Abstract
Objectives:
We investigated sex-based differences in eligibility for and outcomes after receipt of advanced heart failure (HF) therapies.
Background:
Although women are more likely to die from HF than men, registry data suggest that women are less likely to receive heart transplant (HT) or left ventricular assist device (LVAD) for largely unknown reasons.
Methods:
We performed a single-center, retrospective cohort study of patients evaluated for advanced HF therapies from 2012 to 2016. Logistic regression was used to determine the association of sex with eligibility for HT/LVAD. Competing risks and Kaplan-Meier analysis were used to examine survival.
Results:
Of 569 patients (31% women) evaluated, 223 (39.2%) were listed for HT and 81 (14.2%) received destination (DT) LVAD. Women were less likely to be listed for HT (adjusted odds ratio [OR] 0.36, 95% confidence interval [CI] 0.21 – 0.61; P<0.0001), based on allosensitization (P<0.0001) and obesity (P=0.02). Women were more likely to receive DT LVAD (adjusted OR 2.29, 95% CI 1.23 – 4.29; P=0.01). Survival was similar between men and women regardless of whether they received HT, DT LVAD, or were ineligible for therapy.
Conclusion:
Women are less likely to be HT candidates, but more likely to receive DT LVAD.
Keywords: heart failure, heart transplant, left ventricular assist device, women, disparities
INTRODUCTION
Advanced therapies including heart transplantation (HT) and left ventricular assist device (LVAD) have dramatically improved the survival for patients with stage D HF. HT is considered the definitive therapy, with a median survival approaching eleven years.1 However, due to the constant shortage of donor organs for HT, surgical treatment of HF with LVAD has become the standard of care for patients who clinically deteriorate while awaiting HT, or are not HT candidates.2,3 Accordingly, utilization of HT and LVAD have increased steadily over the last two decades.4
Although women make up more than half of patients with HF and are more likely to die from HF than men, there are differences in utilization of advanced HF therapies based on sex.5–7 Despite increasing numbers of patients being listed for HT since the year 2004, the proportion of women candidates has remained relatively static, with approximately 24% of listed patients being women.8 Similarly, though the number of LVAD implants continues to grow, the proportion of women recipients has declined compared to the mid-1990s.9 Since most large HF databases show that women represent at least 30–40% of patients with HF with reduced ejection fraction (HFrEF)10,11, there is a suggestion that women may be underrepresented as candidates for advanced HF therapies. However, there is little published data that explains why this might be. The most obvious explanation is that the incidence of HF with preserved ejection fraction (HFpEF) is higher in women, and thus women may be less suitable for advanced therapies based on differences in HF epidemiology. Prior data also suggest sex-based differences in treatment preference, with a decreased willingness of women to undergo HT.12 Still, the questions that remain are whether there are sex-based differences in recognition of and referral for advanced HF care, as well as differences in comorbidities that might affect eligibility during the evaluation process.
Since most large datasets (i.e. Scientific Registry of Transplant Recipients [SRTR], Interagency Registry for Mechanically Assisted Circulatory Support [INTERMACS]) only contain data on HF patients who received advanced therapies, there is little published data on HF patients who were evaluated but rejected as candidates for advanced therapies. Thus, the objective of this single center retrospective cohort study was to examine sex differences in receipt of advanced HF therapies, and eligibility for advanced HF therapies related to medical and psychosocial comorbidities.
METHODS
Study population.
We retrospectively examined all patients evaluated for advanced HF therapies at Emory University Hospital from January 1, 2012, to December 31, 2016 (N=574). All patients evaluated for HT and LVAD are recorded in the Emory HT database, and all decisions made regarding final candidacy for HT or LVAD are documented in the medical record. Patients who had previously received HT and were evaluated for retransplant during this period were excluded from the study (N=5). This study was approved by the Emory University Institutional Review Board.
Study end-points.
The primary end-point for this analysis was eligibility for HT/LVAD. Eligibility for HT or LVAD is determined using criteria specified in the Emory University Hospital Guidelines for Recipient Candidacy for HT and LVAD based on international guidelines.13,14 Decisions regarding eligibility are made by an advanced HF therapeutics committee, which includes HF/transplant cardiologists and surgeons, HT and LVAD nurse coordinators, biomedical engineers, a pharmacist, social workers, financial counselors, dieticians, and a physical therapist. Transplant centers are required to provide a letter to all patients evaluated for HT that documents the specific reasons that a patient is not considered a HT candidate; these reasons are documented in the medical record. Those candidates who meet the specified criteria are “listed” for HT, while those candidates who do not meet the specified criteria for HT are considered for DT LVAD. All other patients are considered ineligible for advanced HF therapies. Patients were censored at the time of loss to follow-up or at the last date of follow-up on November 1, 2018.
Demographic and clinical covariates.
Information on demographic and clinical covariates was documented at the time of the HT/LVAD evaluation. The primary exposure of interest was defined as sex. Covariates of interest included all of the demographic and clinical variables included in Table 1. Specific reasons that a patient was not considered to be a HT or LVAD candidate were also considered as covariates, including allosensitization defined by panel-reactive antibodies (PRA) levels, requirement for home inotropes, and obesity.
Table 1.
