Abstract
Background
A higher proportion of circulating memory CD4+ T cells is associated with prevalent diabetes mellitus in the general population. Given the broad changes in adaptive immunity, including memory T-cell expansion, and rising prevalence of diabetes in the human immunodeficiency virus (HIV) population, we assessed whether similar relationships were present in persons with HIV (PWH).
Methods
Multiple CD4+ and CD8+ T-cell subsets were measured by flow cytometry, and prevalent diabetes cases were adjudicated by 2 physicians for PWH and HIV-negative participants in the Veterans Aging Cohort Study. Multivariable logistic regression models evaluated the association of T-cell subsets and diabetes stratified by HIV status, adjusted for cytomegalovirus serostatus and traditional risk factors.
Results
Among 2385 participants (65% PWH, 95% male, 68% African American), higher CD45RO+ memory CD4+ T cells and lower CD38+ CD4+ T cells were associated with prevalent diabetes, and had a similar effect size, in both the PWH and HIV-negative (P ≤ .05 for all). Lower CD38+CD8+ T cells were also associated with diabetes in both groups.
Conclusions
The CD4+ and CD8+ T-cell subsets associated with diabetes are similar in PWH and HIV-negative individuals, suggesting that diabetes in PWH may be related to chronic immune activation.
Keywords: HIV, metabolic disease, systemic inflammation, T lymphocytes, type 2 diabetes mellitus
T-cell alterations, including memory CD4+ T-cell expansion, are associated with diabetes in both persons with HIV and HIV-negative individuals, suggesting that changes to the T-cell compartment is related to or possibly mediates diabetes in persons with HIV.
Persons with human immunodeficiency virus (PWH) on long-term antiretroviral therapy (ART) are at risk for developing diabetes, and an increasing proportion of older and overweight individuals will likely contribute to a higher prevalence of metabolic disease in the future [1–5]. The prevalence of diabetes is increasing in PWH [6], although assessing the independent effect of human immunodeficiency virus (HIV) on diabetes risk is complicated by shifting age, overweight and obesity prevalence, and sociodemographic patterns in the HIV population [7, 8]. Furthermore, prior analyses from the Veterans Aging Cohort Study (VACS) demonstrated that the risk of incident diabetes attributable to a 5 pound weight gain was greater among PWH than HIV-negative persons [9].
Monocyte activation and systemic inflammation have been associated with diabetes in both PWH and HIV-negative individuals [10–14]. Compared with HIV-negative individuals, PWH have high circulating concentrations of inflammatory markers including high-sensitivity C-reactive protein, interleukin-6 (IL-6), soluble CD14 (sCD14), and D-dimer, even after suppression of plasma viremia on ART [15, 16]. In the SMART and ESPRIT HIV cohorts, enrollment plasma IL-6 levels were associated with incident diabetes several years before clinical disease [10]. Likewise, in HIV-negative persons, sCD14, which binds circulating lipopolysaccharide, serves as a marker of monocyte activation and correlates with several cardiometabolic disease risk factors and prevalent diabetes [17].
Several studies in the general population have identified higher proportions of circulating proinflammatory and memory CD4+ T cells in diabetics compared with nondiabetics. In one study, the proportion of regulatory (Treg) CD4+ T cells was lower and the ratio of Treg to T-helper cell type 1 (TH1) and type 17 (TH17) was decreased in diabetics compared with nondiabetics, suggesting a shift towards a proinflammatory CD4+ T-cell profile [18]. In a separate study, the proportion of circulating Treg cells inversely correlated with C-reactive protein and hemoglobin A1c (HbA1c) levels and identified metabolically unhealthy obese subjects [19]. Likewise, a decreased proportion of circulating naive CD4+ T cells and increased percentage of memory cells was observed in diabetic patients, suggesting that chronic antigen stimulation may be related to the development of glucose intolerance [20].
Human immunodeficiency virus infection causes significant changes to the adaptive immune system including naive CD4+ T-cell depletion, expansion of memory cells, CD4+ and CD8+ T-cell activation, and accelerated T-cell immunosenescence, which persist despite suppression of plasma viremia with ART [21]. Furthermore, cytomegalovirus (CMV) infection is common among PWH [22], and it can dramatically reshape the T-cell compartment [23]. Previous studies have linked CMV seropositivity to cardiovascular disease and diabetes in both PWH and HIV-negative individuals [22, 24, 25]. Several of these alterations in circulating T cells observed in PWH overlap the subsets associated with diabetes in the general population, raising the possibility that glucose intolerance in PWH may be related to similar immune mechanisms.
The VACS is a longitudinal, observational study of PWH and HIV-negative persons of similar age, race, and geographic location receiving care in the Veterans Affairs (VA) healthcare system [26]. The VA is the largest single provider of HIV care in the United States, and VACS has been previously used to assess the burden of metabolic comorbidities, including diabetes, in PWH and HIV-negative persons [5, 9, 27]. Using VACS-archived samples and clinical records, we assessed the relationship between T-cell subsets and prevalent diabetes according to HIV status independent of CMV serostatus and other common risk factors for metabolic disease.
METHODS
Cohort Description
This is a cross-sectional study using the VACS Biomarker Cohort (VACS-BC), a prospective cohort of 2385 veterans (65% PWH) that is part of the larger VACS. The VACS has been described in detail in previous publications [26]. In brief, PWH and age-, race-, ethnicity-, and site-matched HIV-negative comparator individuals were enrolled starting in June 2002 at 8 VA facilities in the United States from the infectious diseases (PWH) and general medicine (HIV-negative) clinics at each site. All patients completed standardized semiannual surveys to assess multiple domains of health including activity level, substance use, demographic data, comorbidities, and family history of diabetes mellitus and other diseases. Participants had complete blood count, renal and liver panels, glucose, HbA1c, lipid panel, and hepatitis serologies performed at enrollment, and additional clinical data (eg, body mass index [BMI], laboratory values) were extracted from the electronic medical record. The VA Immunology Case Registry provides HIV-specific data including longitudinal CD4+ T-cell counts, serum HIV-1 ribonucleic acid (RNA) quantification, and antiretroviral regimen.
