Abstract
Background
It is unclear whether human immunodeficiency virus (HIV) infection results in permanent loss of T-cell memory or if it affects preexisting antibodies to childhood vaccinations or infections.
Methods
We conducted a matched cohort study involving 50 pairs of HIV-infected and HIV-uninfected women. Total memory T-cell responses were measured after anti-CD3 or vaccinia virus (VV) stimulation to measure T cells elicited after childhood smallpox vaccination. VV-specific antibodies were measured by means of enzyme-linked immunosorbent assay (ELISA).
Results
There was no difference between HIV-infected and HIV-uninfected study participants in terms of CD4+ T-cell responses after anti-CD3 stimulation (P = .19) although HIV-infected participants had significantly higher CD8+ T-cell responses (P = .03). In contrast, there was a significant loss in VV-specific CD4+ T-cell memory among HIV-infected participants (P = .04) whereas antiviral CD8+ T-cell memory remained intact (P > .99). VV-specific antibodies were maintained indefinitely among HIV-uninfected participants (half-life, infinity; 95% confidence interval, 309 years to infinity) but declined rapidly among HIV-infected participants (half-life; 39 years; 24–108 years; P = .001).
Conclusions
Despite antiretroviral therapy–associated improvement in CD4+ T-cell counts (nadir, <200/μL; >350/μL after antiretroviral therapy), antigen-specific CD4+ T-cell memory to vaccinations or infections that occurred before HIV infection did not recover after immune reconstitution, and a previously unrealized decline in preexisting antibody responses was observed.
Keywords: HIV, ART, antiretroviral therapy, smallpox, vaccination, immunological memory
Despite successful immune reconstitution after antiretroviral therapy, virus-specific CD4+ T-cell memory and antiviral antibody responses after childhood smallpox vaccination were found to be preferentially lost among women with human immunodeficiency virus (HIV) infection compared with matched HIV-uninfected controls.
(See the Editorial Commentary by Nilsson and Chiodi, on pages 176–9.)
Suppression of human immunodeficiency virus (HIV) replication using active antiretroviral therapy (ART) has allowed for restoration of immune function and many opportunistic infections, once common are now rare [1]. Despite these advancements, HIV-infected individuals continue to suffer from a 4-fold greater incidence of varicella zoster [2] and other virus-associated ailments [3], leading to the possibility that immune function may still not be fully optimal. Although ART improves many aspects of immunological function [4], there is continuing debate regarding its ability to restore T-cell memory to vaccines or infections that were encountered before HIV infection. Findings of some studies indicate that preexisting antigen-specific T-cell memory is either lost [5–7] or restored [5, 8–10] after ART-associated immune reconstitution. Furthermore, little is known about the durability of preexisting serum antibody responses after HIV/ART [11, 12]. Because HIV-infected individuals often demonstrate immunological characteristics that are more commonly associated with an aging immune system [13], this raises questions regarding whether HIV infection exacerbates immune senescence in part by decreasing protective immunological memory to vaccinations or infections that occurred in the distant past.
Vaccinia virus (VV; used during smallpox vaccination) represents an ideal antigen for determining the duration of immunity in the absence of reinfection, given that (1) the last case of smallpox in the United States occurred in 1949 [14, 15], (2) routine civilian smallpox vaccination was discontinued in 1972 [16], and (3) there are no cross-reactive orthopoxviruses in the United States that commonly infect humans. Moreover, VV has the added advantage of inducing strong antiviral CD4+ and CD8+ T-cell memory and readily infects primary monocytes [17], antigen-presenting cells found among peripheral blood mononuclear cells (PBMCs) that can present virus-specific peptides to both CD4+ and CD8+ T cells, and thus allows direct quantitation of virus-specific memory T cells. In the current study, we measured immune responses after smallpox vaccination as a well-characterized and robust model to determine the persistence of virus-specific T-cell memory and antibody responses after childhood vaccination among HIV-uninfected and HIV-infected women who underwent successful immune reconstitution after ART.
METHODS
Study Participants and Study Design
This was an observational 1-to-1 matched cohort study involving 50 pairs of HIV-infected study participants and HIV-uninfected case controls (100 participants total) enrolled in the Women’s Interagency HIV Study (WIHS) (Table 1). The WIHS enrolled 4137 women, of whom 3067 (74.1%) were HIV-infected and 1071 (25.9%) were HIV-uninfected at study entry. Enrollees are followed up every 6 months for interviews, physical examinations, and specimen acquisition [18]. Study protocols were approved by the individual institutional review boards, and informed consent was obtained from all participants (see Supplementary Methods).
Table 1.
Demographics and Clinical Characteristics of Women’s Interagency HIV Study Cohort Participants
| Characteristic | HIV-Infected Participants (n = 50) | HIV-Uninfected Participants (n = 50) |
|---|---|---|
| Age at enrollment, ya | ||
| Mean (SD) | 38 (6) | 38 (6) |
| Median (SD) | 38 (6) | 37 (6) |
| Race, no. (%) | ||
| African American | 28 (56) | 28 (56) |
| White | 15 (30) | 15 (30) |
| Hispanic | 7 (14) | 7 (14) |
| Nadir CD4+ T-cell count, mean (SD) | 88 (67) | NA |
| Duration of ART, yb | ||
| Mean (SD) | 6.4 (3.1) | NA |
| Median (SD) | 5.3 (3.1) | NA |
| T cells in PBMCs, mean (SD), % | ||
| CD4+ | 18 (7) | 35 (10) |
| CD8+ | 27 (8) | 15 (5) |
| VV antibody titer at baseline, mean (SD), VV-specific ELISA units | 2938 (4022) | 2943 (3423) |
| HBV status at baseline, no. (%) | ||
| Active or resolved infection | 10 (20) | 8 (16) |
| Negative | 39 (78) | 42 (84) |
| Unknown | 1 (2) | 0 (0) |
| HCV status at baseline, no. (%) | ||
| Active or resolved infection | 17 (34) | 17 (34) |
| Negative | 33 (66) | 33 (66) |
| HIV exposure category, no. (%) | ||
| Heterosexual risk | 29 (58) | 15 (30) |
| Intravenous drug use | 13 (26) | 15 (30) |
| No identified risk | 7 (14) | 17 (34) |
| Transfusion risk | 0 (0) | 3 (6) |
| Unknown | 1 (2) | 0 (0) |
Abbreviations: ART, antiretroviral therapy; ELISA, enzyme-linked immunosorbent assay; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; NA, not applicable; PBMCs, peripheral blood mononuclear cells; SD, standard deviation; VV, vaccinia virus.
