Abstract
Objectives
Snow fungus or snow mushroom or white jelly mushroom (Tremella fuciformis), the edible mushroom, was formulated into hand sanitizer in form of moisturizing alcohol‐based hand rub (ABHR) gel.
Methods
The stable base ABHRs were developed. The preferred bases were incorporated with various concentrations of snow mushroom extract. The stable and preferred snow mushroom ABHR was moisturizing and sanitizing efficacies evaluated in 20 human volunteers in comparison with its placebo.
Results
The stable hand sanitizer gel bases containing 66.5% of ethanol and 0.3% of triclosan were developed and incorporated with the extract of snow mushroom polysaccharide. Of which, the preparations containing 10% of snow mushroom and 0.3% of gelling agent gained the highest preferences as assessed in 20 Thai volunteers. The snow mushroom hand sanitizer was proved to be none irritated in the same group of the volunteers as was the placebo. The snow mushroom gel significantly (P < .05) moist the skin better than the placebo at all time of the interval assessment until the end of the study at 180 minutes. The hand sanitizers were confirmed on their anti‐septic, at which the efficacies of the active and placebo ABHR were comparable (P = .90).
Conclusions
Snow mushroom ABHR gel with its confirmed moisturizing and sanitizing efficacies is presented. It is meetings with the recommendation on hand hygienic improvement to combat the infections of diseases spreading. The preparation can be frequency applied with its proved skin hydrating efficacy co‐contributes in a good condition of hand hygiene.
Keywords: alcohol‐based hand rub, hand hygiene, hand sanitizer, moisturizer
1. INTRODUCTION
Due to the pandemic of the coronavirus disease 2019 (COVID‐19), World Health Organization (WHO) has addressed the importance of hand hygiene in preventing the spread of the COVID‐19 virus. Hand hygiene facilities are included alcohol‐based hand rubs (ABHR) or alcohol‐based hand sanitizer (ABHS). 1 ABHR/ABHS is regarded as the most effective practice to reduce the spread of infections 2 than soap and water washing for at least 20 seconds. 3 In addition, rubbing hand with ABHR/ABHS is more practically to be applied than washing hands with soap and water. 1 The sanitizing activity of ABHR/ABHS is increased with the proportion of ethanol. However, high dose of alcohol or high frequency of ABHR/ABHS application increase skin dryness causing skin burning exacerbates high risk of skin infection 4 , 5 that drawbacks the objective for hand hygienic improvement. The sanitizing efficacy of ABHR/ABHS is recommended at a dose of ethanol of at least 60%. 6 Nevertheless, the serious outbreak of the disease may drive the expectation of ABHR/ABHS with a higher dose of ethanol in spite of the shortage supply to fit with a dramatically increasing of demand worldwide. An additional antimicrobial agent is therefore the promising choice achieving hygienic condition of hands with a reliable booth up antimicrobial efficacy of ABHR/ABHS at a sufficient dose of ethanol. In regard with a more often hand sanitizer apply than our previous normal on the basis of infectious protection awareness. Skin hydrating or moisturizing agents are the key solution to suppress dryness of skin highly exposure with ABHR/ABHS. Furthermore, the ingredients are additional function on lubricity improvement during rubbing. 7
In regard with a shortage, availability of ethanol as well as accessing on the sanitizer products are difficulties. The efficient and cost‐effective innovative ABHR/ABHS are strongly encouraged to be developed pursuing hand hygiene and limit disease spreading. Thus, natural moisturizer derived from the available agricultural crop was chosen to be developed into the safe and efficient ABHR/ABHS accordingly.
Snow fungus or snow mushroom or white jelly mushroom (Tremella fuciformis), the edible mushroom, is one of the important source of polysaccharide beneficial for health 8 and applicable for anti‐aging 9 and moisturizing cosmetics. 10 This mushroom is easily to be cultivated and commonly found in Asian countries. This mushroom is therefore applicable to be developed into the safe and efficient moisturizing ABHR/ABHS.
2. MATERIALS AND METHODS
All of the ingredients and reagents used were all cosmetic or pharmaceutical grades.
