Abstract
Background
Data on bone health and renal impairment in people with human immunodeficiency virus (HIV) in resource-limited settings are limited. The primary aim of this study was to investigate the potential role of calcaneal quantitative ultrasonography (QUS) in predicting bone mineral density (BMD) reduction in a population of Ugandan HIV-infected individuals receiving long-term antiretroviral therapy; the secondary end point was to assess the prevalence of proximal tubular dysfunction and the correlation between elevated urinary retinol-binding protein–urinary creatinine ratio (uRBP/uCr) and reduced BMD.
Methods
We conducted a cross-sectional study at the Infectious Diseases Institute, Kampala, Uganda. We included 101 HIV-infected adults who had been receiving continuous antiretroviral therapy for ≥10 years and had undergone dual-energy x-ray absorptiometry (DXA) during the previous 12 months. All patients underwent calcaneal QUS evaluation and urine sample collection.
Results
DXA BMD measurements were significantly associated (P < .01) with calcaneal speed of sound, broadband ultrasound attenuation, and QUS index. Forty-seven individuals (47%) had abnormal uRBP/uCr values. A significant inverse correlation was observed between uRBP/uCr and DXA T scores (lumbar [P = .03], femoral neck [P < .001], and total hip [P = .002]).
Conclusions
Calcaneal QUS results showed a moderate correlation with DXA outputs. The identified high prevalence of subclinical tubular impairment also highlights the importance of expanding access to tenofovir disoproxil fumarate–sparing regimens in resource-limited settings.
Keywords: bone metabolic diseases, HIV, calcaneal ultrasound, tubular impairment, resource-limited setting
Quantitative calcaneal ultrasonography and urinary retinol-binding protein showed a moderate correlation with dual-energy x-ray absorptiometry–measured bone mineral density in people with human immunodeficiency virus receiving long-term antiretroviral therapy in Uganda: longitudinal studies are needed for evaluating whether fragility fractures may be predicted and avoided.
Data from developed countries consistently showed that people with human immunodeficiency virus (HIV) (PWH) may develop low bone mineral density (BMD) and fragility fractures more frequently than the general population. The pathogenesis of reduced BMD in PWH seems to be multifactorial and likely represents a complex interaction between HIV infection, high prevalence of established osteoporosis risk factors (eg, smoking, alcohol abuse, physical inactivity, low body weight, nutritional deficiencies, and hypogonadism ), factors related to antiretroviral therapy (ART), and immunological status [1–4].
In particular, the initiation of combination ART, particularly tenofovir disoproxil fumarate (TDF), has been repeatedly associated with a decrease in BMD [5–7]. Protease inhibitor (PI) use has also been linked to bone loss, although this effect has not been clearly confirmed [6, 8–11]. The exact mechanism by which TDF reduces bone mass is yet to be elucidated, but some authors have linked this effect to TDF-induced renal proximal tubular dysfunction [12–14]. Urinary retinol-binding protein (uRBP) is a marker of renal tubule dysfunction because it is a low-molecular-weight protein (21 kDa) that is reabsorbed in the presence of normal tubulus function. uRBP has been shown to be frequently elevated in individuals on TDF-containing ART [15–17]; in addition, an elevated uRBP–urinary creatinine ratio (uRBP/uCr) has been independently associated with lower lumbar spine BMD in patients receiving TDF-based regimens [18, 19].
Despite growing concern about metabolic bone disease in aging PWH, bone status is poorly documented in resource-limited settings, with an extreme shortage of solid epidemiological data among seropositive persons, as well as in the general population [20, 21]. This situation is partly due to the scarcity of specific equipment for BMD assessment with dual-energy x-ray absorptiometry (DXA), which represents the standard reference method for diagnosing osteoporosis or osteopenia: DXA devices are expensive, nonportable and, when available, generally concentrated in urban areas, beyond the reach of a large proportion of the population [22].
Several authors have highlighted alternative methods to identify individuals at higher risk of fragility fracture in such settings, including risk-assessment tools and quantitative ultrasonographic (QUS) devices [23, 24]. In particular, numerous studies have showed that calcaneal QUS, an ultrasonography-based, inexpensive, portable and ionizing-radiation-free method, is a good predictor of osteoporotic fracture risk in both men and women [25–27], with most of the evidence concerning postmenopausal women and elderly men. Despite being an attractive tool, especially where the use of DXA may be unfeasible, its use in clinical practice is still not well defined. Moreover, only a limited number of studies have investigated the role of QUS in HIV populations in low-income settings, and, to our knowledge, no study has assessed the correlation between QUS and DXA in such a context [20, 21, 28].
In addition, scarce data are available on the effect of TDF-based ART regimens on BMD in developing areas, despite the widespread use of this molecule in low-income countries, where the World Health Organization (WHO) recommends TDF as the preferred nucleoside reverse-transcriptase inhibitor for treating HIV-infected adults and adolescents [21, 29, 30]. The main aim of this study was to investigate the potential role of calcaneal QUS and uRBP as screening tools to predict subclinical bone and tubular disorders in a resource-limited setting.
METHODS
Study Setting and Population
A cross-sectional study was conducted between May and July 2018 at the Infectious Diseases Institute (IDI), a center of excellence for HIV treatment and prevention located in Kampala, Uganda [31]. The primary aim was to compare DXA and calcaneal QUS results in the evaluation of BMD in a population of Ugandan HIV-infected individuals receiving long-term ART; the secondary end point was to assess the prevalence of abnormal uRBP/uCr and the correlation among increased uRBP/uCr and reduced BMD in the study group.
We included HIV-infected individuals >18 years of age, who had been receiving ART for ≥10 years and had undergone DXA scan during the previous 12 months as part of a partner study protocol on bone health conducted concurrently at IDI (“Bone Mineral Density in HIV-Infected Patients on Long-Term ART in Uganda”). The latter was a case-control cross-sectional study enrolling 447 adult participants (200 PWH receiving >10 years of ART, 200 ART-naive PWH and 47 healthy controls): exclusion criteria were hospitalization (or being bedridden), an AIDS-defining Illness or received chemo- or immune-modulating therapy in the previous 60 days. DXA evaluation was performed using a Hologic Discovery Wi Apex 3.1 device (Hologic Bedford).