aAt the time of T-cell assay, both the mean and median age (SD) was 45 (6) years for HIV-infected and 47 (6) years for HIV-uninfected participants.
bThe mean and median durations of ART were calculated with reference to the number of years before the time point when PBMCs were obtained to perform the T-cell assays.
Inclusion criteria for HIV-infected women included selection of individuals who were born before 1971 and were seropositive at baseline against VV (>200 enzyme-linked immunosorbent assay [ELISA] units), had started ART ≤5 years after joining the study, and had a CD4+ T-cell count nadir <200/μL that improved to >350/ μL after administration of ART. Elite controllers and seroconverters with primary HIV infection were excluded from this study to focus immunological analysis on HIV-infected participants with chronic HIV infection in which immune reconstitution was achieved by ART. Controls were matched based on age (±3 years) at the time point when T-cell responses were measured (±5.5 years at time of enrollment), race, and hepatitis C virus antibody status, and they were seropositive for VV at baseline. Antibody decay rates were based on longitudinal serum samples (9 samples per participant in each group on average) that spanned 10–21 years of time, except in 1 HIV-infected participant who was later found to have samples spanning only a 6-year period before de-enrolling from the study (median, 17.4 years of coverage for HIV-infected participants and 17.8 years for HIV-uninfected participants). Participants achieved the target CD4+ T-cell count of >350/μL by 5.6 years on average after entering the study (range, 1.6–14 years).
Memory T-Cell and Antibody Measurements
Cryopreserved PBMCs were cultured with or without an optimized amount of sucrose-purified live VV (multiplicity of infection, 0.3) or with anti-CD3 (clone HIT3a, 0.05 µg/mL) as described elsewhere [19] (Supplementary Methods). VV readily infects monocytes [20] but typically does not infect primary T cells [21] (data not shown). A detection threshold of ≥20 VV-specific interferon (IFN) γ + tumor necrosis factor (TNF) α + T cells per 106 T cells provides ≥95% sensitivity and ≥95% specificity [19, 22]. Serum antibodies were analyzed for all 50 pairs of samples and viable PBMCs suitable for flow cytometry analysis were recovered from 41 of 50 pairs. VV-specific ELISAs were performed using an optimized concentration of VV-infected cell lysate inactivated with 3% hydrogen peroxide, as described elsewhere [19, 23] (Supplementary Methods).
Statistical Analysis
Group sample sizes of 50 and 50 achieved 80% power to detect a 4-fold difference in virus-specific CD8+ T-cell responses (based on preliminary data that control group mean is 8.530 with standard deviation [SD] of 3.511 in log base 2 scale) and >80% power to identify a difference of ≥4-fold in virus-specific CD4+ T cells (based on preliminary data that control group mean is 7.824 with an SD of 3.001 in log base 2 scale) and antibody levels (based on preliminary data that control group mean is 8.858 with an SD of 2.053 in log base 2 scale). For antibody levels, this is a conservative approach for sample size estimation, because the actual study design was a longitudinal study for antibody responses with 8–10 time points per participant, and a mixed-effects model will be used to analyze the data. The sample size calculations and power analysis was performed using PASS 2008 software (NCSS, LLC). T-cell responses to anti-CD3 stimulation were compared using the nonparametric Wilcoxon signed rank test. The proportions of participants with virus-specific CD4+ and CD8+ T-cell responses were compared using the exact McNemar test.
Longitudinal ELISA data were censored [23] to remove data that dropped below the limits of detection and were deemed equivocal (<200 ELISA units). Acute immune responses resulting in serospikes (ie, doubling of ELISA titers between 2 contiguous points) occurred among 4 of 100 study participants, resulting in an incidence rate of 0.23 events per 100 person-years. Serospikes and data from the next 3 years were removed from the analysis so that rapid decay rates that typically occur after an immunogenic event would not influence the estimated long-term decay rate. Two HIV-infected patients had <3 valid data points owing to antibody titers below the limits of detection, and they and their matched HIV-uninfected patients were not included in the mixed-effects model for comparisons between groups. Rates of antibody decay were estimated using log-transformed data and a longitudinal mixed-effects model. Half-life estimates were obtained by transforming the decay rate and the boundaries of the 95% confidence interval (CI) obtained from the fixed-effects slope component of the model. Analysis was performed with R3.4.4 and SAS9.4 (SAS Institute) software (Supplementary Methods).