Baseline characteristics of the study cohort.
| Female N=178 | Male N=391 | P | |
|---|---|---|---|
| Age | 50.3 ± 13.9 | 52.4 ± 12.1 | 0.1 |
| Race | 0.032 | ||
| • White | 61 (34.3) | 178 (45.5) | |
| • Black | 109 (61.2) | 198 (50.6) | |
| • Hispanic | 3 (1.7) | 2 (0.5) | |
| • Asian | 1 (0.6) | 8 (2.0) | |
| • Unknown | 4 (2.2) | 5 (1.3) | |
| Insurance | 0.6 | ||
| • Private | 83 (46.6) | 199 (50.9) | |
| • Medicare | 73 (41.0) | 149 (38.1) | |
| • Medicaid | 22 (12.3) | 42 (10.7) | |
| Heart failure type | <0.0001 | ||
| • Ischemic | 26 (14.6) | 127 (32.5) | |
| • Nonischemic | 99 (55.6) | 223 (57.0) | |
| • Peripartum | 25 (14.0) | 0 (0.0) | |
| • Restrictive | 2 (1.1) | 0 (0.0) | |
| • Other | 21 (11.8) | 31 (7.9) | |
| • ACHD | 5 (2.8) | 10 (2.6) | |
| Coronary artery disease | 56 (31.5) | 204 (52.2) | <0.0001 |
| Lung disease | 27 (15.2) | 44 (11.3) | 0.2 |
| Diabetes mellitus | 57 (32.0) | 140 (35.8) | 0.4 |
| Hypertension | 83 (46.6) | 257 (65.7) | <0.0001 |
| CKD categories • Stage 1–2 • Stage 3 • Stage 4–5 |
73 (41) 85 (47.8) 20 (11.2) |
184 (47.1) 173 (44.2) 34 (8.7) |
0.3 |
| Left ventricular thrombus | 23 (12.9) | 56 (14.3) | 0.7 |
| Inotropes at discharge | 105 (59.0) | 251 (64.2) | 0.2 |
| PRA Class I | 46.8 ± 28.8 | 26.0 ± 24.5 | 0.1 |
| PRA Class II | 56.2 ± 26.4 | 28.7 ± 20.7 | 0.5 |
| Allosensitization (PRA >10%) | 79 (51.6) | 33 (10.2) | <0.0001 |
| Body mass index (kg/m2) | 29.8 ± 7.6 | 28.9 ± 6.6 | 0.2 |
| Body mass index categories • <18 • 18–24.9 • 25–29.9 • 30–34.9 • ≥35 |
3 (1.7) 53 (30.3) 48 (27.4) 30 (17.1) 41 (23.4) |
5 (1.3) 107 (27.6) 133 (34.3) 87 (22.4) 56 (14.4) |
0.05 |
| Right atrial pressure (mmHg) | 12.6 ± 6.5 | 12.0 ± 6.5 | 0.4 |
| Mean pulmonary artery pressure (mmHg) | 36.6 ± 11.0 | 36.0 ± 11.1 | 0.6 |
| Pulmonary capillary wedge pressure (mmHg) | 24.9 ± 9.7 | 25.2 ± 9.7 | 0.7 |
| Cardiac output (L/min) | 3.2 ± 1.0 | 3.8 ± 1.1 | <0.0001 |
| Cardiac index (L/min/m2) | 1.8 ± 0.6 | 1.9 ± 1.1 | 0.4 |
| Hematocrit (%) | 35.4 ± 4.8 | 37.5 ± 6.1 | <0.0001 |
| Hemoglobin A1C (%) | 6.4 ± 1.1 | 6.5 ± 1.5 | 0.6 |
| Total bilirubin (mg/dL) | 1.4 ± 1.2 | 1.7 ± 1.3 | 0.020 |
| Sodium (mmol/L) | 135.2 ± 4.2 | 134.5 ± 5.1 | 0.1 |
| Blood urea nitrogen (mg/dL) | 26.2 ± 17.1 | 30.1 ± 20.0 | 0.027 |
| Creatinine (mg/dL) | 1.3 ± 0.5 | 1.6 ± 0.8 | <0.0001 |
| Estimated glomerular filtration rate (ml/min/1.73 m2) | 56.1 ± 22.7 | 60.5 ± 24.4 | 0.044 |
| Albumin (g/dL) | 3.5 ± 0.6 | 3.4 ± 0.6 | 0.7 |
Values are mean ± standard deviation, N(%), or median (interquartile range).
Psychosocial covariates.
The Stanford Integrated Psychosocial Assessment for Transplantation (SIPAT) scoring system was used to quantitatively assess psychosocial variables in patients who were ineligible for heart transplant (N=346).15 Briefly, the score has four subscales: A = patient’s readiness for transplant, B = social support system, C = psychosocial stability, D = substance abuse and lifestyle. Total scores can be categorized as excellent candidate (0–6), good candidate (7–20), minimally acceptable candidate (21–39), high risk candidate (40–68), and poor candidate (≥69). Patients with missing SIPAT scores were excluded from this analysis (N=15)
Statistical analysis.
Data are presented as mean ± standard deviation (SD), median (interquartile range [IQR]), or N (%) of patients. Baseline characteristics were compared between patients based on sex using Student’s t-test for continuous variables and the chi-square or Fisher’s exact test for categorical variables. Differences in outcomes of the evaluation (HT, DT LVAD, or ineligible) and reasons for ineligibility were compared by sex using the chi-square or Fisher’s exact test. The association of sex with eligibility for HT and DT LVAD was examined using multivariable logistic regression, adjusted for age, race, HF etiology, history of hypertension, CKD stage, serum albumin, serum total bilirubin, hematocrit, insurance, and the reasons for ineligibility for HT or LVAD. Sensitivity analyses were performed to examine eligibility for HT with stratification according to degree of allosensitization. In patients who were listed for HT, we used the Fine and Gray model of competing risks to estimate the association of sex with survival taking into account the competing risk of HT.16 In patients who received DT LVAD or who were ineligible for advanced therapies, the Kaplan-Meier method was used to estimate the association of sex with unadjusted survival rates. Data were analyzed with the use of SAS statistical software version 9.4 (SAS Institute Inc, Cary, NC, USA).
RESULTS
Baseline Characteristics.
