Abstract
Purpose:
Strabismus or anisometropia disrupts binocularity and results in fixation instability, which is increased with amblyopia. Fixation instability has typically been assessed for each eye individually. Recently, vergence instability was reported in exotropic adults and monkeys during binocular viewing. We evaluated fixation instability during binocular viewing in children treated for anisometropia and/or strabismus.
Methods:
160 children age 4–12 years with treated esotropia and/or anisometropia (98 amblyopic, 62 nonamblyopic) were compared to 46 age-similar controls. Fixation instability was recorded during binocular fixation of a 0.3 deg diameter dot for 20 s using a 500 Hz remote video binocular eye tracker (EyeLink 1000; SR Research). The bivariate contour ellipse area (BCEA; log deg2) for fixation instability was calculated for each eye (nonpreferred, preferred) and for vergence instability (left eye position – right eye position). Best-corrected visual acuity, Randot Preschool stereoacuity, and extent of suppression scotoma (Worth 4-Dot) were also obtained.
Results:
When binocularly viewing, both amblyopic and nonamblyopic children treated for anisometropia and/or strabismus had larger fixation instability and vergence instability than controls. Amblyopia primarily added to the instability of the nonpreferred eye. Anisometropic children had less nonpreferred eye instability and vergence instability than those with strabismus or combined mechanism. Nonpreferred eye instability and vergence instability were related to poorer stereoacuity and a larger suppression scotoma. Preferred eye instability was not related to any visual outcome measure. No relationships were found with visual acuity.
Conclusions:
Fixation instability and vergence instability during binocular viewing suggests that discordant binocular visual experience during childhood, especially strabismus, interferes with ocular motor development. Amblyopia adds to instability of the nonpreferred eye. Vergence instability may limit potential for recovery of binocular vision in these children.
Keywords: Fixation instability, Vergence, Amblyopia, Strabismus, Anisometropia, Ocular motor development
1. Introduction
Binocularly discordant visual experience in infancy or early childhood from anisometropia (unequal refractive error), strabismus (misalignment of the visual axis), or a combination of both can hinder visual maturation during this critical period of rapid brain development. Even when treated with optical correction and/or surgical alignment, these pediatric eye conditions are associated with a constellation of visual deficits that include amblyopia (i.e., decreased visual acuity in one eye - ‘lazy eye’) (Birch, 2013), binocular dysfunction (i.e., impaired stereoacuity, interocular suppression) (Birch, 2003, 2013; Birch and Stager, 2006; Birch et al., 2016; Li et al., 2011), and ocular motor impairment (i.e., abnormal saccade initiation and execution) (Niechwiej-Szwedo et al., 2012; Niechwiej-Szwedo et al., 2010), reduced vergence (Kelly et al., 2016), and fixation instability (Birch et al., 2013; González et al., 2012; Subramanian et al., 2013).
During fixation, the eyes are constantly moving, with subtle, rapid flicks of the eye (i.e., microsaccades), slow drifts, and small, quick oscillations (i.e., tremors) (Otero-Millan et al., 2014; Martinez-Conde, 2006). Remarkably, these fixational eye movements do not disrupt binocularity, indicating a tight link between the visual and motor systems. However, when these involuntary eye movements become excessive, fixation is unstable and visual function is disrupted. Fixation instability has been observed in visual disorders including age-related macular degeneration (Markowitz and Steinbach, 2008; Tarita-Nistor et al., 2011), infantile nystagmus (Felius et al., 2011), and amblyopia (Birch et al., 2013; González et al., 2012; Subramanian et al., 2013; Chung et al., 2015; Shaikh et al., 2016).
Fixation instability is a hallmark of strabismus, anisometropia, and amblyopia. Instability is due to increased drift, saccadic oscillations, disconjugacy of fixational saccades, and fusion maldevelopment nystagmus (FMNS; a repeated pattern of a slow nasalward drift, with a rapid refixating temporalward saccade) (Birch et al., 2013; Otero-Millan et al., 2014; Martinez-Conde, 2006; Ciuffreda et al., 1979, 1980; Shi et al., 2012; Tychsen, 2007; Ghasia et al., 2018). Research to date has typically focused on fixation instability under monocular viewing conditions. Instability in the nonpreferred eye is larger than in the preferred eye and compared to control eyes (González et al., 2012; Subramanian et al., 2013; Chung et al., 2015; Shaikh et al., 2016); however, some have found that the preferred eye may also show in stability (Shaikh et al., 2016; Economides et al., 2016). While fixation instability is increased in amblyopia (Birch et al., 2013; González et al., 2012; Subramanian et al., 2013; Chung et al., 2015), amblyopia is not a necessary condition (Birch et al., 2013; Subramanian et al., 2013; Ciuffreda et al., 1979; Ghasia et al., 2018; Economides et al., 2016), suggesting that instability is the consequence of discordant binocular visual experience during visual development.
Only a handful of studies have examined fixation instability during binocular viewing in non-human primate models of strabismus and in strabismic and amblyopic adults (González et al., 2012; Ciuffreda et al., 1979; Tychsen, 2007; Upadhyaya et al., 2017). Ciuffreda et al. (1979) found that even during binocular viewing, fixation instability was present in strabismic adults (Ciuffreda et al., 1979), but no control group was assessed. Gonzalez et al. (González et al., 2012) found that amblyopic eye instability was larger than fellow eye instability, and larger than right eye instability of controls during binocular viewing, while fellow eye instability did not differ from right eye instability of controls. Recently, vergence instability (i.e., variability in ocular alignment over time) during binocular viewing was reported in exotropic monkeys and adults with large deviations, suggesting that a lack of binocularity may be associated with disconjugate eye movements even with binocular fixation (Economides et al., 2016; Upadhyaya et al., 2017).
This study is the first to evaluate fixation instability and vergence instability during binocular viewing in children treated for anisometropia and/or strabismus with or without amblyopia. We also assessed factors that may be associated with instability, such as etiology, presence of amblyopia, severity of amblyopia, and binocularity (i.e., stereoacuity, suppression). As amblyopia is increasingly being appreciated as a binocular rather than monocular disease (Birch, 2013; Hess and Thompson, 2015), it is important to assess how this pediatric eye condition affects children during a more natural, binocular viewing condition.
2. Material and methods
2.1. Participants
A total of 160 children age 4–12 years treated for strabismus, anisometropia, or both (i.e., combined mechanism), with amblyopia (n = 98) or without amblyopia (n = 62), were referred to the Retina Foundation by 18 pediatric ophthalmologists in the Dallas-Fort Worth area. Amblyopia was defined as an interocular difference in visual acuity ≥0.2 logMAR, best-corrected visual acuity in the amblyopic eye ≥0.2 logMAR (20/32 or worse), and best-corrected visual acuity in the fellow eye ≤0.1 logMAR (20/25 or better; 0.2 logMAR [20/32] for age 4 years). Anisometropic children were diagnosed with hyperopic and/or astigmatic anisometropia ≥1.0 diopter spherical equivalent or astigmatism with or without microtropia. Strabismic children were initially diagnosed with esotropia, but were aligned with surgery and/or spectacle correction within 6 prism diopters of orthotropia at near and at distance. A group of 46 age-similar, normal control children were also enrolled who had no history of vision disorders, normal visual acuity, and normal Randot® stereoacuity. None of the children were born preterm (< 32 weeks postmenstrual age), or had co–existing ocular or systemic disease, congenital infections/malformations, or developmental delay. English was the primary language for all children.
2.2. Ethics
The research protocol observed the tenets of the Declaration of Helsinki, was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center and conformed to the requirements of the United States Health Insurance Portability and Privacy Act. Informed consent was obtained from a parent or legal guardian and assent was obtained from children ≥10 years of age prior to testing and after explanation of the study.
