from serum and liver of mice following sequential administration of Propionibacterium acnes in 1989.2 At first, it was believed that this purified cytokine was IL-12, however, by molecular cloning, it was found that this cytokine was different and then named as IL-18.2 After that, IL-18 became a new member of the IL-1 family of cytokines since its structure is relevant to IL-1, especially IL-1β. Firstly, a 24 kDa precursor (IL-18) is synthesized. Then, it is cleaved into an 18 kDa bioactive form by caspase-1 which can be secreted from different cell types.3 Various immune cells including T cells, B cells, natural killer cells, monocytes, dendritic cells (DCs), macrophages, and neutrophils can produce it.4 Interleukin 18 acts as a regulator in the immune response and participates in the immune escape of carcinoma cells, inflammatory reaction, and autoimmune responses lying on the host environment.5,6 Different types of carcinomas produce IL-18, which induces cell migration, invasion, and spread, leading to increased metastasis and tumor growth.6-8 Recently, the prognostic role and value of tumor-infiltrating lymphocytes (TILs) have been widely researched in various types of cancers.9-11 In many clinical studies, IL-18 was discovered to be highly expressed in many malignant tumors, such as laryngeal squamous cell carcinoma (LSCC),12 colorectal cancer (CRC),13 multiple myeloma (MM),14,15 pancreatic cancer (PC),16 hepatocellular carcinoma (HCC),17,18 gastric cancer (GC),19-21 prostate cancer (PrC),22 diffuse large B-cell lymphoma (DLBCL),23 lung cancer (LC),24,25 epithelial ovarian carcinoma (EOC),26 esophageal carcinoma (EC).27 However, significance about the prognostic role of IL-18 remains controversial.
In this article, we conducted a meta-analysis to study the hazard ratios (HR) of IL-18 expression for overall survival (OS) in patients with tumors and further evaluated whether it can be used as a biomarker. We also discussed the relation between IL-18 and radiation injuries.
Materials and Methods
Search Methods
We reported and conducted this systematic meta-analysis and followed the PRISMA statement.28 A systematic search was performed in PubMed, EMBASE, and Cochrane library for qualified studies assessing the prognostic role of IL-18 expression in patients with various types of cancers. We combined search terms: (Interleukin-18 OR IL-18 OR CXCL18) and (neoplasia OR neoplasias OR neoplasm OR tumors OR tumor OR cancer OR cancers OR malignancy OR malignancies OR malignant neoplasms OR malignant neoplasm) and (prognosis OR predict OR prognostic OR survival OR overall survival OR survival rate). We also comprehensively examined the selected studies and reviews. Animal studies, review articles, letters, and case reports were excluded. Two authors (Zhen Yao and Guangyu Gao) examined the articles by their own, and if we disagree with the conclusion, agreement will be reached after consultations.
Data Extraction and Synthesis
Our 2 independent reviewers extracted the data from the primary studies by using a predefined form. The data which we collected included the year of publication, author’s name, nationality of samples, number of patients, HR, and type of tumor. We calculated HRs depending on the data in the primary studies if the data was not given directly. We plotted the Kaplan-Meier curves with Engauge Digitizer (version 4.1).29,30 Then, the collected data was input into RevMan 5.3 software for further analysis.
Quality Evaluation
We used Newcastle-Ottawa Scale (NOS)31 to assess the cohort studies. If the score of study is bigger than 6, it would be considered as high quality.32
Statistical Analysis
The prognostic value of IL-18 expression in patients with cancer was calculated by estimating the HR between groups with IL-18 high expression and low expression. We assessed the heterogeneity and measured the related 95% CI between eligible articles with P value and I 2. In the study, it was believed that there exists an obvious heterogeneity if I 2 ≥ 50% or P ≤ .10. If the heterogeneity was meaningful, we will use a random-effect (RE) model, otherwise we will use a fixed-effect model. Furthermore, we assessed the potential publication bias by visual inspection since asymmetric plot can suggest possible publication bias.
Results
Study Selection and Characteristics
Totally, 279 articles were researched from the above databases by using search strategies which were described above; 263 articles were finally excluded because (a) HR cannot be obtained from enrolled articles directly; (b) repetition of data or experiments on animals; (c) the article type was case report, review, meta-analysis; (d) insufficient data for quantitative analysis. Finally, a total of 16 studies fulfilling our criteria were enrolled in this systematic review and meta-analysis (Figure 1).
Figure 1.
The flow chart of the publication selection process in our meta-analysis.