We excluded patients with acute or chronic renal impairment (Chronic Kidney Disease–Epidemiology Collaboration calculated estimated glomerular filtration rate <60 mL/min), diabetes mellitus, recent insurgence (<3 months) of cardiopathy or acute myocardial infarction, history of trauma or surgery at the calcaneal QUS measurement site, concomitant diseases that could affect the primary or secondary outcomes, concomitant nephrotoxic drug use, hospitalized or bedridden status, and pregnancy. Patients were screened for inclusion and exclusion criteria using information collected in the IDI routine medical files. Eligible participants gave informed consent and underwent study-related clinical assessment, calcaneal QUS evaluation, and urine sample collection and analysis. IDI experimentation guidelines were followed in the conduct of the clinical research. The Uganda National Council for Science and Technology and the School of Biomedical Sciences Higher Degree Research and Ethics Committee, Kampala, approved the study.
Clinical Assessment
Clinical and laboratory data were partly collected during a face-to-face interview with the patient and partly abstracted from the IDI medical records and the partner study’s case report form. The collected information included demographic data, past medical history (including history of previous bone fractures), family history of hip fracture, behavioral risk history (smoking, alcohol intake, and substance abuse, defined as current if active at the time of data collection), most recent serum creatinine level, menopausal status (defined as no menstruation for 6 months unrelated to pregnancy or postpartum amenorrhea), age of menopause and concomitant drug use. As for HIV history, nadir CD4+ T-lymphocyte count, WHO clinical stage, antiretroviral history, most recent CD4+ T-lymphocyte count, and most recent viral load were collected. DXA scan results were evaluated; BMD (grams per centimeter squared), T score, and Z score, assessed with lumbar spine, total hip, neck femur and whole-body scans, were reported.
Measurement of uRBP
Urine samples were collected for dipstick analysis and measurement of uCr and uRBP uCr and uRBP were assessed at the Makerere University–Johns Hopkins University Core Laboratory and at the Translational Laboratory, respectively, both located at IDI. uCr was measured using Roche Diagnostics clinical biochemistry instruments (COBAS C311 analyzer). uRBP was measured on spot urine samples using an enzyme immunoassay kit (Arbor Assays) with a limit of detection of 4.09 ng/mL. The uRBP values were corrected for uCr; uRBP/uCr normality ranges were provided by the kit manufacturer (<130 μg/g in patients aged <50 years and <172 μg/g in those aged ≥50 years).
Calcaneal QUS
All study participants underwent calcaneal QUS performed with the Sahara Hologic device, which consists of 2 transducers, one acting as a transmitter and the other as a receiver. The transducers are acoustically coupled to the heel using rubber pads and oil-based coupling gel [32]. The machine measures broadband ultrasound attenuation (in decibels per megahertz) and speed of sound (meters per second) at a fixed region of interest in the midcalcaneus of the nondominant foot. The device subsequently combines the broadband ultrasound attenuation and speed of sound results to provide the QUS index (QUI) or stiffness index, using the formula QUI = 0.41 (speed of sound) + 0.41 (broadband ultrasound attenuation) − 571, and the estimated BMD (eBMD, in grams per centimeter squared), using the formula eBMD = 0.0025926 (broadband ultrasound attenuation + speed of sound) − 73.687. In addition to broadband ultrasound attenuation, speed of sound, QUI, and eBMD, calcaneal QUS results were also expressed as the corresponding T and Z scores, which were calculated according to the manufacturers’ instructions.
Statistical Analysis
Descriptive statistics included medians with interquartile range (IQR) for continuous variables and numbers and percentages of patients for categorical variables. The association between calcaneal QUS parameters, as well as uRBP/uCr, with DXA measurements was determined using the Spearman correlation ρ coefficient (and P values). Receiver operating characteristic (ROC) curve analysis was used to assess the performance of the different calcaneal QUS parameters in the identification of patients with a DXA T score ≤1. This threshold was chosen for its effect in increasing the risk of osteporotic fractures in postmenopausal women [33].
Data were analyzed using SPSS software (version 22.0; IBM). The study sample size was determined according to the primary objective and based on the work by Clò et al [32]. To detect a correlation between calcaneal QUS and DXA results, ≥69 patients were needed to show the predicted association (r = 0.380), with a power of 90% and an α valueof 5%.
RESULTS
Clinical Characteristics of the Study Population
Of 109 screened patients, 101 were enrolled. The reasons for exclusion were diabetes (n = 5), kidney failure (n = 1), age <18 years (n = 1), and pregnancy (n = 1). All participants were black, and 52.5% were female (n = 53); the median (IQR) age and body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) were 37.9 years (31.1–41.0) years and 23 (20.5–25.9), respectively [Table 1]. More than three-quarters of the patients presented at an advanced WHO clinical stage (24.8% stage III and 57.4% stage IV). The median (IQR) nadir and current CD4 cell counts were 48 (12.3–139.3) and 468 (326.5–674.0) cells/μL. The median (IQR) ART duration was 12.2 (10.6–13.1) years, and 61% of patients were receiving TDF-based regimens. Eighty patients (79.2%) had an HIV RNA level <20 copies/mL. None of the patients had experienced a fragility fracture or had a family history of hip fracture. Five of 53 women (9.4%) were menopausal. No patient reported prolonged steroid use (defined as ≥5 mg prednisone or equivalent daily for ≥3 months).
Table 1.
