Abstract
With the extensive application of radiotherapy in various cancers, its side effects in tissues adjacent to cancers are garnering much attention. Intestines are sensitive to irradiation due to its rapid proliferation, and irradiation-induced enteric inflammation is common in patients with pelvic peritoneal tumors. Sirt1, class III protein deacetylase, could lead to transcriptional repression of various inflammation-associated genes, and our previous study has proved its relationship with interleukin (IL)-1β. Here we show that resveratrol, the activator of Sirt1, could alleviate the bowel inflammation induced by irradiation and the expression of Sirt1 is consistent with the inflammation level. We further identified in vivo that Sirt1 repress the expression of IL-1β by the repression of NLR Family, Pyrin Domain Containing protein 3 (NLRP3) expression. In conclusion, this study confirms resveratrol acts against radiation-induced inflammatory bowel disease via NLRP-3 inflammasome repression in mice and supports Sirt1 as a potential biomarker and therapy target in intestinal radiation protection.
Keywords: radiation, biomarker, resveratrol, SIRT1, NLRP3
Introduction
More than half of patients with cancer received radiotherapy in the course of treatment for the disease.1 But the side effects of radiation therapy limit the effective radiation dose that is delivered to the tumor.2 Gastrointestinal systems are highly sensitive to ionizing radiation (IR) due to its rapid proliferation.3-5 After receiving IR, an acute inflammatory response associated with immune cellular infiltrating occurred, thus leading to intestinal damage.6 A prospective trial found that young cancer survivors treated with abdominal radiation therapy endure high prevalence of adenomatous colorectal polyps.6 In order to ameliorate radiation-induced inflammatory bowel disease, great efforts have been made to protect against radiation enteritis.7,8 However, it requires further explorations to develop more effective and safe treatment to protect intestines from radiation.
Resveratrol (3,4,5-trihydroxy-trans-stilbene) is a natural non-flavonoid polyphenol mainly present in grapes and wine.1 It has been widely proven that resveratrol can expand life span in lower organisms,9,10 protect vascular system,11 benefit metabolic health,12 and act as anticancer drugs.13,14 Moreover, it has been reported that resveratrol has the ability to protect against ultraviolet damage in vitro and in vivo,15-18 reduce radiation-induced chromosome aberration, and ameliorate acute irradiation-induced salivary gland dysfunction, and hepatic and ileal damage in mice.
Resveratrol activates silent information regulator T1 (SIRT1), an NAD+-dependent protein deacetylase. SIRT1 directly couples NAD+ to the chromatin structure through the deacetylation of histones, transcription factors, and transcription co-factors, and this coupling has been reported to be related to metabolic disease,19 inflammation,20 neurodegeneration,21 cardiovascular disease,22 and tumorigenesis.23 SIRT1 was also found to play an important role in modulating the development and progression of inflammation through deacetylating histones and critical transcription factor such as nuclear factor kappa-B (NF-κb) and activator protein 1, thus leading to transcriptional repression of various inflammation-related genes. What is more, it is reported that Sirt1 can also interact with the p65 subunit of NF-κb and inhibit transcription by deacetylating p65 at Lys310 and then suppressing the inflammatory factor.24,25 This interaction indicates that the anti-inflammatory and protective effects of Sirt1 may prove useful in treating radiation enteritis.
Interleukin (IL)-1β is a potent pro-inflammatory cytokine that promotes a variety of innate immune processes associated with infection, inflammation, and autoimmunity.1 As is known to all, IR can increase reactive oxygen species levels and IL-1β expression, and the increasing expression of IL-1β leads to cell apoptosis and tissue damage.
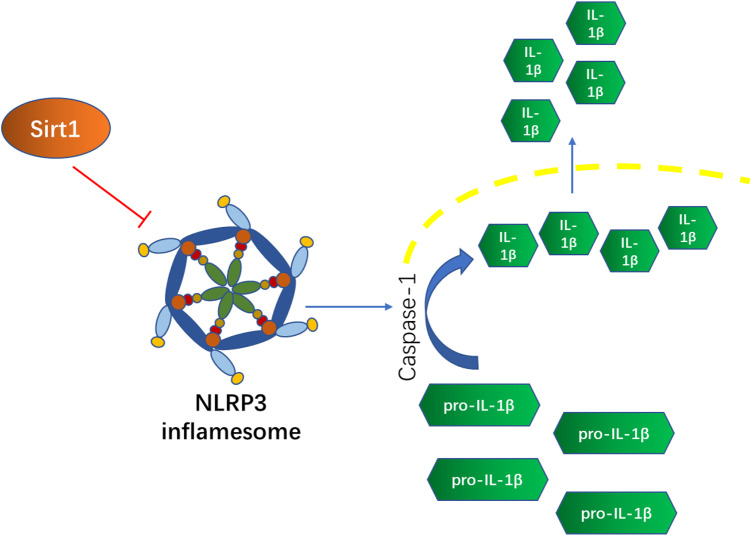
Unlike other secreted proteins, both the activation and the secretion of IL-1β typically requires secondary proteolytic cleavage induced by the NLRP3 inflammasome, one of the subfamilies of nucleotide-binding oligomerization domain-like receptor.26 In our previous study, we found that radiation significantly increased IL-1β expression level in Mesenchymal Stem Cells (MSCs) and that administration of resveratrol in advance could inhibit the IL-1β expression induced by radiation in a concentration-dependent manner. This effect was attributed to Sirt1-mediated inhibition of NLRP-3 inflammasome activation through deacetylating the transcription factor NF-κb. A recent study reported that the NLRP3 inflammasome would be activated under low-dose radiation in vitro and in vivo and the inhibition of NLRP3 would alleviate radiation-induced pneumonitis.27 There are also reports showing that NLRP3 inflammasome overexpression exists in intestinal inflammatory injuries.28,29 There is a simple schematic diagram showing the relationship between Sirt1, NLRP3 inflammation, and IL-1β in Figure 1. And these findings led us to further investigate whether resveratrol can ameliorate IR-induced inflammatory bowel disease by activating Sirt1 and limiting NLRP-3 inflammasome activation in mice.
