Abstract
Objective
The role and mechanism of tetrathiomolybdate (TM) in cancer-associated fibroblasts (CAFs) in colon cancer using three-dimensional (3D) culture were investigated, and the associations between the focal adhesion kinase (FAK) pathway and epithelial–mesenchymal transition (EMT) in CAFs were explored.
Methods
A 3D co-culture model of colon cancer LOVO cells with CAFs and normal fibroblasts (NFs) was established using Matrigel as a scaffold material. The differential expression of LOXL2 (lysyl oxidase-like 2) in the supernatant of CAFs and NFs was determined using ELISA, and expression levels of EMT-related proteins and FAK signaling pathway-related proteins were determined using western blot.
Results
LOXL2 levels secreted by CAFs were higher compared with that secreted by NFs. In the CAF + LOVO group, compared with the LOVO group, E-cadherin expression decreased significantly, while N-cadherin and F-PAK expression increased significantly. TM results were opposite compared with the above results.
Conclusions
CAFs stimulate EMT in human colon cancer LOVO cells by secreting LOXL2 to activate the FAK signaling pathway, thereby promoting tumor metastasis. TM inhibited the occurrence of EMT in the CAF-induced colon cancer LOVO cell line, thereby reducing the invasion and metastasis of colon cancer cells.
Keywords: Three-dimensional culture, cancer-associated fibroblasts, colon cancer, epithelial–mesenchymal transition, focal adhesion kinase signaling pathway, tumor metastasis, tetrathiomolybdate
Introduction
Colorectal cancer (CRC) ranks third in terms of incidence, but second in terms of mortality throughout the world.1 Although significant progress has been made in the early diagnosis and treatment of CRC, the local invasion and distant metastasis of cancer cells leads to death in many cancer patients.2 Cancer cell metastasis undergoes detachment, migration, invasion, and adhesion. These four important steps are related, and they are influenced and regulated by a variety of biomolecules and factors in the process of tumor metastasis.3 Epithelial–mesenchymal transition (EMT), one of the main mechanisms of CRC metastasis, is conducive to cell detachment and migration into surrounding tissues and the formation of new tumor tissue at distant sites.4 Therefore, it is of great importance to study the molecular mechanism of EMT in CRC cells.5
Fibroblasts, the main stromal cells in the tumor microenvironment, play an important role in CRC invasion and metastasis.6 However, the mechanism of these effects has not been clarified. Fibroblasts in the tumor microenvironment, which are known as a cancer-associated fibroblasts (CAFs) or myofibroblasts, are the most abundant type of stromal cells that surround cancer cells.7 CAFs are a diverse population of cells with different origins and phenotypes. In different tumor types, the composition and function of CAFs and normal fibroblasts (NFs) are different.7,8 Circulating tumor cells (CTC) originate from either the primary or metastatic sites, while the importance of detecting CTCs is associated with active disease, tumor progression, and metastatic potential, making them a strong prognostic biomarker and providing the advantage of avoiding a new biopsy.9 Therefore, further elucidating the mechanism of colon cancer metastasis is key to reducing postoperative recurrence and metastasis and improving prognosis.
In addition, copper is a trace element that is necessary for the basic biological processes of all organisms. Copper provides and accepts electrons and facilitates the switch between a reduction and oxidation state, which causes copper to act as a catalytic cofactor for many key functional enzymes in the body, and thus, it plays an important biological role.10 Moreover, copper is a component of many important enzymes. Copper ions are involved in the synthesis of many important enzymes in the human body, such as cytochrome oxidase, which is an electron transport enzyme that is involved in cell respiration; superoxide dismutase, which participates in free radical detoxification; and lysine oxidase (lox) and tyrosine enzymes, which synthesize connective tissue.11 However, free copper ions produce high levels of reactive oxygen species (ROS) because of their redox activity. When the copper content is too high in an organism, copper binds excessively to proteins and nucleic acids or causes lipid oxidation of various membranes to damage cells. Therefore, the lack of copper or excessive copper accumulation in cells can cause serious diseases, such as Menkes and Wilson (WD) diseases, which are genetic diseases.12 For example, dietary copper affects the regulation of peripheral blood flow and blood vessel integrity, while copper sufficiency restores the normal antioxidant function in blood vessels. Moreover, dietary copper deficiency may manifest as increased formation of advanced glycation end-products (AGEs), which in turn may result in vascular injury and increased susceptibility to ischemic heart disease (IHD) especially in diabetes patients and those with metabolic syndrome.13
Recent studies have shown that there is a close link between copper and the development of cancer.14 Ishida et al.14 noted that changes in the copper element in the tumor microenvironment can partially mediate the tumor metabolic phenotype; when the concentration of copper in the drinking water was too high, tumor growth was promoted in a mouse model of pancreatic cancer. Copper has been used as a central regulator of normal and tumor angiogenesis to regulate many angiogenesis reactions.15 Angiogenesis and the acquisition of angiogenic phenotypes are very important for the proliferation of cancer cells.16 Copper promotes angiogenesis through various pathways; for example, copper improves the growth and development of vascular endothelial cells and regulates the synthesis and secretion of angiogenesis-promoting mediators (such as fibroblast growth factor and vascular endothelial growth factor).17 Copper can bind directly to angiogenin to enhance its activity.17–19 Copper may also affect cancer cell invasion of surrounding tissues or transfer to distant organs. Herein, lysine oxidase contributes to the remodeling of the extracellular matrix and the establishment of the microenvironment at the metastasis site, and its activity is dependent on copper.20 Tetrathiomolybdate (TM) is a highly specific copper chelating agent that inhibits tumor angiogenesis.12,21
Currently, traditional two-dimensional cell culture technology is a common method of cell culture that is used for in vitro tumor research because the technique is easy to use, economical, and well established.22 However, the two-dimensional cell culture system lacks a three-dimensional (3D) scaffold that is composed of extracellular matrix, and the dynamic spatial structure of cell–cell and cell–extracellular matrix interactions, and the overall microenvironment that is required for cell growth and differentiation cannot be formed.23 Because the biological response and biological function that are reflected in studies using the two-dimensional cell culture techniques are probably different from those of tissue cells in vivo, 3D cell culture may solve this problem. 3D cell culture technology allows cells to grow in a 3D space and form a specific 3D structure where they can interact with the surrounding cells and extracellular matrix and other components of the microenvironment in a 3D manner.24 Therefore, in this study, we established a 3D cell co-culture model to determine the role and mechanism of TM in CAFs in colon cancer and to explore the associations between the focal adhesion kinase (FAK) pathway and EMT in CAFs. Our study provides a new way to study the biological behavior of tumors.
Materials and methods
Cell source
Human colon cancer LOVO cells were purchased from the Cell Resource Center of Shanghai Academy of Science, Chinese Academy of Science (Shanghai, China). The fresh colon cancer tissue and the normal colon tissue with a diameter of about 10 cm were removed from the same patient under sterile conditions. The NFs used experiment were originally isolated from the same patient. The investigation project and its related consent forms have been examined and certified by Ethics Committee of The Affiliated Hospital of Inner Mongolia Medical University, which approved the study. Patients provided verbal consent to participate in the study. CAFs were induced from the co-culture of human colon cancer LOVO cells and NFs.
