Abstract
Aims
Diffuse alveolar damage (DAD) is a ubiquitous finding in inpatient coronavirus disease 2019 (COVID‐19)‐related deaths, but recent reports have also described additional atypical findings, including vascular changes. An aim of this study was to assess lung autopsy findings in COVID‐19 inpatients, and in untreated severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)‐positive individuals who died in the community, in order to understand the relative impact of medical intervention on lung histology. Additionally, we aimed to investigate whether COVID‐19 represents a unique histological variant of DAD by comparing the pathological findings with those of uninfected control patients.
Methods and results
Lung sections from autopsy cases were reviewed by three pulmonary pathologists, including two who were blinded to patient cohort. The cohorts included four COVID‐19 inpatients, four cases with postmortem SARS‐CoV‐2 diagnoses who died in the community, and eight SARS‐CoV‐2‐negative control cases. DAD was present in all but one SARS‐CoV‐2‐positive patient, who was asymptomatic and died in the community. Although SARS‐CoV‐2‐positive patients were noted to have more focal perivascular inflammation/endothelialitis than control patients, there were no significant differences in the presence of hyaline membranes, fibrin thrombi, airspace organisation, and ‘acute fibrinous and organising pneumonia’‐like intra‐alveolar fibrin deposition between the cohorts. Fibrinoid vessel wall necrosis, haemorrhage and capillaritis were not features of COVID‐19‐related DAD.
Conclusions
DAD is the primary histological manifestation of severe lung disease in COVID‐19 patients who die both in hospital and in the community, suggesting no contribution of hyperoxaemic mechanical ventilation to the histological changes. There are no distinctive morphological features with which to confidently differentiate COVID‐19‐related DAD from DAD due to other causes.
Keywords: autopsy, COVID, diffuse alveolar damage
Introduction
As the coronavirus disease 2019 (COVID‐19) pandemic sweeps across nations, there has been a general lack of consensus in the rapidly expanding clinical literature about the extent to which acute respiratory distress syndrome (ARDS) suffered by patients infected with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is distinct from ARDS due to other causes. Whereas some patients are described as having typical ARDS, others are thought to have atypical presentations or to represent a new pathobiology altogether. 1 , 2 , 3 , 4 Similarly, whereas the pathological descriptions of pulmonary changes in patients who suffer from fatal COVID‐19 apply the overarching diagnosis of diffuse alveolar damage (DAD), some authors emphasise peculiarities, such as prominent fibrinous airspace exudates, variably conspicuous lymphocytic infiltrates with descriptions of endothelialitis, and a range of other vascular changes that include fibrinoid necrosis of small vessels, haemorrhage and vasculitis, and small‐vessel and arterial thrombosis. 1 , 5 , 6 , 7 , 8 , 9
To our knowledge, uncontrolled case reports and series of COVID‐19‐related lung pathology almost exclusively include patients who die in hospital, often after prolonged supportive care. As the histological descriptions of pulmonary changes in COVID‐19‐related ARDS come largely from inpatient data, it is unknown how or whether hyperoxaemic mechanical ventilation and other medical interventions may account for some of the histological findings.
Here, we aimed to assess morphological differences in the lungs of SARS‐CoV‐2‐positive patients who die in hospital and those who die in the community without premortem diagnoses of COVID‐19. Additionally, to better understand whether COVID‐19 represents a histological variant of DAD, we systematically compared the pathological findings of SARS‐CoV‐2‐positive patients with those of uninfected control cases.
Materials and methods
Patient Selection
This autopsy study was exempt from institutional review board approval. The University of Michigan’s Wayne County Medical Examiner Office (WCMEO) performs postmortem questionnaire screening for COVID‐19 on all cases of non‐traumatic death that occur outside of hospital. Screening criteria for SARS‐CoV‐2 testing are based on Centers for Disease Control and Prevention recommendations. 10 Testing may also be pursued when no information surrounding the death of the deceased is known/available. When testing criteria are met, SARS‐CoV‐2 nasopharyngeal swabs are collected at the time of autopsy and processed with real‐time reverse transcriptase polymerase chain reaction (Molecular Simplexa COVID‐19 Direct real‐time RT‐PCR assay; DiaSorin, Cypress, CA, USA). Complete and limited postmortem examinations are performed by American Board of Pathology‐certified forensic pathologists according to standard procedures. Representative sections of tissue, including lung, are submitted for tissue processing, and haematoxylin and eosin‐stained slides are made.