RESULTS
Quantitation of Memory T Cells
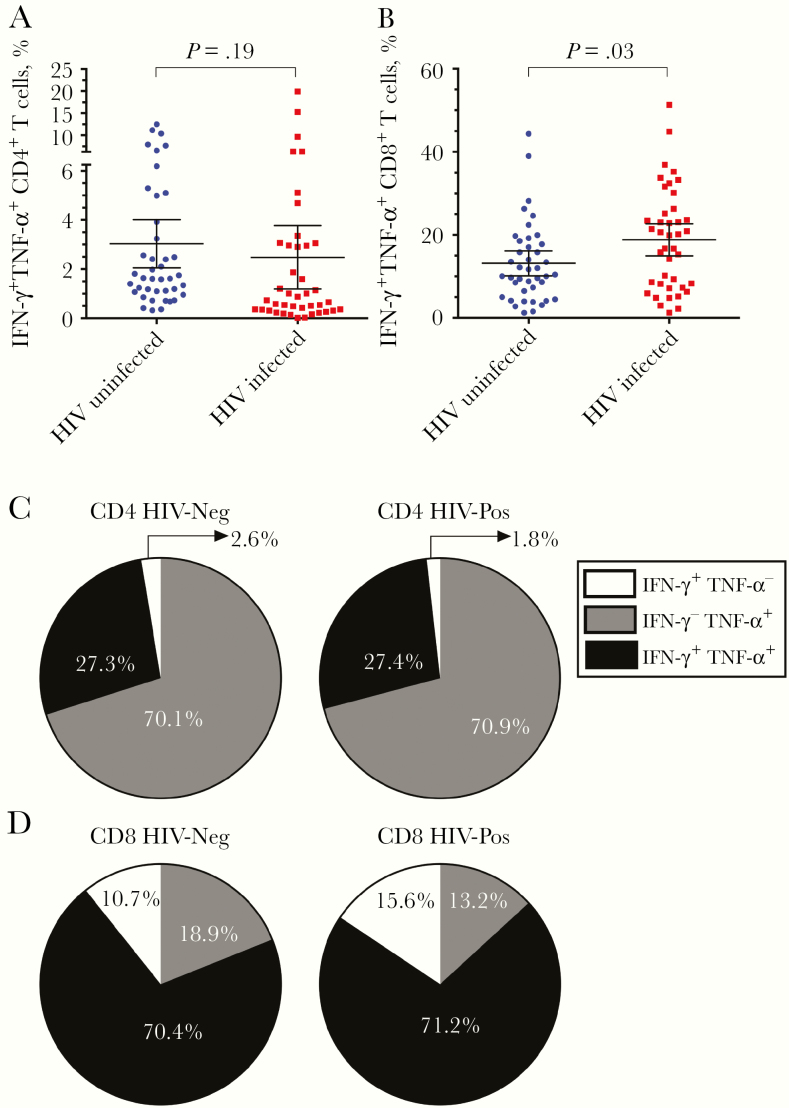
We used intracellular cytokine staining analysis to measure the frequencies of functional CD4+ and CD8+ T cells among HIV-infected participants at a single time point after immune reconstitution (mean [SD], 6.4 [3.1] years after ART) in comparison with HIV-uninfected case controls after direct ex vivo stimulation with anti-CD3 (Figure 1 and Table 1). This approach for T-cell stimulation preferentially activates memory T cells to produce inflammatory cytokines, including IFN-γ and TNF-α, regardless of their antigen specificity. We found that 2.5% (95% CI, 1.2%–3.7%) of CD4+ T cells from HIV-infected participants produced both IFN-γ and TNF-α after stimulation and that this did not differ significantly from the results in HIV-uninfected controls (3.0% IFN-γ +TNF-α +; 95% CI, 2.1%–4.0%, P = .19, Wilcoxon signed rank test) (Figure 1A).
Figure 1.
Cytokine production by CD4+ and CD8+ T cells after polyclonal anti-CD3 stimulation. A, B, Frequency of functional memory CD4+ (A) and CD8+ (B) T cells from human immunodeficiency virus (HIV)–infected and HIV-uninfected participants that respond directly to anti-CD3 stimulation by simultaneously producing 2 antiviral cytokines, interferon (IFN) γ and tumor necrosis factor (TNF) α, as determined by intracellular cytokine staining and flow cytometry. The mean frequency of responsive T cells is plotted, with error bars representing 95% confidence intervals. C, D, Proportions of anti-CD3-responsive CD4+ (C) and CD8+ (D) T cells that produce both IFN-γ and TNF-α (IFN-γ +TNF-α +) or that produce only IFN-γ (IFN-γ +TNF-α −) or only TNF-α (IFN-γ −TNF-α +) after direct ex vivo stimulation with anti-CD3. All values are background subtracted (medium alone), and P values were determined by means of Wilcoxon signed rank test.
Approximately 18.8% (95% CI, 15.1%–22.6%) of CD8+ T cells from HIV-infected participants were IFN-γ +TNF-α + after anti-CD3 stimulation. This value was significantly higher than that in HIV-uninfected controls (13.1%; 95% CI, 10.2–16.0%; P = .03, Wilcoxon signed rank test) (Figure 1B). Overall, cytokine profiles, including IFN-γ +TNF-α +-, IFN-γ +TNF-α −-, or IFN-γ −TNF-α +-expressing T-cell subsets were similar between HIV-infected and HIV-uninfected cohorts after anti-CD3 stimulation of CD4+ (Figure 1C) or CD8+ (Figure 1D) T cells. This indicates that the frequency of anti-CD3 responsive T cells among the HIV-infected participants was equal to, or higher than, that observed in HIV-uninfected controls, and the 2 groups were similar in their overall cytokine profiles.