Cell culture
Cells were cultured in 10% FBS, 89% DMEM (Biological Industries, Kibbutz Beit Haemek, Israel), and 1% streptomycin double antibody complete culture solution (Invitrogen, Carlsbad, CA, USA). Cell cultures were incubated in a humidified atmosphere of 95% air and 5% CO2 at a constant temperature of 37°C.
Co-culture of human colon cancer LOVO cells and NFs
LOVO cells (100 µL, approximately 1.2 ×106 cells/mL) were inoculated into a 0.4-µm upper co-culture chamber, and 600 µL of NFs (approximately 6.7 × 104 cells/mL) were inoculated into the lower co-culture chamber. The cells in the upper and lower chambers were added at the same time, and the indirect culture conditions were maintained for 5 days. The morphology of the cells in the lower chamber was observed by inverted microscopy and photographed, and the cells in the lower chamber were collected and identified in the next step.
Identification of cancer-associated fibroblasts
Immunohistochemical
The cover slide was immersed in the configured polylysine (Beijing Boosen, Beijing, China) working solution for 5 minutes and put it in a 6-well plate. NF cells and co-cultured NFs (about 1 × 106 cells) were cultured in a constant-temperature incubator to 50 to 70% confluence. The original medium was discarded and the cells were washed twice with PBS. Paraformaldehyde (2 mL) was then added for 20–30 minutes to terminate the culture for immunocytochemical staining of α-smooth muscle actin (α-SMA) using the S-P kit (Fuzhou Maixin Co., Ltd., Fuzhou, China) method. Finally, the proportion of cells marked by α-SMA protein in the total number of cells that were observed in the microscopic field of vision was calculated.
Immunofluorescence
The cells in each group were spread well, and they were fixed in 4% paraformaldehyde for 10 minutes and rinsed three times for 5 minutes each with phosphate buffered saline (PBS). The cell membranes were then permeabilized with 0.5% Triton X-100 (Fuzhou Maixin Co., Ltd.), and the cells were rinsed three times for 5 minutes each with PBS. Goat serum was added and the cells incubated for 30 minutes at room temperature. Then, an α-SMA antibody was added and the cells incubated overnight at 4°C. PBS was used to rinse the cells three times for 5 minutes each, and a fluorescent secondary antibody was incubated with the cells at 37°C for 1 to 2 hours. The cells were then washed with PBS for 5 minutes, stained with DAPI for 5 minutes, and washed with PBS for 5 minutes. Finally, images were collected under a fluorescence microscope.
For a description of the protein expression level (α-SMA) methods, see the description of the western blotting below.
NF cell cycle and co-culture using flow cytometry
Based on the cell cycle kit instructions (BD Biosciences, San Jose, CA, USA), each cell sample was incubated with 250 µL trypsin buffer at room temperature (20–25°C) for 10 minutes. Then, the mixture of 200 µL trypsin inhibitor and RNase buffer was incubated at room temperature for 10 minutes. The pre-cooled (2–8°C) PI solution was added and incubated at 4°C for 10 minutes. The NF cell cycle and co-culture were analyzed using flow cytometry, and the results were analyzed using the cell cycle fitting software ModFit (Verity Software House, Topsham, ME, USA).
Detection of cell proliferation activity by MTT assay
NFs in logarithmic growth were used to create a 100-µL/well cell suspension (1.5 × 104 cells/mL) and inoculated on a 96-well cell culture plate; the cells were divided into two groups, NFs and co-culture NFs. Cells were analyzed every day from the second day after inoculation. Then, 10 µL of MTT reagent was added to each well. After 4 hours of culture in an incubator, the A570 value was detected, and the mean values were calculated from three replicates in the experiment.
Scratch injury test
LOVO cells (1 mL/well; approximately 5 × 105/mL) that were in the logarithmic growth phase were inoculated onto a six-well plate, and vertical scratches were made with aseptic 20-µL pipette tips after the cells covered the wells. Then, DPBS was used to rinse the wells three times to remove the non-adherent cells from the scratches. Two milliliters of NF-conditioned medium was added before and after co-culture in each group, and three replicates were performed. After 24 and 48 hours, the cells were observed using inverted phase contrast microscopy. Image-Pro Plus 6.0 software (Version X; Media Cybernetics, Silver Springs, MD, USA) was used to measure and calculate the width and cell mobility. The mean values of the experiment were determined from three replicates.
Migration ability of NFs to LOVO cells before and after co-culture
Matrigel glue was defrosted overnight in a refrigerator at 4°C and diluted in with serum-free medium at a 1:8 ratio. After dilution, 30 µL of Matrigel was added to the upper chamber of the 8-µm Transwell system. The chamber was placed in a cell culture box for at least 30 minutes until the Matrigel was solidified. One hundred microliters of colon cancer LOVO cells (approximately 1 × 106/mL) were inoculated into the upper chamber of the Transwell cells, and 600 µL of NFs (approximately 1 × 105/mL) were added to the lower chamber for co-culture. Cell culture medium was used as a negative control. The upper and lower chamber cells were cultured in complete medium containing 10% serum. The cells were cultured at 37°C for 24 hours in a 5% CO2 cell incubator, and the upper chamber was used for staining. The cells were fixed with 4% paraformaldehyde for 20 to 30 minutes, and a cotton swab was used to gently remove the Matrigel glue and cells. DPBS was used to wash the chamber twice for 3 minutes each, and the cells were stained with 0.1% crystal violet for 15 minutes. Next, the chamber was washed twice with DPBS for 3 minutes each. It was then inverted, allowed to dry naturally, and observed under an inverted microscope. The migration ability of LOVO colon cancer cells toward NFs and co-cultured NFs in the matrix environment was analyzed by counting the cells that were attached to the bottom chamber of the membrane without migrating through to the lower chamber. The mean values of the experiment were from three replicates.
Establishment of a three-dimensional cell co-culture model
Matrigel (8–11 mg/mL, Corning Inc., Corning, NY, USA) was added to a 0.4-µm pre-cooled Transwell chamber. The upper and lower chambers were 100 µL and 200 µL wells, respectively. The cells were incubated at 37°C for 30 minutes until the Matrigel solidified, and 80/90% logarithmic growth cells were used for routine digestion, resuspension, and counting. The cell density in each group was adjusted to 3 × 105/mL, and resuspended LOVO cells were inoculated in the lower chambers at 250 µL/well. Resuspended CAFs and NFs were inoculated into the upper chambers at 100 µL/well. Then, the cells were incubated for 30 minutes at 37°C. Precooled medium was mixed with Matrigel at 10:1 (final concentration, 0.8–1.1 mg/mL). Mixed medium amounts of 100 µL and 250 µL were added to the upper and lower chambers, respectively, and replaced every 2 days.
Determination of trace elements in cell supernatants by flame atomic absorption spectrometry
The supernatants in each group were collected and centrifuged at 2264 ×g for 10 minutes, and the supernatants were retained. Levels of trace elements (Cu, Zn, Ca, Mg, Fe) were determined by BH550s atomic absorption spectrometry.
Detection of LOXL2 by ELISA
The supernatant from CAFs and NFs were collected to detect the level of LOXL2 that was secreted by these cells in accordance with the LOXL2 assay kit manufacturer’s instructions. The reagents were allowed to equilibrate at room temperature, and the samples, standard samples, and HRP-labeled antibody were incubated at 37°C for 60 minutes. The plates were then washed five times, chromogenic liquid was added, and optical density (OD) values were measured at a 450-nm wavelength.