During the study period, 569 patients were evaluated for primary HT and/or LVAD implantation. The baseline characteristics of the cohort are displayed in Table 1. The mean age was 51.7 ± 12.7 years and 178 (31.2%) patients were women. Compared to men, women were more likely to be black, and more likely to have nonischemic HF etiology. Women were less likely to have coronary artery disease (CAD), CKD, and hypertension, but more likely to have class 2 or greater obesity. Women had a lower hematocrit, bilirubin, and creatinine, but higher PRA values. There was no difference by sex in inotrope dependence or hemodynamics at the time of evaluation.
Association of sex with eligibility for advanced HF therapies.
Overall, 223 (39.2%) patients were listed for HT, 81 (14.2%) received destination (DT) LVAD, and 265 (46.6%) were ineligible for advanced therapies. The outcome of the evaluation for HT/LVAD varied according to sex (Figure 1). On univariate analysis, women were less likely to be listed for HT (odds ratio [OR] 0.30, 95% confidence interval [CI] 0.20 – 0.46; P<0.0001). Reasons for ineligibility for HT varied according to sex (Table 2). Women were less likely than men to be eligible for HT due to medical comorbidities including allosensitization and obesity. The only factor that made men less likely to be eligible for HT than women was substance abuse. After excluding patients who were not inotrope dependent (N=70) or people who died during the evaluation process (N=25), women remained less likely than men to be listed for HT (adjusted OR 0.21, 95% CI 0.13 – 0.35; P<0.0001). Further adjustment for allosensitization and obesity attenuated the lower odds of listing for HT associated with female sex somewhat (adjusted OR 0.36, 95% CI 0.21 – 0.61; P<0.0001). All patients considered ineligible for HT were considered for DT LVAD. Women were more likely to receive DT LVAD (adjusted OR 2.29, 95% CI 1.23 – 4.29; P=0.01).
Figure 1.
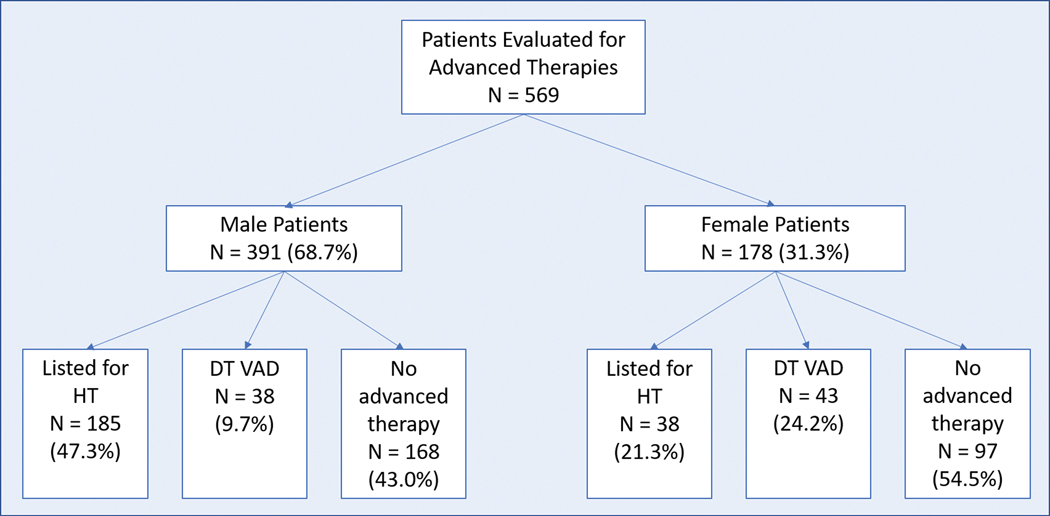
Outcomes of evaluation for advanced heart failure therapy by sex
Table 2.
Primary reasons for ineligibility for heart transplant in patients evaluated for advanced heart failure therapies according to sex.
| Female N=140 | Male N=206 | P | |
|---|---|---|---|
| Age | 4 (2.2) | 11 (2.8) | 0.8 |
| Compliance | 1 (0.6) | 6 (1.5) | 0.3 |
| Substance Abuse | 4 (2.2) | 30 (7.7) | 0.01 |
| Inadequate financial resources | 2 (1.1) | 6 (1.5) | 0.9 |
| Inadequate social support | 11 (6.2) | 26 (6.6) | 0.8 |
| Psychosocial | 6 (3.4) | 9 (2.3) | 0.5 |
| Too sick or death during evaluation | 6 (3.4) | 19 (4.9) | 0.4 |
| Not inotrope dependent | 27 (15.2) | 43 (11.0) | 0.1 |
| Patient preference | 6 (3.4) | 9 (2.3) | 0.5 |
| • Medical contraindications | |||
| • Severe allosensitization (cPRA >75%) | 38 (21.3) | 3 (0.8) | <0.0001 |
| • Obesity | 31 (17.4) | 40 (10.2) | 0.016 |
| • Liver Disease | 3 (1.7) | 6 (1.5) | 0.9 |
| • Neuropathy | 2 (1.1) | 6 (1.5) | 0.7 |
| • Lung Disease | 1 (0.6) | 6 (1.5) | 0.4 |
| • Malignancy | 5 (2.8) | 7 (1.8) | 0.4 |
| • Diabetes with complications | 11 (6.2) | 28 (7.2) | 0.7 |
| • Renal Disease | 6 (3.4) | 22 (5.6) | 0.2 |
| • Pulmonary Hypertension | 0 (0) | 5 (1.3) | 0.3 |
Association of sex with allosensitization and eligibility for HT.