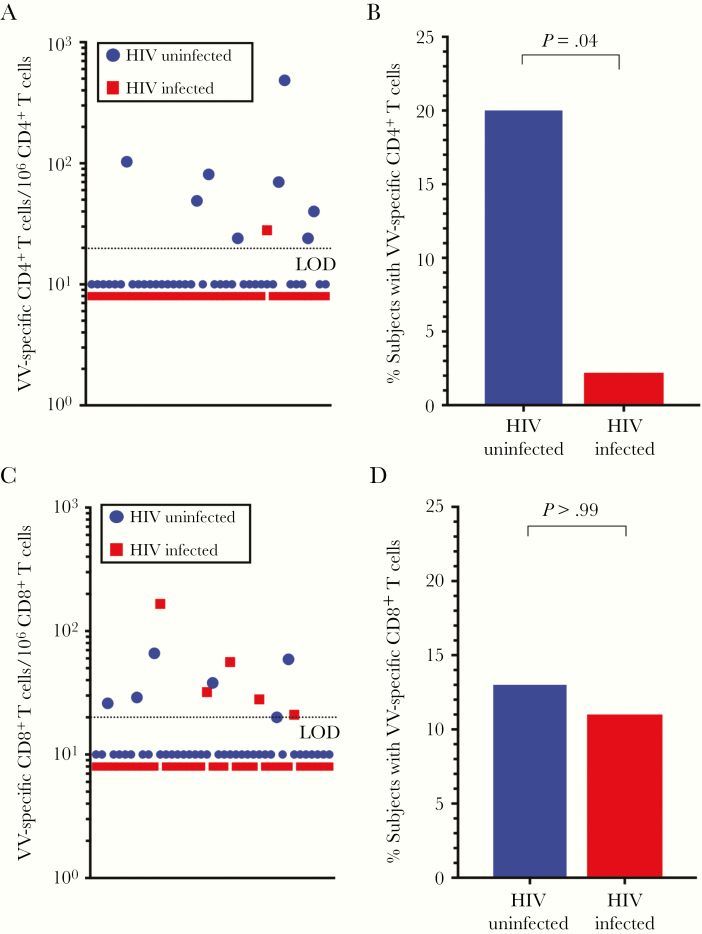
To determine whether immune-reconstituted HIV-infected adults maintained specific T-cell memory against a viral infection that occurred before HIV acquisition, we examined antiviral CD4+ and CD8+ T-cell responses induced by childhood smallpox vaccination (Figure 2). We have previously optimized this assay to measure VV-specific T-cell responses by using intracellular cytokine staining to measure dual production of IFN-γ and TNF-α, and using a detection threshold of ≥20 VV-specific IFN-γ +TNF-α + T cells per 106 T cells provides ≥95% sensitivity and ≥95% specificity [19, 22]. Previous findings indicate that VV-specific T-cell memory declines with a half-life of approximately 8–15-years, resulting in only a subpopulation of vaccinated individuals retaining detectable memory T-cell responses when these are measured 20–40 years after immunization [19, 24–26].
Figure 2.
Quantitation of vaccinia virus (VV)–specific CD4+ and CD8+ T-cell memory. A, B, The quantitation of VV-specific CD4+ T cells (A) and the percentage of human immunodeficiency virus (HIV)–infected and HIV-uninfected participants with detectable VV-specific CD4+ memory T cells (B) were determined after direct ex vivo stimulation with VV. C, D, The frequency of VV-specific CD8+ T-cell responses (C) and the percentage of HIV-infected and HIV-uninfected participants with detectable VV-specific CD8+ T cells (D) were determined after stimulation with VV in the same assays. A frequency of ≥20 virus-specific interferon γ + tumor necrosis factor α + T cells per million T cells is considered a positive antiviral T-cell response. P values were determined using the exact McNemar test. Abbreviation: LOD; limit of detection.
In these current studies, the frequency of measurable VV-specific memory CD4+ T cells was estimated at 109 per 106 CD4+ T cells (median, 59, range; 24–485) among the HIV-uninfected cohort, whereas the 1 HIV-infected participant with a detectable VV-specific CD4+ T-cell response had a score of 28 per 106 CD4+ T cells—ie, near the minimum threshold for detecting a positive T-cell response (Figure 2A). These data indicate that 20% of HIV-uninfected participants maintained VV-specific CD4+ T-cell memory above the detection threshold (≥20 per 106 CD4+ T cells), compared with only 2.4% of HIV-infected participants (P = .04, exact McNemar test) (Figure 2B).
The frequency of VV-specific memory CD8+ T cells was determined within the same assays (Figure 2C). The frequency of VV-specific memory CD8+ T cells among the HIV-uninfected cohort was estimated at 40 per 106 CD8+ T cells (median, 34; range, 20–66), similar to the mean frequency of 61 virus-specific CD8+ T cells per 106 CD8+ T cells (median, 32; range, 21–166) among the HIV-infected cohort. Unlike the virus-specific CD4+ T-cell responses, there was no significant difference between the percentage of HIV-uninfected or HIV-infected participants who maintained antiviral CD8+ T-cell memory (15% vs 12%, respectively; P > .99, exact McNemar test) (Figure 2D). This indicates that after HIV infection and successful immune reconstitution after ART, preexisting CD8+ T-cell memory to an unrelated infection encountered during childhood (VV) seemed to remain intact, whereas preexisting CD4+ T-cell memory to the same pathogen was preferentially lost despite normal numbers of functional CD4+ T cells in circulation (Figure 1).
