Congenital myopathies (CMs) are a group of rare inherited myopathies most commonly presenting in infancy with hypotonia and weakness. They have been classified on the basis of distinctive myopathologic features. Mutations in more than 25 genes can cause CM.1
Recently, truncating mutations of MYO18B have been found to cause nemaline myopathy with cardiomyopathy or Klippel-Feil syndrome (KFS).2–4 KFS is characterized by the presence of congenital synostosis of some or all cervical vertebrae.
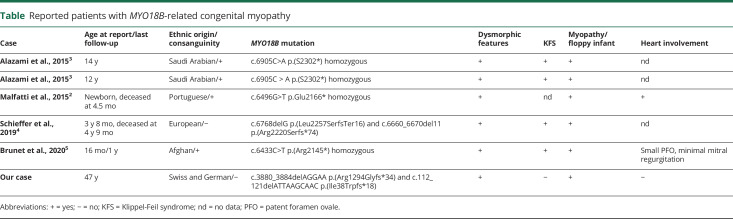
Here, we report the sixth known patient with CM due to 2 novel truncating mutations of MYO18B. Unlike the previously reported patients, our Swiss patient has neither KFS nor cardiomyopathy.
The patient was the third child to healthy nonconsanguineous German mother and Swiss father. The patient's elder siblings were not affected. The patient had delayed motor development. He was able to walk at 1.5 years. Generalized muscle weakness and atrophy were observed since early infancy. He presented at our department at 47 years and reported a slow progression of his muscle weakness. On clinical examination, he had the following dysmorphic features: low-set ears, micrognathia with small teeth, thin lips, high-arched palate, short stature, and bilateral pes planus. He was operated because of strabismus in childhood. He had facial weakness, nasal speech, bilateral steppage gait, mild proximal upper and lower limb weakness, and pronounced distal leg weakness. There were no contractures, nor rigid spine. Tendon reflexes were diminished. There were no cognitive, sensory, coordination, or bladder disturbances. The CK level was normal. Heart ultrasound and Holter ECG were unremarkable. Lung function tests disclosed restrictive respiratory impairment. Needle EMG showed myopathic changes without fibrillation potentials or myotonic discharges. Brain MRI was normal. X-ray of the neck did not find KFS.
We suspected CM and proceeded with next generation sequencing panel (supplementary material, links.lww.com/NXG/A271). We found 2 new frameshift mutations of MYO18B: NM_032608.6:c.3880_3884delAGGAA/p.(Arg1294Glyfs*34) and NM_032608.6:c.112_121delATTAAGCAAC/p.(Ile38Trpfs*18).
The new variants were confirmed using targeted Sanger sequencing. The patient is compound heterozygous for the mutations. His mother carries p.(Ile38Trpfs*18) heterozygously, and his father is a heterozygous carrier of p.(Arg1294Glyfs*34). His asymptomatic sister carries p.(Arg1294Glyfs*34) heterozygously.
The newly identified mutations segregate with disease phenotype and were not found in the current public databases (gnomAD, ESP5400, and G1000).
Our patient demonstrates similar phenotype as the previously reported patients2–5 (table). However, he has no KFS. In contrast to the Portuguese patient,2 our patient did not have cardiomyopathy. Of interest, he demonstrated predominant distal weakness. All previously reported patients presented at birth as floppy infant with general weakness. Apparently, the predominant distal weakness is not a common feature or at least is not characteristic at the beginning.
Table.
Reported patients with MYO18B-related congenital myopathy
It had been speculated that cardiomyopathy could be mutation-specific because truncated myosin 18B protein was found in muscle extracts from the patient reported by Malfatti et al., whereas the mutation in Saudi patients was shown to involve nonsense-mediated messenger RNA decay.2,3 However, the frameshift mutations of another MYO18B patient without cardiomyopathy did not undergo nonsense-mediated decay,4 casting doubt about this hypothesis.
MYO18B (myosin XVIIIB) represents an unconventional myosin family protein. Deficiency of Myo18B caused an embryonic lethality in mice accompanied by the disruption of myofibrillar structures in cardiac myocytes.6 Zebrafish models of Myo18B loss-of-function mutants revealed that Myo18B is essential for coordinating the integration of preformed thick and thin filaments into the sarcomere.7 Based on these data, it is not surprising that the muscle and cardiac involvement is caused by Myo18B deficiency in humans. Because KFS was found in at least 4 MYO18B mutant patients, MYO18B might also play a role in somite segmentation.
All previously reported mutations are located in the C-terminus in the second large unknown domain of Myo18B. The novel mutation p.(Ile38Trpfs*18) is located in the N-terminus. N‐terminal unique domain of Myo18B is crucial for its localization to F‐actin.6 Our second mutation p.(Arg1294Glyfs*34) is located in the myosin head domain containing a guanosine triphosphate/adenosine triphosphate binding site.
The 2 unique N- and C-terminal extension distinguish Myo18B from the conventional myosins. Of interest, apart from p.(Arg1294Glyfs*34), all reported disease-causing mutations cluster in these 2 domains with still unclarified function.
Our case further supports that loss-of-function mutations of MYO18B lead to CM with facial dysmorphism but are not uniformly associated with KFS. This suggests that MYO18B should be included in gene panels for CM. Our case demonstrates the delay in diagnosing CM because of the slow progression and mild symptoms, as previously reported.1 Finding additional patients and analyzing muscle biopsies would be helpful to clarify still largely unknown MYO18B function.
Appendix. Authors
Study funding
No targeted funding reported.
Disclosure
The authors have no disclosures. Go to Neurology.org/NG for full disclosures.
References
- 1.Nicolau S, Liewluck T, Tracy J et al. Congenital myopathies in the adult neuromuscular clinic: diagnostic challenges and pitfalls. Neurol Genet 2019;5:e341 doi: 10.1212/NXG.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malfatti E, Böhm J, Lacène E, et al. A premature stop codon in MYO18B is associated with severe nemaline myopathy with cardiomyopathy. J Neuromuscul Dis 2015;2:219–227. 10.3233/JND-150085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alazami AM, Kentab AY, Faqeih E, et al. A novel syndrome of Klippel-Feil anomaly, myopathy, and characteristic facies is linked to a null mutation in MYO18B. J Med Genet 2015;52:400–404. 10.1136/jmedgenet-2014-102964. [DOI] [PubMed] [Google Scholar]
- 4.Schieffer KM, Varga E, Miller KE, et al. Expanding the clinical history associated with syndromic Klippel-Feil: a unique case of comorbidity with medulloblastoma. Eur J Med Genet 2019;62:103701 10.1016/j.ejmg.2019.103701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunet T, Westphal D, Weber S, et al. A novel pathogenic variant in MYO18B associating early-onset muscular hypotonia, and characteristic dysmorphic features, delineation of the phenotypic spectrum of MYO18B-related conditions. Gene 2020;742:144542 10.1016/j.gene.2020.144542. [DOI] [PubMed] [Google Scholar]
- 6.Ajima R, Akazawa H, Kodama M, et al. Deficiency of Myo18B in mice results in embryonic lethality with cardiac myofibrillar aberrations. Genes Cells 2008;13:987–999. 10.1111/j.1365-2443.2008.01226.x. [DOI] [PubMed] [Google Scholar]
- 7.Berger J, Berger S, Li M, et al. Myo18b is essential for sarcomere assembly in fast skeletal muscle. Hum Mol Genet 2017;26:1146–1156. 10.1093/hmg/ddx025. [DOI] [PubMed] [Google Scholar]