Target protein expression in cells
Western blot
Cells were collected and added to RIPA lysate buffer (plus 100:1 phenylmethanesulfonyl fluoride (PMSF) and phosphatase inhibitor) for protein extraction, and a bicinchoninic acid (BCA) protein concentration kit (Beyotime, Jiangsu, China) was used to determine the protein concentrations. Equal amounts of protein samples were subjected to SDS-PAGE, transferred to nitrocellulose (NC) filter membranes, and blocked using 5% skim milk powder. After washing the membranes, α-SMA antibody (Proteintech, Rosemont, IL, USA), E-cadherin (1:1000; Affinity Biosciences, Cincinnati, OH, USA; AF0131), N-cadherin (1:5000; Abcam ab76011, Cambridge, MA, USA), FAK (1:1000; Abcam ab40794), P-FAK (1:1000; Abcam ab81298), and glyceraldehyde-3-phosphate (GAPDH) (1:5000; Shanghai Dianyin Biotechnology Co., Ltd., Shanghai, China) antibodies were incubated overnight at 4°C. The membranes were washed again and incubated with secondary antibody (EarthOx Life Sciences, Millbrae, CA, USA) for 1 hour at room temperature. The membranes were washed and detected using an ODYSSEY fluorescence imaging system (LI-COR, Lincoln, NE, USA). Finally, the OD values for each group were analyzed using ImageJ image analysis software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
The data were analyzed using SPSS version 22.0 software (IBM Corp., Armonk, NY, USA). The data are expressed as the mean ± standard deviation. Two samples were tested using an independent sample t-test. One-way analysis of variance (ANOVA) was used to compare the means of multiple groups. The number of permeable cells containing LOVO cells in each group (invasion test) were analyzed using a Chi-square test. The significance between groups was evaluated using one way ANOVA followed by a least-significant difference post-test. The results were considered to be statistically significant at P < 0.05.
Results
Morphology before and after co-culture with NFs from colon cancer
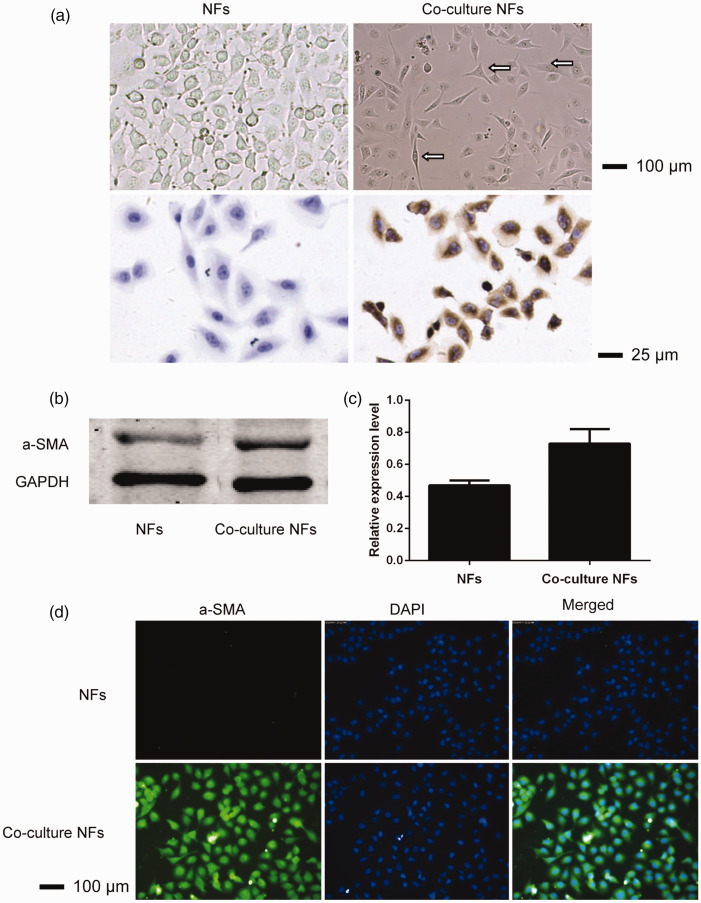
Under an inverted microscope, NFs were ovoid, uniform in size and regular in arrangement. After co-culture, NFs were spindle-shaped or polygon-shaped, with different cell sizes, and a regular arrangement, and they had binucleated and multinucleated cells and sunken or notched nuclei. Figure 1a shows that α-SMA staining of NFs cells was negative and stained blue under a microscope. α-SMA staining of co-culture NFs cells was positive, and more than 90% of cells were stained brown and yellow. These results provide preliminary evidence indicating that co-culture NFs can be activated to CAFs. Morphological observation and identification NFs and co-cultured NFs from colon cancer showed that α-SMA protein expression was significantly higher compared with that in NFs (P < 0.05) (Figure 1b and c). To further compare the α-SMA protein expression in NFs and co-cultured NFs from colon cancer, a cellular immunofluorescence technique was used. The results showed that the fluorescence intensity of α-SMA in the cytoplasm was significantly higher in co-culture NFs compared with NFs (Figure 1d).
Figure 1.
Morphological analysis (first line, ×100) and chemical staining (second line, ×400) of NFs and co-culture with NFs from colon cancer (a). NFs showed fusiform or star-shaped morphology of the same size, while after co-culture, the morphology of the NFs was fusiform or a polygon with different sizes. This could be observed in the binuclear and polynuclear cells, while α-SMA expression was negative in NFs and positive in co-culture NFs. α-SMA protein expression in NFs and co-cultured NFs from colon cancer was determined using SDS-PAGE and an ODYSSEY fluorescence imaging system (b); calculations were performed using ImageJ image analysis software (c); and the cellular immunofluorescence results are presented (d).
NF, normal fibroblasts; α-SMA, α-smooth muscle actin; SDS-PAGE, sodium dodecyl (lauryl) sulfate-polyacrylamide gel electrophoresis.