Studies and Patients’ Characteristics
The selected studies were all published between 2001 and 2020 including 1376 patients, and the sample size in each study ranged from 33 to 154. Totally, there were 11 different cancer types fulfilling our criteria in our meta-analysis, comprising 1 LSCC, 1 CRC, 2 MM, 1 PC, 2 HCC, 3 GC, 1 PrC, 1 DLBCL, 2 LC, 1 EOC, 1 EC, detailed information from the researches were revealed in Table 1.
Table 1.
Characteristics of the Selected Studies.
| Author (year) | Region | Cancer type | Sample sizes | HR (95% CI) | NOS |
|---|---|---|---|---|---|
| Xue, et al (2019) | China | LSCC | 104 | 2.20 (1.18-4.08) | 6 |
| Li, et al (2019) | China | CRC | 146 | 2.23 (1.57-3.17) | 7 |
| Nakamura, et al (2018) | Australia | MM | 145 | 1.84 (1.15-2.94) | 8 |
| Usul Afsar, et al (2017) | Turkey | PC | 33 | 1.14 (0.47-2.14) | 7 |
| Markowitz, et al (2016) | America | HCC | 92 | 1.82 (1.21-3.71) | 6 |
| Tas, et al (2015) | Turkey | GC | 63 | 1.28 (0.49-3.36) | 6 |
| Dwivedi, et al (2011) | India | PrC | 71 | 8.33 (3.13-12.5) | 7 |
| Goto, et al (2011) | Japan | DLBCL | 154 | 2.44 (1.14-5.20) | 7 |
| Okamoto, et al (2009) | Japan | NSCLC | 79 | 3.99 (1.48-10.78) | 7 |
| Tangkijvanich, et al (2007) | Thailand | HCC | 70 | 2.11 (1.30-3.42) | 8 |
| Ye, et al (2007) | China | GC | 50 | 2.20 (0.61-7.91) | 6 |
| Naumnik, et al (2004) | Poland | LC | 71 | 1.14 (0.61-2.21) | 7 |
| Akahiro, et al (2004) | Japan | EOC | 69 | 2.25 (1.15-4.51) | 6 |
| Tsuboi, et al (2004) | Japan | EC | 70 | 1.14 (0.55-3.79) | 8 |
| Alexandrakis, et al (2004) | Greece | MM | 65 | 2.13 (1.08-4.21) | 7 |
| Kawabata, et al (2001) | Japan | GC | 94 | 2.77 (1.14-6.74) | 7 |
Abbreviations: CRC, colorectal cancer; DLBCL, diffuse large B-cell lymphoma; EC, esophageal carcinoma; EOC, epithelial ovarian carcinoma; GC, gastric cancer; HCC, hepatocellular carcinoma; HR, hazard ratios; LC, lung cancer; LSCC, laryngeal squamous cell carcinoma; MM, multiple myeloma; NCSLC, non-small cell lung carcinoma; NOS, Newcastle-Ottawa Scale; PC, pancreatic cancer.
Meta-analysis
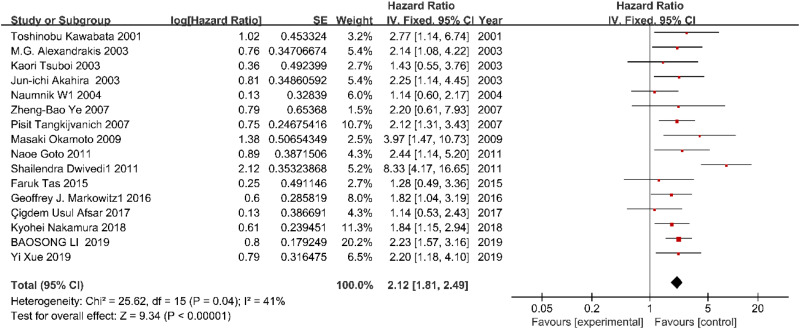
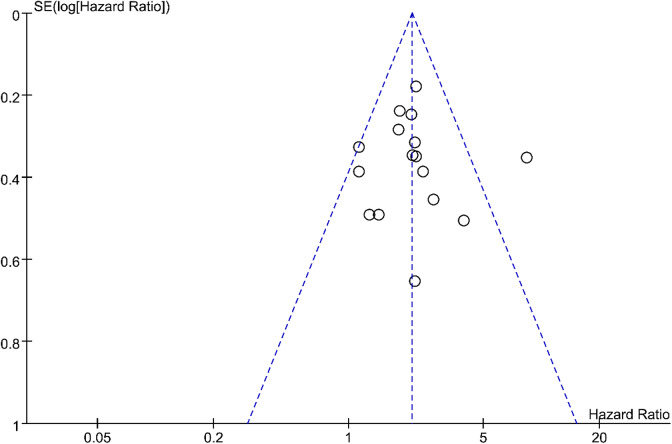
A total of 1376 patients with tumor were retrieved from 16 qualified articles, and we found that high expression of IL-18 was obviously related to a poor OS with a combined HRs of 2.12 (HR: 2.12; 95% CI: 1.81-2.49; P = .04; Figure 2). A RE model was applied because we discovered the heterogeneity among researches on the relationship between IL-18 expression and OS of patients (I 2 = 41%; Q = 25.62; degrees of freedom = 15; P < .00001). In our meta-analysis, the publication bias of the included literature was estimated by Begg’s funnel plot, and the shapes of the funnel plots in our study implied no obvious asymmetry (Figure 3).
