Abstract
Rationale
Whether asthma constitutes a risk factor for coronavirus disease‐2019 (COVID‐19) is unclear. Here, we aimed to assess whether asthma, the most common chronic disease in children, is associated with higher COVID‐19 risk or severity in pediatric populations.
Methods
We performed a systematic literature search in three stages: first, we reviewed PubMed, EMBASE, and CINAHL for systematic reviews of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and COVID‐19 in pediatric populations, and reviewed their primary articles; second, we searched PubMed for studies on COVID‐19 or SARS‐CoV‐2 and asthma/wheeze, and evaluated whether the resulting studies included pediatric populations; third, we repeated the second search in BioRxiv.org and MedRxiv.org to find pre‐prints that may have information on pediatric asthma.
Results
In the first search, eight systematic reviews were found, of which five were done in pediatric populations; none of the 67 primary studies included data on pediatric asthma as a comorbidity for COVID‐19. In the second search, we found 34 results in PubMed, of which five reported asthma in adults, but none included data on children. In the third search, 25 pre‐prints in MedRxiv included data on asthma, but none on children. We found one report by the US Centers for Disease Control and Prevention stating that 40/345 (~11.5%) children with data on chronic conditions had “chronic lung diseases including asthma,” and one from a tertiary hospital in New York that reported asthma in 11/46 (~23.9%) children hospitalized for COVID‐19.
Conclusion
There is scarcely any data on whether childhood asthma (or other pediatric respiratory diseases) constitute risk factors for SARS‐CoV‐2 infection or COVID‐19 severity. Studies are needed that go beyond counting the number of cases in the pediatric age range.
Keywords: asthma & early wheeze, SARS‐CoV‐2, COVID‐19
1. INTRODUCTION
The current outbreak of coronavirus disease‐2019 (COVID‐19), caused by the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), started in or around December 2019, in Wuhan. 1 On 30th January 2020, the World Health Organization (WHO) declared COVID‐19 a pandemic health emergency. 2 Since then, COVID‐19 has continued to spread quickly and has now become the most dangerous pandemic in over 100 years.
An interactive real‐time COVID‐19 reporting system set up by the Center for Systemic Science and Engineering at Johns Hopkins University 3 shows, as of the time of this writing, more than 5.8 million confirmed cases and over 361 000 deaths worldwide (led by the United States, with ~30% of all cases and ~28% of all deaths). Globally, this corresponds to a ~6% case fatality rate, although rates vary widely among countries and subpopulations and it is difficult to ascertain both the true numerator (active unresolved cases may eventually become deaths) and the true denominator (many true cases may go untested or undetected).
The first pediatric case in the literature was reported on January 2020 in 10‐year‐old boy from Shenzhen, China, whose family had visited Wuhan. 4 All epidemiological evidence to date suggests that SARS‐CoV‐2 infection is less severe in children than in adults. In the latest and largest study in the UK, including 16 749 patients hospitalized for COVID‐19, only 239 (2%) were less than 18 years of age (including 139 who were <5 years old). 5 Large studies in Italy and China have also shown very low case‐fatality rates in children and adolescents. 6 Understandably, most studies have focused on adult populations, with very few studies and reviews in children. Moreover, accumulating data points to risk factors for severity and mortality in adults (eg, older age, cardiovascular disease, diabetes, cancer, immunosuppression, obesity, tobacco smoking, etc), 5 , 6 but there is very scarce evidence on whether or which risk factors exist in children. While COVID‐19 is a multisystem disease, it predominantly effects the lungs, and thus it is critically important to understand whether chronic lung diseases place children at higher risk.
The main objective of this study was to identify whether asthma, the most common chronic respiratory disease in children, is a risk factor for SARS‐CoV‐2 infection or COVID‐19 severity in the pediatric population.