Cell cycle of NFs before and after co-culture with colon cancer LOVO cells
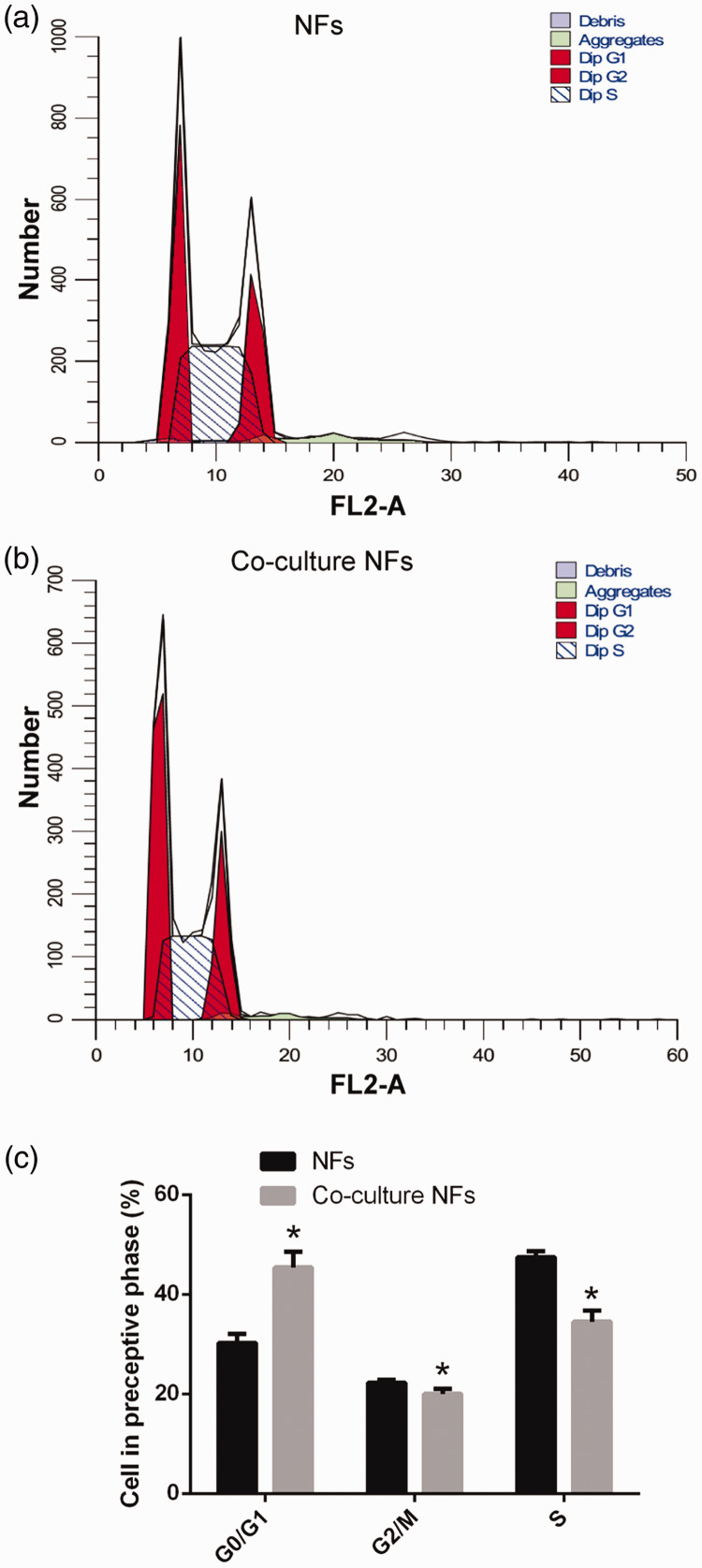
The flow cytometry experiments showed that the number of cells in the two groups (NFs and co-cultured NFs) was different, but the percentage of cells in the two groups was 100%. The original data are described as follows: 1) NFs (Diploid; Dip), 100.00%; Dip G1, 28.30% at 6.68; Dip G2, 22.75% at 13.36; Dip S, 48.95%; G2/G1, 2.00; %CV, 3.88; and 2) Co-cultured NFs (Dip), 100.00%; Dip G1, 41.77% at 6.52; Dip G2, 21.21% at 13.04; Dip S, 37.02%; G2/G1, 2.00; %CV, 4.51 (Figure 2).
Figure 2.
Cell cycle of human colon NFs and co-culture NFs were analyzed using flow cytometry. NFs (a), co-cultured NFs (b), and cells in the respective cell cycle phase (c). Compared with NFs, *, P<0.05.
NF, normal fibroblasts.
Flow cytometry results showed that before and after co-culture with colon cancer LOVO cells, the percentage of NF cells in the G0/G1 phase was 30.28 ±1.75% and 45.41 ± 3.19%, the percentage in G2/M phase was 22.28 ± 0.56% and 20.05 ± 1.02%, and the percentage in S phase was 47.43 ± 1.20% and 34.54 ±2.18%, respectively (Figure 2a, b and c). Co-cultured NFs at different stages of cell cycle were significantly different compared with NFs (P < 0.05). These results indicate that NF cells after co-culture with LOVO cells are significantly different in the cell cycle. The experimental results confirm the successful establishment of our co-culture model, and lay a solid experimental foundation for the next study.
Effect of NFs on the migration of LOVO cells before and after co-culture
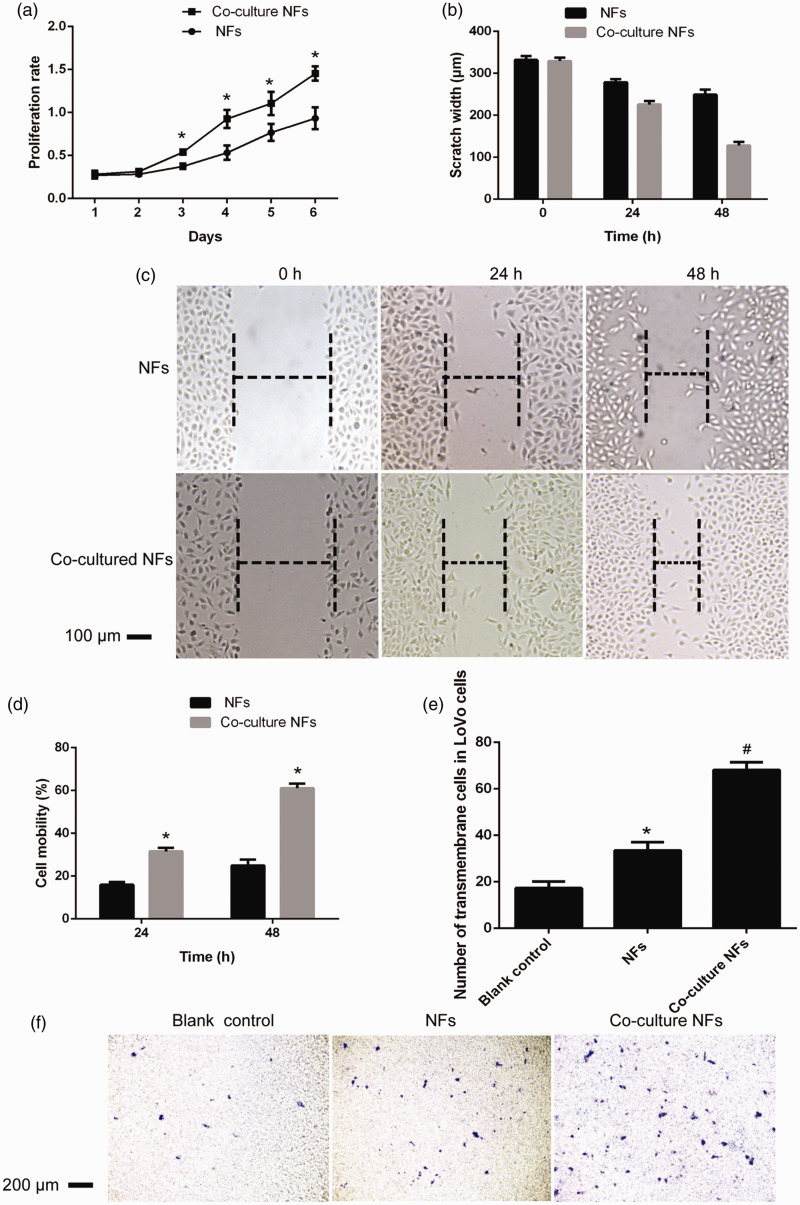
Cell proliferation activity was detected using a 3-(4,5)-dimethylthiahiazo(-z-y1)-3,5-di-phenytetrazoliumromide (MTT) assay and is shown in Figure 3a. Compared with that of NFs, the proliferation activity of co-culture NFs increased significantly from the second day, and this difference was statistically significant. As shown in Figure 3b, c, and d, the cell migration rates before and after co-culture of LOVO cells in the NF group at 24 hours were 16.0 ± 1.2% and 31.6 ± 1.6%, respectively. Moreover, the migration rate of LOVO cells in the NFs group was 25.0 ± 2.8%, and that of LOVO cells in the co-cultured NFs group was 61.1 ± 2.0% (P < 0.01). The results showed that the migration ability of the NF group was significantly higher after co-culture of NFs, and the difference was statistically significant. The effect of NFs on the invasion ability of LOVO cells before and after co-culture is shown in Figure 3e and f; the number of invaded cells was 17.33 ± 2.80 in the blank group and 33.50 ± 3.62 in the NF group, and this difference was significantly different (P < 0.001). Additionally, the number of invaded cells in the NF co-culture group was 68.16 ± 3.31, which was significantly higher compared with the NFs group (P < 0.001) and that in the blank group (P < 0.001).