Figure 2.
The correlation between IL-18 expression and OS in human cancers. OS indicates overall survival.
Figure 3.
Begg’s funnel plots for the studies involved in the meta-analysis.
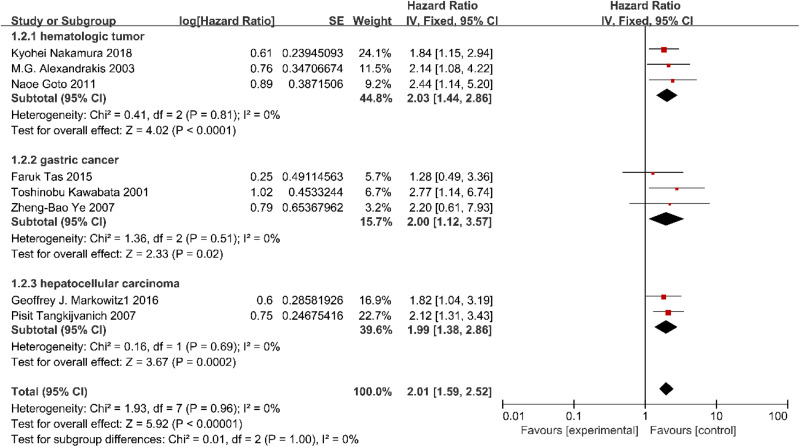
Since 16 qualified articles were used, we set a subgroup meta-analysis to find if there was significant heterogeneity between different kinds of tumors. Considering the number of articles, only hematologic tumor including MM and DLBCL, GC and HCC were analyzed in subgroup. There are 3 studies supplied an OS for hematologic tumor, 2 for HCC and 3 for GC in the stratified analysis by tumor kinds. In conclusion, high expression of IL-18 was significantly related to poorer OS of the patients with hematologic tumor (HR: 2.03, 95% CI: 1.44-2.86, I 2 = 0%, P < .0001; Figure 4), HCC (HR: 1.99, 95% CI: 1.38-2.86, I 2 = 0%, P = .0002; Figure 4), and GC (HR: 2.00, 95% CI: 1.12-3.57, I 2 = 0%, P = .02). However, 2 articles (Tas et al, 2015 and Ye et al, 2007) showed that the IL-18 expression level may have no clinical significance as a prognostic factor in patients with gastric cancer. Considering the publication time, sample size, experimental method, and credibility of the article, we deserted the article (Kawabata et al, 2001) and got a new result (HR: 1.56, 95% CI: 0.72-3.37, I 2 = 0%, P = .061; Figure 4), we believed the prognostic value of IL-18 in gastric carcinoma remains controversial.
Figure 4.
Forest plot demonstrating subgroup analysis of the relationship between IL-18 expression with OS in patients with breast cancer, lung cancer, or gastric cancer. OS indicates overall survival.