Men were more likely than women to have zero class 1 (85.7% vs. 51.6%, P<0.001) or class 2 (94.7% vs 70.6%, P<0.001) PRA. Among those candidates with any sensitization, the distribution of class 1 and 2 PRA is displayed in Figure 2. When stratifying the cohort by those with PRA ≤10% vs. >10%, women remained less likely to be listed for HT among those with PRA ≤10% (adjusted OR 0.50, 95% CI 0.26 – 0.96), and among those with PRA >10% (adjusted OR 0.02, 95% CI 0.003 – 0.15, P=0.017 for sex*PRA interaction).When stratifying the cohort by those with PRA ≤75% vs. >75%, women were still less likely to be listed for HT among those with PRA ≤75% (adjusted OR 0.30, 95% CI 0.17 – 0.53), and there were no women listed for HT who had PRA >75%. Among the patients with extreme sensitization, there was one male with PRA >75% who had an A blood type. Among the women with PRA >75%, 52% were O blood type, 22% were B blood type, and 26% were A blood type.
Figure 2.
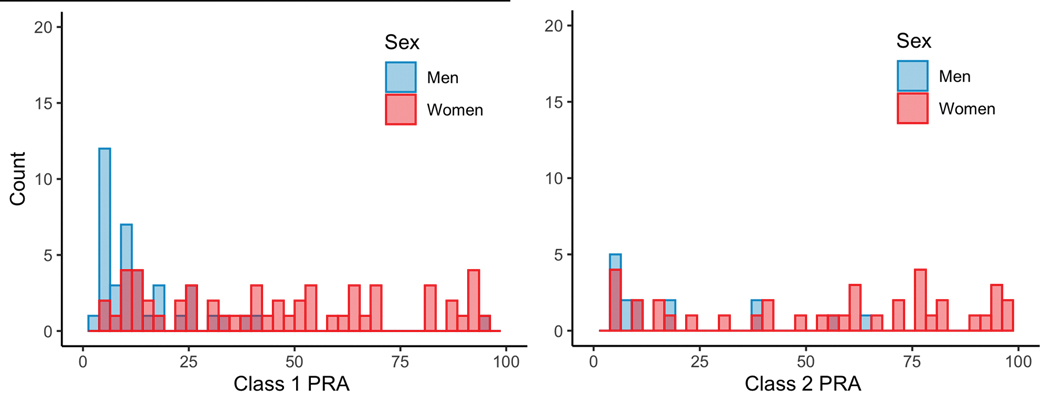
Distribution of class 1 and 2 PRA by sex among those patients with any allosensitization
Psychosocial characteristics of patients considered ineligible for heart transplant.
Among patients considered ineligible for HT, patients who were acceptable for DT LVAD had lower SIPAT scores than patients who were ineligible for any advanced HF therapies (Figure 3). The proportion of patients considered minimally acceptable or worse did not differ by sex (men 14.4% vs. women 10.3%, P=0.2). Total SIPAT scores were higher for men than women (11 [6–17] vs. 8 [4–13.5], P=0.009), driven primarily by the substance abuse (men 5 [3–7] vs. women 3 [3–6], P<0.0001) and social support (men 0 [0–5] vs. women 0 [0–3], P=0.06) subcomponents. Higher scores on the substance abuse subcomponent were observed for men who received DT LVAD and who were ineligible for advanced HF therapies (Table 3).
Figure 3.
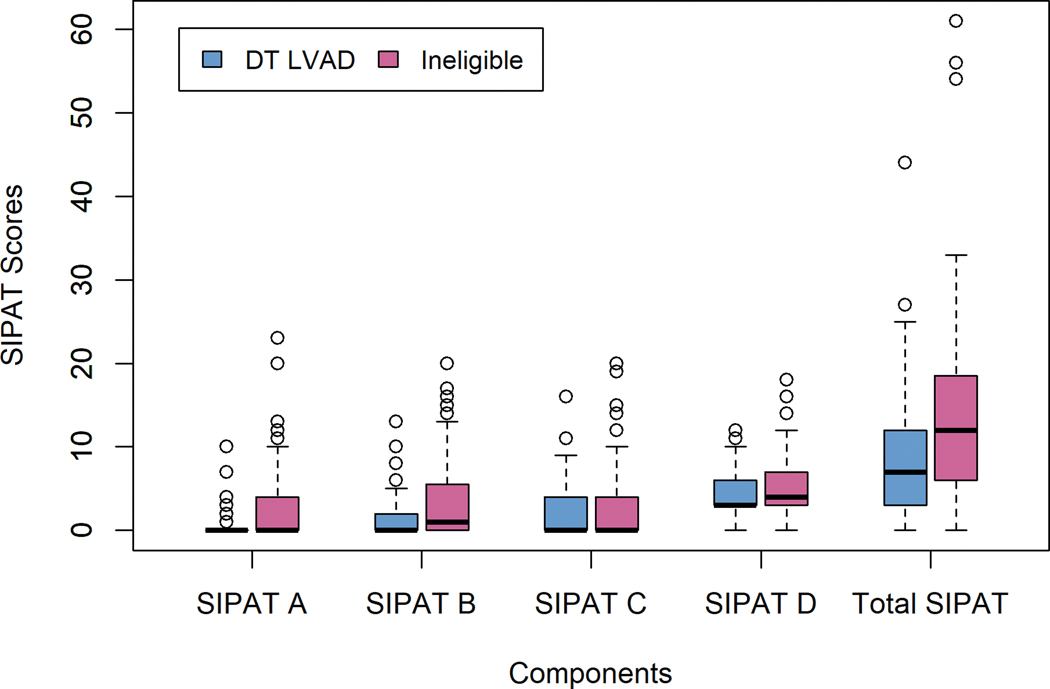
Overall SIPAT and subcomponent scores in patients who received DT LVAD and those who were ineligible for advanced HF therapies
Table 3.