Flow Cytometry
Peripheral blood mononuclear cells (PBMCs) were collected and cryopreserved from VACS-BC participants in 2005–2007. We performed flow cytometry on archived specimens to measure T-cell subsets including the following: CD38+ and senescent (CD57+) CD4+ and CD8+ T cells; CD4+ TH1 (INFγ +), TH2 (IL4+), TH17 (IL17+), and Treg cells (CD25+Foxp3+); and CD8+ and CD4+ naive and memory T cells (including central, effector, transitional, and effector RA+ memory subsets; see Supplementary Materials and Supplementary Tables 1 and 2 for staining protocols, surface marker phenotypes, and reagents). These T-cell subsets were selected because each has been shown to be altered in HIV and/or associated with chronic diseases, including diabetes, in the general population. Due to sample quality and viability of cells, not all participants contributed to cellular phenotyping. However, more than 90% of participants contributed to surface marker phenotyping, with the majority of subsets including more than 96% of participants.
A full description of methods is available in the Supplementary Materials. In brief, PBMCs were thawed rapidly and diluted 10-fold, centrifuged, and washed, and the final pellet was resuspended in supplemented media and filtered through a 70-µm filter.
For functional assays (TH1, TH2, TH17), the sample was stimulated with phorbol myristate acetate (PMA)/ionomycin in the presence of brefeldin A for 3 hours as previously described [28], and stained for viability, surface proteins, and intracellular cytokines, as detailed in the Online Supplement. FoxP3+ Treg cells were stained for viability, surface stained for CD4, and intracellularly stained for FoxP3 as detailed Online.
For surface labeling, samples were centrifuged and resuspended in phosphate-buffered saline pH 7.4 and stained with live/dead stain. Stain was removed by centrifugation, and antibodies or isotype-matched control antibodies were added (see Supplementary Table 1).
Flow cytometry was performed with an MQ10 (Miltenyi Biotec) and analyzed utilizing the MACS Quantity software package. The machine was calibrated daily with calibration beads. Single color compensation controls prepared contemporaneously with the samples were used for each assay to set compensation. Isotype controls were used to set negative gates.
Plasma Biomarkers and Cytomegalovirus Status
Please see Supplementary Material for full methods. In brief, IL-6 was measured using a chemiluminescent immunoassay (QuantiGlo IL-6 immunoassay; R&D Systems, Minneapolis, MN), D-dimer was measured using the STAR automated coagulation analyzer (Diagnostica Stago) and using an immunoturbidometric assay (Liatest D-DI; Diagnostica Stago, Parsippany, NJ), and sCD14 was measured with an enzyme-linked immunosorbent assay (ELISA) (Quantikine sCD14 Immunoassay; R&D Systems). Cytomegalovirus immunoglobulin G concentration was determined using an ELISA from Diamedix (Miami Lakes, FL), and <8 EU/mL was considered to be CMV negative.
Definition of Prevalent Diabetes Mellitus
Potential diabetes cases among VACS-BC participants were identified in the VA electronic medical record utilizing an algorithm that identified candidate charts for review based on glucose and HbA1c laboratory values, pharmacy data, and International Classification of Diseases, Ninth Revision codes (see Supplementary Materials). Potential diabetes cases identified by the algorithm were manually reviewed and adjudicated by 2 physicians with expertise in endocrinology to reduce misclassification. If there was discrepancy between the 2 physician adjudicators, a third physician adjudicator determined classification of the case.
Statistical Methods
We compared patient characteristics by prevalent diabetes status using Wilcoxon rank-sum for continuous variables and χ 2 test for categorical variables separately for PWH and HIV-negative individuals. CD4+ and CD8+ T-cell subsets are reported as median percentages and interquartile ranges. We performed multivariable logistic regression models with prevalent diabetes as the outcome and T-cell subsets as the main predictors. Model 1 adjusted for age, sex, BMI, and viral suppression (PWH models only). Model 2 also adjusted for race, hepatitis C virus serostatus, smoking status, alcohol use, and CMV serostatus. Model 3 further adjusted for plasma levels of IL-6, D-dimer, and sCD14 (measured at the time of PBMC collection). We included only complete cases in the model. Missing values affected 2% or less of non-T-cell variables included in the model. We modeled T-cell subsets as continuous values using 2 steps. First, we fit a spline with 4 knots separately among PWH and HIV-negative participants. If the spline showed a nonlinear association (ie, the spline term was overall statistically significant), we presented the spline plot to graphically display the association between the T-cell subset and odds of prevalent diabetes. If the spline did not show an association significantly different from a straight line, we then modeled the T-cell subset as linear and reported the odds ratio per standard deviation increase in the T-cell percentage, in addition to presenting the plot showing the association of the T-cell subset and risk of prevalent diabetes. In addition, for those T-cell subsets associated with prevalent diabetes in either PWH or HIV-negative participants, we tested for an interaction between HIV status and the T-cell subset. Analyses were conducted using STATA (StataCorp, College Station, TX). Two-sided P < .05 was used to determine significance. This study was reviewed and approved by the Veterans Affairs Institutional Review Board. All study participants provided written informed consent.
RESULTS
Cohort Characteristics
A total of 2385 Veterans (1545 PWH and 840 HIV-negative) were included in the analysis. At the time of collection, PWH were 97% male and 69% black with a median age and BMI of 52 years and 25 kg/m2, respectively. Human immunodeficiency virus-negative participants were 90% male and 67% black with a median age and BMI of 53 years and 30 kg/m2, respectively. Diabetes was present in 281 (18%) PWH and 248 (30%) HIV-negative participants. Persons with HIV with diabetes had similar absolute CD4+ T-cell counts, HIV-1 RNA copies, and proportion on ART compared with those without diabetes (Table 1). Persons with HIV and HIV-negative individuals who were diabetic were older and had a higher prevalence of comorbid conditions including cardiovascular disease, renal insufficiency, hypertension, and obesity compared with nondiabetics (Table 1).
Table 1.