Four persons who died in the Wayne county community, outside of a hospital setting and without premortem diagnoses of COVID‐19, underwent full autopsies at WCMEO, and were found to have positive postmortem SARS‐CoV‐2 test results. Our database of SARS‐CoV‐2‐positive inpatient autopsies performed at Michigan Medicine’s University Hospital was queried to identify a cohort that had the best possible match, by patient demographics, to the SARS‐CoV‐2‐positive community death cohort. One patient in our inpatient cohort has been previously reported. 11 Our autopsy databases were then searched from 1 January 2016 to 31 December 2019 for inpatient and non‐hospitalised community cases diagnosed as ‘diffuse alveolar damage’. As these deaths occurred prior to the first reported case of COVID‐19 in the USA, 12 the cases were considered to be SARS‐CoV‐2‐negative (subsequently referred to as the ‘control cohort’). Patients were excluded if they were lung transplant recipients or had received extracorporeal membrane oxygenation during any part of their hospitalisation. Cases with Pneumocystis pneumonia were also excluded, as the frothy exudate characteristic of Pneumocystis can mimic the appearance of intra‐alveolar fibrin deposition. SARS‐CoV‐2‐negative inpatient control cases were selected on the basis of the best possible match of ventilator days with the SARS‐CoV‐2‐positive inpatient cohort. Only four SARS‐CoV‐2‐negative non‐hospitalised community control cases were identified that met the inclusion criteria, and all cases were included. Pertinent patient data were collected for all cases.
Histological Assessment
All lung slides were independently reviewed by three pulmonary pathologists (K.E.K., C.F.F., and J.L.M.) without knowledge of the gross findings, including lung weights. Two reviewers (C.F.F. and J.L.M.) were blinded to the patient cohorts. Prior to slide review, a reference image bank was circulated for standardisation of diagnostic criteria. Reviewers scored the cases on a 0–3 scale (0 = absent; 1 = rare/focal; 2 = patchy; 3 = diffuse) for each of the following: hyaline membranes (Figure 1A), fibrin thrombi (Figure 1B), airspace organisation (Figure 1C), ‘acute fibrinous and organising pneumonia’ (AFOP)‐like intra‐alveolar fibrin deposition (Figure 1D), acute bronchopneumonia (Figure 1E), perivascular inflammation and/or endothelialitis (Figure 1F,G), fibrinoid vessel wall necrosis (Figure 1H), and haemorrhage and/or capillaritis (Figure 1I). Histological diagnoses were rendered in all cases, and other pathological findings noted.
Figure 1.
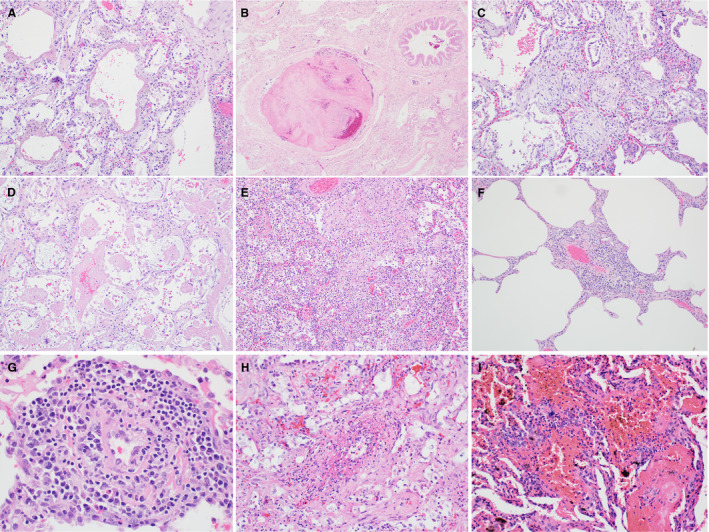
Standard reference image bank. A, Hyaline membranes. B, Fibrin thrombus. C, Airspace organisation. D, ‘Acute fibrinous and organising pneumonia’‐like intra‐alveolar fibrin. E, Acute bronchopneumonia. F, Perivascular inflammation. G, Endothelialitis. H, Fibrinoid vessel wall necrosis. I, Haemorrhage and capillaritis.
