Abstract
Beta-cyclodextrin (β-CD) is an oligosaccharide commonly used to improve the aqueous solubility of lipophilic drugs (e.g., dexamethasone, DEX). Here we present the development of a drug delivery system to provide sustained release of DEX by β-CD-inclusion complex (IC) to amplify the mineralization capacity of stem cells from human-extracted deciduous teeth (SHEDs) as a potential direct pulp capping strategy. First, IC of DEX (DEX-CD-IC) was synthesized with β-CD. To confirm DEX-CD-IC complex formation, X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR) analyses were performed. XRD data indicated that IC formation was achieved due to formation of a new crystalline structure, whereas FTIR revealed the presence of the IC from the shifting of the peaks of each component in DEX-CD-IC. Then, electrospun poly(lactic-co-glycolic acid, PLGA) fibers (PLGA/DEX-CD-IC) were processed by varying the concentration of DEX-CD-IC (5%, 10%, and 15%). The release of DEX from fibers was determined by ultraperformance liquid chromatography for 28 days. Thanks to the solubility enhancement of DEX by IC, electrospun PLGA/DEX-CD-IC fibers released DEX in a more sustained fashion compared to PLGA/DEX fibers. No deleterious effect was found in terms of SHEDs’ proliferation when cultured with or on electrospun fibers, regardless of the IC presence. Importantly, a more pronounced odontogenic differentiation was stimulated by electrospun fibers loaded with the lowest DEX-CD-IC concentration (5%), as a result of the sustained DEX release. In sum, PLGA/DEX-CD-IC fibers have great potential in vital dental pulp therapy, owing to its sustained DEX release, cytocompatibility, and odontogenic differentiation capacity.
Keywords: electrospinning, fibers, drug delivery, dentin, regeneration, dental pulp, endodontics
Graphical Abstract

1. Introduction
Trauma, caries, or overzealous removal of deep decay, may ultimately result in dental pulp exposure [1]. To preserve dental pulp vitality and stimulate tertiary dentin formation through a calcified barrier, a clinical procedure termed “direct pulp capping” needs to be performed [2,3]. To that end, calcium hydroxide (Ca[OH]2) and mineral trioxide aggregate (MTA) are generally applied over the exposed surface of the pulp, aiming to stimulate the formation of a new reparative dentin layer [4,5]. However, drawbacks associated with Ca[OH]2 (chronic pulp tissue inflammation and development of porous dentin bridge) and MTA (handling difficulty, prolonged setting time, and tooth discoloration) limit their clinical outcomes [2,6–8].
Dexamethasone (DEX) is a synthetic glucocorticoid, known not only to support dental stem cells’ (e.g., dental pulp [DPSCs] and periodontal ligament [PDLSCs]) differentiation towards osteo/odontogenic lineages [9,104/3/2020 10:33:00 AM, but also for its immunosuppressive activity attenuating inflammatory responses [11]. Regrettably, cell toxicity has been shown in a dose dependent fashion. Indeed, it has been reported that the viability of human PDLSCs and trabecular meshwork cells showed a gradual decrease in their viabilities when exposed to DEX at high concentrations[12,13]. Thus, due to severe side-effects related to DEX exposure, it is essential to minimize not only the therapeutic dosage/concentration, but more importantly to design a more suitable mechanism of localized delivery to enhance its initial solubility without drug overloading.
Cyclodextrins (CDs) are cyclic oligosaccharides composed of glucopyranose units that have relatively hydrophobic central cavity and hydrophilic outer surfaces [14]. CDs have a distinctive ability as molecular containers to entrap guest molecules as a result of their molecular shape and structure[15,16]. The complexation of molecules and CDs through non-covalent interaction is a dynamic process by which the guest molecule (e.g., DEX) continuously associates and dissociates from the host CD [17]. For example, aiming to reduce the treatment schedule of eye drops after cataract surgery, CDs were reported to increase DEX aqueous solubility and stability, which in turn enhanced drug absorption and reduce local irritation [18].
High-voltage driven polymer electrospinning is a facile, nanotechnology-enabled technique to generate fibrous-based scaffolds for applications in drug delivery and regenerative medicine. Thus, pursuing the development of a delivery system of DEX-β-CD inclusion complex and electrospun fibers would enhance initial DEX release. The purpose of this investigation was to engineer novel biodegradable electrospun polymeric fibers to provide the sustained release of DEX by β-CD-inclusion complex (IC) to amplify SHEDs’ mineralization capacity as a potential direct pulp capping strategy. We hypothesized that the successful formation of such a delivery system would minimize the DEX dose needed to induce osteo/odontogenic differentiation and decrease the associated cell toxicity side effects.
2. Experimental section
2.1. Materials
Dexamethasone (DEX > 99%, Tokyo Chemical Industry Co., Ltd., Tokyo, Japan), beta-cyclodextrin (β-CD, Sigma-Aldrich, St. Louis, MO, USA), chloroform (> 99.8%, Alfa Aesar, Ward Hill, MA, USA), methanol (Thermo Fisher Scientific Inc., Hampton, NH, USA), 1,1,1,3,3,3 hexafluoroisopropanol (HFIP, 99%, Beantown Chemical Co., Hudson, NH, USA), Hexamethyldisilazane (> 99%, Sigma-Aldrich), hexcetylpyridinium chloride monohydrate (CPC, Sigma-Aldrich), β-glycerophosphate (Sigma-Aldrich), ascorbic acid (Sigma-Aldrich), paraformaldehyde (PFA, Sigma-Aldrich) and 75:25 poly(DL-lactide-co-glycolide acid) (PLGA, inherent viscosity: 0.55–0.75 dL/g in CHCl3, Lactel Absorbable Polymers, Durect Corporation, Birmingham, AL, USA) were purchased and used as-received. Distilled-deionized (DI) water from a Millipore Milli-Q ultrapure water system was used in the experiments.