Discussion
Nowadays, inflammation is considered a major sign of carcinoma.33 As early as 1863 Rudolph Virchow34 deduced a relationship between carcinoma and inflammation through observing the leukocyte infiltration in human breast carcinoma. It is widely acknowledged that up to 25% of malignant tumors are associated with both chronic infection and chronic inflammation.35 In the process of tumor initiation, tumor-infiltrating immune cells induce oxidative molecules including active oxygen species and reactive nitrogen species which induce epigenetic alterations in oncogenes or cancer suppressor genes, ultimately promoting carcinogenesis.36 It is significant to clarify the molecular mechanisms between inflammation and cancer risk for cancer prevention and treatment. Tas et al33 found the levels of IL-18 and IL-1β were significantly elevated in various types of malignant tumors. These cytokines can trigger the secretion of vascular endothelial growth factor, fibroblast growth factor-2, and signal transducer and activator of transcription-3, which have procarcinogenic activity, subsequently, support cancer survival and metastasis. High IL-18 expression was associated with adverse performances such as advanced tumor stage and lymph node metastasis. It should also be noted that IL-18 not only play a role as a new prognostic biomarker in tumor but also as a novel potential therapeutic target.
This article is a meta-analysis composed of 16 controlled studies and it is so far the only study that has comprehensively evaluated the published studies on IL-18 expression and patient survival. The survival data for 1376 patients with different tumors including solid tumors and hematologic tumors were systematically analyzed. Among tumors in which high expression of IL-18 is commonly observed (LSCC, CRC, MM, PrC, PC, HCC, GC, DLBCL, LC, EOC, EC), there was a strong link between high expression of IL-18 and adverse consequence, in terms of OS (HR: 2.13; 95% CI: 1.71-2.65; P = .04). Subgroup analysis suggested that high expression of IL-18 was also obviously related with worse prognosis in HCC (HR: 1.99, 95% CI: 1.38-2.86, I 2 = 0%, P = .0002) and hematologic tumor (HR: 2.03, 95% CI: 1.44-2.86, I 2 = 0%, P < .0001). However, only 2 types of hematologic tumors including DLBCL and MM were analyzed, the mechanisms remained unknown and more studies about other types of hematologic tumors are needed. Furthermore, among GC with high expression of IL-18, Kawabata et al21 think the expression level of preoperative IL-18 has obvious significance for the prognosis evaluation in patients with gastric cancer, but Tas et al19 think IL-18 may only play a diagnostic role in patients with gastric carcinoma and its prognostic and predictive values need further research. On account of the limited research evaluating GC and the scale of this research, it is difficult to make a conclusion here, but it is worth investigating the potential mechanism for the different performance.
In 2013, Dinarello et al37 concluded that IL-18 secreted from macrophage, monocyte, and DCs plays a key role in radiation injuries through an inflammasome Toll-like receptors signal transduction pathway. Various kinds of radiation stress response factors such as c-Jun N-terminal kinase, nuclear factor kappa-B, activator protein 1, and mitogen-activated protein kinase are involved in this pathway. In 2014, Ha et al38 found that the increase in circulating IL-18 level after radiation can proportionally reflect the severity of radiation injuries and the radiation dose through the experiments on animals (mice, minipigs, and nonhuman primates). It can be used as a potential biomarker for classification, and can also be used to track casualties and therapeutic effects after radiation accidents. In 2016, Xiao et al39 considered that keeping the balance between IL-18 and IL-18BP may significantly reduce the radiation injuries in human and animals. In 2019, Wei et al40 reviewed the role of NLRP3 inflammasome in radiation injuries and came to the conclusion that the response of inflammation is vital to untoward effect of radiation injuries. The IL-18 released through the activation of NLRP3 plays an important role in radiation injuries and may provide a new biomarker for disease caused by radiation injuries. However, due to limited number of related studies, we can’t draw a conclusion here for the prognostic value of IL-18 in radiation injuries is worthy of further research.
Our study is rigorously designed and conducted. First, we applied the study inclusion criteria strictly to ensure the quality of the enrolled studies. Second, we strictly followed inclusion criteria and filtrated relevant articles from Medline (PubMed), EMBASE, and Cochrane Library when we performed the study. Third, a subgroup analysis was conducted to validly reduce the heterogeneity among the selected studies and to further explore the scope of application for IL-18 as a biomarker of prognosis for malignancy. Fourth, if the included studies have no publication bias, the points on the funnel chart are distributed symmetrically around the estimated actual values of the independent research effect points. The Begg’s funnel plot showed no significant publication bias in our meta-analysis. Furthermore, a comprehensive and detailed literature search was conducted to minimize publication bias. In the end, to avoid choice biases and ensure the comparability and quality of studies, we conducted a methodological assessment of the studies.
However, our current meta-analysis still has some limitations. First, the number of enrolled studies was relatively small, only 16 articles were selected, which lead to the relative insufficiency in subgroups. Second, since cutoff point of IL-18 was not acknowledged, we just used the cutoff point the original articles provided for evaluation of diagnostic value. Third, most HRs are not provided directly, they were calculated by extracting the statistics from the survival curves according to existing data. However, the HRs we calculated might be not as accurate as those retrieved directly from published articles.
