This article refers to ‘Angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers are not associated with severe COVID‐19 infection in a multi‐site UK acute hospital trust’ by D.M. Bean et al., published in this issue on pages 967–974.
The COVID‐19 global pandemic has shaped the last month of discourse in hospitals worldwide. The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has been shown to exploit the angiotensin‐converting enzyme 2 (ACE2) receptor in order to enter the cell: this receptor is homologous to ACE, the better‐known receptor commonly targeted by hypertension treatments, and similar to ACE, ACE2 is expressed in tissues outside of renal and cardiovascular tissues as well. 1 One of its functions is counterbalancing ACE by facilitating conversion of angiotensin II into angiotensin 1–7, turning a vasoconstrictor into a dilator. 2 Previous studies suggest that ACE inhibitors (ACEi) and angiotensin receptor blockers (ARBs) may stimulate viral replication by upregulating the expression of the ACE2 receptor necessary for it to infect a cell. 3 , 4 However, other studies have suggested rather the opposite, 5 , 6 and the use of ACEi or ARBs in heart failure was not associated with higher ACE2 levels. 7 In this issue of the Journal, Bean et al. 8 report the results of their multicentre cohort study aimed at investigating whether chronic treatment with ACEi and ARBs would increase the severity of COVID‐19 infections. The authors note that data on the clinical characteristics of hospitalized COVID‐19 patients show an overrepresentation of cardiovascular comorbidities, which suggests an increased risk of severe disease progression among this population. The widespread usage of ACEi and ARBs in western countries is mentioned by the authors as an important reason for investigating the existence of a relationship between chronic usage of these drugs and COVID‐19 severity, and whether this relationship is a harmful one.
The authors enrolled a cohort of 1200 COVID‐19 adult inpatients at King's College Hospital and Princess Royal University Hospital who all tested positive for SARS‐CoV‐2 by reverse transcriptase polymerase chain reaction. Patients were only included if they were symptomatic at presentation, and the primary endpoint was defined as either death or admission to a critical care unit within 21 days of symptom onset. Clinical notes, letters, and medication orders were reviewed to determine whether patients were chronically treated with ACEi or ARB, and the primary endpoint was manually verified by a clinician. Of the 1200 patients enrolled, 75% had at least one comorbidity and 33.2% were chronically treated with an ACEi or ARB. While the crude analysis did not show an effect of these drugs on disease severity [odds ratio (OR) 0.83, 95% confidence interval (CI) 0.64–1.07], adjusting for age and sex revealed a significant effect: the patients on ACEi and ARBs appeared to achieve better outcomes than the patients not receiving these drugs (OR 0.70, 95% CI 0.53–0.91; P < 0.01), and additional adjustments based on comorbidities strengthened this effect even further (OR 0.63, 95% CI 0.47–0.84; P < 0.01). Results are summarized in Figure 1 . As the authors note, these findings suggest chronic treatment with ACEi and ARBs does not harm COVID‐19 patients and may even have a beneficial effect on disease progression. This is important information: discontinuation of these medications due to fears of increasing the patients' susceptibility to COVID‐19 cannot be recommended and may not be without risk.
Figure 1.
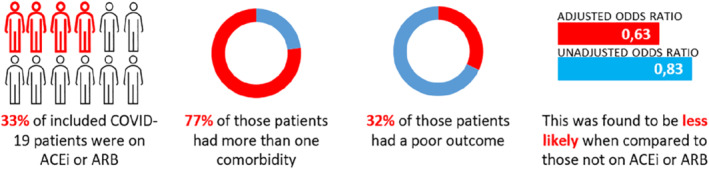
A substantial proportion of the patients in the study of Bean et al. 8 used angiotensin‐converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs), but the use of ACEi or ARBs in COVID‐19 patients was not associated with an overall unfavourable outcome.