Figure 1.
A simple schematic diagram of relationships among Sirt1, NLRP3, and IL-1β. Sirt1 could inhibit the activation of NLRP3 inflammasome and then further restrain the activation and secretion of IL-1β. IL-1β, Interleukin-1β.
Material and Methods
Reagents and Ionizing Radiation
Resveratrol (purity = 97%) was purchased from Tianjin Jianfeng Natural Product Co., Ltd, dissolved in 0.1% carboxymethyl cellulose sodium (CMC) to concentrations of 25 mg/mL, and was stored at 4 °C shielded from light. Rabbit polyclonal CIAS1/NALP3, rabbit polyclonal IL-1β (ab9722), and mouse monoclonal β-actin antibodies (mAbcam 8226) were purchased from Abcam. Rabbit polyclonal SIRT1 was purchased from Santa Cruz Biotechnology.
Animals were exposed to IR in a Cammacell-40 137Cesium γ irradiator (Atomic Energy of Canadian Inc.) at a dose rate of 0.71116 Gy/min.
Mice and Experimental Groups
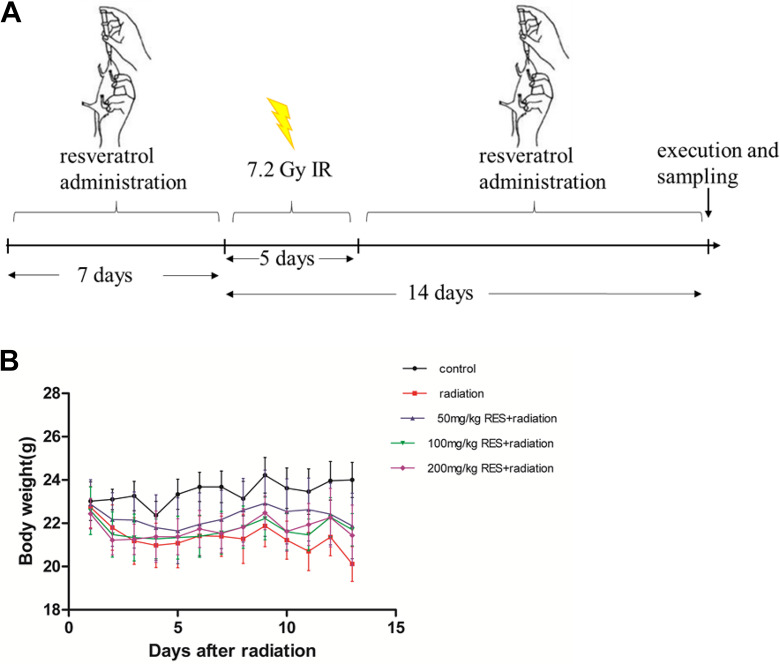
Male C57/6 mice were obtained from the Institute of Laboratory Animal Sciences (PUMC). And they were kept at the certified animal care facility in the Institute of Radiation Medicine of PUMC. The average age of these mice under our treatments was among 8 to 10 weeks. The experimental manipulations in this study were approved by the Ethical Committee of the University. The mice were randomly divided into 5 groups: (1) control (n = 6); (2) radiation (n = 6): vehicle + total body irradiation (TBI); (3) low-dose resveratrol (n = 6): 50 mg/kg resveratrol + TBI; (4) medium-dose resveratrol (n = 6):100 mg/kg resveratrol + TBI; and (5) high dose resveratrol (n = 6):200 mg/kg resveratrol +TBI. Mice were given vehicle (0.1% CMC) or resveratrol at the dose of 50, 100, or 200 mg/kg by gavage every day for 7 days before irradiation and then 14 days after irradiation, as shown in Figure 2A. The mice in both radiation and resveratrol groups received 7.2 Gy radiation. Control group were sham-irradiated.
Figure 2.
The sketch of experimental treatments and the changes in body weight of C57BL / 6 mice in different treatment groups. (A) Mice were given vehicle (0.1% carboxymethyl cellulose sodium) or resveratrol at the dose of 50, 100, or 200 mg/kg by gavage every day for 7 days before irradiation and then 14 days after irradiation After irradiation, the body weight of C57BL / 6 mice in each group was measured daily for 13 days. (B) Body weight of C57BL/6 mice in different treatment groups (mean ± standard deviation, n = 6).