Conclusion
In summary, high IL-18 expression is significantly related to poor prognosis in various malignant tumors. The relation between high expression of IL-18 and poor prognosis in GC needs further study. Interleukin 18 might be a clinically useful biomark to identify high-risk patients and a potential target for tumor therapy.
Footnotes
Authors’ Note: Z.Y. contributed to the design of the project. Y.L. contributed to the administrative support. G.G., J.Y., Z.W., and M.Z. contributed to the collection and assembly of data. Z.Y. and M.Z. contributed to data analysis. All authors taken part in the final approval of the manuscript. Z.Y. and M.Z. contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
ORCID iD: Zhen Yao  https://orcid.org/0000-0002-9959-6881
https://orcid.org/0000-0002-9959-6881
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2. Okamura H, Nagata K, Komatsu T, et al. A novel costimulatory factor for gamma interferon induction found in the livers of mice causes endotoxic shock. Infect Immun. 1995;63(10):3966–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Puren AJ, Fantuzzi G, Dinarello CA. Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1β are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proc Natl Acad Sci USA. 1999;96(5):2256–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Okamura H, Tsutsi H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378(6552):88–91. [DOI] [PubMed] [Google Scholar]
- 5. Lebel-Binay S, Berger A, Zinzindohoue F, et al. Interleukin-18: biological properties and clinical implications. Eur Cytokine Netw. 2000;11(1):15–25. [PubMed] [Google Scholar]
- 6. Park S, Cheon S, Cho D. The dual effects of interleukin-18 in tumor progression. Cell Mol Immunol. 2007;4(5):329–335. [PubMed] [Google Scholar]
- 7. Vidal-Vanaclocha F, Fantuzzi G, Mendoza L, et al. IL-18 regulates IL-1beta-dependent hepatic melanoma metastasis via vascular cell adhesion molecule-1. Proc Natl Acad Sci USA. 2000;97(2):734–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang Y, Cheon S, Jung MK, et al. Interleukin-18 enhances breast cancer cell migration via down-regulation of claudin-12 and induction of the p38 MAPK pathway. Biochem Biophys Res Commun. 2015;459(3):379–386. [DOI] [PubMed] [Google Scholar]
- 9. Lee KH, Kim EY, Yun JS, et al. The prognostic and predictive value of tumor-infiltrating lymphocytes and hematologic parameters in patients with breast cancer. BMC Cancer. 2018;18(1):938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee N, Zakka LR, Mihm MC, Schatton T. Tumour-infiltrating lymphocytes in melanoma prognosis and cancer immunotherapy. Pathology. 2016;48(2):177–187. [DOI] [PubMed] [Google Scholar]
- 11. Mahmoud SM, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29(15):1949–1955. [DOI] [PubMed] [Google Scholar]
- 12. Xue Y, Du HD, Tang D, et al. Correlation between the NLRP3 inflammasome and the prognosis of patients with LSCC. Front Oncol. 2019;9:588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li BS, Wang FX, Ma C, et al. Predictive value of IL-18 and IL-10 in the prognosis of patients with colorectal cancer. Oncol Lett. 2019;18(1):713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nakamura K, Kassem S, Cleynen A, et al. Dysregulated IL-18 is a key driver of immunosuppression and a possible therapeutic target in the multiple myeloma microenvironment. Cancer Cell. 2018;33(4):634–648. [DOI] [PubMed] [Google Scholar]
- 15. Alexandrakis MG, Passam FH, Sfiridaki K, et al. Interleukin-18 in multiple myeloma patients: serum levels in relation to response to treatment and survival. Leuk Res. 2004;28(3):259–266. [DOI] [PubMed] [Google Scholar]
- 16. Usul Afsar C, Karabulut M, Karabulut S, et al. Circulating interleukin-18 (IL-18) is a predictor of response to gemcitabine based chemotherapy in patients with pancreatic adenocarcinoma. J Infect Chemother. 2017;23(4):196–200. [DOI] [PubMed] [Google Scholar]