Cohort Characteristics by HIV and Diabetes Statusa
| Characteristics | HIV Positive | HIV Negative | ||||
|---|---|---|---|---|---|---|
| Variable | Diabetic | Nondiabetic | P Value | Diabetic | Nondiabetic | P Value |
| N | 281 | 1264 | 248 | 592 | ||
| Demographics | ||||||
| Age, years | 55.7 (50.1–59.5) | 51.5 (46.2–57.0) | <.001 | 54.3 (50.9–59.6) | 52.3 (47.1–58.0) | <.001 |
| Body mass index, kg/m2 | 26.9 (23.9–30.9) | 25.0 (22.4–27.7) | <.001 | 32.3 (28.3 36.4) | 28.7 (25.3–32.8) | <.001 |
| Male | 278 (98.9) | 1225 (96.9) | .06 | 228 (91.9) | 531 (89.7) | .32 |
| Race | ||||||
| White | 41 (14.6) | 251 (19.9) | .06 | 52 (21.0) | 123 (20.8) | .23 |
| Black | 196 (69.8) | 872 (69.0) | 161 (64.9) | 404 (68.2) | ||
| Hispanic | 30 (10.7) | 97 (7.7) | 20 (8.1) | 47 (7.9) | ||
| Other | 14 (5.0) | 44 (3.5) | 15 (6.0) | 18 (3.0) | ||
| Comorbid diseases | ||||||
| Cardiovascular | 89 (31.7) | 191 (15.1) | <.001 | 99 (39.9) | 148 (25.0) | <.001 |
| Hypertension | 203 (72.2) | 515 (40.7) | <.001 | 217 (87.5) | 358 (60.5) | <.001 |
| COPD | 46 (16.4) | 178 (14.1) | .33 | 53 (21.4) | 89 (15.0) | .03 |
| Tobacco use | ||||||
| Nonsmoker | 65 (23.1) | 306 (24.3) | .004 | 65 (26.3) | 133 (22.5) | .001 |
| Current | 122 (43.4) | 653 (51.7) | 92 (37.3) | 303 (51.3) | ||
| Past | 94 (33.5) | 303 (24.0) | 90 (36.4) | 155 (26.2) | ||
| Obesity (BMI ≥30 kg/m2) | 85 (30.3) | 165 (13.1) | <.001 | 156 (62.9) | 235 (39.7) | <.001 |
| Current alcohol use, hazardous | 92 (33.5) | 496 (40.4) | .03 | 83 (33.7) | 261 (44.8) | .003 |
| HCV antibody positive | 128 (45.6) | 495 (39.2) | .05 | 57 (23.0) | 175 (29.6) | .05 |
| LDL cholesterol ≥160 mg/dL | 17 (6.1) | 57 (4.7) | .30 | 10 (4.1) | 46 (8.2) | .03 |
| HDL cholesterol <40 mg/dL | 148 (53.2) | 511 (41.7) | <.001 | 106 (42.9) | 174 (30.9) | .001 |
| Current statin use | 149 (53.0) | 313 (24.8) | <.001 | 170 (68.6) | 190 (32.1) | <.001 |
| eGFR < 60 mL ∙ min-1 ∙ 1.73 m−2 | 29 (10.3) | 85 (6.7) | .04 | 41 (16.5) | 43 (7.3) | <.001 |
| CMV antibody positive | 254 (91.0) | 1151 (92.2) | .51 | 171 (70.4) | 419 (73.0) | .44 |
| HIV characteristics | ||||||
| CD4+ at enrollment, cells/µL | 409 (285–604) | 397 (245–585) | .19 | |||
| HIV RNA at enrollment, copies/mL | 75 (50–2076) | 75 (50–4318) | .10 | |||
| ART at enrollment | 234 (83.3) | 1023 (80.9) | .36 |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CMV, cytomegalovirus; COPD, chronic obstructive pulmonary disease; eGRF, estimated glomerular filtration rate; HCV, hepatitis C virus; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; LDL, low-density lipoprotein; RNA, ribonucleic acid.
aContinuous variables shown as median values with interquartile range (parentheses). Categorical variables are shown as total “n” and column percentage. Some variables are missing 4% of participants or less.
Univariate Analysis of T-cell Subsets and Soluble Immune Biomarkers Associated With Prevalent Diabetes
Table 2 shows the T-cell subsets and soluble biomarkers that differed significantly between persons with and without diabetes, stratified by HIV status. CD45RO+CD4+ memory T-cell populations were expanded in diabetics compared with nondiabetics among both PWH (46.8% vs 42.8% [P < .001], respectively) and HIV-negative (54.3% vs 50.5% [P = .02], respectively). The proportion of naive CD4+ T cells was reduced in diabetics compared with nondiabetics among both PWH (32.4% vs 37.3% [P = .05], respectively) and HIV-negative negative (30.7% vs 36.6% [P < .001], respectively). The proportion of CD38+CD4+ T cells was reduced in diabetics compared with nondiabetics among PWH only (39.0% vs 43.7% [P < .001], respectively), whereas the proportion of CD57+CD4+ T cells was reduced in diabetes compared with nondiabetics among HIV-negative individuals only (15.1% vs 17.9% [P = .01], respectively).
Table 2.