2.3. Procedure
2.3.1. Vision assessment
Vision assessments were conducted prior to fixation testing, and included: 1) Crowded monocular visual acuity using the EVA e-ETDRS protocol (Beck et al., 2003; Cotter et al., 2003) for children ≥7 years of age, or the Amblyopia Treatment Study HOTV protocol for children < 7 years of age (Moke et al., 2001; Holmes et al., 2001). 2) Stereoacuity using Randot Preschool and Randot Butterfly tests (Stereo Optical Co., Inc., Chicago, IL, USA). 3) Extent of suppression scotoma using the Worth 4-Dot test at 7 different distances. A flashlight with 4 equidistant lights (one white, one red, two green) was shown at 7 different distances (300 cm, 200 cm, 100 cm, 67 cm, 50 cm, 33 cm, 16 cm) while the child wore red-green anaglyph glasses. The maximum distance at which the child could see all four lights (fusion) was noted, providing an estimate of suppression scotoma size (log deg). (Rosenbaum and Santiago, 1999; Kelly et al., 2018).
2.3.2. Apparatus and eye movement recording
Testing took place in a dimly lit room and children wore habitual optical correction during testing, if required. Stabilization of the child’s head was accomplished using a chin/forehead rest. A liquid-crystal display (LCD) projector was used to present stimuli on a rear-projection screen (27°) at a viewing distance of 115 cm. Distance viewing was chosen to avert vergence dampening of FMNS that can occur at near (Gradstein et al., 1998). Eye positions were recorded with a 500 Hz high-speed video binocular eye tracker (EyeLink 1000; SR Research, Ontario, Canada). Rather than using the built-in EyeLink 1000 calibration sequence, a custom 5-point (0°, ± 10° vertical, ± 10° horizontal presented for 4 s each at location) monocular calibration was performed for each eye using a small white dot (0.3° diameter) presented on a black background. Monocular occlusion during calibration was implemented using a Hoya R72 infrared filter (IR; blocks < 720 nm; LED dominant wavelength was 625 nm). This allowed the EyeLink 1000 eye tracker to record movements of the occluded eye in addition to the fixating eye. The custom calibration was used to avoid issues that may occur during calibration in children who have microtropia, large fixation instability and/or nystagmus as a result of amblyopia, and has been used by our lab to assess stability in children with dense congenital or infantile cataracts (Birch et al., 2012). Following calibration, fixation instability during binocular viewing was recorded while the child viewed the same small white 0.3° diameter dot at primary position (X, Y = 0°, 0°) for 20 s. A sound was presented every 4 s to keep the child’s attention during fixation. Both eyes were recorded simultaneously.
2.4. Data processing
All data were exported from the EyeLink Data Viewer and were processed using custom software written in MATLAB (Mathworks, Natick, MA) that included a graphical user interface to visualize the data. Horizontal and vertical monocular calibration values were applied to the eye position data for each eye. Artifacts, large saccades (> 5°), and blinks detected by EyeLink were removed prior to analysis.
Fixation instability was defined by the size of the bivariate contour ellipse area (BCEA), an area within which 68% of all the x,y-coordinates of fixation data points occurred (González et al., 2012). A smaller BCEA indicates more stable fixation whereas a larger BCEA indicates more instability. The BCEA for fixation instability during binocular viewing was calculated separately for the nonpreferred eye, for the preferred eye, and for vergence instability (left eye XY position – right eye XY position) (Upadhyaya et al., 2017). The nonpreferred eye was defined as the amblyopic eye or the previously amblyopic eye. For anisometropic children with no history of amblyopia, the eye that was at-risk for developing amblyopia (i.e., the eye with the higher refractive error) was chosen as the nonpreferred eye. For strabismic children with no history of amblyopia and control children, the right eye was chosen arbitrarily as the nonpreferred eye. All BCEAs were normalized by using a log10 transformation (González et al., 2012).
2.5. Statistical analyses
Mixed-model analyses of variance (ANOVAs) were conducted to determine differences in fixation instability per group and between eyes. Significant interactions and main effects were followed with posthoc pairwise comparisons. When interactions were not significant, main effects were reported. One-way ANOVAs were conducted to determine group differences in vergence instability. Significant one-way ANOVAs were followed with posthoc pairwise comparisons. For all posthoc comparisons, Bonferonni-corrected alphas ranged from 0.025 to 0.017. For primary analyses, patients were categorized as amblyopic or nonamblyopic, regardless of etiology (strabismus, anisometropia, combined mechanism) to compare against controls. For secondary analyses, patients were categorized into subgroups based on etiology (strabismus, anisometropia, combined mechanism) and degree of stereoacuity (bifixation, ≤60 arcsec; monofixation, 100–800 arcsec; nil > 800 arsec). Pearson r correlations were conducted to determine relationships of fixation instability and vergence instability with vision assessment outcomes (visual acuity, stereoacuity, and extent of suppression scotoma).
3. Results
Groups did not differ in age (amblyopic, mean age ± SD = 8.3 ± 2.3 years; nonamblyopic, 8.4 ± 2.6 years; control, 8.3 ± 2.7 years; F2,203 = 0.05, p = 0.95). Mean nonpreferred eye visual acuity ± SD was 0.48 ± 0.22 logMAR for amblyopic children, 0.06 ± 0.09 logMAR for nonamblyopic children children, and −0.05 ± 0.07 logMAR for control children. Of the patients, 64 children had anisometropia, 44 children had strabismus, and 52 children had both. Group characteristics are found in Table 1. Mean BCEA ± SD for fixation instability and vergence instability per eye per group can be found in Table 2. Fig. 1 shows representative eye position traces and fixation and vergence BCEAs from one child per group.
Table 1.
Group characteristics.
| Amblyopic (n = 98) | Nonamblyopic (n = 62) | Control (n = 46) | |
|---|---|---|---|
| Sex: F, n (%) | 35 (36) | 33 (53) | 23 (50) |
| Age (years), n (%) | |||
| 4–7 | 46 (47) | 27 (44) | 20 (43) |
| 8–10 | 37 (38) | 23 (37) | 16 (35) |
| 11–13 | 15 (15) | 12 (19) | 10 (22) |
| Mean ± SDa (years) | 8.31 ± 2.29 | 8.43 ± 2.57 | 8.31 ± 2.74 |
| Nonpreferred eye visual acuity (logMARb), n (%) (Snellen equivalent) | |||
| No amblyopiac | 0 (0) | 62 (100) | 46 (100) |
| −0.1–0.2 (20/16–20/32) | |||
| Mild to moderate amblyopiad | 76 (78) | N/A | N/A |
| 0.2–0.6 (20/32–20/80) | |||
| Severe amblyopiad | 22 (22) | N/A | N/A |
| 0.7–0.8 (20/100–20/125) | |||
| Mean ± SD (Snellen equivalent) | 0.48 ± 0.22 (20/60 ± 2.2 lines) | 0.06 ± 0.09 (20/23 ± 0.9 lines) | −0.05 ± 0.07 (20/18 ± 0.7 lines) |
| Preferred eye visual acuity (logMAR), n (%) (Snellen equivalent) | −0.02 ± 0.08 | −0.01 ± 0.09 | −0.04 ± 0.07 |
| Mean ± SD (Snellen equivalent) | (20/19 ± 0.8 lines) | (20/20 ± 0.9 lines) | (20/18 ± 0.7 lines) |
| Etiology, n (%) | |||
| Anisometropia | 49 (50) | 15 (24) | N/A |
| Strabismus | 15 (15) | 29 (47) | N/A |
| Combined mechanism | 34 (35) | 18 (29) | N/A |
SD, standard deviation.
logMAR, logarithm of the minimum angle of resolution.
0.1 or leslogMAR interocular difference.
0.2 or greater logMAR interocular difference.
Table 2.
Fixation instability and vergence instability mean ± SD BCEAs for each group, and each etiology, and stereoacuity subgroup for children treated for anisometropia, strabismus, or both.
| Group | Nonpreferred eye BCEAa (log deg2) | Preferred eye BCEA (log deg2) | Vergence BCEA (log deg2) |
|---|---|---|---|
| Amblyopic (n = 98) | −0.02 ± 0.46 | −0.21 ± 0.40 | −0.02 ± 0.48 |
| Nonamblyopic (n = 62) | −0.07 ± 0.51 | −0.16 ± 0.43 | 0.00 ± 0.47 |
| Control (n = 46) | −0.35 ± 0.23 | −0.39 ± 0.29 | −0.38 ± 0.29 |
| Etiology | |||
| Anisometropic (n = 64) | −0.23 ± 0.43 | −0.28 ± 0.43 | −0.17 ± 0.48 |
| Strabismic (n = 44) | 0.09 ± 0.46 | −0.08 ± 0.39 | 0.06 ± 0.47 |
| Combined mechanism (n = 52) | 0.10 ± 0.46 | −0.17 ± 0.38 | 0.11 ± 0.44 |
| Stereoacuity category | |||
| Bifixation (≤60 arcsec; n=15) | −0.35 ± 0.40 | −0.29 ± 0.42 | −0.29 ± 0.43 |
| Monofixation (100–800 arcsec, n = 47) | −0.15 ± 0.48 | −0.17 ± 0.47 | −0.08 ± 0.53 |
| Nil (> 800 arsec, n = 98) | 0.07 ± 0.45 | −0.18 ± 0.38 | 0.06 ± 0.44 |
BCEA, bivariate contour ellipse area.