Figure 3.
Growth curve for human colon NFs and co-culture NFs (a). Compared with the NFs, before co-culture, the scratch width of LOVO cells in human colon NF group at 0, 24, and 48 hours, *, P<0.05; **, P < 0.01 (b); migration width (×40) of LOVO cells in human colon NF group and co-culture NFs (c); comparison of the LOVO cell migration rate in the human colon NF group and co-culture NFs at 24 and 48 hours (d); compared with the NFs, the number of transmembrane LOVO cells in each group; *, P < 0.05; **, P < 0.01 (e); and membrane permeability of LOVO cells in different groups in the invasion assay (× 40) (f). Compared with the blank control, *, P < 0.05; **, P < 0.01; compared with the pre-co-culture, #, P < 0.05; ##, P < 0.01.
NFs, normal fibroblasts.
Changes in copper elements in the tumor microenvironment
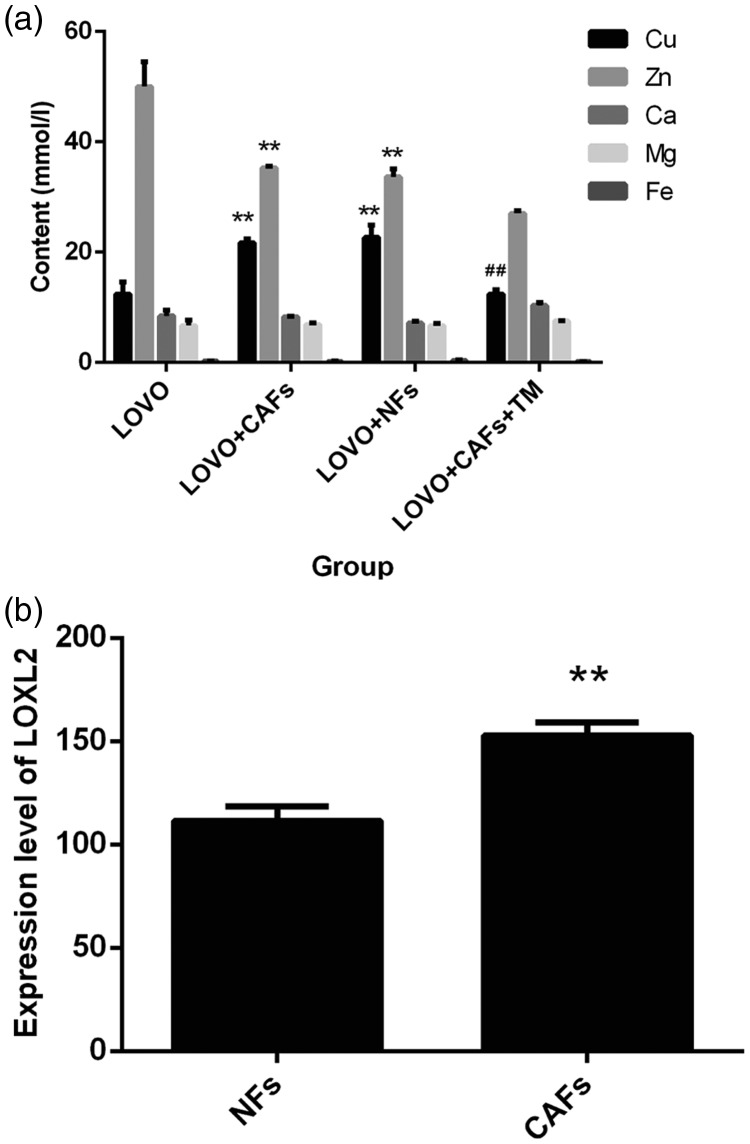
There is a close relationship between copper and the occurrence and development of cancer. Thus, ammonium TM, a highly specific copper chelator, was used to investigate the anti-proliferative activity and role of copper in the tumor microenvironment for its potential application in cancer treatment. Trace elements (including Cu, Zn, Ca, Mg, and Fe) in the cell supernatants were determined using flame atomic absorption spectrometry. As shown in Figure 4a, we observed that the copper content in the LOVO group was significantly lower compared with the LOVO+CAF group and the LOVO+NF group; the opposite results were found for the zinc content, and the differences were statistically significant (PCu = 0.001; PZn = 0.001). Additionally, there was no significant difference between the concentrations of calcium, magnesium, and iron. The content of copper in the TM group was significantly lower compared with the LOVO+CAF group (t = 15.553, P < 0.01). These results suggested that TM could chelate copper in the tumor microenvironment.
Figure 4.
Trace elements in cell supernatants were determined using flame atomic absorption spectrometry, compared with the LOVO, *, P<0.05; **, P < 0.01 (a); compared with the LOVO+NFs, #, P < 0.05; ##, P < 0.01. LOXL2 expression levels were determined in co-culture models (b). Compared with the NFs, *, P < 0.05; **, P < 0.01.
NFs, normal fibroblasts.
LOXL2 expression
Cryopreserved CAFs retain their biological characteristics and can be used for follow-up experimental studies. We next established a co-culture model and detected the LOXL2 expression level in the tumor microenvironment. In Figure 4b, LOXL2 levels that were secreted by NFs and CAFs, as determined using an ELISA kit, were 111.40 ± 7.03 pg/mL and 152.69 ± 6.36 pg/mL, respectively, which were significantly higher compared with NFs (t = 8.713, P<0.01). The above results indicate that copper and LOXL2 levels in the microenvironment of LOVO colon cancer cells are much higher compared with those in the normal tissue microenvironment or single tumor cell microenvironment, and these components may be necessary for tumor cell invasion and metastasis. Thus, LOXL2 also plays an important role in a variety of biological reactions.