Psychosocial variables among subjects considered ineligible for HT.
| SIPAT Component | Women | Men | P |
|---|---|---|---|
|
DT LVAD (N=79, 53.1% women) • Readiness • Social Support • Psychiatric stability • Substance Use • Total score |
0 (0–1) 0 (0–2) 0 (0–4) 3 (3–4) 7 (3–9) |
0 (0–0) 0 (0–4) 0 (0–4) 4 (3–7) 8 (3.5 – 13.5) |
0.6 0.7 0.7 0.01 0.2 |
|
Ineligible for advanced therapies (N=252, 36.6% women) • Readiness • Social Support • Psychiatric stability • Substance Use • Total score |
0 (0–3) 0 (0–3) 2 (0–4) 3 (3–6) 10 (5–15) |
0 (0–3) 0 (0–5) 0 (0–4) 6 (3–7) 11 (6–18) |
0.7 0.1 0.3 <0.0001 0.1 |
Association of sex with survival.
For the entire cohort, the median follow-up time was 808 (IQR 182 – 1379) days. Follow-up time was longer for patients listed for HT (median 1270, IQR [679 – 1918] days) than for patients who received DT LVAD (median 909, IQR [538 – 1201] days) or who were ineligible for advanced HF therapies (median 350, IQR [63 – 925] days). Among patients listed for HT, wait list mortality was similar between women and men (21.1% vs. 27.8%, P=0.4) (Figure 4A & B). The use of bridge-to-transplant (BTT) LVAD was higher for men (37.3% vs. 21.1%, P=0.02), but the overall rate of transplant was higher for women (76.3% vs. 65.8%, P=0.002). Among patients who received DT LVAD there were 43 deaths (53.1%), and there were 162 deaths (61.1%) among patients who were ineligible for advanced HF therapies. Survival was similar between men and women regardless of whether they received DT LVAD, or were ineligible for advanced HF therapies (Figure 4C & D).
Figure 4.
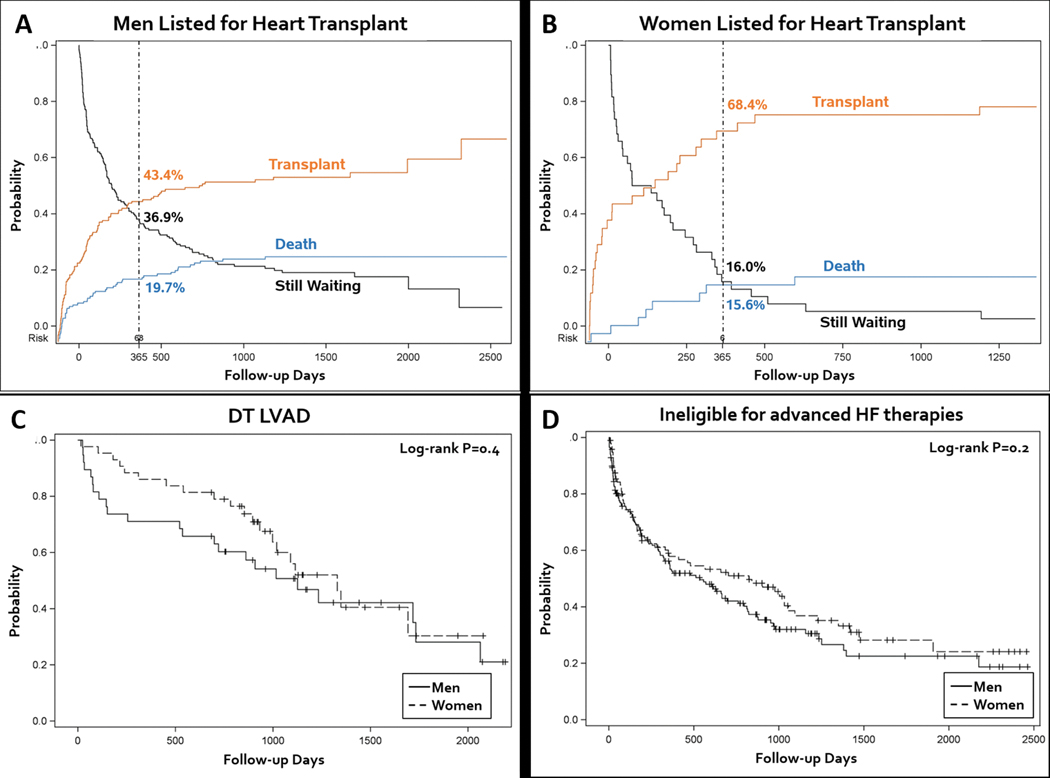
Survival analysis examining competing risk of death and transplant for men (A) and women (B) who were listed for heart transplant, and Kaplan-Meier survival analysis of men and women who received destination therapy LVAD (C), or who were ineligible for advanced therapies (D)
DISCUSSION
In this single center, retrospective cohort study, we confirmed important differences in eligibility for advanced HF therapies between men and women. In our program, women were less likely to be candidates for HT due to medical comorbidities including allosensitization and obesity, but were more than twice as likely to receive DT LVAD compared to men. The only factor that made men less likely than women to be candidates for HT was substance abuse. Among patients who were ineligible for advanced therapies, men had higher SIPAT scores than women, driven primarily by substance abuse. These data suggest that psychosocial factors (including readiness for advanced therapies, social support and psychiatric stability) are not significant contributors to the reasons that women are less likely than men to be listed for HT. Large public datasets (i.e. SRTR, INTERMACS) suggest the proportion of women who receive advanced HF therapies is lower than expected based on HF epidemiology. Although analysis of these datasets would be valuable to determine whether our findings are center-specific, or reflect a national trend, these datasets do not capture data on patients who are evaluated vs those who actually receive advanced HF therapies. Thus, our data are novel since they address the reasons that eligibility for advanced HF therapies may differ between the sexes.