2.1. Formulation of ABHR gels
Gelling agent, Carbopol Ultrez® 10 (Noveon), was dispersed in DI water prior to mix with antibacterial triclosan (Namsiang) which acted as a self‐preserving agent with the main antimicrobial 95% ethanol (Mallinckrodt). Triethanolamine (Namsiang) in DI water was poured into the obtaining gel as a pH adjuster. Thereafter, moisturizing effect was exhibited by polysaccharide (>100% mg polysaccharide) of snow mushroom extract (Specialty Natural Product) as shown in Table 1.
Table 1.
Development of ABHR gel base
| Ingredients (% w/w) | Formula | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| DI water |
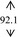
|
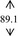
|
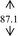
|
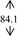
|
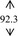
|
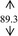
|
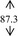
|
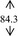
|
| 95% ethanol | ||||||||
| Carbopol ultrez® 20 | 0.5 | 0.5 | 0.5 | 0.5 | 0.3 | 0.3 | 0.3 | 0.3 |
| Triclosan | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Triethanolamine | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Glycerin | 7 | 10 | 12 | 15 | 7 | 10 | 12 | 15 |
| Initial | ||||||||
| pH | 6.52 ± 0.03 | 6.32 ± 0.01 | 6.60 ± 0.01 | 6.59 ± 0.01 | 6.37 ± 0.01 | 6.33 ± 0.01 | 6.29 ± 0.01 | 6.29 ± 0.01 |
| Viscosity (cps) | 4905.00 ± 3.46 | 6402.00 ± 8.08 | 4891.00 ± 0.00 | 5383.00 ± 1.03 | 2883.00 ± 38.15 | 1649.00 ± 1.15 | 1367.00 ± 1.15 | 1134.00 ± 0.57 |
| Heat‐cool cycles | ||||||||
| pH | 6.59 ± 0.00 | 6.49 ± 0.01 | 6.67 ± 0.00 | 6.68 ± 0.01 | 6.39 ± 0.01 | 6.25 ± 0.00 | 6.37 ± 0.02 | 6.33 ± 0.00 |
| Viscosity (cps) | 4907.00 ± 3.46 | 7610.00 ± 9.20 | 4918.00 ± 6.92 | 5359.00 ± 9.81 | 2829.00 ± 3.46 | 1648.00 ± 1.15 | 1439.00 ± 1.15 | 1108.00 ± 3.05 |
2.2. Stability of the formulated ABHR gels
Physicochemical characters of the 16 formulated hand sanitizers were determined using pH meter (Qis B200) and viscometer DV‐II+ Pro. Stability tests were performed by an observation of appearance, color, odor, and texture. These tests included pH (at 25°C) and viscosity (spindle no. 21, 20 rpm, 25°C). The measurements were conducted at the baseline and following accelerated tests by means of centrifugation at 2800 g for 30 minutes and 6 cycles of heating and cooling at 4°C and 45°C for 48 hours. 11
2.3. Sensorial assessment of the formulated ABHR gels
Sensorial of the formulated hand sanitizers were preliminary undertaken by the formulators by the hedonic scales ranging from 1 to 5 (poor‐excellent) during the course of the product developments. The acceptable texture preparations were incorporated with the snow mushroom extract at different contents. The snow mushroom hand sanitizers were thereafter sensorial assessed in 20 volunteers by the interview questionnaires. The preference score was summarized and calculated into percentage. 12
2.4. In vivo efficacy evaluation
2.4.1. Volunteer recruitment
Healthy Thai volunteers aged between 20 and 40 years old having none of skin disease were enrolled in this study. Those of hypersensitive skin and allergy history were excluded including who were pregnant or lactating or dieting. All recruited subjects are informed about the study both in writing and verbally and signed a written consent form which was approved by the ethical committee of the Mae Fah Luang University prior to enrollment.