2. METHODS
We performed a systematic literature research in three stages (see Figure 1): first, we searched PubMed, EMBASE, and CINAHL using the following terms: “(((SARS‐CoV‐2) OR (COVID‐19)) AND ((systematic review)) AND ((children 0‐18 years of age)))” to find systemic reviews on the topic, and then reviewed the primary studies included in those reviews. Second, we searched PubMed for “(((COVID‐19) OR (SARS‐CoV‐2)) AND ((asthma) OR (wheezing))),” to directly find any studies on asthma/wheezing and COVID‐19 (without an age filter), and evaluated whether they included pediatric populations. Third, we repeated search #2 in BioRxiv.org and MedRxiv.org to evaluate whether existing pre‐prints may have relevant pediatric asthma information. The last update of the searches was on 6 May 2020 (see Figure 1).
Figure 1.
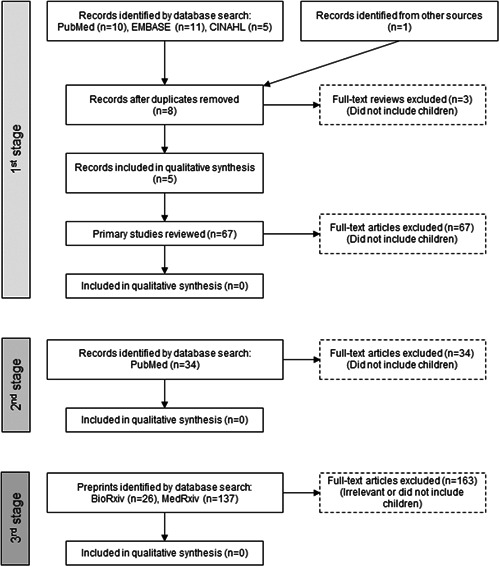
Process of study selection
Both authors (JCR and EF) independently screened and retrieved articles. The same investigators independently assessed full texts of those primary studies included in the systematic review identified. Any discrepancies were resolved by discussion and consensus. If sufficient studies with relevant data were found, the plan was to perform a meta‐analysis by asthma status. The review was performed following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines. 7
3. RESULTS
After removing duplicates, the first search yielded eight systematic reviews. 5 , 8 , 9 , 10 , 11 , 12 , 13 , 14 Three of them were eliminated because they did include information on clinical characteristics in children. 8 , 9 , 10 Therefore, we evaluated five systematic reviews done at different periods during the pandemic and thus including somewhat different primary studies. 11 , 12 , 13 , 14 Castagnoli et al 11 included 18 articles, 17 from China and one from Singapore (444 patients < 10 years old and 553 aged 10‐19 years), published up to 3 March 2020. Choi et al 12 included seven articles from China (225 pediatric patients) up to 12 March 2020. Chang et al 13 included nine studies from China (93 pediatric patients) up to 15 March 2020. The review by Ludvigsson 14 included 45 studies from China (the total number of patients was not described) up to 19 March 2020. And Streng et al 15 included eight studies from China (ranging from 6 to 2143 patients) and one survey from Germany (33 patients) in hospitalized children, up to 31 March 2020. After excluding duplicates, we identified and reviewed 67 primary studies included in those five reviews (see Table S1).
None of the primary studies reviewed reported asthma or recurrent wheezing as a comorbidity or risk factor for COVID‐19. Instead, some of those studies reported young age (especially children <1 year of age) as a group with more severe COVID‐19. One large Chinese study 16 reported nonrespiratory chronic conditions (hydronephrosis, leukemia receiving chemotherapy, and intussusception) among three children who required intensive care unit (ICU) support and mechanical ventilation. Among them, one death occurred in a 10‐month old child with intussusception. Another study reported a patient who developed shock with metabolic acidosis requiring ICU. 17 Yet another report from China 18 described one patient aged 10 to 19 years who died, without other clinical information; that death probably represents the same 14‐year‐old boy described by Dong et al 19 Unfortunately, the two larger studies in Chinese pediatric patients, Dong et al 19 (2413 children) and Wu and McGoogan 20 (965 children) did not report enough clinical data to identify comorbidities or risk factors for COVID‐19 severity. In the German survey of 33 hospitalized children, four out of 22 (18%) children with clinical information had “respiratory comorbidities” without further details. 15
Our second search yielded 34 results in PubMed. Of those, five were primary studies that reported on asthma in adults 21 , 22 , 23 , 24 , 25 ; one other was a guidance statement 26 that referenced a primary report that also included information on asthma in adults. 27 No studies from that search included information on asthma in children, although one case series reported two young children (ages 2 and 3 years) with history of atopic dermatitis and allergic rhinitis, who were hospitalized with COVID‐19; both patients recovered. 28
Our third search yielded 26 pre‐prints in BioRxiv and 137 in MedRxiv. None of the BioRxiv posts were relevant to our topic. Of the 137 pre‐prints in MedRxiv, 23 nonduplicate studies included information on asthma, 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 but none of them included specific information in children.