Preparation of Tissue Samples
The mice were killed by cervical dislocation at the 14th day after irradiation. The whole spleens and thymuses were removed, weighed, immediately frozen in liquid nitrogen for the real-time polymerase chain reaction and western blot assay. To prepare specimens for histologic and immunohistochemical analysis, the intestinal tissues were fixed in 10% buffered formalin phosphate immediately.
To give a quantitative assessment of the immune response, spleen and thymus indexes (Sx) can be defined as: Sx =[Weight of experiment organ (mg)]/ [Weight of experiment animal (g)].
Enzyme-Linked Immunosorbent Assay
Blood samples were collected when the mice were killed. After centrifugation at 3000 rpm for 10 minutes, the serum was isolated and used to determine the level of IL-1β and tumor necrosis factor (TNF)-α using a competitive enzyme-linked immunoassay kit according to the manufacturer’s instructions (R&D Systems). All assays were performed in triplicate. Protein levels were calculated as pg/mg of total protein.30
Pathological Analysis and Immunohistochemistry
Paraffin-embedded tissues were cut in 4 µm sections at the maximum cross-section. Sections were stained with hematoxylin-eosin and observed under a microscope (BX51; Olympus). The 4-µm thick sections from paraffin-embedded tissues were deparaffinized and rehydrated, respectively. Sections were then boiled for 30 minutes in 10 mmol/L citrate buffer solution (pH 6.0) for antigen retrieval. Slides were incubated with 5% skimmed milk for 20 minutes and incubated with rabbit anti-mouse protein antibody (Santa Cruz Biotechnology, 1:600) at 37 °C for 1 hour and visualized using PV-6001 Polymer Detection System (Golden Bridge International Inc.) following the manufacturer’s instructions. Subsequently, slides were incubated with 3′-diaminobenzidine tetrahydrochloride-H2O2 solution for visualization and counterstained with hematoxylin. For analysis, 4 views at 400-fold magnification were chosen randomly on each slide. The images were captured and positive staining was quantified objectively by IPP software.
Quantitative Reverse Transcription–Polymerase Chain Reaction
The specific experimental process followed the methods of Fu et al.30 The specific primer pairs used were as follows:
Sirt1, 5′-CCTTGGAGACTGCGATGTTA-3′ (forward) and
5′-GGCGTGGAGGTTTTTCAG-3′ (reverse);
NLRP3, 5′-TTGCCCATACCTTCAGTCTTG-3′ (forward) and
5′-CTGCCACAAACCTTCCATCT-3′ (reverse);
IL-1β, 5′-TACAAGGAGAACCAAGCAACG-3′ (forward) and
5′-GCCGTCTTTCATTACACAGGA-3′ (reverse);
GAPDH, 5′-GGTGAAGGTCGGTGTGAACG-3′ (forward) and
5′-CTCGCTCCTGGAAGATGGTG-3′ (reverse).
Western Blot Analysis
The total protein of the spleen and thymus tissues was extracted using the 1 step animal tissue active protein extraction Kit (Sangon Biotech) according to the manufacturer’s instructions. After centrifugation at 14,000 rpm, the protein concentration was determined using the Bradford assay. The specific experimental process followed the methods of Fu et al.30
Statistical Analysis
Data were expressed as the mean ± standard error of the mean and were subjected to one-way analysis of variance (ANOVA), followed by an LSD test. A P value of no more than .05 in Student test or ANOVA test was considered to be statistically significant.
Results
Changes in Body Weight of Mice in Different Treatment Groups
The body weight changes of C57BL/6 mice in different treatment groups were observed 13 days after irradiation, and the radiation protection effect of resveratrol on C57BL/6 mice was evaluated (Figure 2B). The weight of C57BL/6 mice in the control group has been maintained above 23 g, and with time the weight has slowly increased; while the irradiation group and the resveratrol group have been exposed to 7.2 Gy for 5 days. The weight of C57BL / 6 mice in each group treated with resveratrol gradually increased from the 6th day after irradiation, while the weight of C57BL/6 mice in the irradiation group still showed a downward trend. This result indicates the radiation protection effect of resveratrol.