HT remains the therapy of choice for advanced HF, with consistently demonstrated improvements in survival, quality of life and functional status.1,17 Accordingly, the number of new active listings for HT increased 57% from 2005 to 2016, however the proportion of women listed for HT remained static during that time.5 Since 2005, most HT recipients have been white men, with no substantial change over the past decade. It remains unclear if the lower proportion of women listed for HT is related to differences in medical comorbidities or biases in physician referral or selection, since few prior studies have identified what factors may have a differential association with the likelihood of HT listing between men and women. Aaronson et al. investigated whether sex bias occurred in the HT candidate selection process at a single center.18 Women were less likely to be accepted as candidates for HT, however the reason for rejection was due to patient preference, with a higher rate of patient self-refusal among women. However, a recent report by Stewart et al. reported that women were more likely than men to be willing to consider LVAD therapy.19 Our analysis shows no difference between sexes in patient preference for HT, but that women are less likely to be eligible for HT because of medical comorbidities.
In our cohort, allosensitization and obesity were the most common comorbidities that made women ineligible for HT. Over the past decade, 1 in 5 adult HT candidates globally were allosensitized (PRA >10%).1 Patients who are allosensitized experience longer waiting times, and have a higher risk of rejection, cardiac allograft vasculopathy, and allograft failure post-transplant.20,21 Moreover, there is currently no prioritization given to sensitized patients in the recently revised UNOS adult heart allocation policy, despite strong expert consensus that these patients represent a disadvantaged subgroup of HT candidates.22 Although desensitization can be used as a treatment strategy to reduce HLA antibody in order to facilitate transplantation, existing data are mostly observational and limited by small sample size and variability in treatment protocols.23 Preliminary data from newer biologic agents appear promising, however larger randomized trials are needed to definitively determine if desensitization strategies can be used widely and effectively. Interestingly, women remained less likely than men to be listed for HT even after our cohort was stratified according to the level of allosensitization. There was some evidence that at least half of the women with PRA >75% also had an O blood type; the combination of a high degree of allosensitization with an unfavorable ABO status (i.e. O blood type) may have influenced clinicians to recommend LVAD as the therapy of choice. Still, although our analysis demonstrates that allosensitization is an important factor, this “risk factor” alone does not fully explain why women are less likely to be listed for HT than men. Obesity is more common in women with HF, and may be a stronger risk factor for HF with preserved ejection fraction than HF with reduced ejection fraction in women.24 Still, pre-transplant BMI >35 m/kg2 is associated with longer wait times, and an increase in morbidity and mortality post-transplant.25,26 Thus, on the basis of these data and published guidelines, our program requires BMI ≤35 m/kg2 for HT listing.13
Once patients are listed for HT, multiple studies have confirmed that wait list outcomes are worse for women as compared to men. While pre-transplant mortality has declined in most subgroups since 2005, women experienced an increase in pre-transplant mortality after 2010 despite a wait time that is 3 months shorter than the average wait time for men.5 Even after adjusting for over 30 baseline variables in the SRTR database, female sex was associated with a significant risk of death among UNOS status 1A (adjusted hazard ratio [HR]: 1.20; 95% CI: 1.05 to 1.37, p = 0.01). Another analysis of SRTR data also confirmed a higher risk of wait-list removal at one year for death or deterioration for women compared to men, but showed the use of BTT LVAD in the current era eliminated the higher risk of associated with female sex.27 However, both analyses confirm that BTT LVAD is used less in women compared to men. Our single-center experience confirms the findings of lower use of BTT LVAD in women, but a higher rate of transplantation for women on the wait-list and similar wait-list mortality between men and women. Comorbidities such as elevated PRA and obesity may play a role in these disparities, since sensitized and/or obese patients may wait longer for a suitable allograft. Hsich et al. examined the SRTR data to examine risk factors associated with wait list mortality that are different for women vs. men.28 There were sex interactions with body mass index for death and HT, however these associations varied based on initial listing status.
In our program, women were twice as likely as men to receive DT LVAD. This was a surprising finding given that most large clinical DT LVAD trials have included approximately 80% men.29–31 Even recent trials examining shared decision making for DT LVAD have included predominantly male subjects.32,33 Newer, smaller profile continuous-flow LVADs are easily implanted into women with small body size compared to older systems. Data from large US and European LVAD registries confirm that women have a higher risk of early mortality after LVAD implantation, and experience higher rates of adverse events including major bleeding, right ventricular failure, and stroke.4,34,35 Survival was similar for men and women post-LVAD in our cohort. However, it is important for future clinical trials to capture the diversity of “real-world” LVAD experience, such that shared decision making surrounding advanced HF therapies can accurately inform female LVAD candidates about their risk for adverse events after implant.
While medical criteria for HT and LVAD candidacy are well recognized, psychosocial criteria are less well established. The SIPAT score has been shown to predict adverse events after HT and LVAD.36,37 However, there is little data to suggest which demographic subgroups may have higher SIPAT scores. Sperry et al. found that patients with Medicaid insurance had higher total and subcomponent SIPAT scores than patients with Medicare or commercial insurance.36 In our cohort, total SIPAT scores were higher for men based primarily on substance abuse. Prior analyses suggest that women were more likely to refuse HT 18, however subcomponent A scores (readiness for HT) were similar between men and women in our cohort. With improved clinical outcomes for both HT and LVAD in the recent era, our experience suggests that women are just as likely as men to be “ready” for advanced therapies to treat their HF.