T-cell Subsets and Soluble Immune Biomarkers and Diabetes Status Stratified by HIV Statusa
| T-cell Subset | HIV Positive | HIV-Negative | ||||
|---|---|---|---|---|---|---|
| Diabetic | Nondiabetic | P Value | Diabetic | Nondiabetic | P Value | |
| CD4+ T-cell Subsets | ||||||
| CD4+ naive | 32.4 (20.8–47.7) | 37.3 (22.9–51.3) | .05 | 30.7 (20.4–43.3) | 36.6 (25.0–49.0) | <.001 |
| CD4+CD38+ | 39.0 (28.6–48.1) | 43.7 (31.5–55.9) | <.001 | 27.1 (17.1–35.4) | 28.0 (19.0–38.7) | .11 |
| CD4+CD57+ (senescent) | 25.7 (15.9–38.4) | 26.9 (17.7–40.9) | .06 | 15.1 (8.6–25.6) | 17.9 (10.9–27.6) | .01 |
| CD4+ CD45RO+ (memory) | 46.8 (37.8–55.7) | 42.8 (34.2–52.5) | <.001 | 54.3 (43.5–63.2) | 50.5 (40.1–60.2) | .02 |
| CD4+ central memory | 27.9 (19.0–34.6) | 24.3 (17.4–31.7) | <.001 | 32.1 (22.5–40.6) | 29.4 (22.8–37.9) | .07 |
| CD4+ transitional memory | 13.9 (9.4–20.6) | 13.3 (8.8–19.0) | .17 | 14.5 (9.9–20.4) | 12.9 (9.1–18.2) | .004 |
| CD4+ TH2 | 4.2 (3.0–6.1) | 3.8 (2.5–5.7) | .04 | 2.7 (1.8–3.8) | 2.7 (1.8–4.0) | .94 |
| CD8+ T-cell Subsets | ||||||
| CD8+ naive | 19.1 (9.2–33.5) | 18.4 (9.4–30.6) | .63 | 25.0 (13.1–36.5) | 29.4 (16.3–46.4) | <.001 |
| CD8+CD38+ | 26.9 (13.5–53.5) | 37.3 (18.0–58.4) | <.001 | 23.9 (11.3–42.5) | 23.2 (11.9–47.0) | .26 |
| CD8+CD45RO+ (memory) | 31.4 (21.3–42.8) | 32.0 (21.5–44.7) | .43 | 25.8 (17.1–37.2) | 23.2 (15.3–33.3) | .01 |
| CD8+ transitional memory | 14.8 (9.3–21.2) | 14.8 (9.3–22.6) | .55 | 12.0 (7.7–19.8) | 10.6 (6.8–15.7) | .004 |
| CD8+CD28− | 53.0 (39.2–66.6) | 56.8 (43.0–70.1) | .009 | 41.5 (29.4–56.1) | 41.2 (28.0–57.1) | .93 |
| Soluble Biomarkers | ||||||
| Interleukin-6 (pg/mL) | 2.5 (1.7–4.1) | 2.0 (1.4–3.3) | <.001 | 2.1 (1.3–3.6) | 1.7 (1.1–2.9) | <.001 |
| Soluble CD14 (µg/mL) | 1852 (1514–2348) | 1694 (1437–2044) | <.001 | 1794 (1565–2063) | 1692 (1438–2019) | .002 |
| D-dimer (µg/mL) | 0.29 (0.19–0.50) | 0.26 (0.15–0.48) | .03 | 0.40 (0.24–0.67) | 0.29 (0.20–0.48) | <.001 |
Abbreviations: HIV, human immunodeficiency virus.
aValues represent the median percentage of parent T-cell population or biomarker concentration and the interquartile range (parentheses). Comparisons with P < .05 are shown in bold. More than 90% of participants contributed samples to each T-cell subset and soluble biomarker.
Among HIV-negative individuals, the proportion of naive CD8+ T cells was reduced and the proportion of CD45RO+CD8+ T cells was increased in diabetics compared with nondiabetics. These changes were not observed in PWH (Table 2). Supplementary Table 3 shows results for all T-cell subsets, and Supplementary Table 4 compares median proportions of each T-cell subset between PWH (stratified by plasma HIV-1 RNA level < or ≥500 copies/mL at the time of PBMC collection) and HIV-negative persons.
Multivariable Regression Analysis of T-cell Subsets and Soluble Immune Biomarkers Associated With Prevalent Diabetes
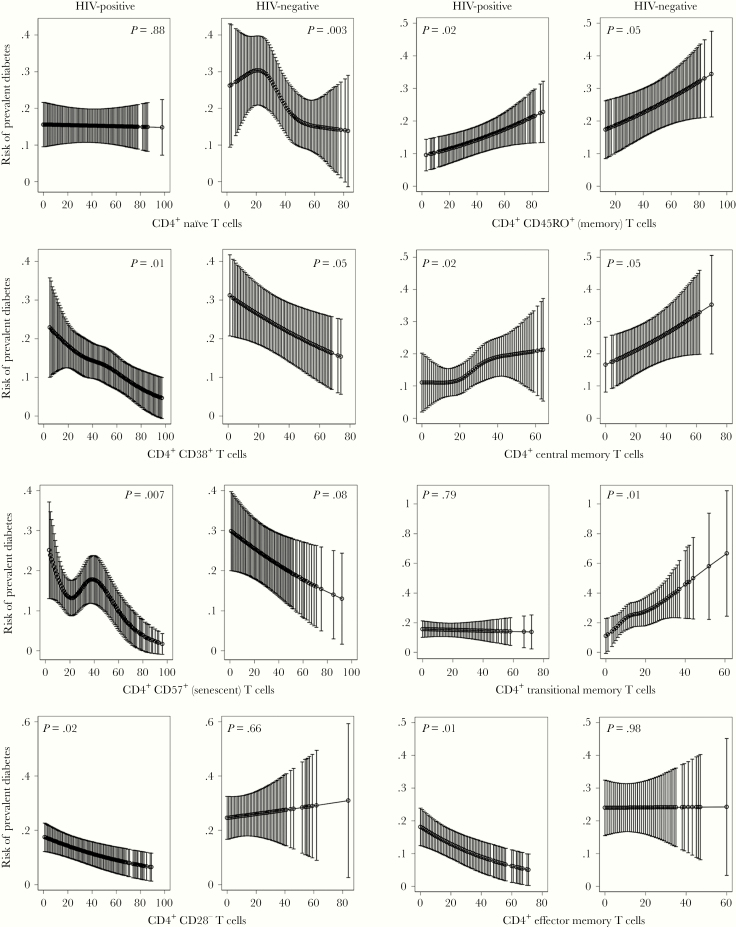
CD4+ T-cell subsets significantly associated with diabetes in model 3 in PWH and/or HIV-negative individuals are shown in Figure 1 and Table 3. A lower percentage of naive CD4+ T cells was associated with prevalent diabetes in the HIV-negative individuals only (P = .003), whereas a lower percentage of senescent CD4+ T cells was associated with diabetes in PWH only (P = .007). In both PWH and HIV-negative individuals, reduced CD38+CD4+ T cells was associated with prevalent diabetes (P = .01 and P = .05, respectively). A higher percentage of memory CD4+ T cells was associated with prevalent diabetes in PWH and HIV-negative individuals (P = .02 and P = .05, respectively). This was mainly driven by expansion of central memory and transitional memory CD4+ T cells in HIV-negative individuals, compared with expansion of central memory with a reduction in effector memory CD4+ T cells in PWH. Finally, a lower proportion of CD4+CD28− T cells was associated with diabetes in PWH (P = .02). We did not observe an association between prevalent diabetes and CD4+ T effector memory RA+, TH1, TH2, TH17, Treg cells, or the TH1/TH2, TH17/TH2, TH1/Treg, or TH17/Treg ratios, in either PWH or HIV-negative individuals.