Resveratrol Attenuates Radiation-Induced Intestinal Injury
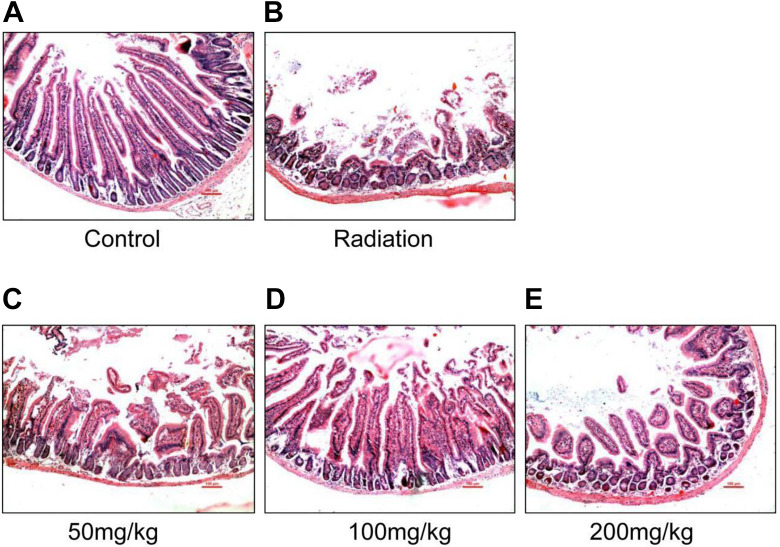
Histological assessment was performed to determine the intestine tissue damage between different groups. As shown in Figure 3, the intestinal injury in the radiation group showed intestinal mucosal edema, immune cellular infiltration with the partial loss or epithelial shedding of the villi compared to the normal intestine with intact villi and crypts. However, pretreatment with resveratrol dramatically attenuates radiation-induced intestinal injury, and we also found that in the medium-dose group (100 mg/kg) resveratrol exerted more protection effect characterized by relatively intact villi and crypts, relatively mild mucosal edema, and immune cellular infiltration compared to the low-dose (50 mg/kg) and high-dose (200 mg/kg) resveratrol groups. These findings suggest that resveratrol pretreatment could prevent intestinal damages by irradiation.
Figure 3.
Hematoxylin-eosin staining of the intestine in different groups. Normal morphology was found in the control group (A), while more severe mucosal injury was observed in the irradiation group (B), resveratrol pretreatment at a dose of 50 mg/kg weight (C), 100 mg/kg weight (D), and 200 mg/kg weight (E) showed relatively intact villi and crypts, relatively mild mucosal edema, and immune cellular infiltration compared to the control group and the 100 mg/kg weight group displayed more significant protective effects; Magnification: ×200; n = 6. Scale bar = 50 μm, Arrow, mucosal injury.
Resveratrol Inhibited Expressions of IL-1β in Intestines
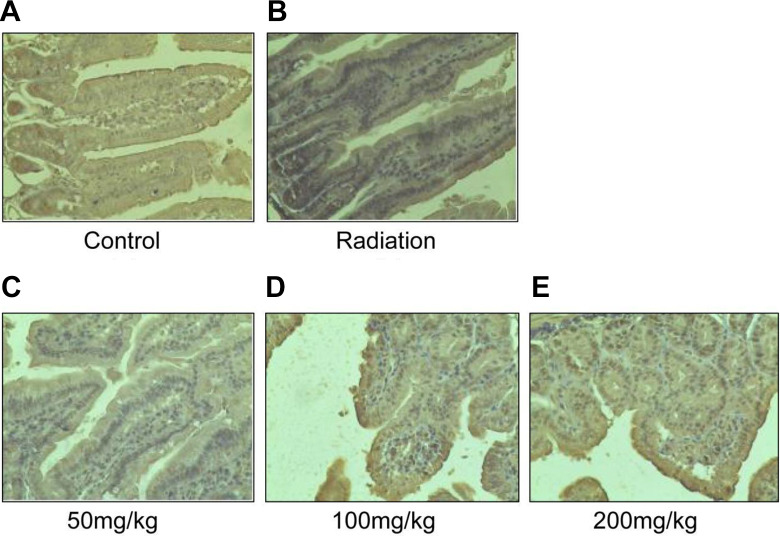
To determine the IL-1β level in the intestinal tissue, we used immunohistochemistry assay. As shown in Figure 4A-E, the expression level of IL-1β protein was low in the control group (Figure 4A). Compared to the control group, there is a stronger IL-1β staining in the cytoplasm of the radiation group (Figure 4B). However, resveratrol pretreatment significantly compromised the irradiation-induced increasing in IL-1β protein expression (Figure 4C-E), and the distribution of the IL-1β was consistent with the pathology results. The findings suggest that resveratrol plays an important role in the reduction of expression and distribution of IL-1β proteins after radiation-induced intestinal injury.
Figure 4.
Immumohistochemical staining of the intestine in different groups. (A) Control group; (B) radiation group; (C) resveratrol pretreatment at a dose of 50 mg/kg weight, (D) 100 mg/kg weight, and (E) 200 mg/kg weight. The IL-1β staining is stronger after radiation. However, pretreatment of resveratrol will compromise the IL-1β protein expression in intestines. Magnification: ×200; n = 6.
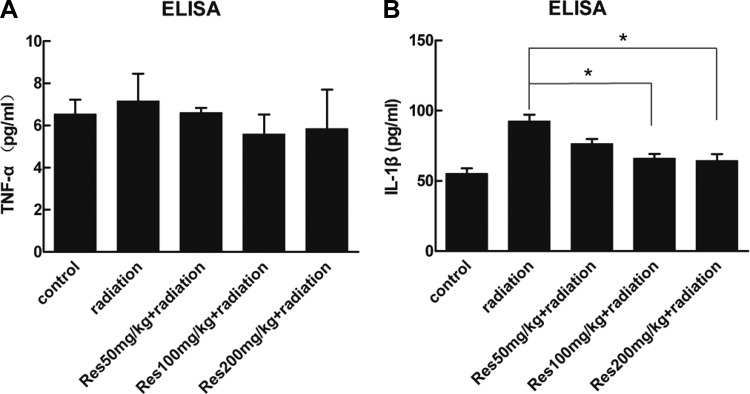
The Serum Levels of IL-1β and TNF-α
Fourteen days after irradiation, serum concentrations of TNF-α and IL-1β were detected. As shown in Figure 5, the serum concentrations of TNF-α showed no significant difference between each group. The serum concentrations of IL-1β were increased after irradiation, while resveratrol pretreatment showed a remission compared to the radiation-alone group with a significant difference at the dose of 100 and 200 mg/kg weight.