Results
Patient Characteristics
Patient characteristics for the SARS‐CoV‐2‐positive inpatient and untreated, community death cohorts are shown in Tables 1 and 2, respectively. Fever and cough were the most common presenting complaints in the SARS‐CoV‐2‐positive inpatient cohort, whereas dyspnoea was more frequently reported in the SARS‐CoV‐2‐positive community cohort (Table 3). Diabetes, chronic lung disease and cardiovascular disease were the most common underlying health conditions in all SARS‐CoV‐2‐positive cases. Obesity was also common, with five of eight SARS‐CoV‐2‐positive cases considered to be obese [body mass index (BMI) of ≥30], and, of these, two were severely obese (BMI of ≥40). Three of the deceased in the non‐hospitalised community cohort died at home; the fourth died in prison, where he reported no symptoms.
Table 1.
Severe acute respiratory syndrome coronavirus 2‐positive inpatient cohort characteristics
| Case | Age (years) | Sex | Race | BMI | Medical conditions | Symptoms at presentation | Ventilator days | Treatment | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 37 | Male | Black | 34.2 | Asthma, DM2 | Fever, cough, SOB, myalgia, HA | 6 | Steroids, hydroxyquinoline, empirical antibiotics | |
| 2 | 46 | Male | Black | 46.6 | Asthma, DM2 | Fever, cough, SOB, myalgia, HA | 8 | Hydroxyquinoline, empirical antibiotics | |
| 3 | 79 | Female | White | 18.5 | DM2, renal transplant, bipolar disorder | Fever, cough, myalgia, diarrhoea | 0* | Tocilizumab, empirical antimicrobials | |
| 4 | 63 | Female | Black | 28.2 | DM2, HTN, CAD | Cough, fever | 16 | Sarilumab trial † | |
BMI, body mass index; CAD, coronary artery disease; DM2, type II diabetes; HA, headache; HTN, hypertension; SOB, shortness of breath.
The patient had a ‘do not intubate’ order.
The patient was enrolled in a phase II/III randomised, double‐blind, placebo‐controlled study assessing the efficacy and safety of sarilumab in hospitalised patients with coronavirus disease 2019. It is unknown whether the patient received sarilumab or placebo.
Table 2.
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)‐positive community cohort characteristics
| Case | Age (years) | Sex | Race | BMI | Medical conditions | Premortem symptoms | Indication(s) for postmortem SARS‐CoV‐2 testing |
|---|---|---|---|---|---|---|---|
| 5 | 49 | Female | Black | 33.7 | CVD | Myalgia, cough, SOB, nausea/vomiting | Symptomatic, sick contacts, healthcare worker |
| 6 | 44 | Male | Black | 48.5 | None | Fever, SOB | Symptomatic |
| 7 | 55 | Male | Black | 34.9 | CVD, DM2, chronic renal failure on HD | None | Sick contacts, prisoner |
| 8 | 67 | Male | Black | 21.7 | Drug abuse disorder | Unknown | Unknown symptoms, contacts, and travel history |
BMI, body mass index; CVD, cardiovascular disease; DM2, type II diabetes; HD, haemodialysis; SOB, shortness of breath.
Table 3.