Demographic, Anthropometric, Behavioral, Clinical, and Human Immunodeficiency Virus–Related Characteristics
| Parameter | Enrolled Patients, No. (%)a (n = 101) |
|---|---|
| Age, median (IQR), y | 37.9 (IQR 31.1–41.0) |
| Female sex | 53 (52.5) |
| BMI, median (IQR)b | 23.1 (20.3–25.9) |
| BMI ≤18.5b | 6 (5.9) |
| History of fracture | 9 (8.9) |
| History of fragility fracture | 0 (0) |
| Family history of hip fracture | 0 (0) |
| Current smoking | 2 (2) |
| Current alcohol use | 15 (14.9) |
| Previous long-term steroid use | 0 (0) |
| Findings in women, no. (% of female population) | |
| Menopausal status | 5 (9.4) |
| Hormonal contraceptive use | 13 (24.5) |
| DMPA use | 4 (7.5) |
| WHO clinical stage | |
| I | 1 (1) |
| II | 17 (16.8) |
| III | 25 (24.8) |
| IV | 58 (57.4) |
| CD4 cell count, median (IQR), cells/μL | |
| Nadir | 48 (12.3–139.3) |
| Current | 468 (326.5–674.0) |
| Current HIV load <20 copies/mL | 80 (79.2) |
| ART duration, median (IQR), y | 12.2 (10.6–13.1) |
| TDF treatment | |
| Current | 61 (61) |
| Exposure | 65 (65) |
| Duration, median (IQR), mo | 48.3 (32.8–86.8) |
| PI treatment | |
| Current | 44 (44) |
| Exposure | 44 (44) |
| Duration, median (IQR), mo | 65.7 (35.9–97.0) |
| Current serum creatinine, median (IQR), mg/dL | 0.7 (0.6–0.9) |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; DMPA, depot medroxyprogesterone acetate; HIV, human immunodeficiency virus; IQR, interquartile range; PI, protease inhibitor; TDF, tenofovir disoproxil fumarate; WHO, World Health Organization.
aData represent no. (%) of patients unless otherwise specified.
bBMI calculated as weight in kilograms divided by height in meters squared.
uRBP/uCr Values
The median (IQR) uRBP/uCr was 119.9 (80–216.3) μg/g, with elevated values in 47 patients (47%). The prevalence of subclinical tubular impairment was 51.2% among patients currently receiving TDF, 46.7% among those currently receiving a PI, and
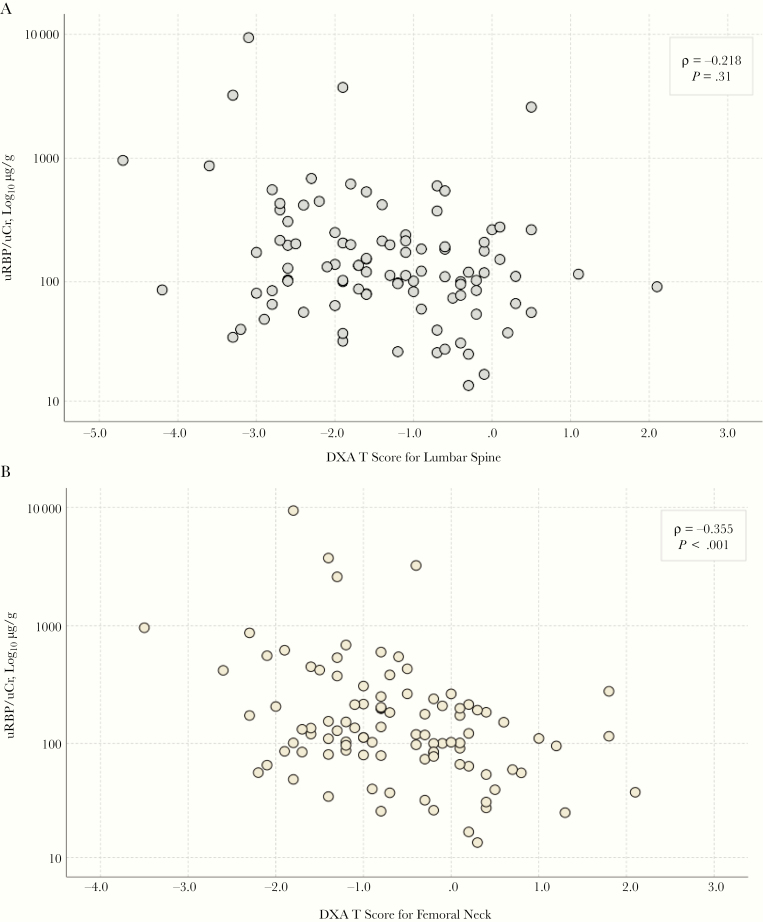
as high as 54.3% among those currently receiving a regimen that included both. In univariate analyses, male sex (P = .044), low BMI (P = .046), and longer TDF exposure (P = .002) were significantly associated with abnormal uRBP/uCr. In multivariate analyses, PI use (adjusted odds ratio [OR], 7.54; 95% confidence interval [CI], 1.74–32.76; P = .007) and years of TDF exposure (1.31; 1.03–1.68; P = .03) were independent predictors in TDF recipients; no factor was identified in participants not receiving TDF. We observed a significant inverse correlation between uRBP/uCr and DXA T scores (lumbar [ρ = −0.218; P = .031], femoral neck [ρ = −0.355; P < .001], and total hip [ρ = −0.303; P = .002]) [Figure 1]; an abnormal uRBP/uCr was associated with greater odds of having a lumbar T score below −1 (OR, 2.35; 95% CI, 1.01–5.35).
Figure 1.
Urinary retinol-binding protein–urinary creatinine ratio (uRBP/uCr) and dual-energy x-ray absorptiometry (DXA) T score correlation at the lumbar spine (A) and femoral neck (B) measurement site. In the statistical analysis, the association between uRBP/uCr and DXA measurements was determined using the Spearman ρ correlation coefficients (and P values).
uRBP/uCr was associated with femoral neck (ρ = −0.248; P = .013) and hip (ρ = −0.216; P = .040) but not lumbar (ρ = −0.167; P = .097) DXA-measured BMD. Linear regression analysis including age, BMI, and uRBP/uCr identified BMI as the only independent predictor of lumbar, femoral, and hip DXA-measured BMD.