Figure 5.
The serum concentrations of (A) TNF-α and (B) IL-1β of different groups. Bars represent mean ± standard deviation. Data were analyzed using analysis of variance with SPSS 13.0. *Represent significant differences from the radiation group.
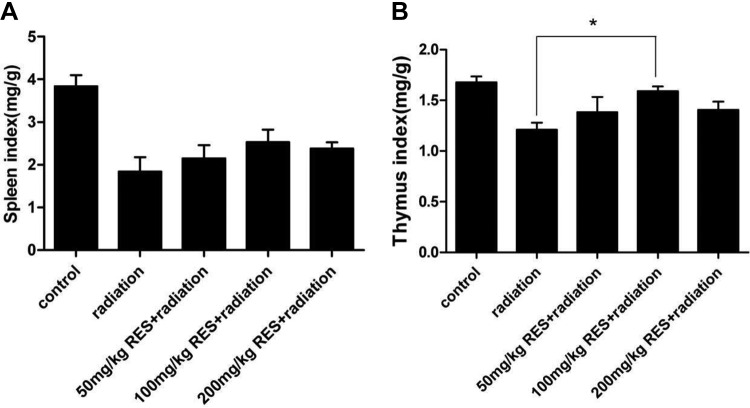
Immune Response
Figure 6 showed the immune response of mice based on spleen and thymus index. We calculated the spleen index and thymus index to examine immune system damage induced by radiation. For the radiation group, the average values of thymus and spleen index were 1.2 and 1.8, respectively. In the resveratrol groups, 50 and 200 mg/kg resveratrol did not induce significant changes both in thymus and spleen index, but 100 mg/kg resveratrol-treated mice presented an increase in thymus index compared to the radiation group (P < .05).
Figure 6.
Spleen index and thymus index of different groups. Bars represent mean ± standard deviation. Data were analyzed using analysis of variance with SPSS 13.0, and the differences between the different groups and control (Con) group for each organ were not significant (P < .05). *Represent significant differences from the radiation group.
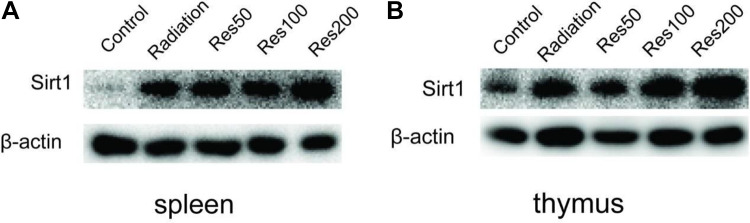
Resveratrol Elevate Sirt1 Level in the Thymus and Spleen of Mice
After investigating the spleen index and thymus index between different groups, the protein expression of Sirt1 was detected. As shown in Figure 7A-B, compared with control group, the radiation group showed more prominent expression of Sirt1 both in the spleen and thymus, and its expression would be further upregulated by additional resveratrol administration in a concentration-dependent manner (50, 100, and 200 mg/kg). That is to say, the Sirt11 expression level is highest when the mice were irradiated combined with resveratrol administration of 200 mg/kg in this research. These findings suggest that resveratrol can distribute in the immune organs of the mice and may exert anti-inflammatory effects.
Figure 7.
The relative expression of Sirt1 in spleen and thymus of mice between different groups was determined by Western blot analysis.
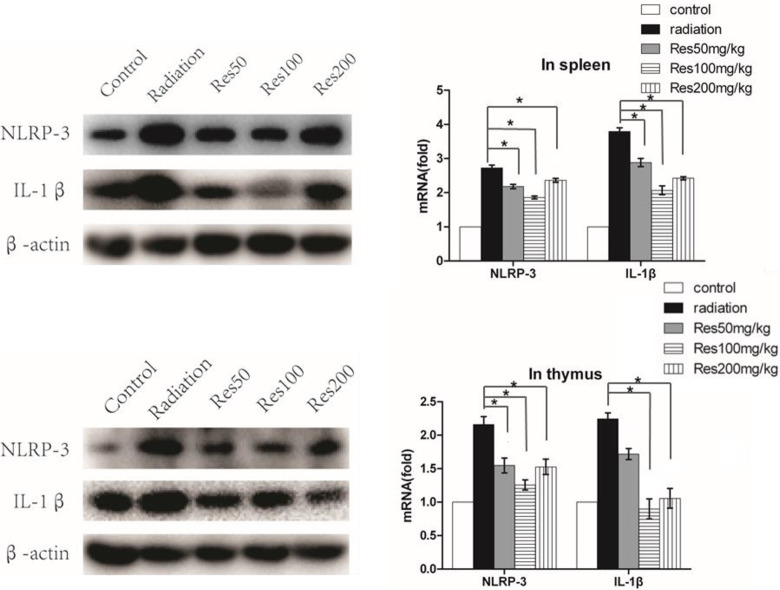
Resveratrol Inhibited the Expression of IL-1β and NLRP-3 in Spleen and Thymus
Because the thymus and spleen were the important immune organs in mice, in order to further illustrate the association between Sirt1 and IL-1β expression, we detected the messenger RNA (mRNA) and protein levels of the IL-1β and NLRP-3 in different groups. As shown in Figure 8A-D, radiation increased the expression of IL-1β and NLRP-3 compared with the control group, while compared to the radiation group, additional treatment with resveratrol attenuated IL-1β mRNA and protein expression in the spleen and thymus of the mice, and the NLRP-3 mRNA and protein expression levels were also decreased. Meanwhile, we found that in the medium dose of resveratrol group, IL-1β and NLRP-3 expression levels were lower than the other 2 resveratrol-treated groups in spleen.