Comparison of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)‐positive inpatient and community cohorts
| SARS‐CoV‐2‐positive inpatient cohort (N = 4) | SARS‐CoV‐2‐positive community cohort (N = 4) | |
|---|---|---|
| Age (years) | ||
| Range (average) | 44–67 (55.5) | 37–79 (56.3) |
| <65 | 3 | 4 |
| ≥65 | 1 | 1 |
| Male/female ratio | 3:1 | 2:2 |
| Black/white ratio | 4:0 | 3:1 |
| BMI range (average) | 21.7–48.5 (34.7) | 18.5–46.6 (31.9) |
| Presenting/premortem symptoms | ||
| Fever | 4 (100) | 1 (25) |
| Cough | 4 (100) | 1 (25) |
| Myalgia | 3 (75) | 1 (25) |
| Dyspnoea | 2 (50) | 2 (50) |
| Headache | 2 (50) | 0 (0) |
| GI complaints | 1 (25) | 1 (25) |
| None/unknown | 0 (0) | 2 (25) |
| Medical conditions | ||
| Diabetes | 4 (100) | 1 (25) |
| Chronic lung disease | 2 (50) | 0 (0) |
| Cardiovascular disease | 1 (25) | 2 (25) |
| Immunocompromised condition | 1 (25) | 0 (0) |
| Chronic renal disease | 0 (0) | 1 (25) |
BMI, body mass index; GI, gastrointestinal.
The inpatient control cohort was composed of four females, two black and two white, ranging in age from 22 years to 80 years (average, 53 years). Ventilator days ranged from zero to 16. All patients died of clinical ARDS. Two patients had underlying sepsis, including one with polymicrobial bacteraemia following acute cholecystitis, and one with candidaemia. One patient had acute exacerbation of idiopathic bronchiectasis and pneumonia, and one suffered an acute intraparenchymal brain haemorrhage. The community death control cohort consisted of two white males, one black female, and one white female, aged 33–63 years (average, 49 years). All died of natural causes, with primary autopsy findings of DAD with or without acute bronchopneumonia.
Pathological Findings
An average of five sections per case (range 3–7) of lung were submitted at the time of autopsy for SARS‐CoV‐2‐positive inpatients. The average for inpatient control cases was 4.8 sections per case (range, 4–5). The averages for non‐hospitalised community cohorts were 3.8 sections per case (range, 3–5) for SARS‐CoV‐2‐positive cases, and 3.5 sections per case (range, 2–5) for SARS‐CoV‐2‐negative control cases.
Definite DAD was identified in all but one (case no. 7) of the SARS‐CoV‐2‐positive cases (Table 4). There was overall agreement in confirming the presence of DAD in the control cases. The lung weights for patients with a consensus diagnoses of DAD ranged from 620 g to 1600 g for the right lung (normal average, 300–350 g) and from 640 g to 1350 g for the left lung (normal average, 250–300 g). The patient without DAD (case no. 7) had lung weights of 450 g each for the right and left lungs.
Table 4.
Pathological findings
| Case | Gross lung weights (g) † | Histological diagnosis(es) | Scoring of histological features*, mean (range) | Underlying chronic changes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hyaline membranes | Fibrin thrombi | Airspace organisation | AFOP‐like fibrin | Acute bronchopneumonia | Perivascular inflammation/endothelialitis | Fibrinoid vessel wall necrosis | Haemorrhage and/or capillaritis | ||||
| SARS‐CoV‐2‐positive inpatient cohort | |||||||||||