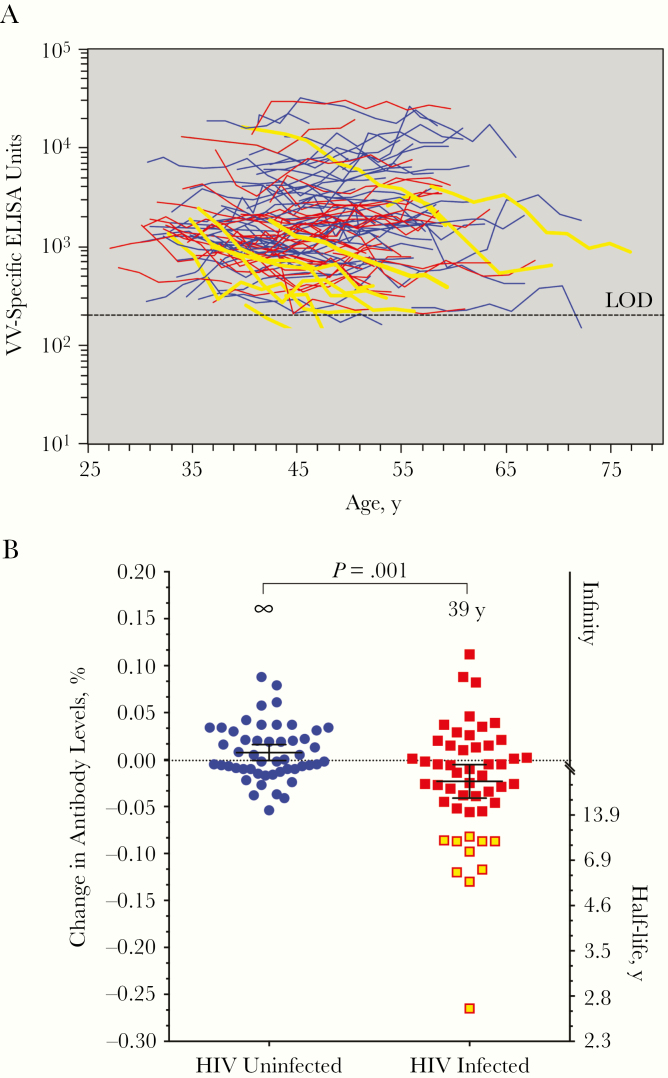
Maintenance of Serum Antibodies
HIV infection causes polyclonal B-cell activation and hypergammaglobulinemia but also induces memory B-cell dysfunction, exhaustion, and death [27–31]. Compared with naive and memory B cells, much less is known about the impact of HIV and ART on plasma cell survival and the maintenance of preexisting serum antibody responses to infections that occurred before HIV infection. To examine this question, we performed longitudinal analysis of antiviral antibody responses after childhood smallpox vaccination (Figure 3A). Findings of prior studies indicate that VV-specific antibody responses are maintained essentially for life, with an estimated half-life of 92 years (95% CI, 46 years to infinity) [23]. Consistent with these findings, we found that VV-specific antibody responses among HIV-uninfected participants were stable, with an estimated half-life of infinity (95% CI, 309 years to infinity) (Figure 3B). In contrast, when VV-specific antibody responses were measured among HIV-infected participants, they declined with a 39-year half-life (95% CI, 24–108 years), and this was significantly more rapid than in HIV-uninfected controls (P = .001) (Figure 3B). The 10 participants with the most rapid antibody decay rates are shown with yellow lines in Figure 3A. Interestingly, they were all from the HIV-infected cohort and demonstrated very rapid antibody half-life estimates of 2.6–8.5 years (Figure 3B).
Figure 3.
Longitudinal analysis of vaccinia virus (VV)–specific antibody responses. A, Longitudinal antibody responses of individual human immunodeficiency virus (HIV)–infected participants (red or yellow lines) and HIV-uninfected participants (blue lines) as a function of age. The dashed line indicates the limit of detection (LOD; 200 enzyme-linked immunosorbent assay [ELISA] units) and values below the LOD are considered equivocal and excluded from analysis. B, Estimated half-life of VV-specific antibody responses based on the individual slopes of each longitudinal antibody response. These values were calculated using the least squares method to show the overall distribution of VV-specific antibody decay rates (percentage change in antibody levels). The mean estimated antibody half-life of each group is shown with error bars representing 95% confidence intervals, and statistical comparisons between groups were determined using a longitudinal mixed-effects model. A mean of 9 serum samples per HIV-infected participant and a mean of 9 serum samples per HIV-uninfected participant were examined over a median period of 17.4 to 17.8 years of time for HIV-infected and HIV-uninfected participants, respectively. Two HIV-infected participants became VV seronegative so quickly that there were not ≥3 data points above the LOD (the minimum for accurate half-life determinations); therefore, their data is presented here graphically but was excluded from the overall group antibody half-life estimation and statistical comparisons. The HIV-infected individuals with the most rapid loss of virus-specific antibodies are shown as yellow lines (A) or yellow-filled symbols (B).
The rapid decay rates observed among these 10 HIV-infected participants were not associated with participant age at enrollment, hepatitis B or hepatitis C virus status, initial HIV RNA viral load, residual HIV RNA viral load after ART, CD4+ T-cell nadir, CD4+/CD8+ T-cell ratio, or time after ART before CD4+ T-cell counts reached >200/μL or >350/μL (data not shown). The rapid loss of VV-specific antibodies among 20% of HIV-infected women does not seem to be directly linked to loss of VV-specific CD4+ T cells, because the other 80% of HIV-infected women seemed to have a relatively normal distribution of antibody decay rates, despite the observation that all but 1 of these women tested negative for virus-specific CD4+ T-cell memory (Figure 2). We also found no correlation between antiviral antibody decay rates and CD4+ T-cell memory among the HIV-uninfected controls (data not shown), which is consistent with our prior studies in which no correlation between CD4+ T-cell memory and antibody titers was observed among VV-immune adults [19]. Unlike their HIV-uninfected counterparts, these studies indicate that 1 in every 5 HIV-infected participants are prone to an accelerated loss of serological memory despite successful immune reconstitution by ART.
DISCUSSION
We examined the durability of antiviral T-cell and antibody responses after childhood smallpox vaccination as a model to determine the impact of HIV and ART on the maintenance of preexisting immunological memory in the absence of reexposure or revaccination. After ART-associated immune reconstitution, HIV-infected women showed no reduction in the percentage of functional, anti-CD3-responsive CD4+ T cells. However, when antigen-specific assays were used to study immunity from smallpox vaccination, we found a nearly complete loss of VV-specific CD4+ T-cell memory even though CD8+ T-cell responses remained largely unchanged compared with HIV-uninfected controls. Analysis of VV-specific antibody responses revealed a significant decline in serological memory despite successful ART-associated maintenance of peripheral CD4+ T cells. The loss of CD4+ T-cell memory and antibody responses to infections encountered before HIV acquisition could have implications with regard to protective immunity to common acute or chronic viral infections.