Fig. 1.
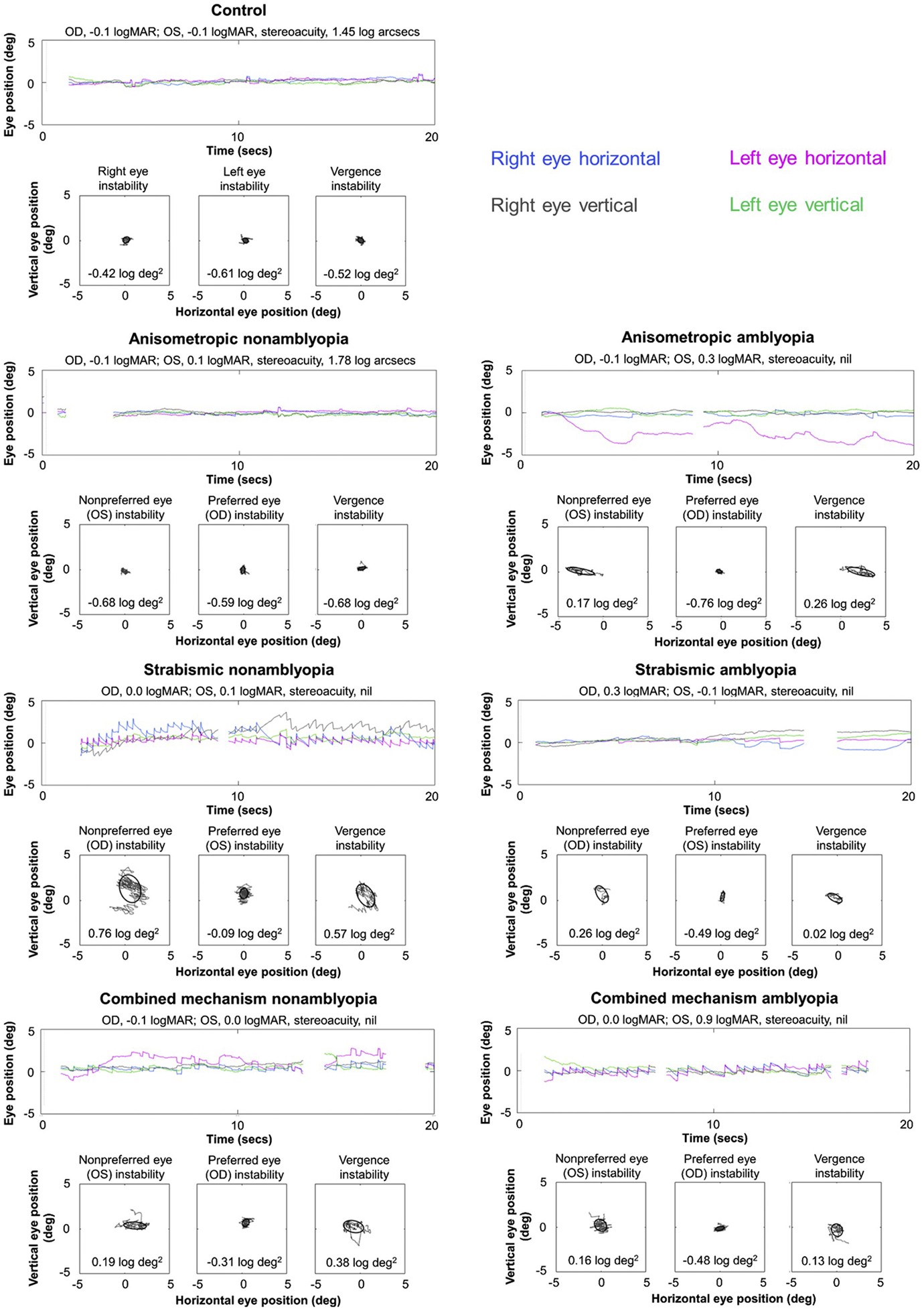
Examples of fixation instability and vergence instability. Representative eye position traces, fixation BCEAs (log deg2; nonpreferred eye, preferred eye) and vergence BCEAs (log deg2) from one child per group (A, control; B, anisometropic nonamblyopia; C, anisometropic amblyopia; D, strabismic nonamblyopia; E, strabismic amblyopia; F, combined mechanism nonamblyopia; G, combined mechanism amblyopia). While the two children with anisometropia (B, C) show little instability and no difference between eyes, children with strabismus and combined menchanism, regardless of amblyopia, have larger instability due to FMNS (D,G), saccadic oscillations (F), dissociated vertical deviation (D), and drift (E).
3.1. Fixation instability during binocular viewing
A 3 × 2 ANOVA of the BCEA (log deg2) with group (amblyopic, nonamblyopic, control) as the between-groups factor and eye (nonpreferred eye, preferred eye) as the within-groups factor revealed no significant group × eye interaction (F2,203 = 2.11, p = 0.1), but did reveal significant main effects of group (F2,203 = 9.84, p = 0.00008) and eye (F1,203 = 11.09, p = 0.001). For the main effect of group, fixation instability was larger in amblyopic children (p = 0.0001) and nonamblyopic children (p = 0.0006) compared to controls, but amblyopic children and nonamblyopic children did not differ (p = 1.00; Fig. 2A). For the main effect of eye, fixation instability was larger in the nonpreferred eye compared to the preferred eye (p = 0.00005).
Fig. 2.
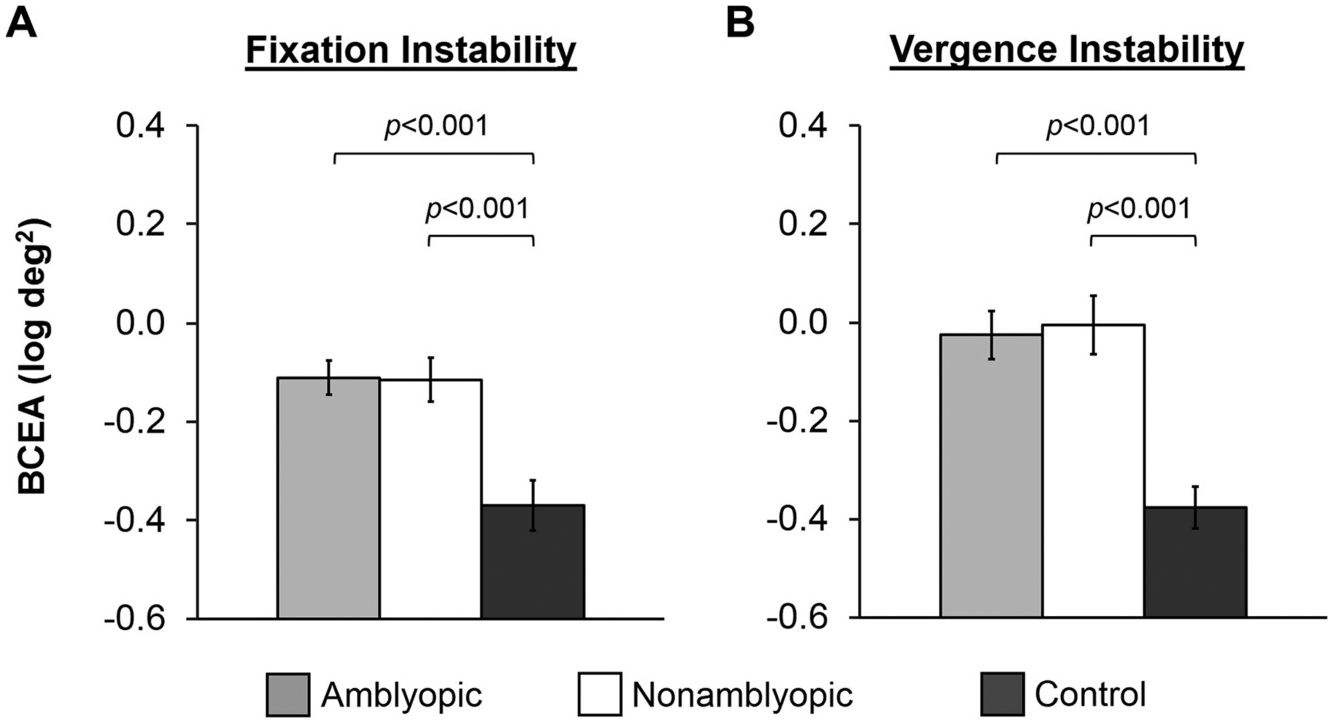
Amblyopia and instability. Bar graphs depicting main effects of group for A) fixation instability (collapsed across eye) and B) vergence instability for amblyopic children (light gray bars), nonamblyopic children (white bars), and normal control children (dark grey bars). Amblyopic and nonamblyopic children exhibited larger fixation and vergence instability compared to controls. Amblyopic and nonamblyopic children did not differ. Error bars represent ± standard error of the mean (SEM).