There are important limitations to our study that are worth noting. This is a retrospective analysis of a single center experience, so our results may not be generalizable to other centers. However, since larger national databases do not capture data on the process of evaluation for advanced HF therapies, we feel our analysis provides unique insights into variables (i.e. allosensitization, obesity) that may influence sex-based disparities in receipt of advanced HF therapies as well as clinical outcomes. Additionally, our analysis does not address differences which may exist in referral to evaluation for advanced therapies, since many patients managed in community centers may not have been referred to our program for evaluation for advanced therapies. Women are diagnosed and referred for advanced HF therapies later in the course of their disease, which may be due to atypical disease presentations, but may also be due to implicit bias on the part of providers.7,38 A recent analysis by Breathett et al. confirms that implicit bias does influence decision making for advanced HF therapies.39 Bias may be particularly important for women who are attempting to access advanced HF therapies, since the psychosocial and socioeconomic determinants of health may have a greater impact on women than men.40,41 For example, women are less likely than men to live with spouses, rendering them more susceptible to biased views of non-traditional forms of social support. In addition, women are less likely to work for income, making them more vulnerable to the financial toxicity associated with underinsurance and health-care associated costs. Finally, we acknowledge that some covariates which may influence patient outcomes are not captured in our medical record, and thus not included in this analysis. Despite these limitations, since our program is the largest HT/LVAD program in the state, we feel that our experience is an important representation of HF care in a region with very high HF morbidity and mortality, and that our results are likely generalizable to other programs around the country.
In summary, women in our program were less likely to be listed for HT, in part due to medical comorbidities including allosensitization and obesity, but were more likely to receive DT LVAD. Men are less likely to be listed for HT due to substance abuse. By examining patients who were evaluated for advanced HF therapies, as opposed to just those patients who received them, our single center experience helps elucidate some of the reasons why women are underrepresented as candidates for HT. Moreover, our finding that women are twice as likely as men to receive DT LVAD highlights the need to ensure adequate representation of women in contemporary LVAD trials. Until we conduct larger, multicenter studies to address these questions, the reasons that eligibility for, or outcomes after receipt of advanced HF therapies may differ by sex will continue to remain unknown.
ACKNOWLEDGMENTS
The project was supported by funding from NIH/NIMHD U54 MD008173. AAM is also supported by funding from NIH/NHLBI K23 HL124287 and the Robert Wood Johnson Foundation (Harold Amos Medical Faculty Development Program).
REFERENCES:
- 1.Lund LH, Khush KK, Cherikh WS, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Heart Transplantation Report—2017; Focus Theme: Allograft ischemic time. The Journal of Heart and Lung Transplantation 2017;36:1037–46. [DOI] [PubMed] [Google Scholar]
- 2.Kirklin JK, Naftel DC, Pagani FD, et al. Sixth INTERMACS annual report: A 10,000-patient database. The Journal of Heart and Lung Transplantation 2014;33:555–64. [DOI] [PubMed] [Google Scholar]
- 3.Boothroyd LJ, Lambert LJ, Sas G, et al. Should Eligibility for Heart Transplantation Be a Requirement for Left Ventricular Assist Device Use? Recommendations Based on a Systematic Review. Canadian Journal of Cardiology 2013;29:1712–20. [DOI] [PubMed] [Google Scholar]
- 4.Kirklin JK, Pagani FD, Kormos RL, et al. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J Heart Lung Transplant 2017;36:1080–6. [DOI] [PubMed] [Google Scholar]
- 5.Colvin M, Smith JM, Skeans MA, et al. OPTN/SRTR 2015 Annual Data Report: Heart. Am J Transplant 2017;17 Suppl 1:286–356. [DOI] [PubMed] [Google Scholar]
- 6.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics−−2012 update: a report from the American Heart Association. Circulation 2012;125:e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsich EM. Sex Differences in Advanced Heart Failure Therapies. Circulation 2019;139:1080–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colvin M, Smith JM, Hadley N, et al. OPTN/SRTR 2016 Annual Data Report: Heart. American Journal of Transplantation 2018;18:291–362. [DOI] [PubMed] [Google Scholar]
- 9.McIlvennan CK, Lindenfeld J, Kao DP. Sex differences and in-hospital outcomes in patients undergoing mechanical circulatory support implantation. The Journal of Heart and Lung Transplantation 2017;36:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hess Paul L, Hernandez Adrian F, Bhatt Deepak L, et al. Sex and Race/Ethnicity Differences in Implantable Cardioverter-Defibrillator Counseling and Use Among Patients Hospitalized With Heart Failure. Circulation 2016;134:517–26. [DOI] [PubMed] [Google Scholar]
- 11.Randolph TC, Hellkamp AS, Zeitler EP, et al. Utilization of cardiac resynchronization therapy in eligible patients hospitalized for heart failure and its association with patient outcomes. American Heart Journal 2017;189:48–58. [DOI] [PubMed] [Google Scholar]
- 12.Aaronson K, Schwartz J, Goin J, Mancini D. Sex differences in patient acceptance of cardiac transplant candidacy. Circulation 1995;91:2753–61. [DOI] [PubMed] [Google Scholar]
- 13.Mehra MR, Canter CE, Hannan MM, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. Journal of Heart and Lung Transplantation 2016;35:1–23. [DOI] [PubMed] [Google Scholar]
- 14.Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: Executive summary. Journal of Heart and Lung Transplantation 2013;32:157–87. [DOI] [PubMed] [Google Scholar]