CD4+ T-cell-mediated cytokine responses after polyclonal anti-CD3 stimulation did not differ significantly between HIV-infected women or HIV-uninfected controls (P = .19) (Figure 1). In contrast, anti-CD3-responsive CD8+ T-cell responses were significantly up-regulated among the HIV-infected cohort (P = .03). These results are consistent with findings of prior studies in which polyclonal stimulation of peripheral T cells from HIV-infected participants showed that CD4+ T-cell responses were equal to or lower than those in HIV-uninfected controls, whereas CD8+ T-cell responses were consistently higher among HIV-infected cohorts [32–34]. Although the reason for increased CD8+ T-cell responses among HIV-infected participants remains unclear, it is believed that immune activation may be due to a decreased ability to control repeated or chronic viral infections, resulting in a state of persistent inflammation and an “inflammaging” phenotype [3].
In our hands, VV-specific memory CD8+ T-cell responses observed among HIV-infected women seemed similar to those observed among HIV-uninfected women in terms of the overall magnitude of the remaining memory T-cell response per participant (Figure 2C) and the proportion of participants who maintained a detectable CD8+ memory T-cell response (Figure 2D). One challenge with interpreting these studies is the low frequency of T-cell memory identified at late time points examined decades after acute VV infection [19], and more studies are needed to determine whether the persistent inflammation and inflammaging phenotype observed among HIV-infected participants might contribute to a more stable frequency of preexisting CD8+ memory T cells or whether the increased frequency of CD8+ T cells with a functional memory phenotype (Figure 1B) is due to recruitment of new T cells into the memory T-cell pool.
Prior studies have indicated that antigen-specific T-cell memory after HIV acquisition and subsequent administration of ART was either lost [5–7] or restored [5, 8–10]. In some cases, the restoration of antigen-specific T-cell responses is likely due to antigenic reexposure after ART (eg, cytomegalovirus, herpes simplex virus, and Candida albicans) [5, 8, 9]. One study showed, lymphoproliferative responses to tetanus toxoid were restored regardless of booster immunization [10]. In another study [9], lymphoproliferative responses to purified protein derivative, influenza, and tetanus toxoid remained persistently weak even after ART. The CD4+ T-cell nadir before ART may influence immune reconstitution, because vaccine-induced CD4+ T-cell memory did not recover among HIV-infected participants with a low CD4+ T-cell nadir of ≤350/μL, whereas those with CD4+ T-cell counts remaining above 350/μL had memory CD4+ T-cell responses that remained intact [6].
In our current studies, we examined the recall responses to VV antigens that are unlikely to be encountered after cessation of routine smallpox vaccination among civilians born after 1972. We enrolled HIV-infected participants who had a CD4+ T-cell nadir of <200/μL that rebounded to >350/μL after ART and found that CD4+ T-cell memory was lost among HIV-infected participants, whereas antiviral CD8+ T-cell memory remained intact (Figure 2). Because CD8+ T-cell responses were determined in the same assays as the CD4+ T-cell responses, this indicates that differences between groups are unlikely to be due to any technical issues or cohort effects and that CD8+ T-cell memory is preferentially retained over CD4+ T-cell memory after HIV acquisition and ART.
Poor antibody responses to vaccination among HIV-infected individuals have been well described [28, 35, 36]. Much less is known about the impact of HIV/ART on the maintenance of preexisting humoral immunity. One study showed, antibody responses to tetanus were maintained among 7 HIV-infected participants taking ART, with a mean half-life of 11 years [11], similar to previous findings in the general population [23, 37]. Differences in antibody decay rates may not have been observed in this small cohort if the most rapid antibody decay rates occur among only 20% of the HIV-infected population, as observed in our study (Figure 3). Alternatively, the antibody decay rates could be different for particular virus or vaccine antigens. One study observed a nonsignificant trend toward more rapid measles antibody decay rates during primary HIV infection [12], but when monitored longitudinally during the chronic phase of infection, the antibody responses seemed stable. However, these longitudinal studies measured just a 24-month span of time, making it difficult to identify broader differences in long-term antibody maintenance.
Further studies are needed to determine whether rapid loss of serological memory among HIV-infected participants is unique to specific viruses or vaccine antigens or whether it represents a more global defect in immune memory among these individuals. For instance, the overall rate of herpes zoster from varicella zoster virus among HIV-infected adults is nearly 4-fold higher than that observed in the general US population [2]. HIV infection is also associated with higher rates of virus-related cancers, including Kaposi sarcoma (human herpesvirus 8), lymphomas (Epstein-Barr virus), anal cancer (human papillomavirus), and liver cancer (hepatitis B and hepatitis C virus). In contrast, there is no association between HIV infection and an increased risk of non–virus-associated cancers, such as breast, prostate, or colorectal cancer [3]. After the introduction of highly active ART, the incidence of certain virus-associated cancers such Kaposi sarcoma and non-Hodgkin lymphoma decreased, whereas the incidence of cervical cancer remained largely unaltered [38], indicating that prolonged immune suppression plays a role in susceptibility to some pathogens but may not completely explain the increased risks associated with HIV infection.
The loss in preexisting serological memory among HIV-infected participants is likely due to the loss of long-lived antibody-secreting plasma cells [39], most of which reside in the bone marrow. Bone and bone marrow abnormalities that may occur after HIV infection or ART include osteoporosis, osteopenia, osteomalacia, osteonecrosis, low bone marrow density, and increased risk of fractures [40]. In addition, HIV infection leads to depletion of hematopoietic progenitor cells [41] and senescence of bone marrow mesenchymal stem cells, resulting in reduced support of hematopoietic stem cells in vitro [42]. HIV infects bone marrow stromal cells and HIV glycoprotein 120 and Gag p55 have been shown to be involved with bone disorders [40]. Moreover, HIV therapies involving tenofovir disoproxil fumurate and protease inhibitors have been directly associated with bone abnormalities [40]. It is possible that HIV, ART, or a combination of these factors may lead to disruption of the bone marrow microenvironment needed to sustain plasma cell survival and long-term antibody responses.