3.2. Vergence fixation instability during binocular viewing
A one-way ANOVA of the BCEA (log deg2) with group (amblyopic, nonamblyopic, control) as the between-groups factor revealed a significant effect of group on vergence instability (F2,203 = 11.84 p = 0.0001). Vergence instability was larger in amblyopic children (p = 0.00004) and nonamblyopic children (p = 0.00007) compared to control children. Amblyopic children and nonamblyopic children did not differ (p = 1.00, Fig. 2B).
3.3. Factors associated with fixation instability and vergence instability during binocular viewing
There was no difference in fixation instability between eyes for the control group (t45 = 1.06, p = 0.29), therefore subsequent analyses excluding controls were conducted to examine factors associated with fixation instability and vergence instability in children treated for anisometropia and/or strabismus.
3.3.1. Etiology
Fixation instability: A 3 × 2 × 2 ANOVA of the BCEA (log deg2) with etiology (anisometropia, strabismus, combined mechanism) and amblyopic category (amblyopic, nonamblyopic) as the between-groups factors and eye (nonpreferred, preferred) as the within-groups factor was conducted. The amblyopic category × eye × etiology (F2,154 = 0.41, p = 0.67) and amblyopic category × etiology (F2,154 = 0.78, p = 0.46) interactions were not significant. The amblyopic category × eye interaction was not significant but there was a potential trend (F1,154 = 3.61, p = 0.06; Fig. 3A). Bonferroni posthoc comparisons show that fixation instability was larger in the nonpreferred eye compared to the preferred eye for amblyopic children (p = 0.0005), but not for nonamblyopic children (p = 0.89). The etiology × eye interaction was significant (F2,154 = 3.28, p = 0.04; Fig. 3B). Bonferroni posthoc comparisons show that fixation instability was larger in the nonpreferred eye compared to the preferred eye for combined mechanism children (p = 0.0008), but not for anisometropic children (p = 1.00) or strabismic children (p = 0.29). Nonpreferred eye instability was larger in combined mechanism children (p = 0.008) and strabismic children (p = 0.02) compared with anisometropic children. Nonpreferred eye instability did not differ between combined mechanism and strabismic children (p = 1.00). Preferred eye instability did not differ between etiologies (p ≥ 0.73 for all three pairwise comparisons).
Fig. 3.
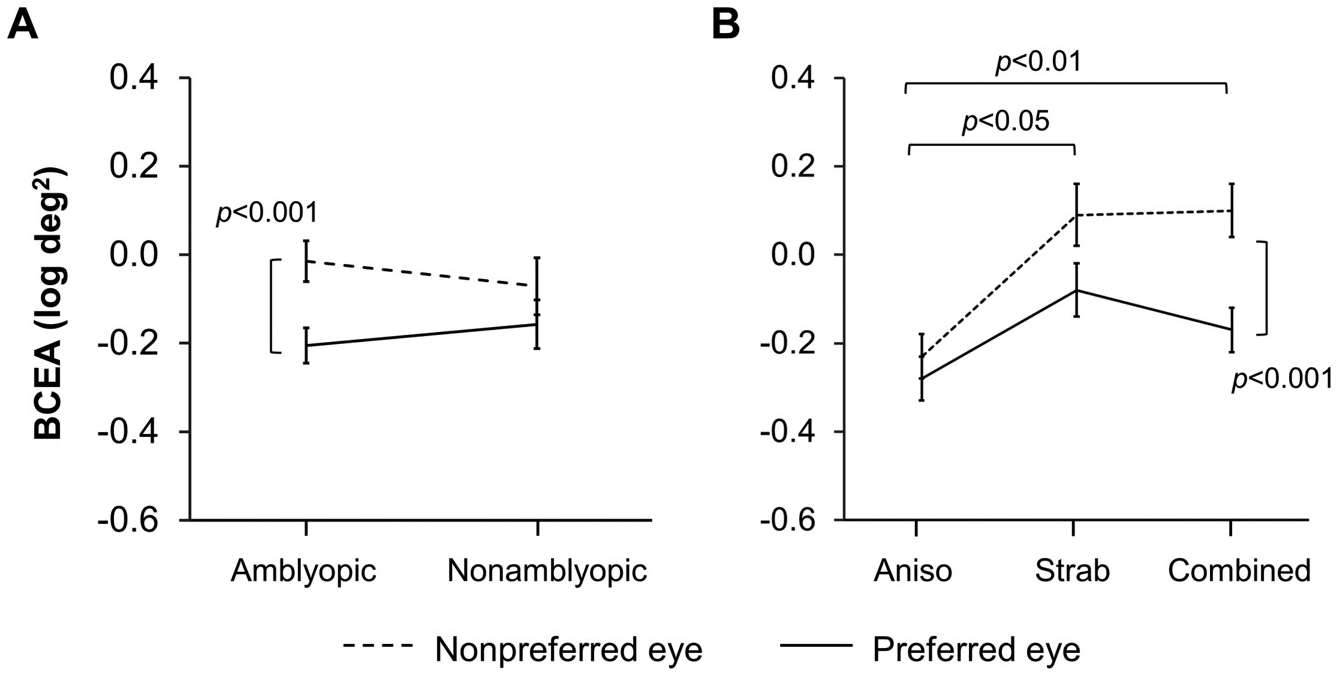
Factors affecting fixation instability; amblyopia and etiology. A) An interaction between eye (nonpreferred eye, dotted line; preferred eye, solid line) and amblyopic category (amblyopic, nonamblyopic) for fixation instability shows that nonpreferred eye instability was larger than preferred eye instability for amblyopic children, but not for nonamblyopic children. B) An interaction between eye (nonpreferred eye, dotted line; preferred eye, solid line) and etiology (anisometropia, strabismus, combined mechanism) for fixation instability shows that children with anisometropia had less nonpreferred eye instability than children with strabismus and children with combined mechanism. Children with combined mechanism had larger nonpreferred eye instability than preferred eye instability. Error bars represent ± SEM.
Vergence instability: A one-way ANOVA of the BCEA (log deg2) with etiology (anisometropia, strabismus, combined mechanism) as the between-groups factor revealed a significant effect of etiology (F2,157 = 6.22, p = 0.003; Fig. 4). Bonferroni posthoc comparisons show that vergence instability was larger in combined mechanism children (p = 0.004) and strabismic children (p = 0.03) compared to anisometropic children. Vergence instability did not differ between strabismic children and combined mechanism children (p = 1.00).
Fig. 4.