2.4.2. Patch test
A single application closed patch test was preliminary performed in 20 volunteers. Finn chambers (8 mm, Smartpractice) were used for skin irritation observation of the test samples (0.1 mL), that is (a) sodium lauryl sulfate 0.5% in water as the positive control, (b) water as the negative control, (c) placebo, and (d) snow mushroom ABHR for a period of 24 hours on volar forearms of the volunteers. Skin irritation severity was graded 0‐4. The obtaining data were gathered for MII (the Mean Irritation Index) calculation. The MII < 0.2 was interpreted as nonirritation. 13
2.4.3. Moisturizing efficacy evaluation
Moisturizing efficacy of the developed snow mushroom hand sanitizers was evaluated. The volunteers were requested not to use moisturizers, body lotions, soap, or occlusive cosmetic preparations on the tested area prior to 12 hours of the in vivo study. All subjects were asked to rest in the controlled room at 25 ± 1°C and 40%‐60% relative humidity for 15 minutes prior to skin moist monitored by using of Moist Sense® (Moritex) on the central of the back of left and right hands. The baseline skin capacitance was recorded, and the formulations (1 g) were singly applied onto the central of back hand (2 × 2 cm) delineated by a randomized‐single blind, placebo‐controlled study. The volunteers were directed to rub 15 seconds of the product onto hand. 14 The volunteers were requested to wait in the environmentally controlled room. Moisturizing efficacy was firstly recorded following 30 minutes of products application and at 90, 120, 150 and 180 minutes, eventually. All of the measurements were done in triplicate, and the efficacy was calculated 15 as followings;
where At = skin capacitance at a specified time.A0 = skin capacitance at baseline.
2.4.4. Sanitizing efficacy evaluation
Microorganisms onto the volunteers' hands were collected before and after (15 minutes) an application of the hand sanitizers by Swab method. Total colony (bacteria and fungi) was counted based on the US Pharmacopeia, 16 and the total colony forming was effectively interpreted regarding USP24‐NF19. 17
2.5. Statistical analysis
Data are presented as the mean ± SD. Statistical analysis was performed using SPSS program version 16.0 for Windows. The parameters were compared and analyzed using one sample t test and ANOVA test with a significance level of P < .05.
3. RESULTS AND DISCUSSION
The pandemic outbreak of COVID‐19 causes several serious issues in our society. Using ABHR/ABHS, maintaining hand hygiene is stated as one of the promising strategies combating the infections of diseases spreading. 1 , 2 However, the availability and accessibility on the safe and efficient products are limited due to shortage of antimicrobial agents especially ethanol, the main active. In addition, high frequency of ABHR/ABHS exposure down‐regulating skin barrier functions exacerbating skin friction, dryness of skin, skin burned, and irritation eventually arising risk of infections. Accordingly, additional antimicrobial agent is the promising choice to enhance sanitizing activity of the preparation at a lower dose but effective ethanol. Thus, ABHR in a dosage form of gel that prolong the contact time of the anti‐septic agents onto hands but not wash off epidermal lipid likes the working action of spray was subjectively to be developed in an order to co‐contribute in the efficacy of hand sanitizer product.
3.1. Formulation of the stable ABHR gels
Ethanol, the major active agent of ABHR, has an anti‐septic activity at a concentration of at least 60%. 2 The sanitizing activity is pronounced with the proportion of ethanol as a dose‐dependent activity. Of which, those of commercial sanitizers mostly contain 60%‐85% ethanol for instance Purell® and Avant original®, etc 18 with their claimed dose effective at 60%‐70% in the formulation. This efficient and cost‐effective dose of ethanol was therefore implied in this present study. Of which, the final concentration of ethanol in the formulated ABHR gel was 66.5%. Taken into account with the serious scene of the wide spreading of the diseases, sanitizing activity solely on the basis of ethanol might be inadequate. The sanitizing activity of the hand sanitizer was designed to be achieved with the addition of triclosan, at the claimed safe and effective concentration of 0.3%. 19 Accordingly, the combination of antimicrobial agents, ethanol, and triclosan at these concentrations were implied in the hand sanitizer development. Gelling agent's concentration (0.5 and 0.3%, w/v) was varied in the course of the gel development in an order to achieve the acceptable texture with the improve lubricity during rubbing hands with the preparation. The base ABHR gels were challenged under the accelerated condition firstly by a centrifugation assay. All base ABHR gels remained homogenous. Thereafter, the preparations were challenged with heating‐cooling assays. The base ABHR gels were proved to be stable as shown in Table 1, and the base gels were therefore developed into snow mushroom hand sanitizers.