The current study has several limitations. We examined immunological memory among HIV-infected individuals who had a CD4+ T-cell count nadir of <200/μL that reconstituted to >350/μL after ART. Although these levels seem adequate to describe a population experiencing severe immunologic damage and significant recovery, additional studies will be needed to determine whether those with a higher nadir or with greater recovery (ie, counts >500/μL) are likely to demonstrate loss of CD4+ T-cell memory (Figure 2) or rapid decline in preexisting antibody responses (Figure 3). As with any long-term cohort study, a proportion of participants are lost to follow-up, owing to death or attrition, and it is unknown if this may affect study results. In addition, the initial study focused on HIV-infected women, and further studies among HIV-infected men are warranted.
Despite effective immune reconstitution with ART, the loss of immunological memory to prior infections or vaccinations may play a previously overlooked role in the chronic inflammation and “accelerated aging” observed among HIV-infected individuals [3]. These data suggest that despite successful use of ART, HIV infection is associated with a significant loss in virus-specific CD4+ T-cell memory and antiviral antibody responses that may leave a sizeable proportion of HIV-infected people at increased risk for virus-associated disease manifestations.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Byung Park for initial power analysis/sample size calculations and Bin Liu for help with sample acquisition for the Women’s Interagency HIV Study (WIHS). Data in this manuscript were collected by the WIHS.
Author contributions. M. H. A. and M. K. S. designed the study, and S. H. was the study coordinator. A. T. and E. H. performed the T-cell and enzyme-linked immunosorbent assays, and A. T. prepared the figures, table, and Methods. L. G. performed the statistical analysis. S. H., K. G. M., M. G., M. C. V., E. T. G., N. R. R., A. L. F., and M. H. A. contributed to the retention of participants and acquisition of samples from participants in the WIHS cohort. M. H. A. and M. K. S. wrote the manuscript, and all authors reviewed the manuscript before submission. All authors had access to the data included in the study, and M. H. A. and M. K. S. had final responsibility for the decision to submit the manuscript for publication.
Disclaimer. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health. The funders of this study played no role in writing the manuscript or reporting/interpreting the data.
Financial support. This work was supported in part by the National Institutes of Health Public Health Service (grant U19 AI109948 to M. K. S.) and the Oregon National Primate Research Center (grant 8P51 OD011092 to M. K. S). The WIHS is supported primarily by the National Institute of Allergy and Infectious Diseases, as follows: Bronx WIHS (principal investigators, Kathryn Anastos and Anjali Sharma; grant U01-AI-035004); Brooklyn WIHS (Howard Minkoff and Deborah Gustafson; grant U01-AI-031834); Chicago WIHS (Mardge Cohen and Audrey French; grant U01-AI-034993); Metropolitan Washington WIHS (Seble Kassaye; grant U01-AI-034994); Connie Wofsy Women’s HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien; grant U01-AI-034989); WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub; grant U01-AI-042590); Southern California WIHS (Joel Milam; grant U01-HD-032632) (WIHS I–WIHS IV). The WIHS receives additional cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Mental Health, with targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research, the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Deafness and other Communication Disorders, and the National Institutes of Health Office of Research on Women’s Health. WIHS data collection is also supported by the National Institutes of Health (grants UL1-TR000004 [University of California, San Francisco, Clinical and Translational Science], P30-AI-050409 [Atlanta Center for AIDS Research], P30-AI-050410 [University of North Carolina Center for AIDS Research], and P30-AI-027767 [University of Alabama at Birmingham Center for AIDS Research]).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kaplan JE, Hanson D, Dworkin MS, et al. . Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin Infect Dis 2000; 30(suppl 1):S5–14. [DOI] [PubMed] [Google Scholar]
- 2. Erdmann NB, Prentice HA, Bansal A, et al. . Herpes zoster in persons living with HIV-1 infection: viremia and immunological defects are strong risk factors in the era of combination antiretroviral therapy. Front Public Health 2018; 6:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nasi M, De Biasi S, Gibellini L, et al. . Ageing and inflammation in patients with HIV infection. Clin Exp Immunol 2017; 187:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deeks SG, Verdin E, McCune JM. Immunosenescence and HIV. Curr Opin Immunol 2012; 24:501–6. [DOI] [PubMed] [Google Scholar]
- 5. Wendland T, Furrer H, Vernazza PL, et al. . HAART in HIV-infected patients: restoration of antigen-specific CD4 T-cell responses in vitro is correlated with CD4 memory T-cell reconstitution, whereas improvement in delayed type hypersensitivity is related to a decrease in viraemia. AIDS 1999; 13:1857–62. [DOI] [PubMed] [Google Scholar]
- 6. Elrefaei M, McElroy MD, Preas CP, et al. . Central memory CD4+ T cell responses in chronic HIV infection are not restored by antiretroviral therapy. J Immunol 2004; 173:2184–9. [DOI] [PubMed] [Google Scholar]
- 7. Puissant-Lubrano B, Combadière B, Duffy D, et al. . Influence of antigen exposure on the loss of long-term memory to childhood vaccines in HIV-infected patients. Vaccine 2009; 27:3576–83. [DOI] [PubMed] [Google Scholar]
- 8. Autran B, Carcelain G, Li TS, et al. . Positive effects of combined antiretroviral therapy on CD4 + T cell homeostasis and function in advanced HIV disease. Science 1997; 277:112–6. [DOI] [PubMed] [Google Scholar]