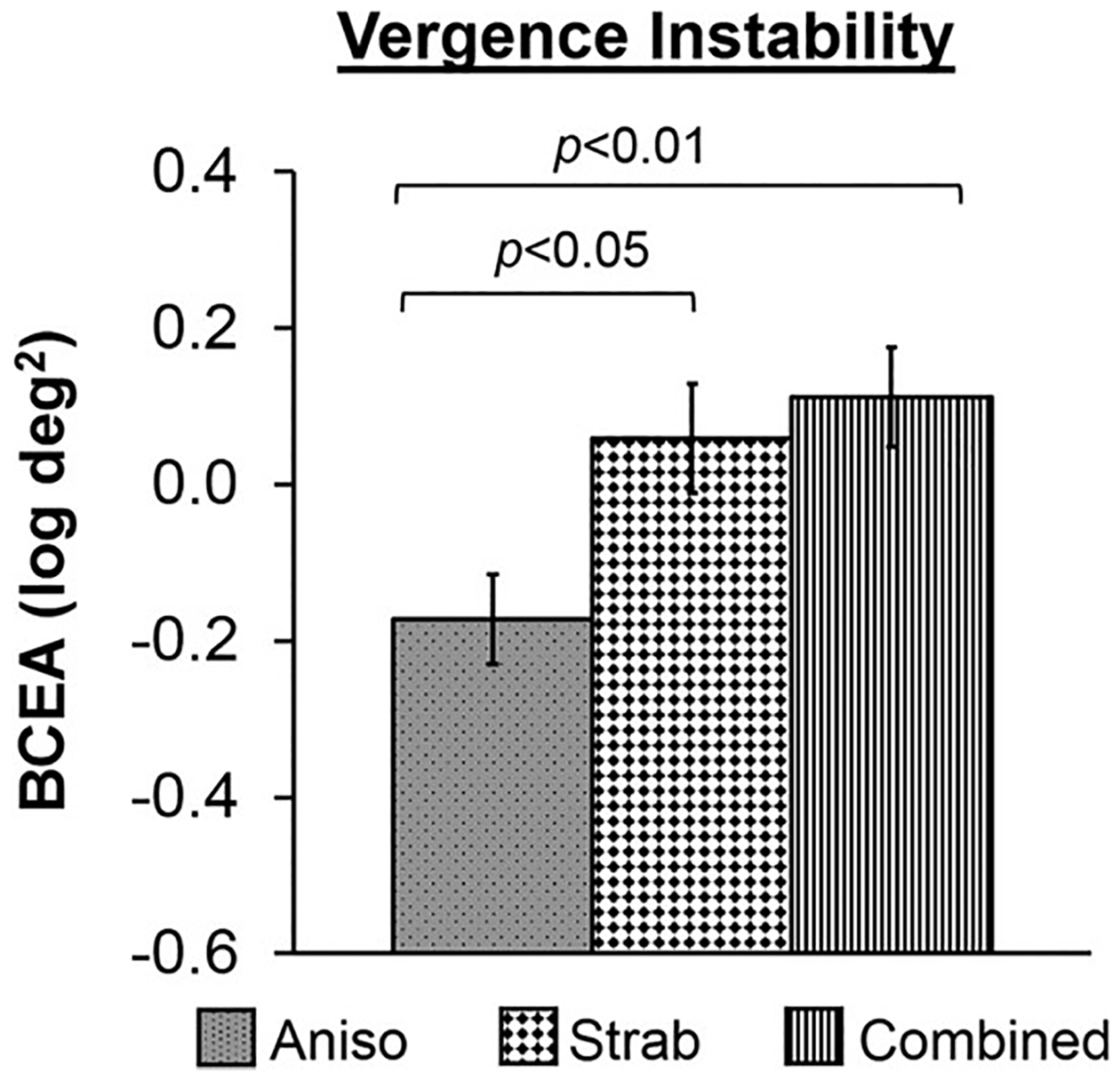
Vergence instability and etiology. Bar graphs depicting group differences in vergence instability for etiology (anisometropia, grey bar with black dots; strabismus, white bar with black diamonds; combined mechanism, white bar with vertical stripes). Children with anisometropia had less vergence instability than children with strabismus and children with combined mechanism. Error bars represent ± SEM.
3.3.2. Stereoacuity
Fixation instability and vergence instability were compared among subgroups of patients based on different levels of stereoacuity (Birch et al., 2013) defined as bifixation (≤60 arcsec, n = 15), monofixation (100–800 arcsec, n = 47), or nil (> 800 arsec, n = 98) stereoacuity.
Fixation instability: A 3 × 2 ANOVA of the BCEA (log deg2) with stereo group as the between-groups factor and eye (nonpreferred eye, preferred eye) as the within-groups factor revealed a significant stereo group × eye interaction (F2,157 = 5.77, p = 0.005; Fig. 5A). Bonferroni posthoc comparisons show that fixation instability was larger in the nonpreferred eye compared to the preferred eye for children with nil stereoacuity (p = 0.000007). There was no difference between nonpreferred and preferred eyes for children with monofixation or for children with bifixation (p = 1.00 for both pairwise comparisons). There was a trend for fixation instability to be larger in the nonpreferred eye in children with nil stereoacuity compared to children with bifixation (p = 0.08), but this was not significant. No other group differences were found (ps ≥ 0.31).
Fig. 5.
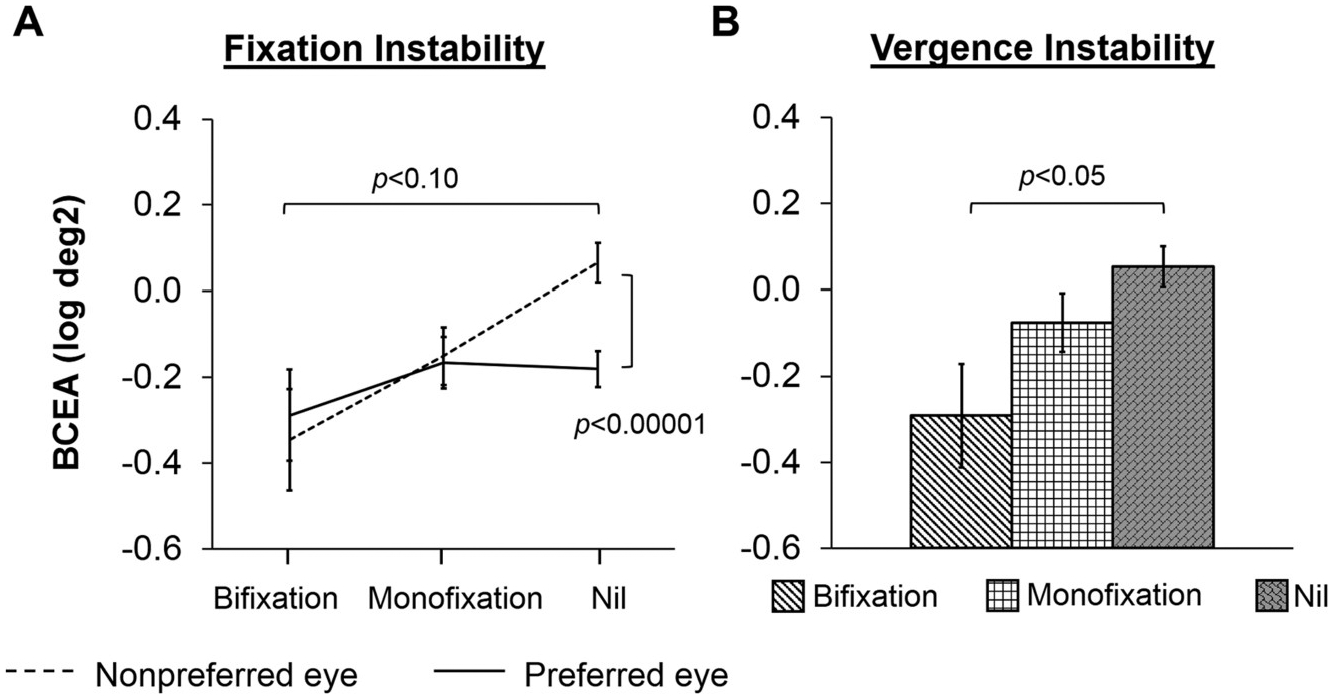
Stereoacuity and instability. A) An interaction between eye (nonpreferred eye, dotted line; preferred eye, solid line) and stereoacuity category (bifxation, monofixation, nil) for fixation instability shows that nonpreferred eye instability was larger than preferred eye instability for children with nil stereoacuity, and that children with nil stereoacuity had larger nonpreferred eye instability than children with bifixation. B) Bar graphs depicting group differences in vergence instability for stereoacuity category (bifxation, white bar with diagonal stripes; monofixation, white bar with squares; nil, grey bar with bricks). Children with nil stereoacuity had larger vergence instability than children with bifxation. Error bars represent ± SEM.