Figure 1.
CD4+ T-cell subsets associated with prevalent diabetes in persons with human immunodeficiency virus (HIV) and HIV-negative individuals using multivariable logistic regression modeling adjusted for age, sex, body mass index, race, hepatitis C virus, and cytomegalovirus serostatus, smoking status, alcohol use, interleukin-6, D-dimer, and soluble CD14. Models for HIV+ also adjusted for plasma HIV-1 ribonucleic acid suppression (<500 copies/mL). T-cell percentage is plotted on the x-axis, and risk of prevalent diabetes is plotted on the y-axis. Error bars represent 95% confidence intervals.
Table 3.
Fully Adjusted Multivariable Model Assessing the Relationship of T-cell Subsets With Diabetes by HIV Status
| T-cell Subset | HIV Positive | HIV Negative | ||
|---|---|---|---|---|
| Odds Ratio (95% CI)a | P Value | Odds Ratio (95% CI)a | P Value | |
| CD4+ T-cell Subsets | ||||
| CD4+ naive | 0.99 (0.86–1.14) | .88 | NL | .003 |
| CD4+CD38+ | NL | .01 | 0.80 (0.64–1.00) | .05 |
| CD4+CD57+ (senescent) | NL | .007 | 0.83 (0.66–1.02) | .08 |
| CD4+CD45RO+ (memory) | 1.19 (1.03–1.38) | .02 | 1.19 (1.00–1.41) | .05 |
| CD4+ central memory | NL | .02 | 1.18 (1.00–1.39) | .05 |
| CD4+ transitional memory | 0.98 (0.85–1.13) | .79 | NL | .01 |
| CD4+ effector memory | 0.81 (0.69–0.95) | .01 | 1.00 (0.80–1.25) | .98 |
| CD4+ TEMRA | 0.87 (0.75–1.00) | .06 | 1.07 (0.83–1.37) | .62 |
| CD4+ TH1 | 1.03 (0.90–1.19) | .64 | 1.10 (0.91–1.32) | .34 |
| CD4+ TH2 | 1.05 (0.92–1.19) | .47 | 0.82 (0.63–1.07) | .14 |
| CD4+ TH17 | 0.97 (0.84–1.12) | .71 | 0.76 (0.52–1.10) | .14 |
| CD4+CD28− | 0.83 (0.71–0.97) | .02 | 1.06 (0.82–1.36) | .66 |
| CD4+CD25+FoxP3+ | 1.01 (0.86–1.18) | .89 | 1.04 (0.91–1.20) | .53 |
| CD8+ T-cell Subsets | ||||
| CD8+ naive | 1.18 (1.00–1.38) | .05 | NL | .02 |
| CD8+CD38+ | NL | <.001 | NL | .03 |
| CD8+CD57+ (senescent) | 1.00 (0.86–1.17) | .97 | 0.84 (0.71–0.98) | .03 |
| CD8+CD45RO+ (memory) | 0.94 (0.81–1.10) | .46 | 1.21 (0.99–1.47) | .06 |
| CD8+ central memory | 1.03 (0.89–1.19) | .67 | 1.12 (0.95–1.32) | .19 |
| CD8+ transitional memory | 0.97 (0.83–1.12) | .67 | 1.34 (1.09–1.65) | .006 |
| CD8+ effector memory | 0.93 (0.80–1.08) | .33 | 0.96 (0.77–1.20) | .73 |
| CD8+ TEMRA | 0.92 (0.79–1.08) | .33 | 0.91 (0.78–1.08) | .28 |
| CD8+ CD28- | 0.83 (0.71–0.98) | .03 | 0.89 (0.74–1.07) | .22 |
| Soluble Biomarkersb | ||||
| Interleukin-6 | NL | .02 | 1.03 (0.91–1.16) | .66 |
| Soluble CD14 | NL | <.001 | NL | .01 |
| D-dimer | 1.02 (0.89–1.18) | .75 | NL | .006 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; NL, nonlinear models; TEMRA, T effector memory RA+.
aOdds ratio only provided for linear models.
bModel 3 excluded the soluble biomarker as a covariate that was also the outcome for the model.
NOTE: P < .05 are bolded. Model 3: adjusted for age, sex, body mass index, viral suppression (HIV + only), race, hepatitis C serostatus, smoking status, alcohol use, cytomegalovirus serostatus, interleukin-6, D-dimer, and soluble CD14.
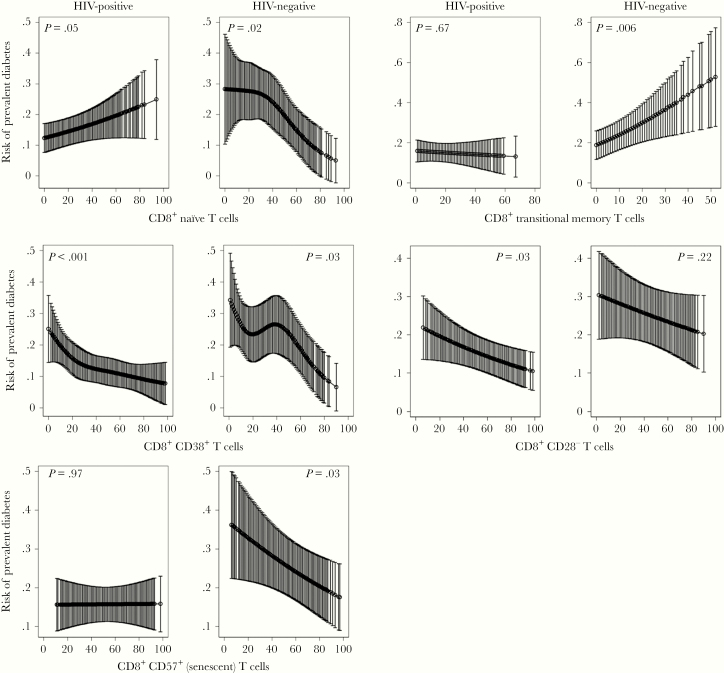
Figure 2 and Table 3 show CD8+ T-cell subsets significantly associated with prevalent diabetes from model 3 in PWH and/or HIV-negative individuals. As observed for CD4+ T cells, a lower proportion of CD38+CD8+ T cells was associated with diabetes in both PWH and HIV-negative individuals (P < .001 and P = .03, respectively). However, the results for naive CD8+ T cells were divergent: in PWH, a higher proportion of naive cells was associated with diabetes (P = .05), whereas in HIV-negative persons, a lower proportion was associated with diabetes (P = .02). A lower proportion of senescent (P = .03) and higher proportion of transitional memory (P = .006) CD8+ T cells were also associated with prevalent diabetes in HIV-negative only. Reduced proportion of CD8+CD28− T cells were associated with diabetes in PWH (P = .03). The interaction term assessing the effect of HIV status on the relationship of each T-cell subset with diabetes was not significant for any of the CD4+ subsets (P > .05) and only significant for naive CD8+ T cells (P = .02) and transitional memory CD8+ T cells (P = .02).