| 1 | R: 1520; L: 1330 | DAD | 2 (2) | 1.7 (1–3) | 0 (0) | 1 (1) | 1.3 (1–2) | 0 (0) | 0 (0) | 0 (0) | – |
| 2 | R: 1300; L: 1100 | DAD | 1 (1) | 2 (2) | 0.7 (0–1) | 1.7 (1‐2) | 1 (0–2) | 0.3 (0–1) | 0 (0) | 0 (0) | – |
| 3 | R: 750; L: 700 | DAD ‡ | 3 (3) | 1 (0–2) | 1.3 (1–2) | 0.7 (0–1) | 1 (0–2) | 0 (0) | 0 (0) | 0 (0) | Diffuse alveolar septal amyloidosis |
| 4 | R: 840; L: 640 | DAD | 1.7 (1–2) | 0.3 (0–1) | 1 (0–2) | 1 (1) | 0 (0) | 0.7 (0–1) | 0 (0) | 0 (0) | – |
| SARS‐CoV‐2‐positive community cohort | |||||||||||
| 5 | R: 800; L: 700 | DAD | 3 (3) | 1 (1) | 0 (0) | 0.7 (0–1) | 0 (0) | 0.3 (0–1) | 0 (0) | 0 (0) | – |
| 6 | R: 750; L: 775 | DAD | 2 (2) | 0.7 (0–1) | 0 (0) | 1 (1) | 0.3 (0–1) | 1 (1) | 0 (0) | 0 (0) | – |
| 7 | R: 450; L: 450 | Focal fibrinous pneumonia ‡ | 0.3 (0–1) | 1 (1) | 0 (0) | 1 (1) | 0 (0) | 0.3 (0–1) | 0 (0) | 0 (0) | Metastatic calcifications |
| 8 | R: 1400; L: 1100 | DAD | 3 (3) | 0 (0) | 0 (0) | 0.3 (0–1) | 1 (1) | 0.3 (0–1) | 0 (0) | 0 (0) | – |
| Control inpatient cohort | |||||||||||
| 9 | R: 980; L: 840 | DAD | 2 (2) | 1.3 (1–2) | 0 (0) | 0.3 (0–1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | – |
| 10 | R: 750; L: 750 | DAD | 2.3 (2–3) | 0.3 (0–1) | 1.3 (1–3) | 1.7 (1–2) | 0 (0) | 0.3 (0–1) | 0 (0) | 0 (0) | – |
| 11 | R: 970; L: 750 | DAD | 2 (2) | 1 (1) | 0 (0) | 2 (2) | 1.7 (1–3) | 0 (0) | 0 (0) | 0 (0) | Bronchiectasis |
| 12 | R: 860; L: 510 | DAD | 1.7 (1–2) | 0 (0) | 0 (0) | 2.7 (2–3) | 1 (0–2) | 0 (0) | 0 (0) | 0 (0) | Metastatic spindle cell neoplasm |
| Control community cohort | |||||||||||
| 13 | R: 620; L: 420 | DAD | 2.3 (2–3) | 0.3 (0–1) | 1 (0–3) | 0.7 (0–1) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | – |
| 14 | R: 1150; L: 1280 | Acute necrotising BP and DAD | 1.7 (1–2) | 1.3 (1–2) | 0 (0) | 1.3 (1–2) | 3 (3) | 0 (0) | 0.7 (0–2) | 0 (0) | – |
| 15 | R: 1150; L: 920 | Acute BP and DAD ‡ | 1.3 (0–2) | 3 (3) | 0 (0) | 1.3 (1–2) | 3 (3) | 0 (0) | 0 (0) | 0 (0) | IV drug abuser’s lung |
| 16 | R: 1600; L: 1350 | DAD | 2 (2) | 0 (0) | 2.3 (2–3) | 2 (2) | 0.3 (0–1) | 0 (0) | 0 (0) | 0 (0) | – |
AFOP, acute fibrinous and organising pneumonia; BP, bronchopneumonia; DAD, diffuse alveolar damage; IV, intravenous; L, left; R, right; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Scoring scale: 0 = absent; 1 = rare/focal; 2 = patchy; 3 = diffuse.
Normal average adult lung weights: R = 300–350 g; L = 250–300 g.
Most common histological diagnosis listed, but no consensus diagnosis.
Among all cases reviewed (n = 16), there was unanimity regarding the presence of hyaline membranes in 14 cases, and unanimity regarding the extent of the hyaline membranes in nine cases. Among the cases without concordance, the majority of disagreements (n = 5) were due to differences in interpretation of the extent; in two cases (no. 7 and no. 15), there was lack of agreement on the presence of hyaline membranes. Case no. 7 was diagnosed as ‘early DAD’ by one reviewer, who identified focal hyaline membranes, whereas the other two reviewers did not see hyaline membranes. Case no. 15 was complicated by diffuse acute bronchopneumonia. Two reviewers also reported patchy hyaline membranes and diagnosed DAD, whereas one reviewer diagnosed only acute bronchopneumonia.