A search of secondary sources and reference lists identified a Morbidity Mortality Weekly Report (MMWR), 52 published by the Centers for Disease Control and Prevention (CDC), that included information from 2572 US children aged less than 18 years. Of those cases, 345 had data on clinical and underlying conditions, and 80 of those children (23%) had at least one underlying condition. The most common underlying conditions were “chronic lung diseases (including asthma)” in 40 children, cardiovascular disease in 25, and immunosuppression in 10; separate information on asthma was not provided. Among the 295 cases for which data on both hospitalization status and underlying medical conditions was available, 28/37 (77%) hospitalized patients had one or more underlying medical condition (including all six patients admitted to an ICU); compared with 30/258 (12%) patients who were not hospitalized. 52 A recently published Italian study, including 100 children seen in 17 emergency departments, reported that 27% had comorbidities without more specifications, and no deaths occurred. 53 And most recently, a study of 46 children hospitalized for COVID‐19 at a tertiary center in New York reported 11/46 (23.9%) had asthma, with no differences in the proportions admitted to the general floor (8/33 or 24.2%) vs PICU (3/13 or 23.1%).
4. DISCUSSION
In a systematic review of the literature, we only found two reports that described asthma or recurrent wheezing as potential risk factors for COVID‐19 in children. Importantly, none of the largest epidemiological studies including children with COVID‐19 reported clinical findings or underlying characteristics to help assess whether asthma, or other chronic lung diseases, constitutes a risk factor for SARS‐CoV‐2 infection or COVID‐19 severity.
COVID‐19 affects primarily the lungs, and accordingly several international guidelines have designated some respiratory conditions as a potential risk factor for severe disease. Chinese guidelines 54 state that “children with a history of contact with severe 2019‐nCoV infected cases, or with underlying conditions (such as congenital heart disease, bronchial pulmonary hypoplasia, respiratory tract anomaly, with abnormal hemoglobin level, and severe malnutrition), or with immune deficiency or immunocompromised status… may become severe cases.” A recent statement from the EAACI Section on Pediatrics 26 declared that “patients with asthma (particularly severe or uncontrolled asthma) and immunodeficiency have also been classified to be at increased risk of developing severe COVID‐19, based more on common sense rather than mounting evidence.” The Global Initiative for Asthma (GINA) recommends avoiding the use of nebulizers due to the increased risk of disseminating COVID‐19 to other patients and health care staff; they thus recommend the use of pressurized metered dose inhalers (pMDI) as the preferred delivery system during asthma attacks. 55 A recent randomized controlled trial 56 showed that even in children with severe asthma exacerbations, administration of albuterol/salbutamol, and ipratropium by MDI with valved‐holding chamber and mask along with oxygen by nasal cannula was more effective than nebulized administration. GINA 55 and the British Thoracic Society 57 do not recommend stopping oral steroids in the patients already taking them for asthma management, and they do not recommend avoiding them for acute asthma attacks even if due to COVID‐19. The US CDC, the Canadian Pediatric Society, and other professional associations have issued guidance for patients with asthma and/or allergies. 58 , 59 , 60 Other professional organizations, such as the American Academy of Pediatrics and the American Thoracic Society, have published interim guidelines that do not specifically address asthma, likely given a paucity of evidence. 61 , 62