Snow mushroom is the edible mushroom that has long been cooking in many Asian cuisines as well as traditional remedies in regard with its taste and pharmacological activities including antioxidation, anti‐inflammatory, and anti‐aging. 8 Of which, the pharmaceutical active ingredient of the mushroom is polysaccharides. The polysaccharides of snow mushroom not only beneficial for health but also for aesthetic proposes. Snow mushroom polysaccharides were exhibited to efficiently reduce water and collagen losses of the skin following UV explosure. 9 In regard with the efficient skin hydrating property of polysaccharides in cosmetics. 10 Snow mushroom polysaccharide is therefore appointed to be incorporated into the sable base ABHR gels and examined on the preparation's efficacy.
Snow mushroom polysaccharide was found compatible with the base sanitizer at all of the mushroom extract concentration (7, 10, 12, and 15%, w/w). The final products were therefore contained more than 0.07, 0.10, 0.12, and 0.15% polysaccharide on the basis of the snow mushroom specification. These concentrations were trialed in an order to compare with the 0.075, 0.105, and 0.150 okra ABHR that was previously reported. 7 All of the snow mushroom formulations were stabled following the accelerated stability tests as undertaken in the base gels as shown in Table 2.
Table 2.
Development of snow mushroom ABHR gels
| Ingredients (% w/w) | Formula | |||||||
|---|---|---|---|---|---|---|---|---|
| 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
| DI water |
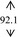
|
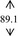
|
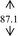
|
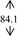
|
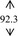
|
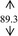
|
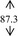
|
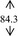
|
| 95% ethanol | ||||||||
| Carbopol ultrez® 20 | 0.5 | 0.5 | 0.5 | 0.5 | 0.3 | 0.3 | 0.3 | 0.3 |
| Triclosan | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Triethanolamine | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Snow mushroom | 7 | 10 | 12 | 15 | 7 | 10 | 12 | 15 |
| Initial | ||||||||
| pH | 7.08 ± 0.00 | 7.05 ± 0.00 | 7.28 ± 0.01 | 7.29 ± 0.02 | 6.86 ± 0.01 | 6.79 ± 0.01 | 6.92 ± 0.02 | 6.70 ± 0.02 |
| Viscosity (cps) | 3791.00 ± 2.88 | 2656.00 ± 3.51 | 2723.00 ± 3.51 | 2740.00 ± 2.30 | 1196 ± 0.57 | 664.00 ± 0.46 | 464.00 ± 0.69 | 338.00 ± 0.23 |
| Heat‐cool cycles | ||||||||
| pH | 7.09 ± 0.00 | 7.17 ± 0.00 | 7.30 ± 0.00 | 7.32 ± 0.01 | 6.89 ± 0.01 | 6.84 ± 0.01 | 6.96 ± 0.01 | 6.73 ± 0.00 |
| Viscosity (cps) | 4078.00 ± 5.77 | 2725.00 ± 2.30 | 2722.00 ± 2.30 | 2724.00 ± 0.57 | 1186.00 ± 4.35 | 648.00 ± 1.84 | 449.00 ± 0.60 | 335.00 ± 4.47 |
Preliminary sensorial evaluated during the course of the sanitizer development, the greater gelling agent resulted in higher viscosity worsen spreadability. Those of hand sanitizers containing 0.3% gelling agent were therefore included for sensorial assessments in the 20 volunteers (Figure 1). Of which, the snow mushroom hand sanitizer with the highest preference, that is F14 containing 0.10% of snow mushroom polysaccharide, was included for in vivo assessments in comparison with its placebo.
FIGURE 1.