Calcaneal QUS Versus DXA
The median (IQR) broadband ultrasound attenuation, speed of sound, QUI, and eBMD were 73.7 (62.7–88.9) dB/MHz, 1546.0 (1523.9–1573.3) m/s, 94.25 (78.33–109.83), and 0.514 (0 .419–0.608) g/cm2 (Table 2). The median (IQR) lumbar, femoral, and total hip T-scores were −1.4 (−2.3 to −0.4), −0.8 (−1.3 to 0.1), and −0.6 (−1.2 to 0.1). T scores <1 were observed in 61 participants (61.6%) for lumbar, 40 (40.4%) for femoral, and 34 (34.3%) for hip DXA, and values <2.5 in 22 (22.2%), 2 (2%), and 2 (2%), respectively.
Table 2.
Calcaneal Quantitative Ultrasonography and Dual-Energy X-ray Absorptiometry Results
| Result | Median Value (IQR) |
|---|---|
| Calcaneal QUS | |
| SOS, m/s | 1546.0 (1523.9–1573.3) |
| BUA, dB/MHz | 73.7 (62.7–88.9) |
| QUI | 94.25 (78.33–109.83) |
| eBM, g/cm2 | 0.514 (0.419–0.608) |
| T score | −0.9 (−1.7 to 0.8) |
| Z score | −0.5 (−1.4 to 0.4) |
| DXA | |
| Lumbar spine | |
| BMD, g/cm2 | 0.907 (0.823–1.004) |
| T score | −1.4 (−2.3 to −0.4) |
| Z score | −1.2 (−2.0 to −0.3) |
| Femoral neck | |
| BMD, g/cm2 | 0.797 (0.712–0.887) |
| T score | −0.8 (−1.3 to 0.1) |
| Z score | −0.4 (−1.1 to 0.3) |
| Total hip | |
| BMD, g/cm2 | 0.906 (0.824–0.992) |
| T score | −0.6 (−1.2 to 0.1) |
| Z score | −0.5 (−1.1 to 0.2) |
| Total body | |
| BMD, g/cm2 | 0.958 (0.915–1.045) |
| T score | −2 (−2.8 to −1.3) |
| Z score | −1.9 (−2.8 to −1.3) |
Abbreviations: BMD, bone mineral density; BUA, broadband ultrasound attenuation; DXA, dual-energy X-ray absorptiometry; eBMD, estimated BMD; IQR, interquartile range; QUI, quantitative ultrasonography index; QUS, quantitative ultrasonography; SOS, speed of sound.
The correlation between calcaneal QUS and DXA measurements was determined using the Spearman test (Table 3). Lumbar, femoral neck, total hip, and total body BMD measurements were significantly associated (all P < .01) with calcaneal speed of sound, broadband ultrasound attenuation, QUI, and eBMD, with the highest r coefficient values obtained when DXA-based BMDs were compared with broadband ultrasound attenuation (total hip BMD, r = 0.482; P < .001). Among the different regions of interest in DXA evaluation, total hip BMD measurements showed the strongest association with calcaneal QUS parameters (speed of sound, r = .413 P < .001; broadband ultrasound attenuation, r = 0.482; QUI, r = 409; eBMD, r = 0.443) (all P < .001). Significant but lower r coefficient values were obtained when QUS parameters were compared with DXA-assessed lumbar spine, femoral neck, and total body BMD measurements.
Table 3.
Correlation Between Dual-Energy X-ray Absorptiometric and Calcaneal Quantitative Ultrasonographic Parameters
| DXA Parameter | Correlation With DXA Parameter by QUS Parameter, Spearman ρ (P Value) | |||||
|---|---|---|---|---|---|---|
| SOS | BUA | QUI | eBMD | Calcaneal T Score | Calcaneal Z Score | |
| Lumbar spine | ||||||
| BMD | 0.281 (.005) | 0.360 (<.001) | 0.309 (.002) | 0.314 (.001) | 0.309 (.002) | 0.314 (.002) |
| T score | 0.267 (.008) | 0.342 (.001) | 0.290 (.004) | 0.301 (.003) | 0.290 (.004) | 0.275 (.008) |
| Z score | 0.231 (.02) | 0.319 (.001) | 0.257 (.01) | 0.266 (.008) | 0.261 (.01) | 0.263 (.01) |
| Femoral neck | ||||||
| BMD | 0.390 (<.001) | 0.461 (<.001) | 0.416 (<.001) | 0.417 (<.001) | 0.429 (<.001) | 0.434 (<.001) |
| T score | 0.335 (.001) | 0.421 (<.001) | 0.363 (<.001) | 0.370 (<.001) | 0.377 (<.001) | 0.344 (.001) |
| Z score | 0.318 (.001) | 0.426 (<.001) | 0.356 (<.001) | 0.357 (<.001) | 0.371 (<.001) | 0.379 (<.001) |
| Total hip | ||||||
| BMD | 0.413 (<.001) | 0.482 (<.001) | 0.409 (<.001) | 0.443 (<.001) | 0.452 (<.001) | 0.477 (<.001) |
| T score | 0.391 (<.001) | 0.482 (<.001) | 0.409 (<.001) | 0.431 (<.001) | 0.433 (<.001) | 0.414 (<.001) |
| Z score | 0.356 (<.001) | 0.460 (<.001) | 0.378 (<.001) | 0.396 (<.001) | 0.404 (<.001) | 0.409 (<.001) |
| Total body | ||||||
| BMD | 0.336 (.001) | 0.380 (<.001) | 0.369 (<.001) | 0.356 (<.001) | 0.368 (<.001) | 0.442 (<.001) |
| T score | 0.346 (.001) | 0.401 (<.001) | 0.382 (<.001) | 0.375 (<.001) | 0.379 (<.001) | 0.404 (<.001) |
| Z score | 0.307 (.002) | 0.380 (<.001) | 0.345 (<.001) | 0.339 (.001) | 0.347 (.001) | 0.398 (<.001) |
Abbreviations: BMD, bone mineral density; BUA, broadband ultrasound attenuation; DXA, dual-energy x-ray absorptiometry; eBMD, estimated BMD; QUI, quantitative ultrasonography index; QUS, quantitative ultrasonography; SOS, speed of sound.