Rather than a risk factor, a recent review of data in adults reported that both asthma and chronic obstructive pulmonary disease appear to be underrepresented in the comorbidities reported for patients with COVID‐19, compared with global estimates of prevalence for these conditions in the general population. 63 This is consistent with individual studies we found during our search, which have shown lower‐than‐expected prevalence of asthma among cases of COVID‐19, 21 , 22 , 23 , 24 , 27 and in contrast to the prevalence of other chronic diseases such as diabetes, which occurred with higher frequency among patients with COVID‐19 than the estimated national prevalence. 63 If asthma is indeed “protective,” this could be due to several factors, including changes in the immune response or decreased risk secondary to chronic medications such as inhaled corticosteroids (ICS). In‐vitro models have shown that ICS may suppress both coronavirus replication and cytokine production. 64 , 65 Analysis of induced sputum samples in a well‐characterized cohort of adults with severe asthma found reduced angiotensin‐converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2) gene expression among patients taking ICS, and especially among those on higher doses 66 ; ACE2 and TMPRSS2 mediate SARS‐CoV‐2 cell infection. Similarly, a recent study (in children and adults) showed that patients with asthma and respiratory allergies had reduced ACE2 gene expression in airway cells, suggesting a potential mechanism of reduced COVID‐19 risk. 67 This is particularly noteworthy considering that one of the potential explanations for children being generally less affected than adults is the hypothesis that children have lower ACE2 receptor expression in alveolar type 2 cells. 68 However, the lower prevalence of asthma among COVID‐19 cases could also stem from bias due to under diagnosis and underreporting, or because patients with chronic lung diseases may be especially cautious in practicing physical distancing and other measures to avoid infection. Finally, it is also conceivable that some milder cases of COVID‐19 might be confused with exacerbations of respiratory disease, and/or that these patients may be reluctant to seek medical care even when sick and are thus never counted.
It is important to note that our understanding of the role of asthma, even in adults, is still incipient. In the largest and most recent analysis to date, UK investigators analyzed data from 17 million adults, including 5683 deaths due to COVID‐19, and reported that both asthma (adjusted hazard ratio, aHR: 1.11 [95% confidence interval, 1.02‐1.20]) and severe asthma (aHR: 1.25 [1.08‐1.44]) were risk factors for COVID‐19 mortality. 51 This study compared COVID‐19 deaths with the general population (regardless of being SARS‐CoV‐2 positive or not), so the estimates combine both risk of infection and risk of death once infected. These results highlight how incomplete our understanding still is. As with most other studies, this large analysis did not include a pediatric population.
5. CONCLUSIONS
After an extensive review of the current literature, only two reports included information on asthma as a potential risk factor for COVID‐19 infection, but not severity or mortality, in children. However, the largest studies to date have been limited to a description of the number of cases by age group, and so it remains unclear whether childhood asthma, or other pediatric respiratory diseases, are associated with COVID‐19 risk or severity.
We, hereby, ask the public health community to move beyond confirming what's already known that the disease affects children and young adults less frequently and severely than older groups, and to study affected pediatric populations in more detail. Does asthma constitute a risk factor for COVID‐19 in children? Do asthma severity or control modify the course of the disease? Are asthma medications (particularly ICS and systemic steroids) or their doses protective or detrimental? Given the limited numbers of pediatric cases in any one given center/country, collaborative international efforts may be the only way to shed light on the topic. Initiatives such as the Pediatric Asthma in Real Life group 69 or a pediatric version of the International Severe Asthma Registry, 70 or efforts coordinated by large professional societies, may be best suited for the task. This will be true not just for childhood asthma but for pediatric diseases in general.
Supporting information
Supporting information
ACKNOWLEDGMENT
Dr. Forno's contribution was partly funded by grant HL149693 from the US National Institutes of Health (NIH).
Castro‐Rodriguez JA, Forno E. Asthma and COVID‐19 in children: A systematic review and call for data. Pediatric Pulmonology. 2020;55:2412–2418. 10.1002/ppul.24909
REFERENCES
- 1. Guan WJ, Ni ZY, Hu Y, et al. China Medical Treatment Expert Group for Covid‐19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . WHO Director‐General's opening remarks at the media briefing on COVID‐19; 11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Accessed May 01, 2020.