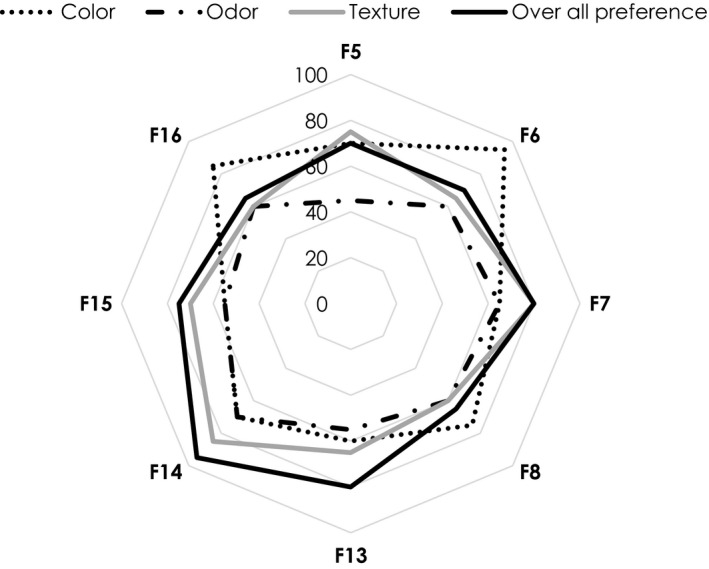
Sensorial evaluations of the developed hand sanitizers
3.2. Skin moisturizing and hand sanitizing efficacies of the developed hand sanitizers
The clinical evaluation was approved by the ethical committee of Mae Fah Luang University with an approval number of REH‐58066. The snow mushroom hand sanitizer and its placebo were consequently challenged on their safety by means of a preliminary skin irritation test in 20 volunteers. An average MII of the sanitizers was similar to that of DI water (MII = 0), which was used as a negative control. The base and snow mushroom ABHR/ABHS gels were therefore proved to be safe and appreciable to be assessed on their efficacies.
Comparative skin moisturizing efficacy evaluation of the snow mushroom sanitizer and its placebo was conducted in 20 volunteers. Rubbing hands with either snow mushroom ABHR gel or its placebo were undertaken for 15 seconds regarding the fact that this rubbing period resulted in the same protection against bacteria. 14 The hand hygienic practice would be more practically fit with the users' behavior nowadays expecting fast/quick but efficient products. The electrical capacitance of skin relating to water content in the stratum corneum was measured by means of moisture meter (Moist Sense®) refereeing skin hydrating efficacy. 10 Skin capacitance at each time interval was compared with the baseline and further expressed as skin hydrating efficacy as shown in Figure 2. The developed hand sanitizers were evidenced moisten the skin. Of which, the snow mushroom sanitizer significantly hydrated skin better than its placebo (P < .05). In addition, skin moist was retained thoroughly 180 minutes of the observation period. It should be noted that moisturizing efficacy of snow mushroom ABHR gel was better than the okra one at all time of the intervals, although that of okra contained greater polysaccharide content (0.15%) that was 13.98, 8.86, 2.45, and 0.32% moisten the hands as examined at 30, 90, 120, 150, and 180 minutes. 7 In addition, the product should be applied every 3 hours or 8 times daily, which less than the recommended frequency (>20 times daily) that induces a significant protective effect against skin dryness. 20
FIGURE 2.
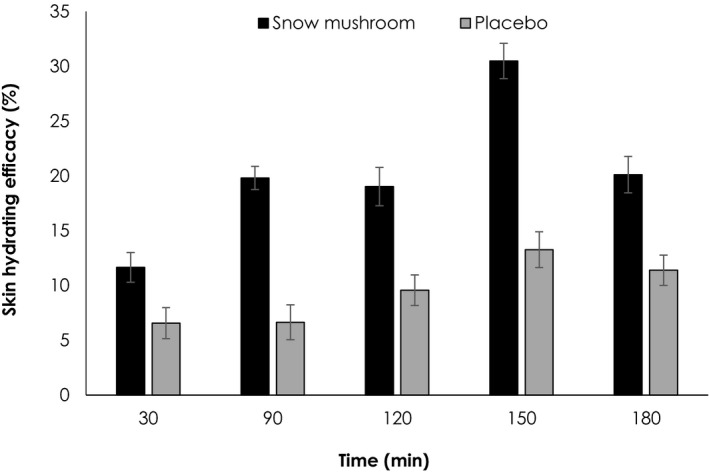
Skin hydrating efficacies of the snow mushroom sanitizer and its placebo
Sanitizing efficacy of the developed hand sanitizers was assuredly examined. Total colony count was undertaken before and after topical application of the sanitizers (Figure 3A). It was clearly evidenced that the sanitizers increased hygienic condition of hands as exhibited in Figure 3B. The sanitizing efficacy of these ABHRs was regulated by the synergistic antimicrobial activities of ethanol and triclosan. Of which, the snow mushroom ABHR slightly better in sanitizing efficacy than the placebo one (88.53 ± 4.21 and 86.90 ± 4.51%, respectively).