This is not the first article on this topic, but we believe this publication to be particularly relevant for practitioners located in Europe: an ethnically mixed population with significant proportions of the various ethnicities helps ward off bias, and we expect the prevalence of chronic ACEi and ARB usage in two large UK‐based hospitals to mirror the usage rates of other western countries. The large cohort size allowed the authors to adjust for age and comorbidities, which allows the results to translate into clinical practice more easily. There are several limitations to this study as well: although the cohort was treated at two different hospitals, both are centres located in London, which makes it difficult to apply the results to other (more rural) regions. Excluding patients not ill enough to require hospitalization also means the investigated drugs' possible effects on the disease progression of less severe COVID‐19 infections will remain unknown. The authors set out to investigate whether there is a harmful relationship between these drugs and COVID‐19 severity however, and their results show that both ACEi and ARBs can be safely continued without increasing the risk of a poor outcome. While the authors are quick to mention these retrospective data should be validated in prospective studies before they can become part of common medical practice, the observation that treatment with ACEi and ARB may result in better outcomes in a ‘respiratory infection’ is still intriguing. At the very least, it challenges the view that COVID‐19 is merely a respiratory disease. The possible beneficial effect of these drugs in patients with acute respiratory distress syndrome, for example due to the hypothesised inhibition of renin–angiotensin system activation, 9 might lead to interesting future ways to treat this syndrome, if supported by future trials.
So on a broader scope, it is our opinion that these results support the inclusion of cardiological expertise to COVID‐19 teams. Due to the prevalence of cardiovascular comorbidities and the frequent usage of cardiac medications in older patients, many clinical decisions for patients suffering from COVID‐19 will impact cardiovascular health status and medications. Complex cardiovascular patients with many comorbidities are difficult to manage as‐is, let alone during a viral pandemic. With routine care being set back, it will likely pay off to invest in these patients.
In more general terms, during the height of the first wave, minimizing infections and maximizing treatment capacity were our top priorities, for good reason. However, we should no longer ignore the collateral damage caused by putting routine care on hold – also since we have more data on the direct effects of scaling down routine care. 10 , 11 We have learned a certain number of deaths cannot be directly attributed to COVID‐19. Though it is possible that part of that excess mortality is still directly caused by COVID‐19 cases – persons who were not tested but were infected – it is likely that allocating resources away from routine care in combination with weeks of government‐issued bulletins telling people to stay away from public areas like hospitals as much as possible may have caused negative effects on public health by itself. For instance, we have seen a substantial reduction in percutaneous coronary intervention lab activations across Europe, 12 , 13 and attributing this decrease to a lower incidence of ST‐elevation myocardial infarction is unrealistic. Similar observations have been made for heart failure: lower admission rates, but a higher incidence of New York Heart Association class III or IV symptoms. 14 , 15
So, now that things have appeared to calm down, we should look at our actions during the first wave and try to learn as much as possible from the decisions we have made. As healthcare practitioners, we should strive to continue regular care, if possible, the second time around. And we plea for COVID (crisis) teams to include cardiological expertise. If a second COVID‐19 wave does eventually arrive – although we all hope this will not happen – we can and must be better prepared learning from the valuable and costly experiences during the COVID‐19 pandemic of 2020.
Conflict of interest: none declared.