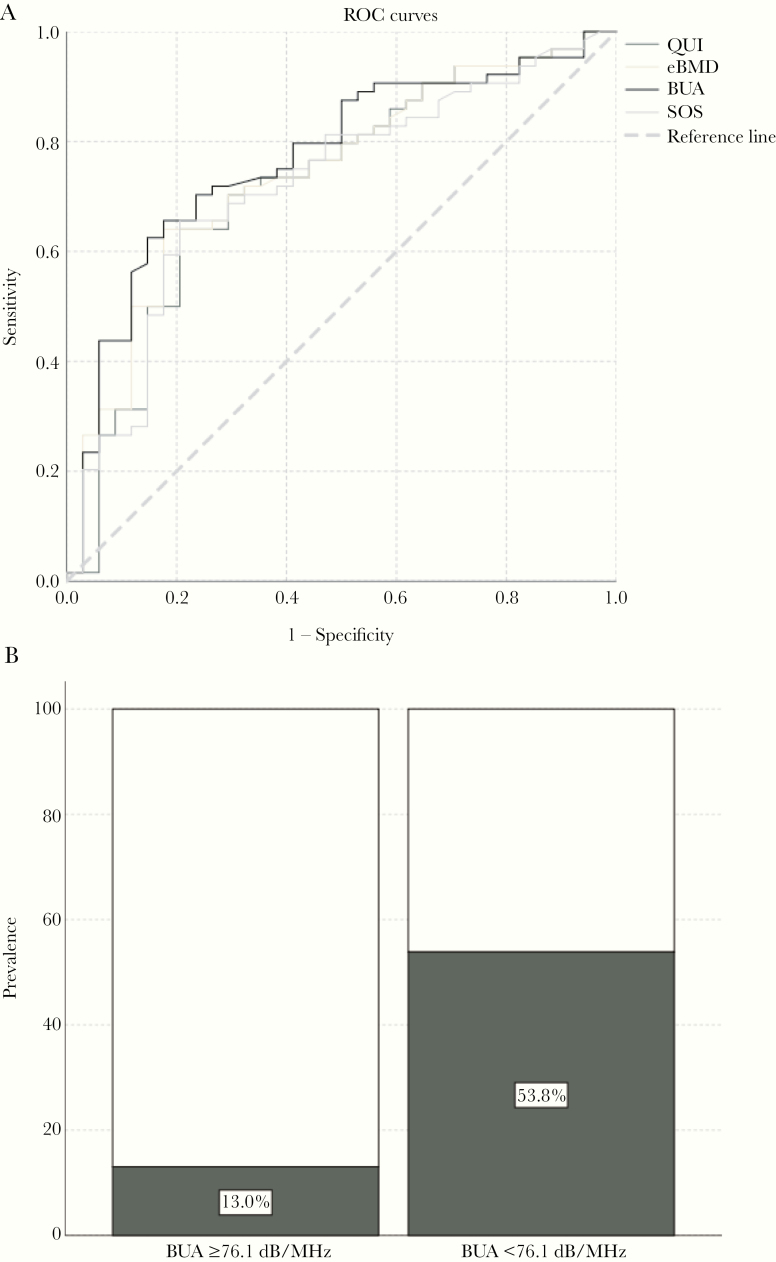
ROC curves identified a broadband ultrasound attenuation <76.1 dB/MHz as a moderately correlated predictor of DXA T scores below −1 (area under ROC, 0.64–0.77; Figure 2). Supplementary Figure 1 plots a Bland-Altman analysis showing the correlation between the difference between calcaneal ultrasound eBMD and DXA measured BMDs. A broadband ultrasound attenuation <76.1 dB/MHz was associated with osteopenia at all studied sites: the best association was observed for total hip score (OR, 7.78 [95% CI, 2.8–21.5]; sensitivity, 82.4%; specificity, 86.9%; P < .001).
Figure 2.
Association between calcaneal quantitative ultrasonographic (QUS) measures and dual-energy x-ray absorptiometric (DXA)–measured T scores. A, Receiver operating characteristic (ROC) curves for calcaneal QUS parameters from patients with DXA-evaluated hip T score below −1 or −1 or above, B, Prevalence of DXA-based hip T score below −1, according to BUA values. Area under the ROC values were 0.723 for the QUS index (QUI), 0.741 for estimated bone mineral density (eBMD), 0.769 for broadband ultrasound attenuation (BUA), and 0.720 for the speed of sound (SOS).
DISCUSSION
In a population of PWH receiving long-term ART in Uganda we observed a high prevalence of subclinical tubular impairment and an association of this abnormality with low BMD; we also report the potential use of calcaneal QUS in screening patients at risk of osteopenia. Few studies have examined the ability of QUS in predicting fragility fractures in PWH, and most of them have been conducted in developed countries [32, 34, 35]. In particular, the ability of calcaneal QUS to accurately screen for bone disease in persons with HIV infection has been investigated by Clò et al [32] in a cross-sectional Italian study carried out in a population of 224 HIV-infected patients; in that study, calcaneal QUS was compared with DXA, the reference standard for diagnosis of osteoporosis or osteopenia, and the results showed a moderate correlation of speed of sound, broadband ultrasound attenuation, and QUI with DXA parameters, especially with total-body BMD (r = of 0.38, 0.4, and 0.42, respectively).
The current study investigated the correlation between the 2 methods (calcaneal QUS and DXA) in a low-resource setting among HIV-infected individuals receiving long-term ART. Interestingly, we described a similar grade of association among QUS parameters (speed of sound, broadband ultrasound attenuation, eBMD, and QUI) and DXA BMD values measured at all regions of interest (lumbar spine, femur neck, total hip, and total body). In our study, this correlation was more consistent when the QUS parameters were compared with total hip BMDs. Among the different QUS parameters, broadband ultrasound attenuation obtained the highest ρ coefficient compared with DXA-assessed lumbar, femoral (femoral neck and total hip), and total body BMD measurements. A fair sensitivity and specificity in detecting osteopenia (DXA T score below −1) were obtained using broadband ultrasound attenuation measurements with a cutoff of 76.1 dB/MHz; nevertheless, we were not able to identify among calcaneal QUS parameters a good predictor of osteoporosis (defined as DXA T score −2.5 or below, according to WHO criteria).