FIGURE 3.
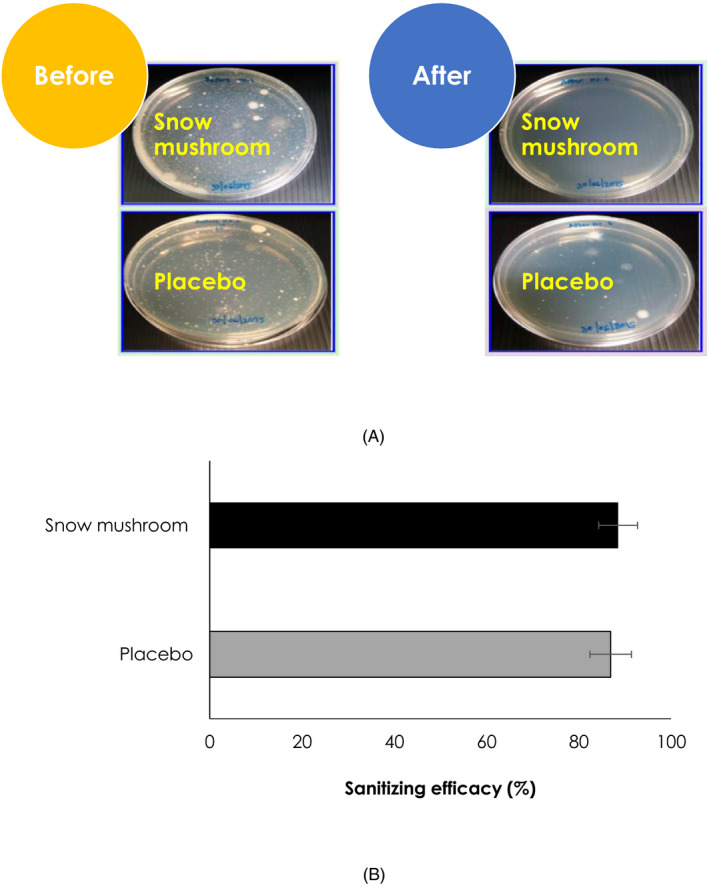
Representatives of colony forming before and after using of the snow mushroom sanitizer in comparison with its placebo (A) and their sanitizing (B) efficacies
4. CONCLUSIONS
Hand hygiene is achieved limiting infectious disease transmission with the developed ABHR gels. In addition, the preparation was evidenced to significantly maintain skin hydration following an application of the snow mushroom ABHR gels thoroughly 180 minutes, which was the observation period. Thus, the product should be applied every 3 hours or 8 times daily to maintain its skin hydrating efficacy. However, it can be applied more frequently pursuing its antimicrobial efficacy. This developed snow mushroom ABHR gel is meeting with the recommendation on hand hygienic improvement to combat the infections of diseases spreading included COVID‐19 protection and improves skin hydration maintaining skin barrier functions that co‐contribute in a good condition of hand hygiene.
ACKNOWLEDGMENTS
The authors would like to thank all of the volunteers on their contributions. Mae Fah Luang University is acknowledged upon facilities supports during this manuscript preparation. The editor and reviewers are acknowledged on their valuable suggestions that make the article more comprehensive.
Lourith N, Pungprom S, Kanlayavattanakul M. Formulation and efficacy evaluation of the safe and efficient moisturizing snow mushroom hand sanitizer. J Cosmet Dermatol.2021;20:554–560. 10.1111/jocd.13543
REFERENCES
- 1. WHO . Interim recommendations on obligatory hand hygiene against transmission of COVID‐19. https://www.who.int/who‐documents‐detail/interim‐recommendations‐on‐obligatory‐hand‐hygiene‐against‐transmission‐of‐covid‐19. 2020. Accessed April 20, 2020.