2.2. Preparation of the inclusion complex (IC)
Inclusion complex of DEX and CD (DEX/CD-IC) was synthesized according to the freeze-drying method [19]. First, β-CD was dissolved in water, then DEX was added to the solution at 1:1 molar ratio and stirred overnight. The formed solution was kept refrigerated at −80°C for 48 h, then the complex was freeze-dried (Labconco FreeZone 2.5L, Labconco Co., Kansas City, MO, USA) for 3 days.
2.3. Preparation of electrospinning solutions
PLGA-DEX and PLGA-DEX/CD-IC fibers were processed via electrospinning. Briefly, in order to prepare PLGA-DEX fibers, DEX (5, 10, or 15% w/w, with respect to polymer weight) was added to a solution of 20% (w/v) PLGA dissolved in chloroform:methanol (3:1) at room temperature (RT). Then, the solutions were stirred overnight prior to electrospinning. Meanwhile, to obtain PLGA-DEX/β-CD-IC fibers, a calculated amount of DEX/β-CD-IC corresponding to 5, 10, or 15% DEX (w/w, with respect to polymer weight) was dispersed in HFIP at RT for 10 min. Then, 15% PLGA (w/v) was added as previously reported [20,21]. The resulting PLGA-DEX/β-CD-IC solutions were stirred for an additional 90 min before electrospinning. For comparison purposes, 22% (w/v) PLGA solution in chloroform:methanol (3:1) was also electrospun. Table 1 summarizes the composition of the PLGA, PLGA-DEX, and PLGA-DEX/CD-IC solutions used to obtain bead-free fibers.
TABLE 1.
Solution compositions and morphological characteristics of resulting electrospun nanofibers for PLGA-MF, PLGA-DEX/β-CD-IC-MF (5% and 15%), and PLGA-DEX-MF (5% and 15%).
| Solutions | %PLGAa (w/v) | % DEXb (w/w) | %β-CDb (w/w) | Average Fiber Diameter (μm) | Fiber Morphology |
|---|---|---|---|---|---|
| PLGA | 22 | - | - | 1.61±0.39 | Bead-free |
| PLGA-DEX (5%) | 18 | 5 | - | 1.36±0.33 | |
| PLGA-DEX (10%) | 18 | 10 | - | 1.28±0.29 | |
| PLGA-DEX (15%) | 18 | 15 | - | 1.09±0.42 | |
| PLGA-DEX/CD-IC (5%) | 15 | 5 | 16.6 | 1.53±0.25 | |
| PLGA-DEX/CD-IC (10%) | 15 | 10 | 39.8 | 2.53±0.32 | |
| PLGA-DEX/CD-IC (15%) | 15 | 15 | 74.5 | 3.56±0.30 |
with respect to solvent and
with respect to polymer
2.4. Electrospinning
PLGA, PLGA-DEX, and PLGA-DEX/CD-IC solutions were loaded into a 5-mL plastic syringe (Becton, Dickson and Company, Franklin Lakes, NJ, USA) with a 27G inner diameter metallic needle (CML Supply LLC., Lexington, KY, USA). Then, at a constant rate (1.5 mL/h), the solutions were pumped via a syringe pump (KDS101; KD Scientific, Holliston, MA, USA). A rotating mandrel at a speed of 120 rpm, covered by a piece of aluminum foil positioned at a distance of 14 cm from the tip of the needle, was used as a collector. A voltage of 15 kV was applied from the high voltage power supply (ES50P-10W/DAM, Gamma High Voltage Research Inc., Ormond Beach, FL, USA). All experiments were carried out at 25°C and 18% relative humidity. The electrospun mats were kept in a vacuum oven for 2 days to ensure evaporation of any remaining solvent, and then stored at 4°C till further analyses.
2.5. Physico-chemical characterizations
The structure and phase composition of DEX, β-CD, DEX/CD-IC, as well as PLGA, PLGA-DEX, and PLGA-DEX/CD-IC electrospun fibers were determined by X-ray diffraction (XRD, Rigaku Ultima IV diffractometer, Rigaku Americas Co., The Woodlands, TX, USA) with Cu Kα (λ = 1.54 angstroms) in Bragg-Brentano geometry. The X-ray source and detector were coupled to scan in a 2 theta (2θ) range from 5° to 45° in a step size of 0.05° at a scan speed of 1°/min. The phase identification was performed using Rigaku’s data analysis software (PDXL Version 2.6.1.2) and ICSD (Inorganic Crystal Structure Database). The infrared spectra of DEX, β-CD, DEX/CD-IC, as well as PLGA, PLGA-DEX, and PLGA-DEX/β-CD-IC electrospun fibers were taken via Fourier transform infrared spectroscopy (FTIR spectrometer, Nicolet iS50 FTIR, Thermo Fisher Scientific Inc., Waltham, MA, USA) and 64 scans were analyzed between 4000 cm−1 and 600 cm−1 for DEX, β-CD, DEX/CD-IC and 2000 cm−1 and 600 cm−1 for electrospun fibers at 4 cm−1 resolution.
2.6. Morphological characterization
The morphology of the processed PLGA, PLGA-DEX, and PLGA-DEX/CD-IC fibers was examined via scanning electron microscopy (SEM, MIRA3, FEG-SEM, TESCAN, Czech Republic). Electrospun samples were mounted on Al stubs using double-sided adhesive carbon tape and then sputter-coated with an Au layer (SPI-Module Carbon/Sputter coater, Thermo Fisher Scientific, West Chester, PA, USA). In order to calculate the average fiber diameter (AFD), ca. 100 fibers were analyzed from three different images using ImageJ software (National Institutes of Health, Bethesda, MD, USA). The results are given as average ± standard deviation.