- 3. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis. 2020;20:533‐534. 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395(10223):514‐523. 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Docherty AB, Harrison EM, Green CA, et al. Features of 16,749 hospitalised UK patients with COVID‐19 using the ISARIC WHO clinical characterisation protocol [published online ahead of print April 28, 2020]. medRxiv. 2020. 10.1101/2020.04.23.20076042 [DOI] [Google Scholar]
- 6. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. JAMA. 2020;323(18):1775‐1776. 10.1001/jama.2020.4683 [DOI] [PubMed] [Google Scholar]
- 7. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 8. Di Mascio D, Khalil A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID 1 ‐19) during pregnancy: a systematic review and meta‐analysis. Am J Obstet Gynecol. 2020:100107. 10.1016/j.ajogmf.2020.100107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Viner RM, Russell SJ, Croker H, et al. School closure and management practices during coronavirus outbreaks including COVID‐19: a rapid systematic review. Lancet Child Adolesc Health. 2020;4(5):397‐404. 10.1016/S2352-4642(20)30095-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chauhan RP, Dessie ZG, Noreddin A, El Zowalaty ME. Systematic review of important viral diseases in Africa in light of the 'One Health' concept. Pathogens. 2020;9(4), 10.3390/pathogens9040301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Castagnoli R, Votto M, Licari A, et al. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection in children and adolescents: a systematic review [published online ahead of print April 22, 2020]. JAMA Pediatr. 2020. 10.1001/jamapediatrics.2020.1467 [DOI] [PubMed] [Google Scholar]
- 12. Choi SH, Kim HW, Kang JM, Kim DH, Cho EY. Epidemiology and clinical features of coronavirus disease 2019 in children. Clin Exp Pediatr. 2020;63(4):125‐132. 10.3345/cep.2020.00535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang TH, Wu JL, Chang LY. Clinical characteristics and diagnostic challenges of pediatric COVID‐19: a systematic review and meta‐analysis. J Formos Med Assoc. 2020;119:982‐989. 10.1016/j.jfma.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ludvigsson JF. Systematic review of COVID‐19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088‐1095. 10.1111/apa.15270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Streng A, Hartmann K, Armann J, Berner R, Liese JG. [COVID‐19 in hospitalized children and adolescents]. Monatsschr Kinderheilkd. 2020:1‐12. 10.1007/s00112-020-00919-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu X, Zhang L, Du H, et al. Chinese Pediatric Novel Coronavirus Study Team . SARS‐CoV‐2 infection in children. N Engl J Med. 2020;382(17):1663‐1665. 10.1056/NEJMc2005073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen F, Liu ZS, Zhang FR, et al. [First case of severe childhood novel coronavirus pneumonia in China]. Zhonghua Er Ke Za Zhi. 2020;58(3):179‐182. 10.3760/cma.j.issn.0578-1310.2020.03.003 [DOI] [PubMed] [Google Scholar]
- 18. Novel Coronavirus Pneumonia Emergency Response Epidemiology T. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID‐19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145‐151. 10.3760/cma.j.issn.0254-6450.2020.02.003 [DOI] [PubMed] [Google Scholar]
- 19. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID‐19 among children in China. Pediatrics. 2020;145:e20200702. 10.1542/peds.2020-0702 [DOI] [PubMed] [Google Scholar]
- 20. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239‐1242. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 21. Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China [published online ahead of print February 19, 2020]. Allergy. 2020. 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 22. Liu C, Jiang ZC, Shao CX, et al. [Preliminary study of the relationship between novel coronavirus pneumonia and liver function damage: a multicenter study]. Zhonghua Gan Zang Bing Za Zhi. 2020;28(2):148‐152. 10.3760/cma.j.issn.1007-3418.2020.02.003 [DOI] [PubMed] [Google Scholar]