The opinions expressed in this article are not necessarily those of the Editors of the European Journal of Heart Failure or of the European Society of Cardiology. doi: 10.1002/ejhf.1924
References
- 1. Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett 2002;532:107–110. [DOI] [PubMed] [Google Scholar]
- 2. Kuba K, Imai Y, Penninger JM. Multiple functions of angiotensin‐converting enzyme 2 and its relevance in cardiovascular diseases. Circ J 2013;77:301–308. [DOI] [PubMed] [Google Scholar]
- 3. Hoffmann M, Kleine‐Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Diaz JH. Hypothesis: angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID‐19. J Travel Med 2020;27:taaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mizuiri S, Aoki T, Hemmi H, Arita M, Sakai K, Aikawa A. Urinary angiotensin‐converting enzyme 2 in patients with CKD. Nephrology (Carlton) 2011;16:567–572. [DOI] [PubMed] [Google Scholar]
- 6. Úri K, Fagyas M, Kertész A, Borbély A, Jenei C, Bene O, Csanádi Z, Paulus WJ, Édes I, Papp Z, Tóth A, Lizanecz E. Circulating ACE2 activity correlates with cardiovascular disease development. J Renin Angiotensin Aldosterone Syst 2016;17:1470320316668435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sama IE, Ravera A, Santema BT, van Goor H, ter Maaten JM, Cleland JG, Rienstra M, Friedrich AW, Samani NJ, Ng LL, Dickstein K, Lang CC, Filippatos G, Anker SD, Ponikowski P, Metra M, van Veldhuisen DJ, Voors AA. Circulating plasma concentrations of angiotensin‐converting enzyme 2 in men and women with heart failure and effects of renin‐angiotensin‐aldosterone inhibitors. Eur Heart J 2020;41:1810–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bean DM, Kraljevic Z, Searle T, Bendayan R, O'Gallagher K, Pickles A, Folarin A, Roguski L, Noor K, Shek A, Zakeri R, Shah AM, Teo JT, Dobson RJ. Angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers are not associated with severe COVID‐19 infection in a multi‐site UK acute hospital trust. Eur J Heart Fail 2020;22:967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin‐angiotensin‐aldosterone system inhibitors in patients with Covid‐19. N Engl J Med 2020;382:1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Docherty KF, Butt JH, de Boer RA, Dewan P, Koeber L, Maggioni AP, McMurray JJ, Solomon SD, Jhund PS. Excess deaths during the Covid‐19 pandemic: an international comparison. medRxiv 2020. 10.1101/2020.04.21.20073114. [DOI] [Google Scholar]
- 11. Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJ. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID‐19: the pandemic response causes cardiac collateral damage. Eur Heart J 2020;41:1852–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Filippo O, D'Ascenzo F, Angelini F, Bocchino PP, Conrotto F, Saglietto A, Secco GG, Campo G, Gallone G, Verardi R, Gaido L, Iannaccone M, Galvani M, Ugo F, Barbero U, Infantino V, Olivotti L, Mennuni M, Gili S, Infusino F, Vercellino M, Zucchetti O, Casella G, Giammaria M, Boccuzzi G, Tolomeo P, Doronzo B, Senatore G, Grosso Marra W, Rognoni A, Trabattoni D, Franchin L, Borin A, Bruno F, Galluzzo A, Gambino A, Nicolino A, Truffa Giachet A, Sardella G, Fedele F, Monticone S, Montefusco A, Omedè P, Pennone M, Patti G, Mancone M, De Ferrari GM. Reduced rate of hospital admissions for ACS during Covid‐19 outbreak in northern Italy. N Engl J Med 2020;383:88–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pessoa‐Amorim G, Camm CF, Gajendragadkar P, De Maria GL, Arsac C, Laroche C, Zamorano JL, Weidinger F, Achenbach S, Maggioni AP, Gale CP, Poppas A, Casadei B. Admission of patients with STEMI since the outbreak of the COVID‐19 pandemic. A survey by the European Society of Cardiology. Eur Heart J Qual Care Clin Outcomes 2020. May 28. 10.1093/ehjqcco/qcaa046 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bromage DI, Cannata A, Rind IA, Gregorio C, Piper S, Shah AM, McDonagh TA. The impact of COVID‐19 on heart failure hospitalization and management: report from a Heart Failure Unit in London during the peak of the pandemic. Eur J Heart Fail 2020;22:978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hall ME, Vaduganathan M, Khan MS, Papadimitriou L, Long RC, Hernandez GA, Moore CK, Lennep BW, McMullan MR, Butler J. Reductions in heart failure hospitalizations during the COVID‐19 pandemic. J Card Fail 2020;26:462–463. [DOI] [PMC free article] [PubMed] [Google Scholar]