To our knowledge, no other study has analyzed the correlation between QUS and DXA in PWH in a low-resource country, and only a limited number of studies have investigated the role of QUS in HIV populations in such a setting [20, 28]. In particular, Cournil et al [20] reported the use of calcaneal QUS in PWH in Senegal, observing a reduced BMD in HIV-infected individuals compared with healthy controls; although no therapeutic risk factor was identified, low BMI was relevant in predicting reduced BMD. Another study [28] focused on a population of ART-experienced, TDF-naive PWH from Senegal, Cameroon, and Burkina Faso, in whom first-line ART failed; that study evaluated changes in calcaneal QUS-assessed bone quality 96 weeks after patients were switched to either tenofovir dIsoproxil fumarate/emtricitabine/lopinavir/ritomavir, tenofovir disoproxil fumarate/emtricitabine/darunavir/ritonavir or abacavir/didanosine/lopinavir/ritonavir, showing a reduction in bone quality after second-line ART initiation, independent of TDF exposure. Owing to differences in study design, our results cannot be compared with those findings apart from calcaneal QUS baseline measurements: our population showed a slightly lower median (IQR) QUI (94.25 [78.33–109.83]) than reported by Kabore et al [28] (101 [87–116]).
Our study showed a good level of association among calcaneal QUS and DXA parameters, confirming the potential role of calcaneal QUS as a tool that, thanks to its affordability and portability, can be used for assessing bone density in HIV-infected patients, especially in resource-limited settings where DXA is not easily available. Even if this is beyond the aim of the current study, assessing the use of calcaneal QUS in aging PWH may better define thresholds for selecting patients in need of a bone health assessment.
Almost half the study population showed subclinical tubular impairment. It must be pointed out that patients with chronic kidney failure or diabetes were not included in the study, to avoid potential confounders. Data on the occurrence of tubular disorders in HIV-infected African populations are limited; in particular, only a few studies have been reported on TDF-related renal adverse events [36]. In the current study, we found an association among longer TDF treatment duration and tubular damage; this association was confirmed with multivariate analysis. The high prevalence of abnormal uRBP/uCr reported in our population suggests the usefulness of implementing access to kidney-sparing antiretroviral drugs, in particular, tenofovir alafenamide (TAF). Indeed, in North America and Western Europe, an alternative formulation of TAF is currently provided, with a reduced potential for bone and renal toxicity; this is not available in resource-limited settings, but there is debate on the harmfulness of TDF when administered with unboosted antiretrovirals [37].
Data on long-term renal safety (based on estimated creatinine clearance values) in limited-resource settings, however, are reassuring [38, 39]. In our cohort, we observed a detrimental effect of TDF/PI coadministration; the reduced screening capacity in low-and middle-income countries prompts a change toward safer regimens that do not require close monitoring. In this context, the switch from TDF to TAF in unboosted regimens was associated with a decrease in renal markers of tubular dysfunction [40]. In this setting, dual TDF-sparing regimens could provide effective and safe options that need to be tested in resource-limited settings with a high burden of resistance-associated mutations [41, 42]. The association between higher uRBP/uCr and reduced BMD confirms the potential link between subclinical tubular toxicity and bone health [18]; a 2019 report confirmed this association but identified BMI as a key risk factor attenuating the strength of the potential link between subclinical tubular toxicity and bone health [19].
The ongoing discussion on the best “global” treatment of HIV is also beneficial in evaluating our results. The ADVANCE trial reported the noninferiority of dolutegravir-based regimens (with either TDF or TAF) compared with efavirenz-based regimens [43]. Despite a slightly more favorable bone and renal profile (in terms of estimated creatinine clearance and the incidence of new hip osteopenia), TAF was associated with weight gain and, potentially, incident obesity. Given the complex interaction between BMI and bone health, it is unclear whether TAF would be the safest option in limited-resource settings.
However, several limitations must be noted. First, the majority of the study participants were premenopausal women and men <50 years old, in whom Z scores are preferred over T scores for BMD assessment conforming to WHO diagnostic criteria [44, 45]. Second, it is essential to mention that, according to the International Society for Clinical Densitometry, heel QUS, in conjunction with clinical risk factors and when DXA devices are not usable, can be used to identify individuals at very low fracture risk, in whom no further diagnostic evaluation may be necessary, or those at sufficiently high risk to warrant the initiation of pharmacological treatment; however, the International Society for Clinical Densitometry officially validates the use of calcaneal QUS for predicting fragility fracture only in postmenopausal women and men >65 years old, who were not numerous in our study population [24].
Furthermore, it is important to emphasize that the present study, like the majority of the studies conducted in low- and middle-income countries on bone health and HIV, has a cross-sectional design. An additional preanalytic limitation is the lack of uRBP standardization in the Ugandan population, thus potentially leading to an overestimation of subclinical impairment. Longitudinal data on the incidence of osteoporotic fractures in the study population could add some evidence on the predictive value of calcaneal QUS parameters and DXA-measured BMD in predicting the risk of fragility fracture [35, 45, 46].
In conclusion, calcaneal QUS results showed a moderate correlation with DXA outputs; a reasonable sensitivity and specificity for osteopenia in this group of relatively young HIV-infected participants support its use as a screening tool in resource-limited settings. However, further studies are needed to identify the most suitable variable among calcaneal QUS parameters, together with a proper cutoff, which could help identify osteoporotic patients with a higher sensitivity. As for the uRBP/uCr analysis, a high prevalence of subclinical tubular impairment was found in our Ugandan cohort of PWH receiving long-term ART. These data suggest the need for a long-term follow-up of renal and bone health in PWH in resource-limited settings, where patients are progressively aging and facing an increase in noncommunicable diseases.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: Conference on Retroviruses and Opportunistic Infections, Seattle, Washington, 4–7 March 2019. Abstract 695.