2.7. In vitro drug release
The amount of DEX released from PLGA-DEX and PLGA-DEX/β-CD-IC electrospun fibers was measured by the Ultra Performance Liquid Chromatography (UPLC, Waters Co., Wilford, MA, USA) using C18 column. The release experiments were carried out by immersing electrospun fibers (5 mg) in phosphate buffered saline (PBS) at 37°C. The aliquots (0.5 mL) were taken at different time intervals for UPLC analysis and replenished with an equivalent amount of fresh PBS. The mobile phase for UPLC was acetonitrile (0.01% formic acid)/water (0.01% formic acid)/ (55/44) at 0.3 mL/min. Column temperature was 50°C. The UV detector was set at 240 nm. Injection volume was 10 μL. A standard curve 0.05 μg/mL - 10 μg/mL was obtained at 240 nm. Analyses were performed in triplicate, and the results are given as an average ± standard deviation.
2.8. Cell culture
2.8.1. Cell harvesting and isolation
The pulp tissues of normal exfoliated human deciduous incisors were collected from children (7- to 8-year of age) according to previously described protocols [22,23]. In brief, the pulp tissues were digested in collagenase type I (Worthington Biochem, Freehold, NJ) and dispase (Roche Molecular Biochemicals) for 1 h at 37°C. Then, single-cell suspensions (SHEDs) were cultured and expanded in basal alfa-Minimum Essential Medium (α-MEM; Gibco, Life Technologies, Inc., Grand Island, NY, USA) supplemented with 15% heat-inactivated fetal bovine serum (Gibco) and 1% antibiotic/antimyotic solution (Sigma-Aldrich) and maintained in an atmosphere of 5% CO2 at 37°C.
2.8.2. Cell proliferation
PLGA-based electrospun scaffolds loaded with 5, 10, and 15 wt.% DEX or DEX/β-CD-IC were cut in a circular shape using a 6-mm-diameter biopsy punch (Acu-Punch, Acuderm Inc., Ft. Lauderdale, FL, USA) and then disinfected by UV-irradiation (30 min each side). SHEDs at passage 4 were seeded at a density of 5×103 cells/well in 24-well plates, meanwhile DEX- or DEX/CD-IC-PLGA-based electrospun scaffolds were mounted on sterile plastic transwell inserts (Corning Life Sciences, Tewksbury, MA, USA) to investigate the effect of DEX release on cell proliferation using AlamarBlue assay (Invitrogen, Thermo Fisher Scientific Inc., Waltham, MA, USA) at 1, 3, and 5 days. On the day of analysis, 10% of the assay reagent was added to 90% of the media in each well and allowed to react for 2 h at 37°C and 5% CO2. Then, the incorporated dye was determined at 560 nm (excitation range is 540–570 nm) and an emission of 590 nm (emission range is 580–610 nm); using a fluorescence-based plate reader (SpectraMax iD3, Molecular Devices, LLC, San Jose, CA, USA). Fresh medium was added after washing the cells with PBS.
2.8.3. Cell/scaffold interaction
Confluent SHEDs at passage 4 were harvested for seeding onto the electrospun PLGA-based fibrous scaffolds. DEX- and DEX/β-CD-IC-loaded PLGA scaffolds (15μ15 mm2) were mounted on CellCrown™ (Scaffdex, Tampere, Finland, Europe) plastic inserts in 24-well plates and seeded with SHEDs at a density of 5×103 cells/scaffold. SHEDs were cultured for 1, 3, and 5 days. At the end of each time point, the medium was aspirated, and the scaffold was gently washed in PBS. Cells were fixed in 4% PFA for 48 h. Then, the scaffolds were dehydrated in an ascending ethanol series (25% – 100 %), followed by incubation in HMDS and allowed to dry overnight. Finally, the samples were coated with an Au layer and SEM images were obtained.
2.9. Odontogenic differentiation assays
Electrospun scaffolds loaded with 5, 10, and 15 wt% DEX or DEX/β-CD-IC were cut in a circular shape using a 6-mm-diameter biopsy punch prior to UV-irradiation for disinfection purposes (30 min/each side). Confluent SHEDs at passage 4 were harvested for seeding at a density of 3×104 cells/well for odontogenic differentiation (alkaline phosphate activity, ALP and alizarin red staining, ARS) assays. SHEDs cultured in basal media were used as a negative control, while SHEDs cultured in odontogenic differentiation media (supplemented with 50 μg/mL ascorbic acid, 10 mM β-glycerophosphate and 10−8 M DEX) were used as a positive control. Cells were seeded on 24-well plates, and the DEX- and DEX/CD-IC-loaded PLGA scaffolds were mounted on sterile plastic transwell inserts to investigate the effect of DEX release on odontogenic differentiation using ALP and ARS assays. Cells were analyzed at 7 and 14 days for ALP, and at 14 and 21 days for ARS.
2.9.1. ALP activity
The colorimetric SensoLyte pNPP ALP kit (AnaSpec, Inc., Freemont, CA, USA) was used to evaluate the ALP activity according to the manufacturer’s recommendations. Cells were washed twice with 1× assay buffer. Then, cells were lysed in 1× assay buffer with 3 mL Triton-X-100. 50-μL supernatant was transferred to a 96-well plate after 10 min incubation at RT. A 50 μL pNPP was added to the supernatant and then allowed to react for 60 min at 25°C. The absorbance was measured at 405 nm. Total ALP activity was calculated based on an ALP standard of known concentration and was normalized to total protein.
2.9.2. Alizarin red staining
Alizarin red staining (ARS, ScienCell Research Laboratories, Corte Del Cedro Carlsbad, CA, USA) was used to investigate mineralized nodule formation. Cells were washed 3× for 15 min at RT with PBS and fixed with 4% formaldehyde. Then, cells were washed with DI water and stained with 1 mL of 40 mM ARS per well for 30 min. DI water was used to wash the cells prior to imaging and quantification. The images of stained samples were taken using Photo Scanner (Epson Perfection V800, Epson, Long Beach, CA, USA). Subsequently, stained cultures were quantified by following a de-staining procedure for 15 min at RT using 10% (w/v) CPC in 10 mM sodium phosphate at pH 7.0. The ARS concentration was measured by absorbance at 562 nm.