- 15.Maldonado JR, Dubois HC, David EE, et al. The Stanford Integrated Psychosocial Assessment for Transplantation (SIPAT): A New Tool for the Psychosocial Evaluation of Pre-Transplant Candidates. Psychosomatics 2012;53:123–32. [DOI] [PubMed] [Google Scholar]
- 16.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association 1999;94:496–509. [Google Scholar]
- 17.Liao L, Anstrom KJ, Gottdiener JS, et al. Long-term costs and resource use in elderly participants with congestive heart failure in the Cardiovascular Health Study. American Heart Journal 2007;153:245–52. [DOI] [PubMed] [Google Scholar]
- 18.Aaronson KD, Schwartz JS, Goin JE, Mancini DM. Sex differences in patient acceptance of cardiac transplant candidacy. Circulation 1995;91:2753–61. [DOI] [PubMed] [Google Scholar]
- 19.Stewart GC, Cascino T, Richards B, et al. Ambulatory Advanced Heart Failure in Women: A Report From the REVIVAL Registry. JACC Heart Fail 2019;7:602–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grupper A, Nestorovic EM, Daly RC, et al. Sex Related Differences in the Risk of Antibody-Mediated Rejection and Subsequent Allograft Vasculopathy Post-Heart Transplantation: A Single-Center Experience. Transplant Direct 2016;2:e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris AA, Cole RT, Veledar E, et al. Influence of race/ethnic differences in pre-transplantation panel reactive antibody on outcomes in heart transplant recipients. J Am Coll Cardiol 2013;62:2308–15. [DOI] [PubMed] [Google Scholar]
- 22.Kobashigawa JA, Johnson M, Rogers J, et al. Report From a Forum on US Heart Allocation Policy. American Journal of Transplantation 2015;15:55–63. [DOI] [PubMed] [Google Scholar]
- 23.Chih S, Patel J. Desensitization strategies in adult heart transplantation—Will persistence pay off? The Journal of Heart and Lung Transplantation 2016;35:962–72. [DOI] [PubMed] [Google Scholar]
- 24.Savji N, Meijers WC, Bartz TM, et al. The Association of Obesity and Cardiometabolic Traits With Incident HFpEF and HFrEF. JACC: Heart Failure 2018;6:701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russo MJ, Hong KN, Davies RR, et al. The Effect of Body Mass Index on Survival Following Heart Transplantation: Do Outcomes Support Consensus Guidelines? Annals of Surgery 2010;251. [DOI] [PubMed] [Google Scholar]
- 26.Weiss ES, Allen JG, Russell SD, Shah AS, Conte JV. Impact of Recipient Body Mass Index on Organ Allocation and Mortality in Orthotopic Heart Transplantation. The Journal of Heart and Lung Transplantation 2009;28:1150–7. [DOI] [PubMed] [Google Scholar]
- 27.Morris AA, Cole RT, Laskar SR, et al. Improved Outcomes for Women on the Heart Transplant Wait List in the Modern Era. Journal of Cardiac Failure 2015;21:555–60. [DOI] [PubMed] [Google Scholar]
- 28.Hsich Eileen M, Blackstone Eugene H, Thuita L, et al. Sex Differences in Mortality Based on United Network for Organ Sharing Status While Awaiting Heart Transplantation. Circulation: Heart Failure 2017;10:e003635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park SJ, Milano CA, Tatooles AJ, et al. Outcomes in advanced heart failure patients with left ventricular assist devices for destination therapy. Circ Heart Fail 2012;5:241–8. [DOI] [PubMed] [Google Scholar]
- 30.Rogers JG, Pagani FD, Tatooles AJ, et al. Intrapericardial Left Ventricular Assist Device for Advanced Heart Failure. N Engl J Med 2017;376:451–60. [DOI] [PubMed] [Google Scholar]
- 31.Mehra MR, Naka Y, Uriel N, et al. A Fully Magnetically Levitated Circulatory Pump for Advanced Heart Failure. N Engl J Med 2017;376:440–50. [DOI] [PubMed] [Google Scholar]
- 32.Savji N, Meijers WC, Bartz TM, et al. The Association of Obesity and Cardiometabolic Traits With Incident HFpEF and HFrEF. JACC Heart Fail 2018;6:701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kostick KM, Bruce CR, Minard CG, et al. A Multisite Randomized Controlled Trial of a Patient-Centered Ventricular Assist Device Decision Aid (VADDA Trial). Journal of Cardiac Failure 2018;24:661–71. [DOI] [PubMed] [Google Scholar]
- 34.Magnussen C, Bernhardt AM, Ojeda FM, et al. Gender differences and outcomes in left ventricular assist device support: The European Registry for Patients with Mechanical Circulatory Support. The Journal of Heart and Lung Transplantation 2018;37:61–70. [DOI] [PubMed] [Google Scholar]
- 35.Morris AA, Pekarek A, Wittersheim K, et al. Gender differences in the risk of stroke during support with continuous-flow left ventricular assist device. J Heart Lung Transplant 2015;34:1570–7. [DOI] [PubMed] [Google Scholar]
- 36.Sperry BW, Perez AL, Alvarez PA, Kendall K, Gorodeski EZ, Starling RC. Medicaid Insurance and Psychosocial Status in Patients Evaluated for Heart Transplantation. J Am Coll Cardiol 2017;70:2727–8. [DOI] [PubMed] [Google Scholar]
- 37.Bui QM, Braun OO, Brambatti M, et al. The value of Stanford integrated psychosocial assessment for transplantation (SIPAT) in prediction of clinical outcomes following left ventricular assist device (LVAD) implantation. Heart Lung 2018. [DOI] [PubMed] [Google Scholar]
- 38.DeFilippis EM, Truby LK, Garan AR, et al. Sex-related differences in use and outcomes of left ventricular assist devices as bridge to transplantation. JACC: Heart Failure 2019;7:250–7. [DOI] [PubMed] [Google Scholar]
- 39.Breathett K, Yee E, Pool N, et al. Does Race Influence Decision Making for Advanced Heart Failure Therapies? Journal of the American Heart Association 2019;8:e013592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaw LJ, Pepine CJ, Xie J, et al. Quality and Equitable Health Care Gaps for Women: Attributions to Sex Differences in Cardiovascular Medicine. Journal of the American College of Cardiology 2017;70:373–88. [DOI] [PubMed] [Google Scholar]
- 41.McSweeney J, Pettey C, Lefler LL, Heo S. Disparities in heart failure and other cardiovascular diseases among women. Womens Health (Lond) 2012;8:473–85. [DOI] [PMC free article] [PubMed] [Google Scholar]