Figure 2.
CD8+ T-cell subsets associated with prevalent diabetes in persons with human immunodeficiency virus (HIV) and HIV-negative individuals using multivariable logistic regression modeling adjusted for age, sex, body mass index, race, hepatitis C virus, and cytomegalovirus serostatus, smoking status, alcohol use, interleukin-6, D-dimer, and soluble CD14. Models for HIV+ also adjusted for plasma HIV-1 ribonucleic acid suppression (<500 copies/mL). T-cell percentage is plotted on the x-axis and risk of prevalent diabetes is plotted on the y-axis. Error bars represent 95% confidence intervals.
Finally, we assessed the relationship of plasma biomarkers with diabetes status. Higher concentrations of sCD14 were associated with diabetes in both PWH (P < .001) and HIV-negative persons (P = .01). Elevated concentrations of IL-6 were associated with diabetes in PWH (P = .02), whereas elevated D-dimer was associated with diabetes in HIV-negative persons (P = .006) only. Supplementary Table 5 provides results for models 1, 2, and 3.
DISCUSSION
The association of systemic inflammation and insulin resistance in both PWH and HIV-negative individuals has been extensively reported in the literature [10, 11, 29, 30], but the adaptive immune system has recently emerged as a potential contributor to metabolic dysregulation [31–33]. In the largest and most comprehensive assessment of T-cell subsets and diabetes to date, we show that expansion of memory CD4+ T cells is associated with prevalent diabetes in both PWH and HIV-negative persons. The striking similarity in the T-cell subsets associated with diabetes after adjusting for systemic inflammation, CMV serostatus, and a range of common risk factors for metabolic disease suggests that chronic antigen stimulation and memory cell expansion may have a role in the development of diabetes in PWH.
A link between expanded CD4+ memory T cells and diabetes is consistent with prior studies of general population cohorts [20, 34]. In the Multi-Ethnic Study of Atherosclerosis (MESA), decreased naive and increased memory CD4+ T cells were associated with prevalent diabetes, although adjusting for CMV seropositivity attenuated this relationship in memory T cells [20]. A similar relationship has been shown in other metabolic diseases including atherosclerosis [35, 36]. Memory T-cell populations reflect a small pool of T effector cells that survive after initial pathogen elimination. The source of expanded memory subsets in diabetes is unclear, but it could reflect antigen stimulation in the peripheral tissues, including adipose tissue, which undergoes marked changes in T-cell populations after HIV infection [37, 38]. Alternatively, expansion of memory T-cell subsets may occur as a consequence of increased infection rate that accompanies diabetes [39].
Human immunodeficiency virus infection alters the adaptive immune system with progressive loss of naive CD4+ T cells and activation of CD4+ and CD8+ T cells [21, 40]. Depletion of naive T cells and chronic antigen stimulation can lead to increased terminally differentiated T-cell subsets with reduced proliferative ability, shortened telomeres, and decreased responsiveness to stimuli [41]. These changes parallel complex changes observed in the aging immune system, wherein naive T cells decrease with expansion of memory T-cell pools that, in the setting of chronic antigen stimulation and innate immune activation, promote T-cell senescence and decreased ability to respond to new pathogens [42]. Persons with HIV are at risk for many of the same diseases observed in the aging population, and HIV-induced immune system changes have been implicated [43].
We found no association between circulating TH1, TH2, TH17, or Treg CD4+ cells and diabetes among PWH and HIV-negative individuals. This was also in contrast to prior studies in HIV-negative individuals [44, 45]. Obese individuals were observed to have (1) a polarization towards a proinflammatory CD4+ helper T-cell phenotype in one study and (2) a higher TH1/TH2 ratio correlated with insulin resistance [44]. In our study, it is important to note that we stimulated cells in a T-cell receptor (TCR)-independent manner using PMA/ionomycin, whereas the cited study used plate-bound antihuman CD3 antibodies. This might account for differences in measured T- helper immune responses, which might require antigen-specific stimulation via TCRs. Similar studies have also reported a proinflammatory T-helper cell polarization in diabetic persons [45, 46]. Furthermore, circulating regulatory T cells, which have an anti-inflammatory immunomodulating role, were reduced in diabetic individuals in some studies [18, 19, 46], although not in others [34]. Notably, we found that a lower level of CD4+CD28− T cells was associated with diabetes in PWH, which was unexpected given the age-inappropriate expansion of CD4+CD28− T cells in patients with a variety of chronic inflammatory diseases [47–49]. These cells can produce large amounts of interferon-γ and tumor necrosis factor-α, have cytotoxic potential, and are thought to contribute to the pathogenesis of several autoimmune diseases and solid organ transplant rejection. Our findings suggest that CD4+CD28− T-cell expansion is not a contributor to the development of type 2 diabetes, although the basis for the observed inverse association is not clear.
Finally, we found that IL-6 and sCD14, markers of innate immune activation, were associated with prevalent diabetes in PWH, consistent with prior studies [10]. More important, even after adjusting for IL-6, sCD14, and D-dimer, changes in CD4+ T-cell subsets were still significantly associated with prevalent diabetes, which confirms that heightened innate immune activation alone does not fully account for the development of diabetes in PWH.