Figure 8.
The relative expression of NLRP-3 and IL-1β in spleen (A) and thymus (B) of mice between different groups were determined by Western blot analysis and quantitative real-time polymerase chain reaction analyses, respectively. The values are presented as the mean ± standard deviation (n = 3). *P < .05, compared with the radiation group.
Discussion
The side effects of radiation on normal tissues especially those adjacent to tumors limit the application of radiation on tumor therapy. The gastrointestinal system is a major target in the irradiation-induced injuries and also responds early to irradiation. And the severe acute intestinal radiation injury includes intestinal inflammation of different extents, mucosal thickening, collagen deposition, and even fibrosis. Our results demonstrate that TBI causes inflammatory bowel disease in mice, and pretreatment with resveratrol could attenuate the disease.
First, histopathologic examination showed that irradiation significantly damaged the intestinal mucosa. Compared to the radiation group, resveratrol preconditioning group witnessed a significant alleviation of intestinal tissue injury. Recently, more and more studies focus on the radiation protection effect of resveratrol. For instance, resveratrol could ameliorate salivary gland, ovarian, hepatic, and ileal damage induced by radiation.31,32 Also Zhang et al. reported that mice treated with resveratrol before radiation had significantly higher survival rates.33 But most research to date have focused on the antioxidant properties of resveratrol, rather than on the effect of resveratrol suppression of inflammation. In our previous studies, we also found that resveratrol could exert radioprotective effect by inhibiting the IL-1β expression in vitro. To the best of our knowledge, IL-1β plays an important role in innate immune processes, and IL-1β is a major pro-inflammatory cytokine influential in inflammatory bowel disease. So we detected the IL-1β expression in the damaged intestinal tissue. As shown in Figure 3, IL-1β staining is stronger in the damaged intestinal tissue of the radiation group, which is consistent with the pathology results. It demonstrated that the intestinal tissue damage was due to the IL-1β overexpression which is induced by radiation. And pretreatment with resveratrol significantly compromised the IL-1β protein expression. These results suggest that resveratrol may exert radioprotective effect by inhibiting the IL-1β expression. Meanwhile, we detected the serum concentrations of TNF-α and IL-1β and have no significant changes between different groups.
In order to further illustrate the mechanism, we focus on the changes between the immune organs of the mice. The indexes of spleen and thymus are typical indicators for the immune system of mice. It is known that resveratrol prefers to accumulate in the spleen. Despite no obvious statistical difference, the resveratrol still caused a change in spleen index. And we also found that 100 mg/kg resveratrol-treated mice presented an increase in thymus index compared to the radiation group (P < .05). These changes impelled us to explore more findings. Because the spleen and thymus were the important immune organs, and they also participated in the most immune response in mice, we focus on the mechanism of inflammatory cytokine expression in spleen and thymus to further illustrate the radioprotective effect of resveratrol.
Resveratrol has been known as a Sirt1 activator.34 To further understand the anti-inflammatory mechanism of resveratrol in IR-induced inflammatory bowel disease, we investigated whether Sirt1 played a key role in resveratrol’s protective effect. Li et al. reported that the major distribution tissues of resveratrol in rats were liver, spleen, heart, and lung. Consistent with Li’s results, our date show that resveratrol significantly elevates the expression of Sirt1 in spleen and thymus (Figure 6). Sirt1 is an NAD+-dependent deacetylase that was found to play an important role in modulating the development and progression of inflammation.15 It has been recently reported that SIRT1 knockdown resulted in enhanced expression of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 in vitro, and in vivo knockdown of SIRT1 also led to macrophage recruitment to adipose tissue.16 Our data show that resveratrol decreased the expression of IL-1β in a Sirt1-dependent manner in thymus, and in spleen, we found that 200 mg/kg resveratrol-treated mice presented an increase in IL-1β compared to the 100 mg/kg resveratrol-treated mice. These results suggest that the overexpression of Sirt1 could inhibit radiation-induced IL-1β expression.