E-cadherin and N-cadherin expression
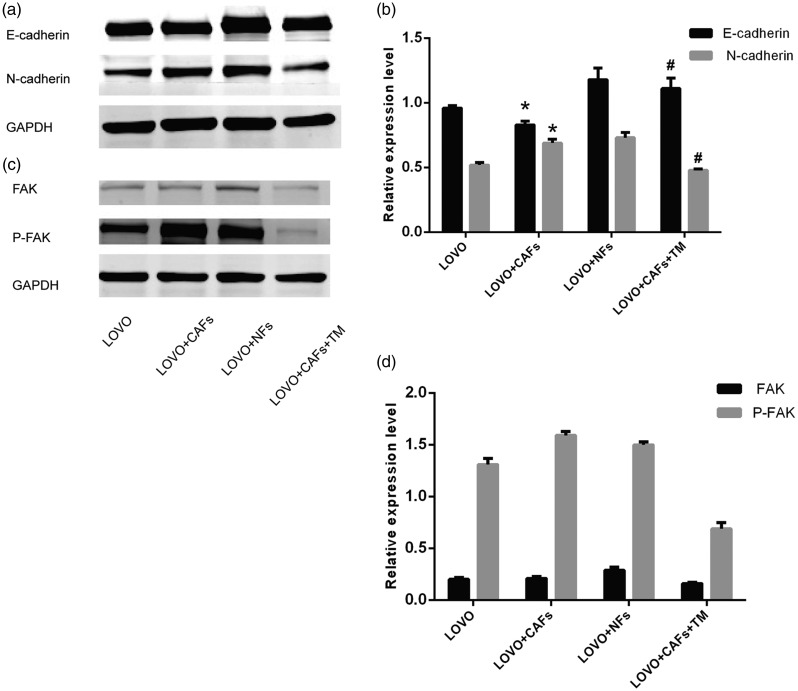
The expression of cell target proteins (E-cadherin and N-cadherin) in each group was determined by western blot. As shown in Figure 5a and b, compared with that in the LOVO group, the E-cadherin expression level in the LOVO+CAF 3D co-culture group was significantly lower (18.48%, P<0.05), and that of N-cadherin was significantly higher (61.525%, P<0.05). The decrease in epithelial cell markers and the increase in stromal cell-related markers suggest that CAFs can promote EMT in LOVO colon cancer cells. Compared with that in the co-culture group LOVO+CAF, the expression of E-cadherin in the TM group increased significantly (P<0.05), while the expression of N-cadherin decreased significantly (P<0.05). The results showed that TM inhibited the epithelial–stromal transformation of colon cancer LOVO cells. FAK is an important molecule for cell migration, and we further examined the expression of members of the FAK pathway by western blot. The expression of FAK in the LOVO group, CAFs co-culture, and TM treatment was 0.20 ± 0.02, 0.21 ± 0.02, and 0.16 ± 0.01, respectively, and the expression of P-FAK was 1.31 ±0.06, 1.59 ± 0.04, and 0.69 ± 0.06, respectively. There was no significant difference in FAK expression between CAF co-culture group and LOVO group, while P-FAK expression was significantly increased in the CAF co-culture group compared with the LOVO group (P < 0.05). FAK and P-FAK in the CAFs co-culture group were significantly higher compared with those in the TM treatment group (P < 0.05). Therefore, above results confirmed that CAFs could promote FAK phosphorylation in LOVO cells, while TM could inhibit FAK phosphorylation in LOVO cells. Figure 5c and d shows that CAFs may be responsible for FAK activation and phosphorylation. Additionally, TM inhibits FAK activation and the occurrence of EMT in colon cancer cells. Therefore, the FAK pathway is involved in EMT in colon cancer cells that are induced by CAFs.
Figure 5.
Effect of interstitial cells in the tumor microenvironment on EMT in LOVO colon cancer cells. Expression levels of cell target proteins (E-cadherin and N-cadherin) were determined by western blot (a, b). Compared with the LOVO, *, P < 0.05; **, P < 0.01; compared with the LOVO+NFs, #, P < 0.05; ##, P < 0.01. The induction of the FAK pathway, which is involved in EMT in colon cancer cells, by CAFs, was analyzed by western blot (c) and (d).
EMT, epithelial–mesenchymal transition; NFs, normal fibroblasts; FAK, focal adhesion kinase; CAFs, cancer-associated fibroblasts.
Discussion
Recently, the tumor microenvironment has become a therapeutic target of solid tumors, and it is very important to the progression of tumors. The interstitial microenvironment, which is composed mainly of CAFs, plays an important role in CRC metastasis.25 In addition to CAFs, endothelial cells and various leukocyte populations such as tumor-associated macrophages, were shown to construct a favorable microenvironment to promote tumor growth and invasiveness. From a diagnostic point of view, the role of the fibrinogen-to-prealbumin ratio (FPR) in CRC has not been completely clarified. However, the combination of the FPR, CEA, and CA19-9 could optimize the ability to discriminate between CRC and benign disease.26
Copper is a necessary trace element in the human body. However, if an imbalance occurs, it will lead to various pathological conditions, including the occurrence and development of tumors.27 Copper is a cofactor for many important proteins, and it plays an important role in tumor angiogenesis and tumor cell migration and metastasis.28 Moreover, TM exhibits a higher potency compared with other chelators29 and functions by both binding dietary copper to prevent copper absorption and forming a complex with free Cu and albumin in the blood.30 Clinical cancer trials of TM, which are partially based on the relationship of Cu with angiogenesis,31,32 reported that TM is well tolerated in patients with advanced malignancies. LOXL2 is a copper-dependent oxidase that can catalyze extracellular matrix collagen to cross-link with elastoprotein, inducing fibrosis and maintaining the integrity of the extracellular matrix structure.33 LOXL2 has been shown to be involved in the progression and metastasis of several tumor types.34 However, LOXL2 can regulate many cellular functions, including tumor angiogenesis and EMT, ultimately leading to tumor invasion and metastasis.35 As a new type of copper chelating agent, TM has significant antiangiogenic36 and antitumor effects, including inhibiting tumor proliferation, inducing tumor cell apoptosis, preventing tumor invasion and metastasis, and changing the activation state of many different tumor signaling pathways, which confirm its therapeutic effect against some tumors in clinical trials.12,36–38
The process of tumor invasion and metastasis is complex, and it consists of several separate steps. The process of metastasis requires that primary tumor cells lose cell–cell connections, acquire the ability to migrate, and form cell–matrix adhesion complexes to form metastatic foci that move through blood vessels or lymphatic vessels into secondary organs.3 Carcinoma-associated fibroblasts influence tumor initiation, progression, and metastasis within the tumor-associated stroma. This suggests that CAFs would be a potential target for tumor therapy. Here, we selected NFs and CAFs in the tumor microenvironment to co-culture with human colon cancer LOVO cells. Because CAFs come from a variety of pathways, local infiltration of fibroblasts is the main source. Colon cancer LOVO cells activate interstitial fibroblasts to CAFs, which were identified by their morphology, growth behaviors, and immunocytochemical staining. CAFs specifically express α-SMA, and the positive α-SMA expression is a CAF activation marker.39 In this study, CAFs were obtained by indirect co-culture of LOVO cells with NFs, and the specific expression of α-SMA was confirmed by a cell immunofluorescence assay and western blot analysis. During co-culture, NFs act as tumor cells through some special molecular mechanisms during activation, and they enhance the metastasis and invasion of tumor cells. In this experiment, NFs were activated in co-culture, and the invasion ability of tumor cells was also enhanced at the same time. Currently, research on breast cancer, pancreatic cancer, prostate cancer, and other cancer tissues has confirmed that CAFs have a strong secretory function compared with NFs, and this function is involved in the activation of endothelial cells, tumor angiogenesis, and tumor metastasis. Here, we used 3D cell culture to simulate the human tumor microenvironment and create a model that is similar to tumor cells that reflects the actual physiological and pathological environment in terms of gene expression, matrix secretion, and cell function.