Fibrin thrombi, involving small precapillary vessels and/or muscular arteries, were identified by at least one reviewer in 13 cases. Fibrin thrombi were not identified in one SARS‐CoV‐2‐positive case and two control cases. There was no difference in the overall extent of fibrin thrombi identified between SARS‐CoV‐2‐positive cases and control cases, with an average extent of 0.9 (on a scale of 0–3) in both aggregated SARS‐CoV‐2‐positive cases and control cases.
Histological evidence of organising DAD was reported by at least one reviewer as focal (score 1) or patchy (score 2) airspace organisation in three of four SARS‐CoV‐2‐positive inpatients. Airspace organisation was scored as present by all three reviewers in only one case (no. 3). In contrast, airspace organisation was not identified by any reviewers in SARS‐CoV‐2‐positive community cases.
Interobserver variability was greatest for AFOP‐like intra‐alveolar fibrin, which was scored as present by at least one reviewer in all cases. This feature was identified by all three reviewers in 11 cases that were equally distributed across all four cohorts, with unanimity regarding extent in six of them. Of the cases for which there was not a consensus on extent, reviewers scored four cases as either 1 (focal) or 2 (patchy), and one was scored as 2 (patchy) or 3 (diffuse). For five cases, spread across all four cohorts, there was lack of agreement on the presence of AFOP‐like intra‐alveolar fibrin, but, when scored as present, it was only a focal finding (score of 1). Overall, the extent of fibrin deposition was not appreciably different between aggregated SARS‐CoV‐2‐positive cases (average 0.9) and control cases (average 1).
There was more acute bronchopneumonia in SARS‐CoV‐2‐positive inpatients (average of 1.5) than in SARS‐CoV‐2‐positive community cases (average of 0.8). Perivascular inflammation was a more common finding in SARS‐CoV‐2‐positive cases (average of 0.4) than in control cases (average of <0.1), but, in SARS‐CoV‐2‐positive cases, it was a focal finding when identified, and only one case was scored as having perivascular inflammation by all three reviewers. Fibrinoid vessel wall necrosis, haemorrhage and/or capillaritis were not features of SARS‐CoV‐2‐positive cases. Viral cytopathic changes were not seen in any SARS‐CoV‐2‐positive cases.
Discussion
To our knowledge, this is the first comparison of autopsy lung findings in SARS‐CoV‐2‐positive medically managed inpatients and untreated, non‐hospitalised deceased persons with those in uninfected historical control cases of DAD. The main limitation of our study is the small number of cases in each of the cohorts. Nonetheless, we think our SARS‐CoV‐2‐positive cases are probably representative of the broader COVID‐19 patient population, as both hospitalised and non‐hospitalised deceased persons had underlying conditions previously identified as risk factors for severe disease, including diabetes, chronic lung disease, cardiovascular disease, 13 and obesity. 14
DAD is ubiquitous at autopsy in hospitalised and non‐hospitalised COVID‐19 patients. DAD was the primary abnormality in all hospitalised COVID‐19 patients, as previously reported by others. 5 , 6 , 7 , 8 , 9 , 15 , 16 DAD was also present in nearly all SARS‐CoV‐2‐positive deceased persons who died without medical intervention. This suggests that COVID‐19‐related lung disease is a common cause of death not only in hospitalised patients, but also in those who die in the community. The single exception was a case that lacked histomorphological features clearly diagnostic of DAD. The lung weights were also relatively normal as compared with all other cases in which DAD was present. All reviewers noted a focal AFOP‐like airspace exudate, similarly to previous descriptions of incidental pulmonary findings in patients later diagnosed with COVID‐19. 17 This person was reportedly asymptomatic prior to being found dead. We conclude that he was either an asymptomatic SARS‐CoV‐2 carrier or suffered from early preclinical or subclinical COVID‐19, for the following reasons: (i) the absence of significant pulmonary changes; and (ii) autopsy evidence of hypertensive heart disease, which probably accounted for his death.