The NLRP3 inflammasome has been associated with a wide range of diseases including autoinflammatory, infectious, and autoimmune disorders. Following activation of the NLRP3 inflammasome, cells secrete large amounts of proinflammatory cytokines, such as IL-1β. In our study, we found that radiation-induced IL-1β expression is coincident with increased NLRP3 expression. This result suggests that radiation-induced increases in IL-1β occur via the NLRP3 pathway. As is known to us, NLRP3 transcription is dependent on NF-κb,35 and the Sirt1 has shown to act on a wide range of histones and non-histone substrates including NF-κb,24,25 so we hypothesized that NF-κb and Sirt1 may functionally interact in vivo. Our previous results suggest that NF-κb was involved in the process of IL-1β secretion and expression. More importantly, studies also show that NF-κb was regulated by post-translational modifications such as the reversible acetylation of p65, which thereby down-regulates the expression of various pro-inflammatory cytokines.17,18
Conclusions
In conclusion, our results demonstrated that resveratrol ameliorates IR-induced inflammatory bowel disease via the inhibition of IL-1β expression. Since our research conformed that resveratrol could distribute in the immune organs of the mice like spleen and thymus, and then elevate the Sirt1 level, which further suppresses NLRP3 inflammasome expression and subsequent IL-1β production and secretion, we conclude that Sirt1 can effectively regulate the NLRP3 inflammasome activation and its downstream inflammatory molecules. Given that resveratrol is an activator of Sirt1, our findings suggest that resveratrol is an effective protection agent for radiation-induced intestinal injury. We also provided a theoretical basis for developing radioprotective drugs that target Sirt1.
Footnotes
Authors Contribution: Hao Sun and Hui Cai contributed equally to this work.
Data Availability: The data used to support the findings of this study are included within the article. Any additional data used to support the findings of this study are available upon request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China [31670859, 31971168, 81772243, 81803172, 81803167, 31800703, 31900891, 81972976]; CAMS Innovation Fund for Medical Science [2017-I2M-1-016, 2019-I2M-2-006]; China Postdoctoral Science Foundation [2018M630106]; Natural Science Foundation of Tianjin [18JCYBJC26800, 18JCQNJC12300, 17JCYBJC42700, 19JCYBJC26600]; the Fundamental Research Funds for the Central Universities [10023201601602, 3332019159, 3332019097]; and the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences [2017-1001-08, 400 2018RC310020].
ORCID iD: Hui Cai  https://orcid.org/0000-0002-0719-1829
https://orcid.org/0000-0002-0719-1829
Ningning He  https://orcid.org/0000-0002-2360-6203
https://orcid.org/0000-0002-2360-6203
Qiang Liu  https://orcid.org/0000-0002-7668-6868
https://orcid.org/0000-0002-7668-6868
References
- 1. Nakata R, Takahashi S, Inoue H. Recent advances in the study on resveratrol. Biol Pharm Bull. 2012;35(3):273–279. [DOI] [PubMed] [Google Scholar]
- 2. Gruber J, Tang SY, Halliwell B. Evidence for a trade-off between survival and fitness caused by resveratrol treatment of Caenorhabditis elegans. Ann N Y Acad Sci. 2007;1100:530–542. [DOI] [PubMed] [Google Scholar]
- 3. Churnratanakul S, Wirzba B, Lam T, et al. Radiation and the small intestine. Future perspectives for preventive therapy. Dig Dis. 1990;8(1):45–60. [DOI] [PubMed] [Google Scholar]
- 4. Keefe DMK. Gastrointestinal mucositis: a new biological model. Support Care Cancer. 2004;12(1):6–9. [DOI] [PubMed] [Google Scholar]
- 5. Jung E, Perrone EE, Brahmamdan P, et al. Inhibition of intestinal epithelial apoptosis improves survival in a murine model of radiation combined injury. PLoS One. 2013;8(10):e77203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Daly PE, Samiee S, Cino M, et al. High prevalence of adenomatous colorectal polyps in young cancer survivors treated with abdominal radiation therapy: results of a prospective trial. Gut. 2017;66(10):1797–1801. [DOI] [PubMed] [Google Scholar]
- 7. Lu L, Jiang M, Zhu C, He J, Fan S. Amelioration of whole abdominal irradiation-induced intestinal injury in mice with 3,3’-Diindolylmethane (DIM). Free Radic Biol Med. 2019;130:244–255. [DOI] [PubMed] [Google Scholar]
- 8. Bhat K, Duhachek-Muggy S, Ramanathan R, et al. 1-(4-nitrobenzenesulfonyl)-4-penylpiperazine increases the number of Peyer’s patch-associated regenerating crypts in the small intestines after radiation injury. Radiotherapy and oncology: journal of the European Society for Therapeutic. Radiol Oncol. 2019;132:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bhullar KS, Hubbard BP. Lifespan and healthspan extension by resveratrol. Biochim Biophys Acta. 2015;1852(6):1209–1218. [DOI] [PubMed] [Google Scholar]
- 10. Li YR, Li S, Lin CC. Effect of resveratrol and pterostilbene on aging and longevity. Biofactors (Oxford, England). 2018;44(1):69–82. [DOI] [PubMed] [Google Scholar]