2.10. Statistical analyses
Statistical analyses were performed using the GraphPad Prism 5 software package (GraphPad Software, San Diego, CA, USA). The statistical difference for both dexamethasone release and osteogenic activity tests were analyzed by one-way ANOVA and Tukey’s multiple comparison post-tests (p<0.05).
3. Results and discussion
3.1. X-ray diffraction
The XRD patterns of DEX, β-CD, and DEX/CD-IC powders, as well as the eletrospan micron-sized fibers (hereafter referred as MF, PLGA-MF, PLGA-DEX-MF, and PLGA-DEX/CD-IC-MF) are given in Fig. 1. DEX is a crystalline compound with characteristic diffraction peaks at 2θ = 6.1 and 15.4 [24]. However, the diffraction peaks attributed to dexamethasone where absent in the DEX/CD-IC; instead, we observed the appearance of new peaks (Fig.1A). This might be due to the inability of DEX to form crystals since they are separated from each other by the cavity of CD molecules. Noteworthy, this finding is in accordance with the literature that suggests a potential change in molecular organization of the cyclodextrin from a cage-type structure to a channel-type structure [26–28]. Therefore, successful formation of the inclusion complex was confirmed. PLGA-MF has a characteristic broad peak at 2θ = 10–25°; whereas, both PLGA-DEX-MF and PLGA-DEX/CD-IC-MF exhibited very similar XRD patterns with PLGA (Fig. 1B). Both DEX and DEX/CD-IC were distributed in PLGA fibers in an amorphous form, which might be related with the quick evaporation of the solvent during electrospinning [21,25].
FIGURE 1.
(A) XRD patterns of DEX, CD, and DEX/β-CD-ICs formed by lyophilization (B) XRD of the electrospun fibers with DEX or IC, indicate that IC was successfully formed and PLGA-DEX/β-CD-IC-MF were amorphous, which is similar to PLGA fibers.
3.2. Fourier-transform infrared spectroscopy (FTIR)
The chemical structure of DEX, β-CD, DEX/β-CD-IC, PLGA-MF, PLGA-DEX-MF, and PLGA-DEX/β-CD-IC-MF are presented in Fig. 2A–B. β-CD exhibited characteristic absorption peaks at around 1028 cm−1, 1082 cm−1, 1157 cm−1, 1638 cm−1, 2925 cm−1, and 3401 cm−1 corresponding to C-C stretching vibration, stretching C-O vibration, asymmetric stretching vibration of the C-O-C glyosidic bridge, H-OH bending, C-H stretching, and O-H stretching, respectively [29]. Absorption bands shifted toward a higher frequency, which proposed obtaining a complex of DEX and β-CDs [30]. A characteristic C=O peak at 1650 cm−1 for DEX was observed in the spectrum of the DEX/β-CD-IC. DEX peaks at ~ 1650 cm−1 in PLGA-DEX-MF and PLGA-DEX/β-CD-IC-MF indicate successful DEX incorporation.
FIGURE 2.
(A) FTIR spectra of DEX, CD, and DEX/β-CD-ICs indicates the presence of the characteristic peaks of DEX within the IC. (B) FTIR spectra indicate the presence of IC and DEX within PLGA fibers.
3.3. Scanning electron microscopy (SEM)
Scanning electron microscopy (SEM) images of PLGA-MF, PLGA-DEX-MF, and PLGA-DEX/β-CD-IC-MF are shown in Fig. 3. Bead-free and uniform micron-sized fibers (MF) were obtained. Figure 3 shows the average fiber diameters (AFD) of PLGA-MF, PLGA-DEX-MF and P LGA-DEX/β-CD-IC-MF. The observed higher diameter of PLGA-DEX/β-CD-IC-MF, as compared PLGA-MF and PLGA-DEX-MF, might be correlated with potential differences in solution viscosity and conductivity, which were not investigated in this study. Nonetheless, it has been reported that the increase in viscosity results in higher chain entanglement, and subsequently, the fiber diameter increases [31,32]. On the other hand, increasing the number of charges leads to considerable stretching of the polymer jet; increases the conductivity of a solution; and subsequently, decreases the diameter of the produced fibers [31,32].
FIGURE 3.
Representative SEM images of the electrospun nanofibers. (A) PLGA-MF, (B) PLGA/DEX5%, (C) PLGA/DEX10%, (D) PLGA/DEX15%, (E) PLGA-DEX/IC5%, (F) PLGA-DEX/IC10% and (G) PLGA-DEX/IC15% at (×5,000 and ×10,000) and their AFD.