SARS‐CoV‐2‐positive inpatient and non‐hospitalised community cases show no meaningful differences in lung histology at autopsy. DAD is the primary abnormality in both, with there being little to distinguish these two populations. Airspace organisation, which is a finding more typical of the organising phase of DAD, was seen only in hospitalised COVID‐19 patients, indicating that they survived longer with their lung disease than their non‐hospitalised counterparts. Neither SARS‐CoV‐2‐infected cohort showed fibrinoid vessel wall necrosis, vasculitis/capillaritis or haemorrhage resembling that previously reported by others. 7 , 8 , 9 We have not observed any of these vascular changes in other hospital‐based COVID‐19 patient autopsies that were not included in this study. Given the morphological similarity between treated COVID‐19 DAD and COVID‐19 DAD in non‐hospitalised patients, we conclude that the DAD seen in the inpatient cohort is directly attributable to viral infection rather than medical therapies, including those that may cause hyperoxaemic lung injury.
DAD in SARS‐CoV‐2‐positive patients is histologically indistinguishable from DAD due to other causes. Although additional clinical correlation is needed to understand whether the ARDS experienced by COVID‐19 patients represents a novel subphenotype, our results suggest that the histological features of COVID‐19‐related DAD are not specific, and indicate that the pathogenesis includes elements common to other causes of DAD. Fibrin thrombi with an average score of at least rare/focal (≥1) were seen in five of eight SARS‐CoV‐2‐positive cases and in four of eight control cases. Furthermore, we have not observed a greater incidence of thrombi in other COVID‐19 inpatient autopsies. These observations underscore the original descriptions of DAD, in which fibrin thrombi were common. 18 Indeed, the term DAD was coined to emphasise the central role of diffuse injury to the epithelium and endothelium constituting the distal pulmonary acinus. AFOP‐like fibrin also occurred with similar frequency in all SARS‐CoV‐2‐infected and uninfected cases. At least focal (mean of ≥1) AFOP‐like changes were identified in five of eight SARS‐CoV‐2‐positive cases and in six of eight control cases. This finding was never more than ‘patchy’ in SARS‐CoV‐2‐positive cases as independently assessed by three reviewers, including two who were blinded to cohort assignment. AFOP‐like fibrin was extensive in only a single non‐hospitalised case that preceded the COVID‐19 pandemic, supporting the non‐specific nature of this histological finding. Variably prominent alveolar fibrin deposition was also included in the original description of DAD, 18 further demonstrating that it is not specific to COVID‐19 or any other variant of DAD. Perivascular inflammation was noted by at least one reviewer in five of eight SARS‐CoV‐2‐positive cases as compared with one of eight COVID‐19‐negative control cases. When present, this finding was, at most, focal (mean of ≤1) and of uncertain significance with regard to the pathogenesis of COVID‐19‐related lung disease. As a diagnostic clue, it is neither specific nor sufficient to reliably separate COVID‐19 from other causes of DAD. Similar patterns of inflammation have been described and illustrated with a variety of terms in other viral pneumonias, including those caused by coronaviruses, human parechovirus, respiratory syncytial virus, human parainfluenza virus 1, and influenza virus. 19 This finding has also been described in non‐viral cases of DAD, further emphasising its non‐specific nature. 18
We had relatively high rates of concordance for the presence or absence of most histological features, and discordance usually involved a difference of only a single grade (e.g. ‘absent’ versus ‘rare/focal’). The rates of discordance were randomly distributed across all four cohorts, and therefore did not significantly affect the final comparisons. Finally, as the numbers of lung sections examined were relatively consistent between cohorts, we think that sampling bias is unlikely to explain our results.
In summary, our blinded comparison of SARS‐CoV‐2‐infected deceased persons affirm previous observations of DAD as the primary histological manifestation of severe lung disease in hospitalised COVID‐19 patients, and further establishes that this is the primary finding in those who die untreated, in non‐hospital settings. We also demonstrate that the histomorphological findings in SARS‐CoV‐2‐infected patients show substantial overlap with those in patients who have DAD from other causes, suggesting common elements in their pathogenesis.
Conflicts of interest
The authors state that they have no conflicts of interest. There are no sources of funding to declare.