The results of cellular immunofluorescence staining showed that α-SMA expression in NFs was negative, while in CAFs, it was highly expressed, and western blot results showed the same results. CAFs express α-SMA specifically, and α-SMA-positive expression is an activation marker CAFs. NFs, however, tended to express low or no α-SMA. α-SMA is a protein with high intracellular expression and low expression on the cell membrane. When the penetration effect of cell membrane is not ideal, the protein expression may be lower compared with the actual content using cellular immunofluorescence. Western blot was performed to determine protein expression in the whole cell. Therefore, these factors may lead to differences.
The flow cytometry experiments were performed after the cells were recovered, and the number of cells in the two groups (NFs and co-cultured NFs) was different, but the percentage of cells in the two groups was 100%. The original data are presented in Figure 2. The results showed that the percentage of cells in the G0/G1 phase, the G2/M phase, and S stage was significantly different between NFs and co-cultured NFs. Among them, the G2/M phase and S phase of NFs were higher compared with co-cultured NFs, but the opposite was found in the G0/G1 stage. The results of flow cytometry also showed that NFs and CAFs are different in their cell cycle, and further identified that NFs can be activated by co-culture of colon cancer LOVO cells, thus obtaining the different biological characteristics of CAFs cells. These biological characteristics may be different from the effects of these two cells on tumor cells. It is suggested that CAFs from the previous experiment maintained their biological characteristics and can be used for related, follow-up experimental studies. Additionally, the results of the cell proliferation test also showed that the proliferation activity of co-culture NFs increased significantly starting at 2 days. The results suggest that cancer cells can promote the proliferation of NFs cells.
From research on the origin of CAFs in CRC, specific α-SMA expression was obtained from indirect co-cultivation of LOVO cells with NFs to obtain CAFs, and the obtained cells were confirmed to be CAFs by immunocytochemical staining, cell immunofluorescence, and western blot. α-SMA is considered to be a specific marker for CAFs. Our results also confirmed whether α-SMA is expressed in the NFs group, but it is highly expressed in the co-culture NF group. In particular, the authors identified α-SMAhigh CAFs that were in direct contact with neoplastic cells.40 Fiori et al.41 reported that CAFs that were generated from normal activated fibroblasts (NAFs) that showed persistent activation by the presence of growth factors to promote tumor initiation. Therefore, the above data concluded that NFs have been converted to CAFs under the effect of certain factors that were secreted by the tumor cells.
We then established a co-culture model and detected copper levels and LOXL2 expression in the tumor microenvironment. The results showed that the copper content was significantly higher in the co-culture group compared with the control group, and the level of LOXL2 secreted by CAFs was higher compared with that secreted by NFs (P<0.05). These results indicate that the levels of copper and LOXL2 in the microenvironment of colon cancer LOVO cells are much higher compared with those in the normal tissue microenvironment or single tumor cell microenvironment. These components may be necessary for the invasion and metastasis of tumor cells. LOXL2 also plays an important role in a variety of biological reactions. Research has also indicated that LOXL2 promoted tumor cell invasion in vitro and increased gastric carcinoma metastasis in vivo.42
EMT has been associated with increased aggressiveness and the acquisition of migratory properties, providing tumor cells with the ability to invade adjacent tissues.43 EMT is a key step in the start of cell invasion because it leads to the damage of cell-to-cell connections and the motility and invasiveness of tumor cells, thus promoting tumor metastasis.44 Another key step in tumor cell migration is the formation of cell–matrix adhesion, which is regulated by two key proteins in the cell: FAK and Src. Inactivation of either of these proteins can lead to a loss of tumor cell mobility. FAK is activated through a series of phosphorylation events and is involved in the activation and regulation of various cell migration and adhesion signaling molecules.45 Barker et al.46 reported that tumor-secreted LOXL2 activates fibroblasts through FAK signaling. We detected E-cadherin and N-cadherin expression and related protein expression such as FAK and P-FAK. CAFs were shown to promote the development of EMT and phosphorylation of FAK in colon cancer LOVO cells, activate the FAK signaling pathway, and eventually promote distant colon cancer metastasis. The same results shows that CAFs play an important role in the development and progression of cancer by inducing EMT. We also found that TM can chelate copper in the tumor microenvironment and inhibit the activation of FAK and the occurrence of EMT in colon cancer cells.
Conclusion
Our results show that TM can be used to regulate the micro-environment of colon cancer and the many key steps of tumor metastasis. TM can significantly inhibit colon cancer cell mobility and invasiveness by chelating copper and inhibiting FAK, and thus, reducing colon cancer cell invasion and metastasis. The results provide evidence that CAFs are a target for cancer therapy and show how copper mediates tumor progression at the molecular level. Therefore, TM, which is a novel agent with many potential anti-tumor effects, specifically inhibited colon cancer cells, and it is a potential new candidate for controlling tumor metastasis in colon cancer patients.
Acknowledgement
We are grateful to all participants in this study.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by Grants from the National Natural Science Foundation of China, No. 81260364 and No. 81860416; the National Clinical Key Specialist Construction Project in China, No. 2018; and the Natural Science Foundation of Inner Mongolian Autonomous Region, No. 2017MS (LH) 0826.