3.4. In vitro drug release
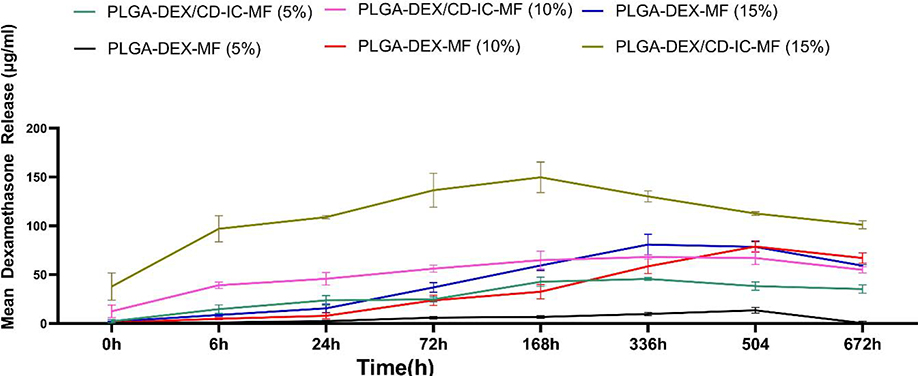
The release profiles of DEX from each group were examined using UPLC. Fig. 4 shows the mean amount of DEX released (in μg/mL) at different time intervals up to 28 days (672 h). In detail, 5 wt.% PLGA-DEX/CD-IC-MF released about six times more drug (43 μg/mL) over the 168-h period when compared to its PLGA-DEX-MF counterpart (~ 7 μg). It is worth mentioning that higher amounts of DEX release from PLGA-DEX/CD-IC-MFs, compared to PLGA-DEX-MF, were observed; in other words, when comparing the mean DEX release at 168 h between 5 and 15 wt.% concentrations for PLGA-DEX/CD-IC-MFs and PLGA-DEX-MFs were 3 and 8 times greater for 15 wt.%, respectively. This might be related to the solubility increment in the drug by complexation in PLGA-DEX/CD-IC-MFs [20]. Interestingly, the fairly constant and sustained release of DEX over 4 weeks holds significant clinical potential, as a delivery system that could ultimately provide resident dental pulp stem cells with the required supplement for predictable odonto/osteogenic differentiation. The release profile of PLGA-DEX/CD-IC-MFs (10%) was similar to PLGA-DEX-MF (15%); whereas, PLGA-DEX-MF (10%) was similar to PLGA-DEX/CD-IC-MFs (5%). Therefore, we sought to examine the effects of this drug delivery system on the behavior of SHEDs focusing on both the highest and lowest release profiles provided by both PLGA-DEX/CD-IC-MFs and PLGA-DEX-MF at 5% and 15%.
FIGURE 4.
Release profiles of DEX from PLGA-DEX/β-CD-IC (5%and15%) and PLGA-DEX-MF (5% and 15%) nanofibers over 672 h.
3.5. Cell studies
3.5.1. Cell proliferation
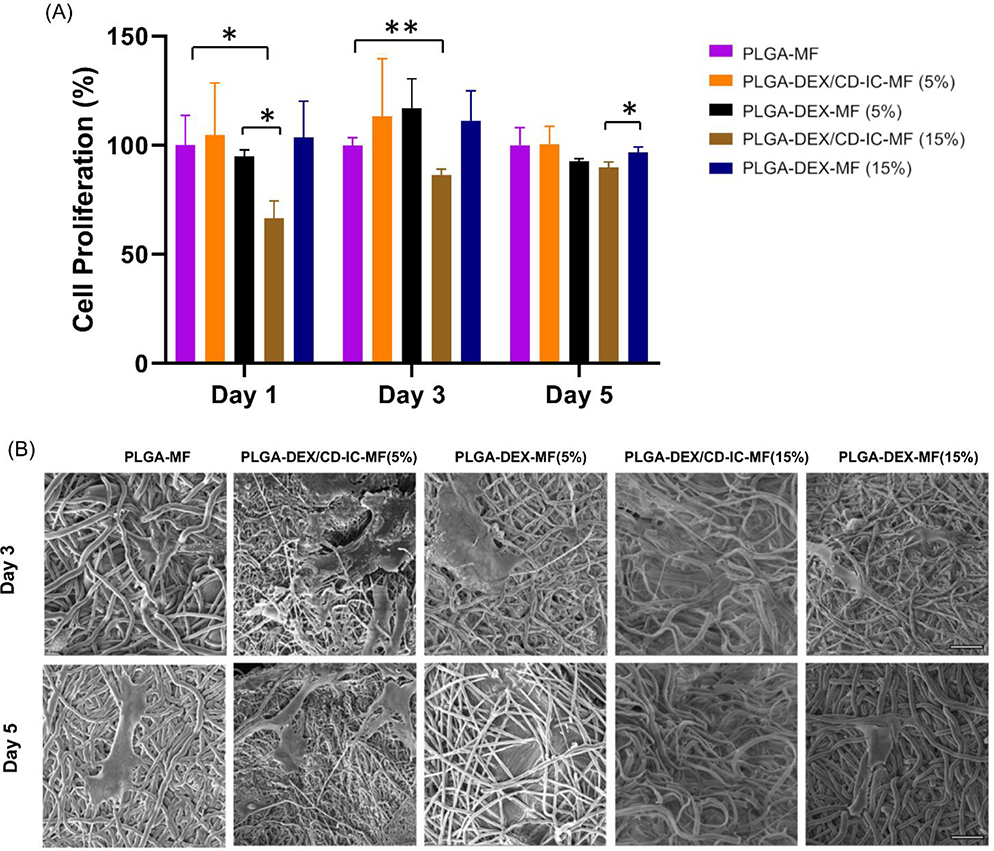
SHEDs have characteristics of stem cells, including differentiation, self-regeneration capacity, clonogenic potential, and expression of numerous mesenchymal stem cells markers [33]. They have also been recognized as analogs of bone marrow mesenchymal stem cells (BMMSCs) [34]. In the present investigation, SHEDs were chosen due to their proven osteo/odontogenic differentiation capacity. Cell proliferation increased from day 1 to day 3 on all scaffolds, which demonstrates the non-toxic characteristic of the synthesized electrospun fibers (Fig. 5A). However, cell proliferation decreased at day 5 in 5% to 15% PLGA-DEX-MF and PLGA-DEX/β-CD-IC-MF. This can be due to the DEX release from the scaffolds, which might induce the early differentiation of SHEDs. However, at a higher level of DEX release, cell proliferation was significantly reduced most likely due to toxic effects of DEX on the cells. This is consistent with previous findings, where cell viability showed a gradual decrease with an increasing concentration of DEX [12,35].
FIGURE 5.