Author contributions
All authors made substantial contributions to the acquisition (T. Nguyen, J. M. Jentzen, O. Rayes, C. J. Schmidt, and A. M. Wilson) or the interpretation (K. E. Konopka, C. F. Farver, and J. L. Myers) of data, participated in the drafting or revision of the work, and gave final approval to the submitted version.
Konopka K E, Nguyen T, Jentzen J M, Rayes O, Schmidt C J, Wilson A M, Farver C F & Myers J L. (2020) Histopathology 77, 570–578. 10.1111/his.14180 Diffuse alveolar damage (DAD) resulting from coronavirus disease 2019 Infection is Morphologically Indistinguishable from Other Causes of DAD
K.E.K and T.N. contributed equally to this work.
References
- 1. Ackermann M, Verleden SE, Kuehnel M et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N. Engl. J. Med. 2020; 383; 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bos LD, Paulus F, Vlaar AP, Beenen LF, Schultz MJ. Subphenotyping ARDS in COVID‐19 patients: consequences of ventilator management. Ann. Am. Thorac. Soc. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marini JJ, Gattinoni L. Management of COVID‐19 Respiratory Distress. JAMA 2020; 323; 2329. [DOI] [PubMed] [Google Scholar]
- 4. Li X, Ma X. Acute respiratory failure in COVID‐19: is it ‘typical’ ARDS? Crit. Care 2020; 24; 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fox SE, Akmatbekov A, Harbert JL, Li G, Brown JQ, Vender Heide RS. Pulmonary and cardiac pathology in African American patients with COVID‐19: an autopsy series from New Orleans. Lancet Respir. Med. 2020; 8; 681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lax SF, Skok K, Zechner P et al. Pulmonary arterial thrombosis in COVID‐19 with fatal outcome: results from a prospective, single‐center, clinicopathologic case series. Ann. Intern. Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Menter T, Haslbauer JD, Nienhold R et al. Post‐mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tian S, Xiong Y, Liu H et al. Pathological study of the 2019 novel coronavirus disease (COVID‐19) through postmortem core biopsies. Mod. Pathol 2020; 33; 1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Varga Z, Flammer AJ, Steiger P et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet 2020; 395; 1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Instructions for completing the human infection with 2019 novel coronavirus (COVID‐19) case report form. Version 1, May 2020. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/coronavirus/2019‐ncov/downloads/COVID‐19‐Persons‐Under‐Investigation‐and‐Case‐Report‐Form‐Instructions.pdf (accessed 1 June 2020).
- 11. Konopka KE, Wilson A, Myers JL. Postmortem lung findings in an asthmatic patient with coronavirus disease 2019. Chest 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holshue ML, DeBolt C, Lindquist S et al. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020; 382; 929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. CDC COVID‐19 Response Team . Preliminary estimates of the prevalence of selected health conditions among patients with coronavirus disease 2019—United States, February 12‐March 28, 2020. Morb. Mortal. Wkly. Rep. 2020; 69; 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Lusignan S, Dorward J, Correa A et al. Risk factors for SARS‐CoV‐2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre Primary Care Network: a cross‐sectional study. Lancet Infect. Dis. 2020; 20; 1034–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schaller T, Hirschbühl K, Burkhardt K et al. Postmortem examination of patients with COVID‐19. JAMA 2020; 323; 2518–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID‐19 autopsies, Oklahoma. USA. Am. J. Clin. Pathol. 2020; 153; 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pathology of early‐phase 2019 novel coronavirus (COVID‐19) pneumonia in two patients with lung cancer. J. Thorac. Oncol. 2020; 15; 700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Katzenstein AL, Bloor CM, Leibow AA. Diffuse alveolar damage—the role of oxygen, shock, and related factors. A review. Am. J. Pathol. 1976; 85; 209–228. [PMC free article] [PubMed] [Google Scholar]
- 19. Ishiguro T, Kobayashi Y, Ryuji U et al. Viral pneumonia requiring differentiation from acute and progressive diffuse interstitial lung disease. Intern. Med. 2019; 58; 3509–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]