- 17. Markowitz GJ, Yang P, Fu J, et al. Inflammation-dependent IL-18 signaling restricts hepatocellular carcinoma growth by enhancing the accumulation and activity of tumor-infiltrating lymphocytes. Cancer Res. 2016;76(8):2394–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tangkijvanich P, Thong-Ngam D, Mahachai V, Theamboonlers A, Poovorawan Y. Role of serum interleukin-18 as a prognostic factor in patients with hepatocellular carcinoma. World J Gastroenterol. 2007;13(32):4345–4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tas F, Tilgen Yasasever C, Karabulut S, Tastekin D, Duranyildiz D. Clinical significance of serum interleukin-18(IL-18) levels in patients with gastric cancer. Biomed Pharmacother. 2015;70:19–23. [DOI] [PubMed] [Google Scholar]
- 20. Ye ZB, Ma T, Li H, Jin XL, Xu HM. Expression and significance of intratumoral interleukin-12 and interleukin-18 in human gastric carcinoma. World J Gastroenterol. 2007;13(11):1747–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kawabata T, Ichikura T, Majima T, et al. Preoperative serum interleukin-18 level as a postoperative prognostic marker in patients with gastric carcinoma. Cancer. 2001;92(8):2050–2055. [DOI] [PubMed] [Google Scholar]
- 22. Dwivedi S, Goel A, Natu SM, Mandhani A, Khattri S, Pant KK. Diagnostic and prognostic significance of prostate specific antigen and serum interleukin 18 and 10 in patients with locally advanced prostate cancer: a prospective study. Asian Pac J Cancer Prev. 2011;12(7):1843–1848. [PubMed] [Google Scholar]
- 23. Goto N, Tsurumi H, Kasahara S, et al. Serum interleukin-18 level is associated with the outcome of patients with diffuse large B-cell lymphoma treated with CHOP or R-CHOP regimens. Eur J Haematol. 2011;87(3):217–227. [DOI] [PubMed] [Google Scholar]
- 24. Okamoto M, Azuma K, Hoshino T, et al. Correlation of decreased survival and IL-18 in bone metastasis. Intern Med. 2009;48(10):763–773. [DOI] [PubMed] [Google Scholar]
- 25. Naumnik W, Chyczewska E, Kovalchuk O, Tałałaj J, Izycki T, Panek B. Serum levels of interleukin-18 (IL-18) and soluble interleukin-2 receptor (sIL-2 R) in lung cancer. Rocz Akad Med Bialymst. 2004;49:246–251. [PubMed] [Google Scholar]
- 26. Akahiro J, Konno R, Ito K, Okamura K, Yaegashi N. Impact of serum interleukin-18 level as a prognostic indicator in patients with epithelial ovarian carcinoma. Int J Clin Oncol. 2004;9(1):42–46. [DOI] [PubMed] [Google Scholar]
- 27. Tsuboi K, Miyazaki T, Nakajima M, et al. Serum interleukin-12 and interleukin-18 levels as a tumor marker in patients with esophageal carcinoma. Cancer Lett. 2004;205(2):207–214. [DOI] [PubMed] [Google Scholar]
- 28. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. [DOI] [PubMed] [Google Scholar]
- 29. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815–2834. [DOI] [PubMed] [Google Scholar]
- 30. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cota GF, de Sousa MR, Fereguetti TO, Rabello A. Efficacy of anti-leishmania therapy in visceral leishmaniasis among HIV infected patients: a systematic review with indirect comparison. PLoS Negl Trop Dis. 2013;7(5):e2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang L, Liu Z, Liu X, Zeng Y, Liu J. The hepatectomy efficacy of huge hepatocellular carcinoma and its risk factors: a meta-analysis. Medicine. 2017;96(52):e9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Z, Wang N, Zheng Y, Wang S. Inflammasome and cancer. Exp Suppl. 2018;108(1):281–302. [DOI] [PubMed] [Google Scholar]
- 34. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. [DOI] [PubMed] [Google Scholar]
- 35. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. [DOI] [PubMed] [Google Scholar]
- 36. Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49(11):1603–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dinarello CA, Novick D, Kim S, Kaplanski G. Interleukin-18 and IL-18 binding protein. Front Immunol. 2013;4:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ha CT, Li XH, Fu D, et al. Circulating interleukin-18 as a biomarker of total-body radiation exposure in mice, minipigs, and nonhuman primates (NHP). PLoS One. 2014;9(10):e109249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xiao M. The role of proinflammatory cytokine interleukin-18 in radiation injury. Health Phys. 2016;111(2):212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wei J, Wang H, Wang H, et al. The role of NLRP3 inflammasome activation in radiation damage. Biomed Pharmacother. 2019;18:109217. [DOI] [PubMed] [Google Scholar]