- 23. Zhang JJ, Cao YY, Dong X, et al. Distinct characteristics of COVID‐19 patients with initial rRT‐PCR‐positive and rRT‐PCR‐negative results for SARS‐CoV‐2 [published online ahead of print April 13, 2020]. Allergy. 2020. 10.1111/all.14316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID‐19 inpatients in Wuhan [published online ahead of print April 12, 2020]. J Allergy Clin Immunol. 2020. 10.1016/j.jaci.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Konopka KE, Wilson A, Myers JL. Postmortem lung findings in an asthmatic with coronavirus disease 2019 (COVID‐19) [published online ahead of print April 28, 2020]. Chest. 2020. 10.1016/j.chest.2020.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brough HA, Kalayci O, Sediva A, et al. Managing childhood allergies and immunodeficiencies during respiratory virus epidemics ‐ the 2020 COVID‐19 pandemic [published online ahead of print April 22, 2020]. Pediatr Allergy Immunol. 2020. 10.1111/pai.13262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory‐confirmed coronavirus disease 2019 ‐ COVID‐NET, 14 States, March 1‐30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458‐464. 10.15585/mmwr.mm6915e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dong X, Cao YY, Lu XX, et al. Eleven faces of coronavirus disease 2019 [published online ahead of print March 20, 2020]. Allergy. 2020. 10.1111/all.14289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ashraf MA, Shokouhi N, Shirali E, et al. COVID‐19 in Iran, a comprehensive investigation from exposure to treatment outcomes [published online ahead of print April 24, 2020]. medRxiv. 2020. 10.1101/2020.04.20.20072421 [DOI] [Google Scholar]
- 30. Auld S, Caridi‐Scheible M, Blum JM, et al. ICU and ventilator mortality among critically ill adults with COVID‐19 [published online ahead of print April 26, 2020]. medRxiv. 2020. 10.1101/2020.04.23.20076737 [DOI] [Google Scholar]
- 31. Barrett ES, Horton DB, Roy J, et al. Prevalence of SARS‐CoV‐2 infection in previously undiagnosed health care workers at the onset of the US COVID‐19 epidemic [published online ahead of print April 24, 2020]. medRxiv. 2020. 10.1101/2020.04.20.20072470 [DOI] [Google Scholar]
- 32. Bello‐Chavolla OY, Bahena‐Lopez JP, Antonio‐Villa NE, et al. Predicting mortality due to SARS‐CoV‐2: a mechanistic score relating and diabetes to COVID‐19 outcomes in Mexico [published online ahead of print May 22, 2020]. medRxiv. 2020. 10.1101/2020.04.20.20072223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burn E, You SC, Sena A, et al. An international characterisation of patients hospitalised with COVID‐19 and a comparison with those previously hospitalised with influenza [published online ahead of print April 25, 2020]. medRxiv. 2020. 10.1101/2020.04.22.20074336 [DOI] [Google Scholar]
- 34. Clark A, Jit M, Warren‐Gash C, et al. How many are at increased risk of severe COVID‐19 disease? Rapid global, regional and national estimates for 2020 [published online ahead of print April 22, 2020]. medRxiv. 10.1101/2020.04.18.20064774 [DOI] [Google Scholar]
- 35. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID‐19 in New York City: a prospective cohort study [published online ahead of print April 20, 2020]. medRxiv. 2020. 10.1101/2020.04.15.20067157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ni Lochlainn M, Lee KA, Sudre CH, et al. Key predictors of attending hospital with COVID19: an association study from the COVID Symptom Tracker App in 2,618,948 individuals [published online ahead of print April 29, 2020]. medRxiv. 2020. 10.1101/2020.04.25.20079251 [DOI] [Google Scholar]
- 37. Paranjpe I, Russak A, De Freitas JK, et al. Clinical characteristics of hospitalized Covid‐19 patients in New York City [published online ahead of print June 11, 2020]. medRxiv. 2020. 10.1101/2020.04.19.20062117 [DOI] [Google Scholar]
- 38. Qian Z, Alaa AM, van der Schaar M, Ercole A. Between‐centre differences for COVID‐19 ICU mortality from early data in England [published online ahead of print April 27, 2020]. medRxiv. 2020. 10.1101/2020.04.19.20070722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vaid A, Somani S, Russak AJ, et al. Machine learning to predict mortality and critical events in COVID‐19 positive New York City patients [published online ahead of print April 28, 2020]. medRxiv. 2020. 10.1101/2020.04.26.20073411 [DOI] [Google Scholar]