(A) Cell proliferation at day 1, 3 and 5 on PLGA-MF, PLGA-DEX/β-CD-IC (5% and15%) and PLGA-DEX-MF (5% and 15%) electrospun fibers. (B) SEM images of SHEDs seeded on PLGA-DEX/CD-IC-MF (5%), PLGA-DEX-MF (5%), PLGA-DEX/CD-IC-MF (15%), PLGA-DEX-MF (15%) PLGA-MF at day 3 and day 5 scale bar 20 μm.
Representative SEM images of SHEDs on the various electrospun scaffolds (Fig. 5B) show the interaction between SHEDs and fibers. The cells were able to integrate into the structure of the scaffold, despite the presence of the inclusion complex, and extend their cellular processes into and around the fibers. Despite differences in the DEX release profile in PLGA-DEX-MFs and PLGA-DEX/β-CD-IC-MFs, SHEDs were able to attach and proliferate well on the scaffolds. Further, there was a pronounced difference in SHEDs’ proliferation ability on the group that released higher amounts of DEX (i.e., PLGA-DEX/CD-IC at 15%), which could be attributed to the amount of DEX that negatively affects SHEDs proliferation ability.
3.5.2. ALP activity
Osteo/odontoblastic differentiation of SHEDS was assessed by ALP activity, a membrane-associated enzyme, and an early marker of osteoblastic differentiation [36]. The results of ALP activity are shown in Fig. 6. SHEDs differentiation was dependent on the DEX concentration; however, the concentration’s maximal effect was not beyond 5% DEX/β-CD-IC. This increase corresponded with the DEX concentration up to 100 nM, which is usually known to support osteogenic differentiation [13]. ALP activity was significantly less in the 15% DEX/β-CD-IC group, compared to the 5% DEX/β-CD-IC group at day 7. The reduced ALP activity in the 15% DEX/β-CD-IC group is in agreement with the findings of a study showing that DEX at high dosages utilizes negative effects on osteoblasts. These effects, highlighted by reduced osteoblasts proliferation, increased apoptosis, and subsequently, decreased in bone formation [13,37,38].
FIGURE 6.
ALP activity indicates early osteogenic differentiation of SHEDs at day 7 and day 14.
3.5.3. Alizarin red staining
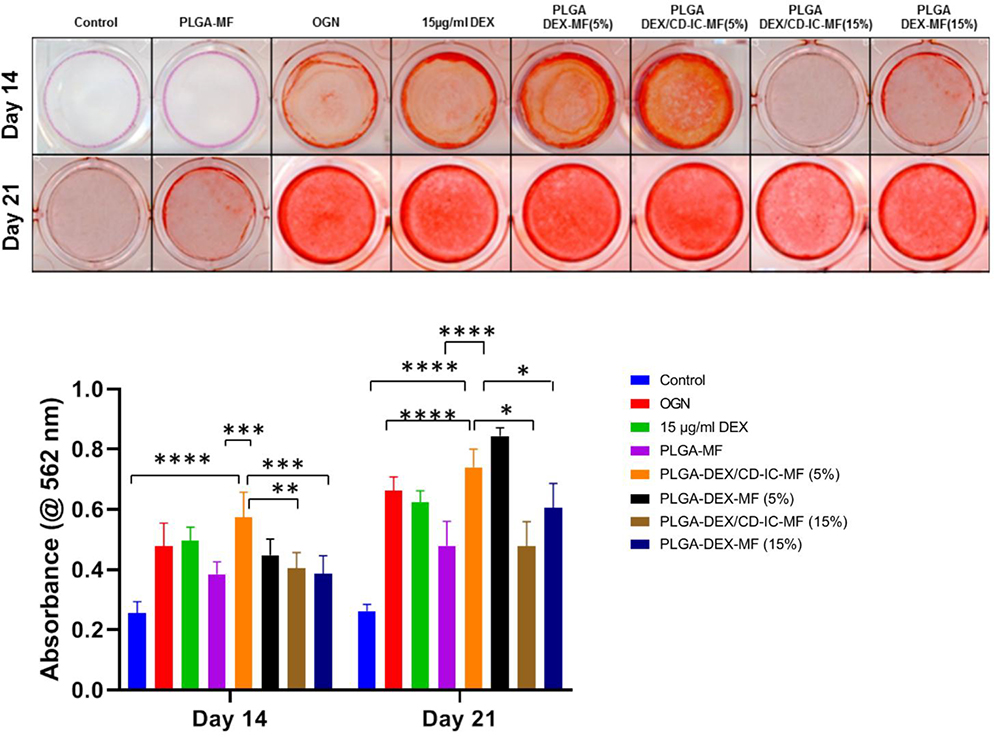
In our experiments, the proposed PLGA-DEX/β-CD-IC scaffold was designed to supply SHEDs with a sustained amount of an osteogenic differentiation molecule (DEX). According to the literature, the effect of DEX on stem cells osteogenic differentiation is dose-dependent [13,39]. Osteogenic differentiation of MSCs is known to be initiated by DEX at an early stage and further maturation at later stages [40]. Thus, alizarin red staining was used to investigate the osteo/odontogenic differentiation of SHEDs in response to DEX release from the scaffolds after 14 and 21 days. Representative images of Alizarin Red stained wells are shown in (Fig. 7A). Alizarin Red staining was intense which indicates calcium deposition in the cultures exposed 5% PLGA-DEX/CD-IC-MF in comparison to DEX-free PLGA-MF at Day 14. At Day 21, calcium deposits were observed in cultures exposed to 5% PLGA-DEX/CD-IC-MF while less deposits were noticed when DEX released at higher levels in 15% PLGA-DEX/CD-IC-MF and 15% PLGA-DEX-MF. Quantitative data (Fig. 7B) demonstrated increased mineralization in cells exposed to 5% PLGA-DEX/CD-IC-MF, which is supported by the drug release profile. At early time point, 5% PLGA-DEX/CD-IC-MF resulted in significantly higher mineralization, which was similar to 15% PLGA-DEX/CD-IC-MF, 15% PLGA-DEX-MFs, 5% PLGA-DEX-MF scaffolds and PLGA-MF. Whereas, there was no statistical difference noticed within groups of 15% PLGA-DEX/CD-IC-MF, 15% PLGA-DEX-MFs and 5% PLGA-DEX-MFs scaffolds, and the control groups. Hence, the solubility increment in the DEX complexation, provides SHEDs with the necessary supplements for differentiation at minimal doses. Similar to previously reported concentration where 100 nM DEX was sufficient to induce differentiation of human BMMSCs into osteoblasts [41–43]. Nonetheless, further steps are needed to validate the clinical efficacy of the proposed DEX/β-CD-IC polymeric electrospun scaffolds using a pre-clinical animal model of vital pulp therapy.