ORCID iD
Xin-Lin Wu https://orcid.org/0000-0002-2860-4477
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Marx V. Tracking metastasis and tricking cancer. Nature 2013; 494: 133–136. [DOI] [PubMed] [Google Scholar]
- 3.Guan X. Cancer metastases: Challenges and opportunities. Acta Pharm Sin B 2015; 5: 402–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcucci F, Rumio C. How tumor cells choose between epithelial-mesenchymal transition and autophagy to resist stress—Therapeutic implications. Front Pharmacol 2018; 9: 714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catalano V, Turdo A, Di Franco S, et al. Tumor and its microenvironment: A synergistic interplay. Semin Cancer Biol 2013; 23: 522–532. [DOI] [PubMed] [Google Scholar]
- 6.Koliaraki V, Pallangyo CK, Greten FR, et al. Mesenchymal cells in colon cancer. Gastroenterology 2017; 152: 964–979. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Deng Z, Zhu G. Emerging platinum(IV) prodrugs to combat cisplatin resistance: From isolated cancer cells to tumor microenvironment. Dalton Trans 2019; 48: 2536–2544. [DOI] [PubMed] [Google Scholar]
- 8.Nair N, Calle AS, Zahra MH, et al. A cancer stem cell model as the point of origin of cancer-associated fibroblasts in tumor microenvironment. Sci Rep 2017; 7: 6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zarkavelis G, Boussios S, Papadaki A, et al. Current and future biomarkers in colorectal cancer. Ann Gastroenterol 2017; 30: 613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denoyer D, Masaldan S, La FS, et al. Targeting copper in cancer therapy: ‘Copper That Cancer’. Metallomics 2015; 7: 1459. [DOI] [PubMed] [Google Scholar]
- 11.Tokuda E, Furukawa Y. Copper homeostasis as a therapeutic target in amyotrophic lateral sclerosis with SOD1 mutations. Int J Mol Sci 2016; 17: E636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baldari S, Di Rocco G, Toietta G. Current biomedical use of copper chelation therapy. Int J Mol Sci 2020; 21: 1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiNicolantonio JJ, Mangan D, O’Keefe JH. Copper deficiency may be a leading cause of ischaemic heart disease. Open Heart 2018; 5: e000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishida S, Andreux P, Poitry-Yamate C, et al. Bioavailable copper modulates oxidative phosphorylation and growth of tumors. Proc Natl Acad Sci U S A 2013; 110: 19507–19512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai H, Xie F, Mulgaonkar A, et al. Bombesin functionalized 64Cu-copper sulfide nanoparticles for targeted imaging of orthotopic prostate cancer. Nanomedicine (Lond) 2018: 13: 1695–1705. [DOI] [PubMed] [Google Scholar]
- 16.Nienhüser H, Schmidt T. Angiogenesis and anti-angiogenic therapy in gastric cancer. Int J Mol Sci 2018; 19: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatori Y, Lutsenko S. The role of copper chaperone Atox1 in coupling redox homeostasis to intracellular copper distribution. Antioxidants 2016; 5: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukai T, Ushio-Fukai M, Kaplan JH. Copper transporters and copper chaperones: Roles in cardiovascular physiology and disease. Am J Physiol Cell Physiol 2018; 315: C186–C201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin XY, Wang YN, Yang XP, et al. Synthesis, characterization, and anticancer activity of two mixed ligand copper(ii) complexes by regulating the VEGF/VEGFR2 signaling pathway. Dalton Trans 2017; 46: 16446–16454. [DOI] [PubMed] [Google Scholar]
- 20.Kober KI, Cano A, Géraud C, et al. Loxl2 is dispensable for dermal development, homeostasis and tumour stroma formation. Plos One 2018; 13: e0199679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antoniades V, Sioga A, Dietrich EM, et al. Is copper chelation an effective anti-angiogenic strategy for cancer treatment? Med Hypotheses 2013; 81: 1159–1163. [DOI] [PubMed] [Google Scholar]
- 22.Haycock JW. 3D cell culture: A review of current approaches and techniques. Methods Mol Biol 2011; 695: 1–15. [DOI] [PubMed] [Google Scholar]
- 23.Maddaly R, Paramesh V, Kaviya SR, et al. 3D cell culture systems: Advantages and applications. J Cell Physiol 2015; 230: 16–26. [DOI] [PubMed] [Google Scholar]
- 24.Tsai S, McOlash L, Palen K, et al. Development of primary human pancreatic cancer organoids, matched stromal and immune cells and 3D tumor microenvironment models. BMC Cancer 2018; 18: 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu S, Dong L, Sun W, et al. Stromal-epithelial crosstalk provides a suitable microenvironment for the progression of ovarian cancer cells in vitro. Cancer Invest 2013; 31: 616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boussios S, Ozturk MA, Moschetta M, et al. The developing story of predictive biomarkers in colorectal cancer. J Pers Med 2019; 9: E12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garber K. Biomedicine. Targeting copper to treat breast cancer. Science 2015; 349: 128–129. [DOI] [PubMed] [Google Scholar]
- 28.Turski ML, Thiele DJ. New roles for copper metabolism in cell proliferation, signaling, and disease. J Biol Chem 2009; 284: 717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brewer GJ, Askari F, Dick RB, et al. Treatment of Wilson’s disease with tetrathiomolybdate: V. Control of free copper by tetrathiomolybdate and a comparison with trientine. Transl Res 2009; 154: 70–77. [DOI] [PubMed] [Google Scholar]
- 30.Kodama H, Fujisawa C, Bhadhprasit W. Inherited copper transport disorders: Biochemical mechanisms, diagnosis, and treatment. Curr Drug Metab 2012; 13: 237–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finney L, Vogt S, Fukai T, et al. Copper and angiogenesis: Unravelling a relationship key to cancer progression. Clin Exp Pharmacol Physiol 2009; 36: 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brady DC, Crowe MS, Turski ML, et al. Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature 2014; 509: 492–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grossman M, Ben-Chetrit N, Zhuravlev A, et al. Tumor cell invasion can be blocked by modulators of collagen fibril alignment that control assembly of the extracellular matrix. Cancer Res 2016; 76: 4249–4258. [DOI] [PubMed] [Google Scholar]
- 34.Cano A, Santamaría PG, Moreno-Bueno G. LOXL2 in epithelial cell plasticity and tumor progression. Future Oncol 2012; 8: 1095–1108. [DOI] [PubMed] [Google Scholar]
- 35.Torres S, Garcia-Palmero I, Herrera M, et al. LOXL2 is highly expressed in cancer-associated fibroblasts and associates to poor colon cancer survival. Clin Cancer Res 2015; 21: 4892–4902. [DOI] [PubMed] [Google Scholar]
- 36.Brewer GJ. The promise of copper lowering therapy with tetrathiomolybdate in the cure of cancer and in the treatment of inflammatory disease. J Trace Elem Med Biol 2014; 28: 372–378. [DOI] [PubMed] [Google Scholar]
- 37.Chan N, Willis A, Kornhauser N, et al. Influencing the tumor microenvironment: A Phase II study of copper depletion using tetrathiomolybdate in patients with breast cancer at high risk for recurrence and in preclinical models of lung metastases. Clin Cancer Res 2017; 23: 666–676. [DOI] [PubMed] [Google Scholar]
- 38.Schneider BJ, Lee JS, Hayman JA, et al. Pre-operative chemoradiation followed by post-operative adjuvant therapy with tetrathiomolybdate, a novel copper chelator, for patients with resectable esophageal cancer. Invest New Drugs 2013; 31: 435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Komohara Y, Takeya M. CAFs and TAMs: Maestros of the tumour microenvironment. J Pathol 2017; 241: 313–315. [DOI] [PubMed] [Google Scholar]
- 40.Öhlund D, Handly-Santana A, Biffi G, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 2017; 214: 579–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fiori ME, Di Franco S, Villanova L, et al. Cancer-associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Mol Cancer 2019; 18: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng L, Ran YL, Hu H, et al. Secreted LOXL2 is a novel therapeutic target that promotes gastric cancer metastasis via the Src/FAK pathway. Carcinogenesis 2009; 30: 1660–1669. [DOI] [PubMed] [Google Scholar]
- 43.Canesin G, Cuevas EP, Santos V, et al. Lysyl oxidase-like 2 (LOXL2) and E47 EMT factor: Novel partners in E-cadherin repression and early metastasis colonization. Oncogene 2015; 34: 951–964. [DOI] [PubMed] [Google Scholar]
- 44.Singh M, Yelle N, Venugopal C, et al. EMT: Mechanisms and therapeutic implications. Pharmacol Ther 2017; 182: 80–94. [DOI] [PubMed] [Google Scholar]
- 45.Roy-Luzarraga M, Hodivala-Dilke K. Molecular pathways: Endothelial cell FAK-A target for cancer treatment. Clin Cancer Res 2016; 22: 3718–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barker HE, Bird D, Lang G, et al. Tumor-secreted LOXL2 activates fibroblasts through FAK signaling. Mol Cancer Res 2013; 11: 1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]