- 2. Allegranzi B, Gayet‐Ageron A, Damani N, et al. Global implementation of WHO's multimodal strategy for improvement of hand hygiene: a quasi‐experimental study. Lancet Infect Dis. 2013;13:843‐851. [DOI] [PubMed] [Google Scholar]
- 3. Grayson ML, Mevani S, Druce J, et al. Efficacy of soap and water and alcohol‐based hand‐rub preparation against live H1N1 influenza virus on the hands of human volunteers. Clin Infect Dis. 2009;48:285‐291. [DOI] [PubMed] [Google Scholar]
- 4. Kaiser NE, Newman JL. Formulation technology as a key component in improving hand hygiene practices. Am J Infect Control. 2006;34:S82‐S97. [Google Scholar]
- 5. Kampf G, Löffler H. Dermatological aspects of a successful introduction and continuation of alcohol‐based hand rubs for hygienic hand disinfection. J Hosp Infect. 2003;55:1‐7. [DOI] [PubMed] [Google Scholar]
- 6. USFDA . Policy for temporary compounding of certain alcohol‐based hand sanitizer products during the public health emergency immediately in effect guidance for industry. https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/policy‐temporary‐compounding‐certain‐alcohol‐based‐hand‐sanitizer‐products‐during‐public‐health. 2020. Accessed April 20, 2020.
- 7. Kanlayavattanakul M, Rodchuea C, Lourith N. Moisturizing effect of alcohol‐based hand rub containing okra polysaccharide. Int J Cosmet Sci. 2012;34:280‐283. [DOI] [PubMed] [Google Scholar]
- 8. Wu YJ, Wei ZX, Zhang FM, Linhardt RJ, Sun PL, Zhang AQ. Structure, bioactivities and applications of the polysaccharides from Tremella fuciformis mushroom: a review. Int J Biol Macromol. 2019;121:1005‐1010. [DOI] [PubMed] [Google Scholar]
- 9. Wen L, Gao Q, Ma CW, et al. Effect of polysaccharides from Tremella fuciformis on UV‐induced photoaging. J Funct Foods. 2016;20:400‐410. [Google Scholar]
- 10. Kanlayavattanakul M, Lourith N. Biopolysaccharides for skin hydrating cosmetics. Ramawat KG, Mérillon JM, eds. Polysaccharides. Switzerland: Springer; 2015:1867‐1892. [Google Scholar]
- 11. Kanlayavattanakul M, Lourith N, Chaikul P. Jasmine rice panicle: A safe and efficient natural ingredient for skin aging treatments. J Ethnopharmacol. 2016;193:607‐616. [DOI] [PubMed] [Google Scholar]
- 12. Ribeiro H, Marto J, Raposo S, et al. From coffee industry waste materials to skin‐friendly products with improved skin fat levels. Eur J Lipid Sci Technol. 2013;115:330‐336. [Google Scholar]
- 13. Schnuch A, Aberer W, Agathos M, et al. Performing patch testing with contact allergens. J Dtsch Dermatol Ges. 2008;6:770‐775. [DOI] [PubMed] [Google Scholar]
- 14. Pires D, Soule H, Bellissimo‐Rodrigues F, Gayet‐Ageron A, Pittet D. Hand hygiene with alcohol‐based hand rub: how long is long enough. Infect Control Hosp Epidermiol. 2017;38:547‐552. [DOI] [PubMed] [Google Scholar]
- 15. Lourith N, Kanlayavattanakul M. Ceylon spinach: a promising crop for skin hydrating products. Ind Crop Prod. 2017;105:24‐28. [Google Scholar]
- 16. USPC . United States Pharmacopoeia: The National formulary/USPC. Rockville: USPC; 2000:1809‐1816. [Google Scholar]
- 17. Lawan K, Kanlayavattanakul M, Lourith N. Antimicrobial efficacy of caprylyl glycol and ethylhexylglycerine in emulsion. J Health Res. 2009;23:1‐3. [Google Scholar]
- 18. Messina MJ, Linsey AB, Brodell RT, Mostow EN. Hand hygiene in the dermatologist's office: to wash or to rub? J Am Acad Dermatol. 2008;59:1043‐1049. [DOI] [PubMed] [Google Scholar]
- 19. Allegranzi B, Pittet D. Role of hand hygiene in healthcare‐associated infection prevention. J Hops Infect. 2009;73:305‐315. [DOI] [PubMed] [Google Scholar]
- 20. Chamorey E, Marcy PY, Dandine M, et al. A prospective multicenter study evaluating skin tolerance to standard hand hygiene techniques. Am J Infect Control. 2011;39:6‐13. [DOI] [PubMed] [Google Scholar]