FIGURE 7.
(A) Alizarin red stained wells shows the effect of DEX induced mineralization in SHEDs at day 14. (B) Quantification results of Alizarin Red S staining showed the effect of different concentration of DEX released from PLGA-DEX-MF, and PLGA-DEX/CD-IC-MF induced matrix mineralization in SHEDs.
4. Conclusion
In conclusion, our data showed a significant improvement of DEX solubility after complex formation with β-CD. The release profile provided by the inclusion complex confirms high release of DEX when compared to non-inclusion complex counterpart. Of note, a decrease in SHEDs toxicity and a significant increase in mineralization was associated with the 5 wt.% DEX/β-CD-IC group, owing to its sustained DEX release, cytocompatibility, and proven odontogenic differentiation capacity.
Highlights.
A drug delivery system to afford sustained release of DEX was achieved by CD-IC.
DEX/CD-IC was successfully incorporated into PLGA fibers via electrospinning.
UPLC revealed the solubility enhancement and sustained release of DEX by CD-IC.
Cell compatibility and proliferation was more favorable in DEX/CD-IC fibers.
Odonto/osteogenic differentiation was enhanced in DEX/CD-IC electrospun fibers.
Acknowledgments
The authors acknowledge technical support from the Michigan Center for Materials Characterization. We also appreciate the help provided by Dr. Zhongrui Li (Department of Earth and Environmental Sciences at the University of Michigan) for performing XRD analysis. The authors deny any conflicts of interest related to this study. M.C.B. acknowledges the National Institutes of Health (NIH)/National Institute of Dental and Craniofacial Research (NIDCR) (R01DE026578). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest
There are no conflicts to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Orhan AI, Oz FT & Orhan K Pulp exposure occurrence and outcomes after 1- or 2-visit indirect pulp therapy vs complete caries removal in primary and permanent molars. Pediatr Dent 32, 347–355 (2010). [PubMed] [Google Scholar]
- [2].Hilton T Keys to Clinical Success with Pulp Capping: A Review of the Literature. Oper Dent 34, 615–625 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Choung HW et al. Tertiary Dentin Formation after Indirect Pulp Capping Using Protein CPNE7. J. Dent. Res. 95, 906–912 (2016). [DOI] [PubMed] [Google Scholar]
- [4].Ricketts D Management of the deep carious lesion and the vital pulp dentine complex. Br Dent J 191, 606–610 (2001). [DOI] [PubMed] [Google Scholar]
- [5].Song M et al. Clinical and Molecular Perspectives of Reparative Dentin Formation: Lessons Learned from Pulp-Capping Materials and the Emerging Roles of Calcium. Dent. Clin. North Am. 61, 93–110 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ghoddusi J, Forghani M & Parisay I New Approaches in Vital Pulp Therapy in Permanent Teeth. Iran Endod J 9, 15–22 (2014). [PMC free article] [PubMed] [Google Scholar]
- [7].Palma PJ et al. Six-Month Color Stability Assessment of Two Calcium Silicate-Based Cements Used in Regenerative Endodontic Procedures. Journal of Functional Biomaterials 10, 14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ramos JC et al. 1-year In Vitro Evaluation of Tooth Discoloration Induced by 2 Calcium Silicate-based Cements. Journal of Endodontics 42, 1403–1407 (2016). [DOI] [PubMed] [Google Scholar]
- [9].Chadipiralla K et al. Osteogenic differentiation of stem cells derived from human periodontal ligaments and pulp of human exfoliated deciduous teeth. Cell Tissue Res. 340, 323–333 (2010). [DOI] [PubMed] [Google Scholar]
- [10].Jaiswal N, Haynesworth SE, Caplan AI & Bruder SP Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J. Cell. Biochem. 64, 295–312 (1997). [PubMed] [Google Scholar]
- [11].Bereshchenko O, Migliorati G, Bruscoli S & Riccardi C Glucocorticoid-Induced Leucine Zipper: A Novel Anti-inflammatory Molecule. Front. Pharmacol. 10, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sharma A et al. Effects of dexamethasone on human trabecular meshwork cells in vitro. Graefes Arch Clin Exp Ophthalmol 251, 1741–1746 (2013). [DOI] [PubMed] [Google Scholar]
- [13].Kim S-M, Kim Y-G, Park J-W, Lee J-M & Suh J-Y The effects of dexamethasone on the apoptosis and osteogenic differentiation of human periodontal ligament cells. J Periodontal Implant Sci 43, 168–176 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tiwari G, Tiwari R & Rai AK Cyclodextrins in delivery systems: Applications. J Pharm Bioallied Sci 2, 72–79 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Palem CR, Chopparapu K, Subrahmanyam P & Yamsani M Cyclodextrins and their Derivatives in Drug Delivery: A Review. Current Trends in Biotechnology and Pharmacy 6, 255–275 (2012). [Google Scholar]
- [16].Stella null, Rao null, Zannou null & Zia null. Mechanisms of drug release from cyclodextrin complexes. Adv. Drug Deliv. Rev. 36, 3–16 (1999). [DOI] [PubMed] [Google Scholar]