Vergence instability: A one-way ANOVA of the BCEA (log deg2) revealed a significant effect of stereo group on vergence instability (F2,157 = 4.08, p = 0.02; Fig. 5B). Bonferroni posthoc comparisons show that vergence instability was larger for children with nil stereoacuity compared to children with bifixation (p = 0.03). No other group differences were found (ps ≥ 0.36).
3.3.3. Correlations
Pearson r correlations excluding control children were conducted to determine whether nonpreferred eye visual acuity, stereoacuity, and extent of suppression scotoma were predictive of fixation instability and vergence instability (Fig. 6). All ps < 0.05 were considered significant.
Fig. 6.
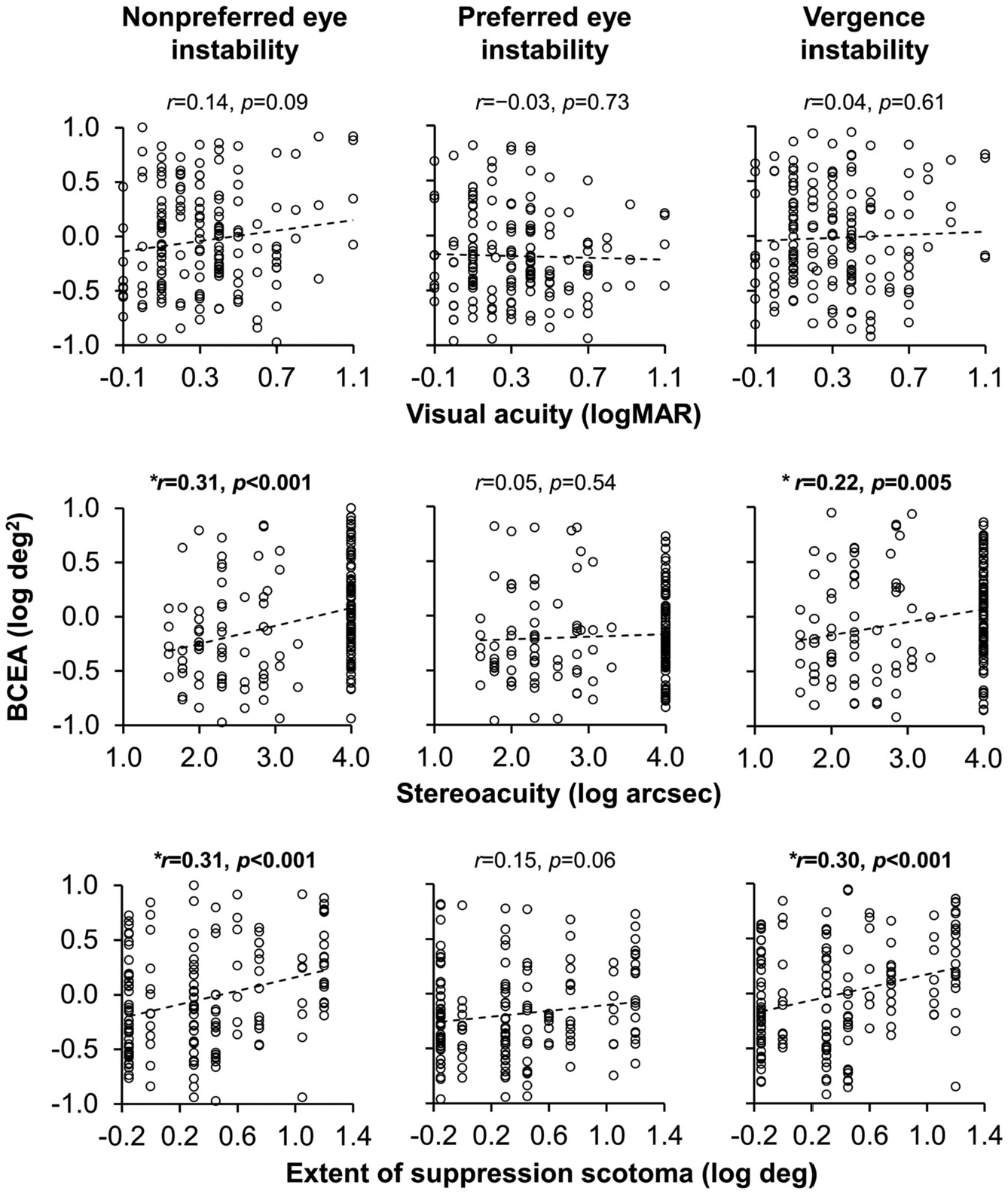
Correlations with instability. Scatterplots depicting correlations between instability (nonpreferred eye, preferred eye, vergence) and visual assessment measures (nonpreferred eye visual acuity, stereoacuity, extent of suppression). Nonpreferred eye instability and vergence instability were associated with worse stereoacuity and larger extent of suppression scotoma. Nil stereoacuity was arbitrarily assigned a value of 4 log arsec. Suppression (no fusion) for the extent of suppression scotoma was arbitrarily assigned a value of 1.2 log deg *denotes a significant correlation p < 0.05.
Fixation instability: Larger nonpreferred eye instability was significantly associated with worse stereoacuity (r = 0.31, CI95 = 0.16 to 0.44, p < 0.001) and larger extent of suppression scotoma (r = 0.31, CI95 = 0.16 to 0.44, p < 0.001), but not nonpreferred eye visual acuity (r = 0.14, CI95 = −0.02 to 0.29, p = 0.09). Preferred eye instability was not significantly associated with any factor (ps ≥ 0.06).
Vergence instability: Larger vergence instability was significantly associated with worse stereoacuity (r = 0.22, CI95 = 0.07 to 0.36, p = 0.005), and larger extent of suppression scotoma (r = 0.30, CI95 = 0.15 to 0.43, p < 0.001), but not nonpreferred eye visual acuity (r = 0.04, CI95 = −0.12 to 0.19, p = 0.61).
4. Discussion
In this study, we report fixation instability and vergence instability during binocular viewing in amblyopic and nonamblyopic children treated for anisomteropia, strabismus, or both. The main findings of our study were:
Binocularly discordant visual experience due to anisometropia and/or strabismus regardless of amblyopia, results in greater fixation instability and vergence instability compared with controls.
Amblyopia adds to the instability of the nonpreferred eye (Fig. 3A).
Children with strabismus and children with combined mechanism had greater nonpreferred eye instability and vergence instability than children with anisometropia.
Nonpreferred eye instability and vergence instability were moderately associated with impaired binocular vision (i.e., stereoacuity, extent of suppression scotoma), but not with nonpreferred eye visual acuity
Fixation instability in anisometropic and strabismic children and adults has been well-documented during monocular viewing (Birch et al., 2013; González et al., 2012; Subramanian et al., 2013; Chung et al., 2015; Shaikh et al., 2016; Ciuffreda et al., 1979; Ghasia et al., 2018). Here, we report fixation instability during binocular viewing in children with anisometropia and/or strabismus, with and without amblyopia. Amblyopia is not a necessary condition for fixation instability during monocular viewing (Birch et al., 2013; Subramanian et al., 2013; Ciuffreda et al., 1979; Ghasia et al., 2018; Economides et al., 2016), or during binocular viewing as evident in the lack of difference in instability between amblyopic and nonamblyopic children in our study. However, amblyopia added to increased fixation instability of the nonpreferred eye asamblyopic children, but not nonamblyopic children, had larger nonpreferred than preferred eye instability (See significant interaction in Fig. 3A). The latter finding is consistent with previous studies showing that the amblyopic eye is more unstable than the fellow eye under monocular and binocular viewing conditions (González et al., 2012; Subramanian et al., 2013; Chung et al., 2015; Shaikh et al., 2016).