- 40. Whitman JD, Hiatt J, Mowery CT, et al. Test performance evaluation of SARS‐CoV‐2 serological assays [published online ahead of print May 17, 2020]. medRxiv. 2020. 10.1101/2020.04.25.20074856 [DOI] [Google Scholar]
- 41. Zhang H, Wang X, Fu Z, et al. Potential factors for prediction of disease severity of COVID‐19 patients [published online ahead of print March 23, 2020]. medRxiv. 2020. 10.1101/2020.03.20.20039818 [DOI] [Google Scholar]
- 42. Wang Z, Weng J, Li Z, et al. Development and validation of a diagnostic nomogram to predict COVID‐19 pneumonia [published online ahead of print April 06, 2020]. medRxiv. 2020. 10.1101/2020.04.03.20052068 [DOI] [Google Scholar]
- 43. Rubin SJS, Falkson SR, Degner N, Blish C. Clinical characteristics associated with COVID‐19 severity in California [published online ahead of print March 30, 2020]. medRxiv. 2020. 10.1101/2020.03.27.20043661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rentsch CT, Kidwai‐Khan F, Tate JP, et al. Covid‐19 testing, hospital admission, and intensive care among 2,026,227 United States veterans aged 54‐75 years [published online ahead of print April 14, 2020]. medRxiv. 2020. 10.1101/2020.04.09.20059964 [DOI] [Google Scholar]
- 45. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospitalization and critical illness among 4,103 patients with COVID‐19 disease in New York City [published online ahead of print April 11, 2020]. medRxiv. 2020. 10.1101/2020.04.08.20057794 [DOI] [Google Scholar]
- 46. Fadel R, Morrison A, Vahia A, et al. Early short course corticosteroids in hospitalized patients with COVID‐19 [published online ahead of print May 15, 2020]. medRxiv. 2020. 10.1101/2020.05.04.20074609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ebinger JE, Achamallah N, Ji H, et al. Pre‐existing characteristics associated with Covid‐19 illness severity [published online ahead of print May 10, 2020]. medRxiv. 2020. 10.1101/2020.04.29.20084533 [DOI] [Google Scholar]
- 48. Carr E, Bendayan R, Bean D, et al. Supplementing the National Early Warning Score (NEWS2) for anticipating early deterioration among patients with COVID‐19 infection [published online ahead of print June 11, 2020]. medRxiv. 2020. 10.1101/2020.04.24.20078006 [DOI] [Google Scholar]
- 49. Benelli G, Buscarini E, Canetta C, et al. SARS‐COV‐2 comorbidity network and outcome in hospitalized patients in Crema, Italy [published online ahead of print April 30, 2020]. medRxiv. 2020. 10.1101/2020.04.14.20053090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Adams ML, Grandpre J, Katz DL. Updated estimates of comorbidities associated with risk for COVID‐19 complications based on US data [published online ahead of print May 06, 2020]. medRxiv. 2020. 10.1101/2020.05.02.20088781 [DOI] [Google Scholar]
- 51. Williamson E, Walker AJ, Bhaskaran KJ, et al. OpenSAFELY: factors associated with COVID‐19‐related hospital death in the linked electronic health records of 17 million adult NHS patients [published online ahead of print May 07, 2020]. medRxiv. 2020. 10.1101/2020.05.06.20092999 [DOI] [Google Scholar]
- 52. Team CC‐R. Coronavirus disease 2019 in Children ‐ United States, February 12‐April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(14):422‐426. 10.15585/mmwr.mm6914e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Parri N, Lenge M, Buonsenso D Coronavirus Infection in Pediatric Emergency Departments Research Group . Children with Covid‐19 in pediatric emergency departments in Italy [published online ahead of print May 01, 2020]. N Engl J Med. 2020. 10.1056/NEJMc2007617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shen K, Yang Y, Wang T, et al. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts' consensus statement [published online ahead of print February 07, 2020]. World J Pediatr. 2020. 10.1007/s12519-020-00343-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. COVID‐19 : GINA answers to frequently asked questions on asthma management 2020 [updated 3/25/2020]. https://ginasthma.org/covid-19-gina-answers-to-frequently-asked-questions-on-asthma-management/. Accessed May 01, 2020.