- 11. Breuss JM, Atanasov AG, Uhrin P. Resveratrol and its effects on the vascular system. Int J Mol Sci. 2019;20(7):1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Springer M, Moco S. Resveratrol and its human metabolites—Effects on metabolic health and obesity. Nutrients. 2019;11(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiang Z, Chen K, Cheng L, et al. Resveratrol and cancer treatment: updates. Ann N Y Acad Sci. 2017;1403(1):59–69. [DOI] [PubMed] [Google Scholar]
- 14. Ko JH, Sethi G, Um JY, et al. The role of resveratrol in cancer therapy. Int J Mol Sci. 2017;18(12):2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yamamoto H, Schoonjans K, Auwerx J. Sirtuin functions in health and disease. Mol Endocrinol. 2007;21(8):1745–1755. [DOI] [PubMed] [Google Scholar]
- 16. Zhu X, Liu Q, Wang M, et al. Activation of Sirt1 by resveratrol inhibits TNF-α induced inflammation in fibroblasts. PLoS One. 2011;6(11):e27081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang L, Zhang J, Yan C, et al. SIRT1 regulates CD40 expression induced by TNF-α via NF-κB pathway in endothelial cells. Cell Physiol Biochem. 2012;30(5):1287–1298. [DOI] [PubMed] [Google Scholar]
- 18. Lei M, Wang J-g, Xiao D-m, et al. Resveratrol inhibits interleukin 1β-mediated inducible nitric oxide synthase expression in articular chondrocytes by activating SIRT1 and thereby suppressing nuclear factor-κB activity. Eur J Pharmacol. 2012;674(2-3):73–79. [DOI] [PubMed] [Google Scholar]
- 19. Nogueiras R, Habegger KM, Chaudhary N, et al. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev. 2012;92(3):1479–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen X, Lu Y, Zhang Z, Wang J, Yang H, Liu G. Intercellular interplay between Sirt1 signalling and cell metabolism in immune cell biology. Immunology. 2015;145(4):455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Donmez G, Outeiro TF. SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO Mol Med. 2013;5(3):344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chong ZZ, Wang S, Shang YC, Maiese K. Targeting cardiovascular disease with novel SIRT1 pathways. Future Cardiol. 2012;8(1):89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Song N-Y, Surh Y-J. Janus-faced role of SIRT1 in tumorigenesis. Ann N Y Acad Sci. 2012;1271(1):10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salminen A, Ojala J, Huuskonen J, Kauppinen A, Suuronen T, Kaarniranta K. Interaction of aging-associated signaling cascades: inhibition of NF-kappaB signaling by longevity factors FoxOs and SIRT1. Cell Mol Life Sci. 2008;65(7-8):1049–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin Q-Q, Yan C-F, Lin R, et al. SIRT1 regulates TNF-α-induced expression of CD40 in 3T3-L1 adipocytes via NF-κB pathway. Cytokine. 2012;60(2):447–455. [DOI] [PubMed] [Google Scholar]
- 26. Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. [DOI] [PubMed] [Google Scholar]
- 27. Li X, Gong Y, Li D, et al. Low-dose radiation therapy promotes radiation pneumonitis by activating NLRP3 inflammasome. Int J Radiat Oncol Biol Phys. 2020;S0360-3016(20)31039–31047. [DOI] [PubMed] [Google Scholar]
- 28. Zhao J, Shen S, Dai Y, Chen F, Wang K. Methamphetamine induces intestinal inflammatory injury via nod-like receptor 3 protein (NLRP3) inflammasome overexpression in vitro and in vivo. Med Sci Monit. 2019;25:8515–8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sabui S, Skupsky J, Kapadia R, et al. Tamoxifen-induced, intestinal-specific deletion of Slc5a6 in adult mice leads to spontaneous inflammation: involvement of NF-kappaB, NLRP3, and gut microbiota. Am J Physiol Gastrointest Liver Physiol. 2019;317(4):G518–G530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fu Y, Wang Y, Du L, et al. Resveratrol inhibits ionising irradiation-induced inflammation in MSCs by activating SIRT1 and limiting NLRP-3 inflammasome activation. Int J Mol Sci. 2013;14(7):14105–14118 doi:10.3390/ijms140714105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Şimşek G, Gürocak S, Karadaǧ N, et al. Protective effects of resveratrol on salivary gland damage induced by total body irradiation in rats. Laryngoscope. 2012;122(12):2743–2748. [DOI] [PubMed] [Google Scholar]
- 32. Simsek Y, Gurocak S, Turkoz Y, et al. Ameliorative effects of resveratrol on acute ovarian toxicity induced by total body irradiation in young adult rats. J Pediatr Adolesc Gynecol. 2012;25(4):262–266. [DOI] [PubMed] [Google Scholar]
- 33. Bradamante S, Barenghi L, Villa A. Cardiovascular protective effects of resveratrol. Cardiovasc Drug Rev. 2004;22(3):169–188. [DOI] [PubMed] [Google Scholar]
- 34. Park S-J, Ahmad F, Philp A, et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148(3):421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bauernfeind FG, Horvath G, Stutz A, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183(2):787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]