Until now, no study has assessed vergence instability during binocular viewing in treated anisometropic and strabismic children. Similar to fixation instability, we found larger vergence instability, regardless of whether amblyopia was present, pointing to disconjugate fixational eye movements. Disconjugacy has been reported during monocular viewing in strabismic children and adults (Ghasia et al., 2018), and during binocular viewing in exotropic monkeys (Upadhyaya et al., 2017) and exotropic adults (Economides et al., 2016). Vergence instability may also reflect larger nonpreferred than preferred eye instability, as seen in our amblyopic children (See Fig. 3A and Section 3.3.1). If vergence instability was primarily due to nonpreferred eye instability, we would expect to see the same pattern in Figs. 3B and 4. Namely, we would expect much less instability in anisometropia compared with strabismus and combined mechanism, which is what we have observed in this study. If vergence instability was primarily due to preferred eye instability, or a combination of nonpreferred and preferred eye instability, we would expect to see similar amounts of instability, regardless of etiology, which was not the case.
Previous monocular fixation studies point to increased drift, saccadic oscillations, disconjugacy of fixational saccades, and FMNS as sources of instability (Birch et al., 2013; Otero-Millan et al., 2014; Martinez-Conde, 2006; Ciuffreda et al., 1979, 1980; Shi et al., 2012; Tychsen, 2007; Ghasia et al., 2018). From Fig. 1, it is evident that these abnormal fixational eye movements also contributed to instability during binocular viewing in our group. For example, the largest source of fixation instability in the child with strabismic nonamblyopia (Fig. 1D) is clearly due to FMNS. Also present in this child is a dissociated vertical deviation (DVD), a condition where one eye drifts upward, that introduces a vertical component to the nystagmus (Irving et al., 1998). Since our focus was on the overall instability (measured by the BCEA) during binocular viewing, we did not categorize waveforms for type/presence of nystagmus, nor did we determine the exact components that may have contributed to fixation and vergence instability in our group. These outcome measures could be a focus of future analysis with our data.
Our analysis of factors associated with increased fixation and vergence instability points to discordant binocular input, not amblyopia, as the key factor in abnormal ocular motor development in these pediatric eye conditions. However, etiology also plays a role, perhaps as a factor that mediates the severity of binocular discordance. We found that fixation instability and vergence instability were larger with strabismus and combined mechanism than anisometropia. Further, children with combined mechanism had larger nonpreferred eye compared with preferred eye instability, whereas the other two groups did not. Indeed, anisometropic children tend to have better stereoacuity, which was related to instability; 43/64 (67%) of anisometropic children had measurable stereoacuity, compared with only 12/52 (23%) and 14/44 (32%) of strabismic and combined mechanism children, respectively. Etiology differences have also been reported for other visual functions in anisometropic children, including shorter interocular latency during saccades, less severe visual acuity deficits and less depth of suppression than other etiologies (Birch et al., 2016; McKee et al., 2003, 2016).
BCEAs for anisometropic children were smaller compared with strabismic and combined mechanism children. Nonetheless, the mean BCEAs fell outside the 95% confidence interval of controls for nonpreferred eye instability (control BCEA CI95 = −0.42 to −0.28 log deg2, anisometropia mean BCEA = −0.23 log deg2) and for vergence instability (control BCEA CI95 = −0.46 to −0.29 log deg2, anisometropia mean BCEA = −0.17 log deg2). This is consistent with previous studies showing unstable fixation during monocular viewing in amblyopic anisometropia (Birch et al., 2013; Subramanian et al., 2013).
Previous studies have been inconclusive as to whether nonpreferred eye visual acuity plays a role in fixation instability (Birch et al., 2013; González et al., 2012; Subramanian et al., 2013; Shaikh et al., 2016; Ciuffreda et al., 1979). In our study, we did not find a significant relationship between the amount of instability (nonpreferred eye, preferred eye, vergence) during binocular viewing and nonpreferred eye visual acuity. However, most of the amblyopic children (78%) in our study had mild to moderate amblyopia. While the severity of amblyopia was not related to the amount of instability in our study, the mere presence of amblyopia added to fixation instability of the nonpreferred eye. This bias during binocular viewing may reflect the dominance of the preferred eye that suppresses the nonpreferred eye (Birch et al., 2016). In fact, bifoveal fixation is significantly improved in strabismic amblyopes when interocular suppression is artificially reduced via contrast-rebalancing (Raveendran et al., 2014).
Although not related to visual acuity, both nonpreferred eye fixation instability and vergence instability were moderately related to impairments in binocularity, i.e., stereo deficits and a larger extent of suppression scotoma. Clearly, the vergence angle must be relatively stable to support single binocular vision. On the other hand, abnormal disparity sensitivity could lead to poor vergence control and increased vergence instability. Larger vergence instability may also be a consequence of poor accommodation that often accompanies strabismus and anisometropia (Ingram et al., 2009), as the vergence and accommodation systems are linked (Fincham and Walton, 1957). Considering that our correlations were only moderate (i.e., ~0.3), factors other than binocular impairments may also be contributing to nonpreferred eye instability and vergence instability.
Target parameters, including size and shape (e.g., dot, circle, cross) can influence fixation instability (Pirdankar and Das, 2016; Thaler et al., 2013). We used a 0.3° diameter dot; smaller target sizes have been shown to yield better stability (Pirdankar and Das, 2016). We were not concerned that the amblyopic children might not be able to detect the dot because they always viewed the dot with both eyes. Others have used small dots for testing fixation instability in amblyopic and strabismic children and adults, and have reported similar values for control BCEA. For example, Shaikh et al., (2016) used a 0.5 deg dot and reported a BCEA of −0.48 log deg2 for controls, compared with a BCEA of −0.39 log deg2 for controls in our study viewing a 0.3 deg dot. Lastly, because we compared data from children with amblyopia, anisometropia, and/or strabismus with data from age-matched controls who viewed the same target, any differences among groups in instability can be attributed to eye condition (amblyopia, strabismus, anisometropia).
5. Conclusions
Instability during binocular viewing in children with anisometropia and/or strabismus suggests that discordant binocular experience during childhood interferes with ocular motor control, especially in strabismus. While no relationship was found with severity of amblyopia, the presence of amblyopia adds to instability of the nonpreferred eye. Vergence instability may limit potential for recovery of binocular vision in children with anisometropia and/or strabismus.
Acknowledgements
Dr. Martin Steinbach played a large role in Dr. Kelly’s academic development. His research interests in amblyopia and eye movements opened the door for numerous research discussions, as well as her participation in his studies, which ignited her interest in amblyopia. As his teaching assistant, she had the privilege of observing his warm and passionate teaching style for the duration of her graduate career. The authors acknowledge Dr. Steinbach’s immense contribution to the field of eye movements, and to amblyopia. The authors would also like to thank Angie De La Cruz, Santoshi Ramachandran, Sarah Morale, and Kirby Mateja for their help with data collection.
Footnotes
This study was conducted at the Retina Foundation of the Southwest and the data were presented, in part, at the Association for Research in Vision and Ophthalmology (ARVO) 2018 Annual Meeting.
Conflicts of interest
None of the authors have a financial conflict of interest with any of the material presented in the manuscript. This work was supported by the National Eye Institute (EY02313 and K99EY028224).