Acknowledgments. The authors thank all of the study participants; IDI staff members for their administrative support and their contributions to patient enrollment and data collection; and the Makerere University–Johns Hopkins University Core Laboratory and Translational Laboratory staff for their support in urine sample management.
Disclaimer. The funding sources had no involvement in the study design, the collection, analysis, and interpretation of data, or the writing of the report.
Financial support. This work was supported by the Unit of Infectious Diseases, Department of Medical Sciences, University of Torino, and by the University of Alabama at Birmingham Center For AIDS Research, a National Institute of Health–funded program (grant P30 AI027767) made possible by the following institutes: National Institute of Allergy and Infectious Diseases, National Cancer Institute, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Heart, Lung, and Blood Institute, National Institute on Drug Abuse, National Institute on Aging, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of General Medical Sciences, and National Institute on Minority Health and Health Disparities.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Borderi M, Gibellini D, Vescini F, et al. Metabolic bone disease in HIV infection. AIDS 2009; 23:1297–310. [DOI] [PubMed] [Google Scholar]
- 2. Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS 2006; 20:2165–74. [DOI] [PubMed] [Google Scholar]
- 3. McComsey GA, Tebas P, Shane E, et al. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis 2010; 51:937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lapadula G, Chatenoud L, Gori A, et al. ; Italian MASTER Cohort Risk of severe non AIDS events is increased among patients unable to increase their CD4+ T-cell counts >200+/μl despite effective HAART. PLoS One 2015; 10:e0124741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stellbrink HJ, Orkin C, Arribas JR, et al. ; ASSERT Study Group Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis 2010; 51:963–72. [DOI] [PubMed] [Google Scholar]
- 6. McComsey GA, Kitch D, Daar ES, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: AIDS Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis 2011; 203:1791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haskelberg H, Mallon PW, Hoy J, et al. Bone mineral density over 96 weeks in adults failing first-line therapy randomized to raltegravir/lopinavir/ritonavir compared with standard second-line therapy. J Acquir Immune Defic Syndr 2014; 67:161–8. [DOI] [PubMed] [Google Scholar]
- 8. Duvivier C, Kolta S, Assoumou L, et al. ANRS 121 Hippocampe study group Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS 2009; 23:817–24. [DOI] [PubMed] [Google Scholar]
- 9. Brown TT, Moser C, Currier JS, et al. Changes in bone mineral density after initiation of antiretroviral treatment with tenofovir disoproxil fumarate/emtricitabine plus atazanavir/ritonavir, darunavir/ritonavir, or raltegravir. J Infect Dis 2015; 212:1241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mondy K, Yarasheski K, Powderly WG, et al. Longitudinal evolution of bone mineral density and bone markers in human immunodeficiency virus-infected individuals. Clin Infect Dis 2003; 36:482–90. [DOI] [PubMed] [Google Scholar]
- 11. Nolan D, Upton R, McKinnon E, et al. Stable or increasing bone mineral density in HIV-infected patients treated with nelfinavir or indinavir. AIDS 2001; 15:1275–80. [DOI] [PubMed] [Google Scholar]
- 12. Labarga P, Barreiro P, Martin-Carbonero L, et al. Kidney tubular abnormalities in the absence of impaired glomerular function in HIV patients treated with tenofovir. AIDS 2009; 23:689–96. [DOI] [PubMed] [Google Scholar]
- 13. Nelson MR, Katlama C, Montaner JS, et al. The safety of tenofovir disoproxil fumarate for the treatment of HIV infection in adults: the first 4 years. AIDS 2007; 21:1273–81. [DOI] [PubMed] [Google Scholar]
- 14. Calcagno A, Cusato J, Marinaro L, et al. Clinical pharmacology of tenofovir clearance: a pharmacokinetic/pharmacogenetic study on plasma and urines. Pharmacogenomics J 2016; 16:514–8. [DOI] [PubMed] [Google Scholar]
- 15. Hall AM, Edwards SG, Lapsley M, et al. Subclinical tubular injury in HIV-infected individuals on antiretroviral therapy: a cross-sectional analysis. Am J Kidney Dis 2009; 54:1034–42. [DOI] [PubMed] [Google Scholar]
- 16. Post FA, Moyle GJ, Stellbrink HJ, et al. Randomized comparison of renal effects, efficacy, and safety with once-daily abacavir/lamivudine versus tenofovir/emtricitabine, administered with efavirenz, in antiretroviral-naive, HIV-1-infected adults: 48-week results from the ASSERT study. J Acquir Immune Defic Syndr 2010; 55:49–57. [DOI] [PubMed] [Google Scholar]
- 17. Calcagno A, Cusato J, Marinaro L, et al. Tenofovir clearance is reduced in HIV-positive patients with subclinical tubular impairment. AIDS 2016; 30:915–20. [DOI] [PubMed] [Google Scholar]
- 18. Hamzah L, Samarawickrama A, Campbell L, et al. Effects of renal tubular dysfunction on bone in tenofovir-exposed HIV-positive patients. AIDS 2015; 29:1785–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alvarez-Barco E, Campbell L, Burling K, et al. Impact of renal tubular function on bone mineral density in older people with HIV [abstract 689]. In: Abstract eBook of the Conference on Retroviruses and Opportunistic Infections (CROI), Boston, March 4–7, 2019. Available at: https://user-degqumh.cld.bz/croi2019-abstract-ebook/266/. Accessed 15 March 2020. [Google Scholar]
- 20. Cournil A, Eymard-Duvernay S, Diouf A, et al. ANRS 1215 Study Group Reduced quantitative ultrasound bone mineral density in HIV-infected patients on antiretroviral therapy in Senegal. PLoS One 2012; 7:e31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matovu FK, Wattanachanya L, Beksinska M, Pettifor JM, Ruxrungtham K. Bone health and HIV in resource-limited settings: a scoping review. Curr Opin HIV AIDS 2016; 11:306–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fuleihan GEH, Adib G, Nauroy L; International Osteoporosis Foundation The Middle East & Africa Regional Audit: epidemiology, costs and burden of osteoporosis in 2011. 2011. Available at: https://www.iofbonehealth.org/sites/default/files/PDFs/Audit%20Middle%20East_Africa/Middle_East_Africa_audit.pdf. Accessed 15 March 2020. [Google Scholar]
- 23. Kanis JA, McCloskey E, Johansson H, Oden A, Leslie WD. FRAX(®) with and without bone mineral density. Calcif Tissue Int 2012; 90:1–13. [DOI] [PubMed] [Google Scholar]
- 24. International Society for Clinical Densitometry. 2019 ISCD official positions—adult Available at: https://www.iscd.org/official-positions/2019-iscd-official-positions-adult/. Accessed 12 August 2019.