- 56. Iramain R, Castro‐Rodriguez JA, Jara A, et al. Salbutamol and ipratropium by inhaler is superior to nebulizer in children with severe acute asthma exacerbation: randomized clinical trial. Pediatr Pulmonol. 2019;54(4):372‐377. 10.1002/ppul.24244 [DOI] [PubMed] [Google Scholar]
- 57. BTS advice for healthcare professionals treating patients with asthma; 2020. https://www.brit-thoracic.org.uk/document-library/quality-improvement/covid-19/bts-advice-for-healthcare-professionals-treating-patients-with-asthma/. Accessed May 01, 2020.
- 58. CDC . COVID‐19: People who are at higher risk – people with asthma; 2020. [updated 04/02/2020]. https://www.cdc.gov/coronavirus/2019-ncov/specific-groups/asthma.html. Accessed May 01, 2020.
- 59. Abrams EM, Jong GW, Yang CL. Asthma and COVID‐19. CMAJ. 2020;192(20):E551. 10.1503/cmaj.200617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shaker MS, Oppenheimer J, Grayson M, et al. COVID‐19: pandemic contingency planning for the allergy and immunology clinic. J Allergy Clin Immunol Pract. 2020;8(5):1477‐1478. 10.1016/j.jaip.2020.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wilson KC, Chotirmall SH, Bai C, Rello J. COVID‐19: interim guidance on management pending empirical evidence. From an American Thoracic Society‐led International Task Force [updated 04/03/2020]. https://www.thoracic.org/covid/covid-19-guidance.pdf. Accessed May 01, 2020.
- 62. Pediatrics AAO . Critical updates on COVID‐19 [updated 04/23/2020]. https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/. Accessed May 01, 2020.
- 63. Halpin DMG, Faner R, Sibila O, Badia JR, Agusti A. Do chronic respiratory diseases or their treatment affect the risk of SARS‐CoV‐2 infection? Lancet Respir Med. 2020;8:436‐438. 10.1016/S2213-2600(20)30167-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yamaya M, Nishimura H, Deng X, et al. Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV‐229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells. Respir Investig. 2020;58:155‐168. 10.1016/j.resinv.2019.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Matsuyama S, Kawase M, Nao N, et al. The inhaled corticosteroid ciclesonide blocks coronavirus RNA replication by targeting viral NSP15 [published online ahead of print March 12, 2020]. bioRxiv. 2020. 10.1101/2020.03.11.987016 [DOI] [Google Scholar]
- 66. Peters MC, Sajuthi S, Deford P, et al. COVID‐19 related genes in sputum cells in asthma: relationship to demographic features and corticosteroids [published online ahead of print April 29, 2020]. Am J Respir Crit Care Med. 2020. 10.1164/rccm.202003-0821OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jackson DJ, Busse WW, Bacharier LB, et al. Association of respiratory allergy, asthma and expression of the SARS‐CoV‐2 receptor, ACE2 [published online ahead of print April 22, 2020]. J Allergy Clin Immunol. 2020. 10.1016/j.jaci.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID‐19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38(1):1‐9. 10.12932/AP-200220-0772 [DOI] [PubMed] [Google Scholar]
- 69. Papadopoulos NG, Custovic A, Deschildre A, et al. Impact of COVID‐19 on pediatric asthma: practice adjustments and disease burden. J Allergy Clin Immunol Pract. 2020;S2213‐2198(20):30599‐7. 10.1016/j.jaip.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bulathsinhala L, Eleangovan N, Heaney LG, et al. Development of the International Severe Asthma Registry (ISAR): a modified Delphi study. J Allergy Clin Immunol Pract. 2019;7(2):578‐588. 10.1016/j.jaip.2018.08.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