This study has several strengths. First, VACS-BC is the largest cohort of PWH and HIV-negative individuals, recruited from a single medical system, with broad immune phenotyping of T-cell subsets and comprehensive medical records. Although prior studies have examined immune cells associated with diabetes in the general population (MESA eg) [20], no studies have investigated these associations in PWH. Second, we were able to utilize multiple data points from surveys and the VA electronic medical record to provide accurate categorization of cases of diabetes, which were adjudicated by physician review. Third, we measured T-cell phenotypes, plasma inflammatory markers, and determined CMV serostatus at the same time point, and we used a model to assess the relationship of each factor with diabetes and investigate whether the adaptive immune system was independently associated with diabetes.
Our study also had several limitations. First, this was a cross-sectional study so we are only able to show association of T-cell subsets with prevalent diabetes, and future analyses will explore the association with incident diabetes. Second, CD38+ is commonly used as a marker for T-cell activation, but it can also be found in naive T cells. Due to our methodology, we do not have other markers of activation (eg, HLA-DR) and we are unable to confirm that CD38+ represents only T-cell activation. Therefore, we reported and analyzed CD38+ T cells without classifying them as activated. Third, HIV-negative individuals had higher prevalence of diabetes than PWH, which may reflect differences in comorbidities and other risk factors. However, this does not affect the validity of our primary results because we compared the association of T-cell subsets with diabetes among PWH and HIV-negative individuals separately. Fourth, this study may not be generalizable because individuals were overwhelmingly male and included a higher proportion of black participants. Fifth, we cannot exclude potential effects related to use of cryopreserved cells rather than fresh whole blood for flow cytometry, although data in healthy individuals indicate little, if any, differences [50]. Finally, this analysis was conducted to discover novel adaptive immune changes associated with diabetes. Therefore, we did not adjust for multiple comparisons and may have found significant associations by chance. However, most of the associations between T-cell subsets and diabetes were observed in both PWH and HIV-negative participants, which argues against spurious findings.
CONCLUSIONS
In summary, we show that T-cell alterations including expansion of memory phenotype CD4+ cells are associated with diabetes in both PWH and HIV-negative individuals, even after adjusting for circulating markers of the innate immune system and CMV serostatus. These changes were broadly similar regardless of HIV status and suggest that diabetes in PWH may be related to, or possibly mediated by, changes in the T-cell compartment. Further work will be necessary to examine potential mechanisms linking chronic immune stimulation and memory cell expansion with metabolic dysfunction.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: ID Week, October 2019, Washington D.C.
Disclaimer. The funders had no role in the study design, data collection and analyses, decision to publish, or preparation of the manuscript. Views presented in the manuscript are those of the authors and do not reflect those of the Department of Veterans Affairs or the United States Government.
Financial support. This work was funded by the National Institute on Alcohol Abuse and Alcoholism by COMpAAAS/Veterans Aging Cohort Study (Grant Numbers U24 AA020794, U01 AA020790, U01 AA020795, U01 AA020799, and U10 AA013566); the National Institute of Diabetes and Digestive and Kidney Diseases (R56-DK108352); the National Heart Lung and Blood Institute (R01 HL125032); and the National Institute of Allergy and Infectious Diseases (Grant Numbers P30 AI110527 and T32 AI00747426; to S. S. B.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Crum-Cianflone N, Roediger MP, Eberly L, et al. . Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS One 2010; 5:e10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown TT, Cole SR, Li X, et al. . Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med 2005; 165:1179–84. [DOI] [PubMed] [Google Scholar]
- 3. Taylor BS, Liang Y, Garduño LS, et al. . High risk of obesity and weight gain for HIV-infected uninsured minorities. J Acquir Immune Defic Syndr 2014; 65:e33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Wit S, Sabin CA, Weber R, et al. . Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Diabetes Care 2008; 31:1224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Butt AA, McGinnis K, Rodriguez-Barradas MC, et al. . HIV infection and the risk of diabetes mellitus. AIDS 2009; 23:1227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duncan AD, Goff LM, Peters BS. Type 2 diabetes prevalence and its risk factors in HIV: a cross-sectional study. PLoS One 2018; 13:e0194199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Samad F, Harris M, Puskas CM, et al. . Incidence of diabetes mellitus and factors associated with its development in HIV-positive patients over the age of 50. BMJ Open Diabetes Res Care 2017; 5:e000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hernandez-Romieu AC, Garg S, Rosenberg ES, Thompson-Paul AM, Skarbinski J. Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009–2010. BMJ Open Diabetes Res Care 2017; 5:e000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herrin M, Tate JP, Akgün KM, et al. . Weight gain and incident diabetes among HIV-infected veterans initiating antiretroviral therapy compared with uninfected individuals. J Acquir Immune Defic Syndr 2016; 73:228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Betene ADC, De Wit S, Neuhaus J, et al. . Interleukin-6, high sensitivity C-reactive protein, and the development of type 2 diabetes among HIV-positive patients taking antiretroviral therapy. J Acquir Immune Defic Syndr 2014; 67:538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001; 286:327–34. [DOI] [PubMed] [Google Scholar]
- 12. Min D, Brooks B, Wong J, et al. . Alterations in monocyte CD16 in association with diabetes complications. Mediators Inflamm 2012; 2012:649083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shikuma CM, Chow DC, Gangcuangco LM, et al. . Monocytes expand with immune dysregulation and is associated with insulin resistance in older individuals with chronic HIV. PLoS One 2014; 9:e90330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, McComsey GA. Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care 2010; 33:2244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Neuhaus J, Jacobs DR Jr, Baker JV, et al. . Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis 2010; 201:1788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sandler NG, Wand H, Roque A, et al. . Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reiner AP, Lange EM, Jenny NS, et al. . Soluble CD14: genomewide association analysis and relationship to cardiovascular risk and mortality in older adults. Arterioscler Thromb Vasc Biol 2013; 33:158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zeng C, Shi X, Zhang B, et al. . The imbalance of Th17/Th1/Tregs in patients with type 2 diabetes: relationship with metabolic factors and complications. J Mol Med (Berl) 2012; 90:175–86. [DOI] [PubMed] [Google Scholar]