References
- Beck R, Moke P, Turpin A, et al. , 2003. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am. J. Ophthalmol 135 (2), 194–205. [DOI] [PubMed] [Google Scholar]
- Birch EE, 2003. Marshall Parks lecture. Binocular sensory outcomes in accommodative ET. J AAPOS 7 (6), 369–373. [DOI] [PubMed] [Google Scholar]
- Birch EE, 2013. Amblyopia and binocular vision. Prog. Retin. Eye Res 33, 67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Stager DR, 2006. Long-term motor and sensory outcomes after early surgery for infantile esotropia. J AAPOS 10 (5), 409–413. [DOI] [PubMed] [Google Scholar]
- Birch EE, Wang J, Felius J, Stager DR, Hertle RW, 2012. Fixation control and eye alignment in children treated for dense congenital or developmental cataracts. J AAPOS 16 (2), 156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Subramanian V, Weakley DR, 2013. Fixation instability in anisometropic children with reduced stereopsis. J AAPOS 17 (3), 287–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Morale SE, Jost RM, et al. , 2016. Assessing suppression in amblyopic children with a dichoptic eye chart. Investig. Ophthalmol. Vis. Sci 57 (13), 5649–5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung STL, Kumar G, Li RW, Levi DM, 2015. Characteristics of fixational eye movements in amblyopia: limitations on fixation stability and acuity? Vis. Res 114, 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffreda KJ, Kenyon RV, Stark L, 1979. Saccadic intrusions in strabismus. Arch. Ophthalmol 97 (9), 1673–1679. [DOI] [PubMed] [Google Scholar]
- Ciuffreda KJ, Kenyon RV, Stark L, 1980. Increased drift in amblyopic eyes. Br. J. Ophthalmol 64 (1), 7–14. 10.1136/bjo.64.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter SA, Chu RH, Chandler DL, et al. , 2003. Reliability of the electronic early treatment diabetic retinopathy study testing protocol in children 7 to < 13 years old. Am. J. Ophthalmol 136 (4), 655–661. [DOI] [PubMed] [Google Scholar]
- Economides JR, Adams DL, Horton JC, 2016. Variability of ocular deviation in strabismus. JAMA Ophthalmol 134 (1), 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felius J, Fu VLN, Birch EE, Hertle RW, Jost RM, Subramanian V, 2011. Quantifying nystagmus in infants and young children: relation between foveation and visual acuity deficit. Investig. Ophthalmol. Vis. Sci 52 (12), 8724–8731. [DOI] [PubMed] [Google Scholar]
- Fincham BYEF, Walton J, 1957. The reciprocal actions of accommodation and convergence. J. Physiol 137, 488–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasia FF, Otero-Millan J, Shaikh AG, 2018. Abnormal fixational eye movements in strabismus. Br. J. Ophthalmol 102, 253–259. [DOI] [PubMed] [Google Scholar]
- González EG, Wong AMF, Niechwiej-Szwedo E, Tarita-Nistor L, Steinbach MJ, 2012. Eye position stability in amblyopia and in normal binocular vision. Invest. Ophthalmol. Vis. Sci 53 (9), 5386–5394. [DOI] [PubMed] [Google Scholar]
- Gradstein L, Goldstein HP, Wizov SS, Hayashi T, Reinecke RD, 1998. Relationships among visual acuity demands, convergence, and nystagmus in patients with manifest/latent nystagmus. J AAPOS 2 (4), 218–229. [DOI] [PubMed] [Google Scholar]
- Hess RF, Thompson B, 2015. Amblyopia and the binocular approach to its therapy. Vis. Res 114, 4–16. [DOI] [PubMed] [Google Scholar]
- Holmes JM, Beck RW, Repka MX, et al. , 2001. The amblyopia treatment study visual acuity testing protocol. Arch. Ophthalmol 119 (9), 1345–1353. [DOI] [PubMed] [Google Scholar]
- Ingram RM, Lambert TW, Gill LE, 2009. Visual outcome in 879 children treated for strabismus: insufficient accommodation and vision deprivation, deficient emmetropisation and anisometropia. Strabismus 17 (4), 148–157. [DOI] [PubMed] [Google Scholar]
- Irving EL, Goltz HC, Steinbach MJ, Kraft SP, 1998. Vertical latent nystagmus component and vertical saccadic asymmetries in subjects with dissociated vertical deviation. J AAPOS 2 (6), 344–350. [DOI] [PubMed] [Google Scholar]
- Kelly KR, Felius J, Ramachandran S, John BA, Jost RM, Birch EE, 2016. Congenitally impaired disparity vergence in children with infantile esotropia. Investig. Opthalmol. Vis. Sci 57 (6), 2545–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KR, Jost RM, Wang Y, et al. , 2018. Improved binocular outcomes following binocular treatment for childhood amblyopia. Investig. Ophthalmol. Vis. Sci 59 (3), 1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Thompson B, Lam CSY, et al. , 2011. The role of suppression in amblyopia. Invest. Ophthalmol. Vis. Sci 52 (7), 4169–4176. [DOI] [PubMed] [Google Scholar]
- Markowitz SN, Steinbach MJ, 2008. Fixation characteristics of patients with macular degeneration recorded with the Mp-1 microperimeter. Retina 28, 125–133. [DOI] [PubMed] [Google Scholar]
- Martinez-Conde S, 2006. Fixational eye movements in normal and pathological vision. Prog. Brain Res 154, 151–176. [DOI] [PubMed] [Google Scholar]
- McKee SP, Levi DM, Movshon JA, 2003. The pattern of visual deficits in amblyopia. J. Vis 3 (5), 380–405. [DOI] [PubMed] [Google Scholar]
- McKee SP, Levi DM, Schor CM, Movshon JA, 2016. Saccadic latency in amblyopia. J. Vis 16 (5), 1–15 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moke PS, Turpin AH, Beck RW, et al. , 2001. Computerized method of visual acuity testing: adaptation of the amblyopia treatment study visual acuity testing protocol. Am. J. Ophthalmol 132 (6), 903–909. [DOI] [PubMed] [Google Scholar]
- Niechwiej-Szwedo E, Goltz HC, Chandrakumar M, Hirji ZA, Wong AMF, 2010. Effects of anisometropic amblyopia on visuomotor behavior, I: saccadic eye movements. Invest. Ophthalmol. Vis. Sci 51 (12), 6348–6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niechwiej-Szwedo E, Chandrakumar M, Goltz HC, Wong AMF, 2012. Effects of strabismic amblyopia and strabismus without amblyopia on visuomotor behavior, I: saccadic eye movements. Invest. Ophthalmol. Vis. Sci 53 (12), 7458–7468. [DOI] [PubMed] [Google Scholar]
- Otero-Millan J, Macknik SL, Martinez-Conde S, 2014. Fixational eye movements and binocular vision. Front. Integr. Neurosci 8, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirdankar OH, Das VE, 2016. Influence of target parameters on fixation stability in normal and strabismic monkeys. Investig. Ophthalmol. Vis. Sci 57 (3), 1087–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raveendran RN, Babu RJ, Hess RF, Bobier WR, 2014. Transient improvements in fixational stability in strabismic amblyopes following bifoveal fixation and reduced interocular suppression. Ophthalmic Physiol. Optic 34 (2), 214–225. [DOI] [PubMed] [Google Scholar]
- Rosenbaum A, Santiago A (Eds.), 1999. Clinical Strabismus Management. WB Saunders, Philadelphia. [Google Scholar]
- Shaikh AG, Otero-Millan J, Kumar P, Ghasia FF, 2016. Abnormal fixational eye movements in amblyopia. PLoS One 11 (3), e0149953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi XFF, Xu LM, Li Y, Wang T, Zhao KX, Sabel BA, 2012. Fixational saccadic eye movements are altered in anisometropic amblyopia. Restor. Neurol. Neurosci 30 (6), 445–462. [DOI] [PubMed] [Google Scholar]
- Subramanian V, Jost RM, Birch EE, 2013. A quantitative study of fixation stability in amblyopia. Invest. Ophthalmol. Vis. Sci 54 (3), 1998–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarita-Nistor L, Brent MH, Steinbach MJ, González EG, 2011. Fixation stability during binocular viewing in patients with age-related macular degeneration. Investig. Ophthalmol. Vis. Sci 52 (3), 1887–1893. [DOI] [PubMed] [Google Scholar]
- Thaler L, Schütz AC, Goodale MA, Gegenfurtner KR, 2013. What is the best fixation target? The effect of target shape on stability of fixational eye movements. Vis. Res 76, 31–42. [DOI] [PubMed] [Google Scholar]
- Tychsen L, 2007. Causing and curing infantile esotropia in primates: the role of decorrelated binocular input. Trans. Am. Ophthalmol. Soc 105, 564–593. [PMC free article] [PubMed] [Google Scholar]
- Upadhyaya S, Pullela M, Ramachandran S, Adade S, Joshi AC, Das VE, 2017. Fixational saccades and their relation to fixation instability in strabismic monkeys. Investig. Opthalmol. Vis. Sci 58 (13), 5743. [DOI] [PMC free article] [PubMed] [Google Scholar]