- 9. Hardy GA, Imami N, Sullivan AK, et al. . Reconstitution of CD4+ T cell responses in HIV-1 infected individuals initiating highly active antiretroviral therapy (HAART) is associated with renewed interleukin-2 production and responsiveness. Clin Exp Immunol 2003; 134:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burton CT, Goodall RL, Samri A, et al. ; INITIO Trial International Co-ordinating Committee Restoration of anti-tetanus toxoid responses in patients initiating highly active antiretroviral therapy with or without a boost immunization: an INITIO substudy. Clin Exp Immunol 2008; 152:252–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bonsignori M, Moody MA, Parks RJ, et al. . HIV-1 envelope induces memory B cell responses that correlate with plasma antibody levels after envelope gp120 protein vaccination or HIV-1 infection. J Immunol 2009; 183:2708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Titanji K, De Milito A, Cagigi A, et al. . Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood 2006; 108:1580–7. [DOI] [PubMed] [Google Scholar]
- 13. Effros RB, Fletcher CV, Gebo K, et al. . Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis 2008; 47:542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Irons JV, Sullivan TD, Cook EBM, Cox GW, Hale RA. Outbreak of smallpox in the lower Rio Grande Valley of Texas in 1949. Am J Public Health 1953; 43:25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968. N Engl J Med 1969; 281:1201–8. [DOI] [PubMed] [Google Scholar]
- 16. Henderson DA, Inglesby TV, Bartlett JG, et al. . Working Group on Civilian Biodefense. Smallpox as a biological weapon: medical and public health management. JAMA 1999; 281:2127–37. [DOI] [PubMed] [Google Scholar]
- 17. Hammarlund E, Dasgupta A, Pinilla C, Norori P, Früh K, Slifka MK. Monkeypox virus evades antiviral CD4+ and CD8+ T cell responses by suppressing cognate T cell activation. Proc Natl Acad Sci U S A 2008; 105:14567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barkan SE, Melnick SL, Preston-Martin S, et al. . WIHS Collaborative Study Group. The Women’s Interagency HIV Study. Epidemiology 1998; 9:117–25. [PubMed] [Google Scholar]
- 19. Hammarlund E, Lewis MW, Hansen SG, et al. . Duration of antiviral immunity after smallpox vaccination. Nat Med 2003; 9:1131–7. [DOI] [PubMed] [Google Scholar]
- 20. Alzhanova D, Edwards DM, Hammarlund E, et al. . Cowpox virus inhibits the transporter associated with antigen processing to evade T cell recognition. Cell Host Microbe 2009; 6:433–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sánchez-Puig JM, Sánchez L, Roy G, Blasco R. Susceptibility of different leukocyte cell types to vaccinia virus infection. Virol J 2004; 1:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hammarlund E, Lewis MW, Carter SV, et al. . Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat Med 2005; 11:1005–11. [DOI] [PubMed] [Google Scholar]
- 23. Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med 2007; 357:1903–15. [DOI] [PubMed] [Google Scholar]
- 24. Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol 2003; 171:4969–73. [DOI] [PubMed] [Google Scholar]
- 25. Combadiere B, Boissonnas A, Carcelain G, et al. . Distinct time effects of vaccination on long-term proliferative and IFN-γ-producing T cell memory to smallpox in humans. J Exp Med 2004; 199:1585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Amara RR, Nigam P, Sharma S, Liu J, Bostik V. Long-lived poxvirus immunity, robust CD4 help, and better persistence of CD4 than CD8 T cells. J Virol 2004; 78:3811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lane HC, Masur H, Edgar LC, Whalen G, Rook AH, Fauci AS. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med 1983; 309:453–8. [DOI] [PubMed] [Google Scholar]
- 28. Moir S, Fauci AS. Insights into B cells and HIV-specific B-cell responses in HIV-infected individuals. Immunol Rev 2013; 254:207–24. [DOI] [PubMed] [Google Scholar]
- 29. Moir S, Ho J, Malaspina A, et al. . Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med 2008; 205:1797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moir S, Malaspina A, Ho J, et al. . Normalization of B cell counts and subpopulations after antiretroviral therapy in chronic HIV disease. J Infect Dis 2008; 197:572–9. [DOI] [PubMed] [Google Scholar]
- 31. Shen X, Tomaras GD. Alterations of the B-cell response by HIV-1 replication. Curr HIV/AIDS Rep 2011; 8:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sousa AE, Victorino RM. Single-cell analysis of lymphokine imbalance in asymptomatic HIV-1 infection: evidence for a major alteration within the CD8+ T cell subset. Clin Exp Immunol 1998; 112:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eylar EH, Lefranc C, Báez I, et al. . Enhanced interferon-γ by CD8+ CD28- lymphocytes from HIV+ patients. J Clin Immunol 2001; 21:135–44. [DOI] [PubMed] [Google Scholar]
- 34. Eylar EH, Lefranc CE, Yamamura Y, et al. . HIV infection and aging: enhanced interferon- and tumor necrosis factor-alpha production by the CD8+ CD28- T subset. BMC Immunol 2001; 2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. French N, Moore M, Haikala R, Kayhty H, Gilks CF. A case-control study to investigate serological correlates of clinical failure of 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults. J Infect Dis 2004; 190:707–12. [DOI] [PubMed] [Google Scholar]
- 36. Malaspina A, Moir S, Orsega SM, et al. . Compromised B cell responses to influenza vaccination in HIV-infected individuals. J Infect Dis 2005; 191:1442–50. [DOI] [PubMed] [Google Scholar]
- 37. Hammarlund E, Thomas A, Poore EA, et al. . Durability of vaccine-induced immunity against tetanus and diphtheria toxins: a cross-sectional analysis. Clin Infect Dis 2016; 62:1111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ghebre RG, Grover S, Xu MJ, Chuang LT, Simonds H. Cervical cancer control in HIV-infected women: past, present and future. Gynecol Oncol Rep 2017; 21:101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hammarlund E, Thomas A, Amanna IJ, et al. . Plasma cell survival in the absence of B cell memory. Nat Commun 2017; 8:1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ahmad AN, Ahmad SN, Ahmad N. HIV infection and bone abnormalities. Open Orthop J 2017; 11:777–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li G, Zhao J, Cheng L, et al. . HIV-1 infection depletes human CD34+CD38- hematopoietic progenitor cells via pDC-dependent mechanisms. PLoS Pathog 2017; 13:e1006505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yuan YH, Zhao SS, Wang XL, Teng ZP, Li DS, Zeng Y. HIV-1 p55-gag protein induces senescence of human bone marrow mesenchymal stem cells and reduces their capacity to support expansion of hematopoietic stem cells in vitro. Cell Biol Int 2017; 41:969–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.