- 19. Wagner NM, Brandhorst G, Czepluch F, et al. . Circulating regulatory T cells are reduced in obesity and may identify subjects at increased metabolic and cardiovascular risk. Obesity (Silver Spring) 2013; 21:461–8. [DOI] [PubMed] [Google Scholar]
- 20. Olson NC, Doyle MF, de Boer IH, et al. . Associations of circulating lymphocyte subpopulations with type 2 diabetes: cross-sectional results from the Multi-Ethnic Study of Atherosclerosis (MESA). PLoS One 2015; 10:e0139962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hazenberg MD, Hamann D, Schuitemaker H, Miedema F. T cell depletion in HIV-1 infection: how CD4+ T cells go out of stock. Nat Immunol 2000; 1:285–9. [DOI] [PubMed] [Google Scholar]
- 22. Lichtner M, Cicconi P, Vita S, et al. . Cytomegalovirus coinfection is associated with an increased risk of severe non-AIDS-defining events in a large cohort of HIV-infected patients. J Infect Dis 2015; 211:178–86. [DOI] [PubMed] [Google Scholar]
- 23. Fülöp T, Larbi A, Pawelec G. Human T cell aging and the impact of persistent viral infections. Front Immunol 2013; 4:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spyridopoulos I, Martin-Ruiz C, Hilkens C, et al. . CMV seropositivity and T-cell senescence predict increased cardiovascular mortality in octogenarians: results from the Newcastle 85+ study. Aging Cell 2016; 15:389–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen S, de Craen AJ, Raz Y, et al. . Cytomegalovirus seropositivity is associated with glucose regulation in the oldest old. Results from the Leiden 85-plus Study. Immun Ageing 2012; 9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Justice AC, Dombrowski E, Conigliaro J, et al. . Veterans Aging Cohort Study (VACS): overview and description. Med Care 2006; 44:S13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Medapalli RK, Parikh CR, Gordon K, et al. . Comorbid diabetes and the risk of progressive chronic kidney disease in HIV-infected adults: data from the Veterans Aging Cohort Study. J Acquir Immune Defic Syndr 2012; 60:393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tracy RP, Doyle MF, Olson NC, et al. . T-helper type 1 bias in healthy people is associated with cytomegalovirus serology and atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc 2013; 2:e000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang M, Gan H, Shen Q, Tang W, Du X, Chen D. Proinflammatory CD14+CD16+ monocytes are associated with microinflammation in patients with type 2 diabetes mellitus and diabetic nephropathy uremia. Inflammation 2012; 35:388–96. [DOI] [PubMed] [Google Scholar]
- 30. Pandzic Jaksic V, Gizdic B, Miletic Z, Trutin-Ostovic K, Jaksic O. Association of monocyte CCR2 expression with obesity and insulin resistance in postmenopausal women. Clin Invest Med 2013; 36:E24–31. [DOI] [PubMed] [Google Scholar]
- 31. Deiuliis J, Shah Z, Shah N, et al. . Visceral adipose inflammation in obesity is associated with critical alterations in Tregulatory cell numbers. PLoS One 2011; 6:e16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Feuerer M, Herrero L, Cipolletta D, et al. . Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 2009; 15:930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nishimura S, Manabe I, Nagasaki M, et al. . CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 2009; 15:914–20. [DOI] [PubMed] [Google Scholar]
- 34. Rattik S, Engelbertsen D, Wigren M, et al. . Elevated circulating effector memory T cells but similar levels of regulatory T cells in patients with type 2 diabetes mellitus and cardiovascular disease. Diab Vasc Dis Res 2019; 16:270–80. [DOI] [PubMed] [Google Scholar]
- 35. Olson NC, Doyle MF, Jenny NS, et al. . Decreased naive and increased memory CD4(+) T cells are associated with subclinical atherosclerosis: the multi-ethnic study of atherosclerosis. PLoS One 2013; 8:e71498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ammirati E, Cianflone D, Vecchio V, et al. . Effector memory T cells are associated with atherosclerosis in humans and animal models. J Am Heart Assoc 2012; 1:27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wanjalla CN, McDonnell WJ, Koethe JR. Adipose tissue T Cells in HIV/SIV infection. Front Immunol 2018; 9:2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Couturier J, Suliburk JW, Brown JM, et al. . Human adipose tissue as a reservoir for memory CD4+ T cells and HIV. AIDS 2015; 29:667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carey IM, Critchley JA, DeWilde S, Harris T, Hosking FJ, Cook DG. Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study. Diabetes Care 2018; 41:513–21. [DOI] [PubMed] [Google Scholar]
- 40. Bofill M, Mocroft A, Lipman M, et al. . Increased numbers of primed activated CD8+CD38+CD45RO+ T cells predict the decline of CD4+ T cells in HIV-1-infected patients. AIDS 1996; 10:827–34. [DOI] [PubMed] [Google Scholar]
- 41. Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol 2008; 214:231–41. [DOI] [PubMed] [Google Scholar]
- 42. Fulop T, Larbi A, Dupuis G, et al. . Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol 2017; 8:1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 2011; 62:141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Viardot A, Heilbronn LK, Samocha-Bonet D, Mackay F, Campbell LV, Samaras K. Obesity is associated with activated and insulin resistant immune cells. Diabetes Metab Res Rev 2012; 28:447–54. [DOI] [PubMed] [Google Scholar]
- 45. Zhao R, Tang D, Yi S, et al. . Elevated peripheral frequencies of Th22 cells: a novel potent participant in obesity and type 2 diabetes. PLoS One 2014; 9:e85770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jagannathan-Bogdan M, McDonnell ME, Shin H, et al. . Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J Immunol 2011; 186:1162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Broux B, Markovic-Plese S, Stinissen P, Hellings N. Pathogenic features of CD4+CD28- T cells in immune disorders. Trends Mol Med 2012; 18:446–53. [DOI] [PubMed] [Google Scholar]
- 48. Schmidt D, Goronzy JJ, Weyand CM. CD4+ CD7- CD28- T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest 1996; 97:2027–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Betjes MG, Huisman M, Weimar W, Litjens NH. Expansion of cytolytic CD4+CD28- T cells in end-stage renal disease. Kidney Int 2008; 74:760–7. [DOI] [PubMed] [Google Scholar]
- 50. Thyagarajan B, Barcelo H, Crimmins E, et al. . Effect of delayed cell processing and cryopreservation on immunophenotyping in multicenter population studies. J Immunol Methods 2018; 463:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.