- 25. Khaw KT, Reeve J, Luben R, et al. Prediction of total and hip fracture risk in men and women by quantitative ultrasound of the calcaneus: EPIC-Norfolk prospective population study. Lancet 2004; 363:197–202. [DOI] [PubMed] [Google Scholar]
- 26. Hans D, Dargent-Molina P, Schott AM, et al. Ultrasonographic heel measurements to predict hip fracture in elderly women: the EPIDOS prospective study. Lancet 1996; 348:511–4. [DOI] [PubMed] [Google Scholar]
- 27. Bauer DC, Glüer CC, Cauley JA, et al. Study of Osteoporotic Fractures Research Group. Broadband ultrasound attenuation predicts fractures strongly and independently of densitometry in older women: a prospective study. Arch Intern Med 1997; 157:629–34. [PubMed] [Google Scholar]
- 28. Kabore FN, Eymard-Duvernay S, Zoungrana J, et al. TDF and quantitative ultrasound bone quality in African patients on second line ART, ANRS 12169 2LADY sub-study. PLoS One 2017; 12:e0186686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martin A, Moore C, Mallon PW, et al. Second Line study team Bone mineral density in HIV participants randomized to raltegravir and lopinavir/ritonavir compared with standard second line therapy. AIDS 2013; 27:2403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2nd ed. 2016 Available at: http://apps.who.int/iris/bitstream/handle/10665/208825/9789241549684_eng.pdf?sequence=1. Accessed 12 August 2019. [PubMed]
- 31. Weissberg D, Mubiru F, Kambugu A, et al. Ten years of antiretroviral therapy: Incidences, patterns and risk factors of opportunistic infections in an urban Ugandan cohort. PLoS One 2018; 13:e0206796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clò A, Gibellini D, Damiano D, et al. Calcaneal quantitative ultrasound (QUS) and dual X-ray absorptiometry (DXA) bone analysis in adult HIV-positive patients. New Microbiol 2015; 38:345–56. [PubMed] [Google Scholar]
- 33. Kanis JA, Johnell O, Oden A, Dawson A, De Laet C, Jonsson B. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int 2001; 12:989–95. [DOI] [PubMed] [Google Scholar]
- 34. Mora S, Viganò A, Cafarelli L, et al. Applicability of quantitative ultrasonography of the radius and tibia in HIV-infected children and adolescents. J Acquir Immune Defic Syndr 2009; 51:588–92. [DOI] [PubMed] [Google Scholar]
- 35. Quiros Roldan E, Brianese N, Raffetti E, et al. Comparison between the gold standard DXA with calcaneal quantitative ultrasound based-strategy (QUS) to detect osteoporosis in an HIV infected cohort. Braz J Infect Dis 2017; 21:581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Villa G, Phillips RO, Smith C, et al. Renal health after long-term exposure to tenofovir disoproxil fumarate (TDF) in HIV/HBV positive adults in Ghana. J Infect 2018; 76:515–21. [DOI] [PubMed] [Google Scholar]
- 37. Hill A, Hughes SL, Gotham D, Pozniak AL. Tenofovir alafenamide versus tenofovir disoproxil fumarate: is there a true difference in efficacy and safety? J Virus Erad 2018; 4:72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. De Waal R, Cohen K, Fox MP, et al. Changes in estimated glomerular filtration rate over time in South African HIV-1-infected patients receiving tenofovir: a retrospective cohort study. J Int AIDS Soc 2017; 20:21317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mulenga L, Musonda P, Mwango A, et al. IeDEA-Southern Africa Effect of baseline renal function on tenofovir-containing antiretroviral therapy outcomes in Zambia. Clin Infect Dis 2014; 58:1473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gupta SK, Post FA, Arribas JR, et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. AIDS 2019; 33:1455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cahn P, Madero JS, Arribas JR, et al. GEMINI Study Team Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet 2019; 393:143–55. [DOI] [PubMed] [Google Scholar]
- 42. Boyd MA, Kumarasamy N, Moore CL, et al. SECOND-LINE Study Group Ritonavir-boosted lopinavir plus nucleoside or nucleotide reverse transcriptase inhibitors versus ritonavir-boosted lopinavir plus raltegravir for treatment of HIV-1 infection in adults with virological failure of a standard first-line ART regimen (SECOND-LINE): a randomised, open-label, non-inferiority study. Lancet 2013; 381:2091–9. [DOI] [PubMed] [Google Scholar]
- 43. Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med 2019; 381:803–15. [DOI] [PubMed] [Google Scholar]
- 44. Focà E, Magro P, Guaraldi G, et al. GEPPO (GEriatric Patients living with HIV/AIDS: a Prospective Multidimensional cOhort) Study Group Elderly HIV-positive women: a gender-based analysis from the Multicenter Italian “GEPPO” Cohort. PLoS One 2019; 14:e0222225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ensrud KE, Crandall CJ. Osteoporosis. Ann Intern Med 2017; 167:ITC17–ITC32. [DOI] [PubMed] [Google Scholar]
- 46. Porcelli T, Gotti D, Cristiano A, et al. Role of bone mineral density in predicting morphometric vertebral fractures in patients with HIV infection. Osteoporos Int 2014; 25:2263–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.