- [17].Gidwani B & Vyas A A Comprehensive Review on Cyclodextrin-Based Carriers for Delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. Biomed Res Int 2015, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Loftsson T & Stefánsson E Cyclodextrins in eye drop formulations: enhanced topical delivery of corticosteroids to the eye. Acta Ophthalmol Scand 80, 144–150 (2002). [DOI] [PubMed] [Google Scholar]
- [19].Aytac Z, Ipek S, Durgun E & Uyar T Antioxidant electrospun zein nanofibrous web encapsulating quercetin/cyclodextrin inclusion complex. J Mater Sci 53, 1527–1539 (2018). [Google Scholar]
- [20].Aytac Z & Uyar T Core-shell nanofibers of curcumin/cyclodextrin inclusion complex and polylactic acid: Enhanced water solubility and slow release of curcumin. Int J Pharm 518, 177–184 (2017). [DOI] [PubMed] [Google Scholar]
- [21].Aytac Z, Sen HS, Durgun E & Uyar T Sulfisoxazole/cyclodextrin inclusion complex incorporated in electrospun hydroxypropyl cellulose nanofibers as drug delivery system. Colloids Surf B Biointerfaces 128, 331–338 (2015). [DOI] [PubMed] [Google Scholar]
- [22].Zhang Z et al. Wnt/β-Catenin Signaling Determines the Vasculogenic Fate of Postnatal Mesenchymal Stem Cells. STEM CELLS 34, 1576–1587 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Miura M et al. SHED: Stem cells from human exfoliated deciduous teeth. PNAS 100, 5807–5812 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ali H, Kalashnikova I, White MA, Sherman M & Rytting E Preparation, characterization, and transport of dexamethasone-loaded polymeric nanoparticles across a human placental in vitro model. Int J Pharm 454, 149–157 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Aytac Z, Kusku SI, Durgun E & Uyar T Quercetin/β-cyclodextrin inclusion complex embedded nanofibres: Slow release and high solubility. Food Chem 197, 864–871 (2016). [DOI] [PubMed] [Google Scholar]
- [26].Sinha VR, Anitha R, Ghosh S, Nanda A & Kumria R Complexation of celecoxib with β-cyclodextrin: Characterization of the interaction in solution and in solid state. Journal of Pharmaceutical Sciences 94, 676–687 (2005). [DOI] [PubMed] [Google Scholar]
- [27].Abarca RL, Rodríguez FJ, Guarda A, Galotto MJ & Bruna JE Characterization of beta-cyclodextrin inclusion complexes containing an essential oil component. Food Chemistry 196, 968–975 (2016). [DOI] [PubMed] [Google Scholar]
- [28].Campos EVR et al. Chitosan nanoparticles functionalized with β-cyclodextrin: a promising carrier for botanical pesticides. Sci Rep 8, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Aytac Z, Celebioglu A, Yildiz ZI & Uyar T Efficient Encapsulation of Citral in Fast-Dissolving Polymer-Free Electrospun Nanofibers of Cyclodextrin Inclusion Complexes: High Thermal Stability, Longer Shelf-Life, and Enhanced Water Solubility of Citral. Nanomaterials (Basel) 8, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Aytaç Z, Ipek S, Erol I, Durgun E & Uyar T Fast-dissolving electrospun gelatin nanofibers encapsulating ciprofloxacin/cyclodextrin inclusion complex. Colloids and surfaces. B, Biointerfaces 178, 129–136 (2019). [DOI] [PubMed] [Google Scholar]
- [31].Aytac Z, Kusku SI, Durgun E & Uyar T Encapsulation of gallic acid/cyclodextrin inclusion complex in electrospun polylactic acid nanofibers: Release behavior and antioxidant activity of gallic acid. Materials Science and Engineering: C 63, 231–239 (2016). [DOI] [PubMed] [Google Scholar]
- [32].Ramakrishna S An Introduction to Electrospinning and Nanofibers. (World Scientific, 2005). [Google Scholar]
- [33].Huang GT-J, Gronthos S & Shi S Mesenchymal Stem Cells Derived from Dental Tissues vs. Those from Other Sources. J Dent Res 88, 792–806 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Seo B et al. SHED repair critical-size calvarial defects in mice. Oral Dis 14, 428–434 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kuppermann BD, Zacharias LC & Kenney MC Steroid Differentiation: The Safety Profile of Various Steroids on Retinal Cells in Vitro and their Implications for Clinical Use (An American Ophthalmological Society Thesis). Trans Am Ophthalmol Soc 112, 116–141 (2014). [PMC free article] [PubMed] [Google Scholar]
- [36].Shen Q, Zhu S, Hu J, Geng N & Zou S Recombinant human bone morphogenetic protein-4 (BMP-4)-stimulated cell differentiation and bone formation within the expanding calvarial suture in rats. J Craniofac Surg 20, 1561–1565 (2009). [DOI] [PubMed] [Google Scholar]
- [37].Hayami T, Zhang Q, Kapila Y & Kapila S Dexamethasone’s enhancement of osteoblastic markers in human periodontal ligament cells is associated with inhibition of collagenase expression. Bone 40, 93–104 (2007). [DOI] [PubMed] [Google Scholar]
- [38].Cheng SL, Zhang SF & Avioli LV Expression of bone matrix proteins during dexamethasone-induced mineralization of human bone marrow stromal cells. J. Cell. Biochem. 61, 182–193 (1996). [DOI] [PubMed] [Google Scholar]
- [39].Ghali O et al. Dexamethasone in osteogenic medium strongly induces adipocyte differentiation of mouse bone marrow stromal cells and increases osteoblast differentiation. BMC Cell Biol 16, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Schacke H, Döcke WD & Asadullah K Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Ther. 96, 23–43 (2002). [DOI] [PubMed] [Google Scholar]
- [41].Oshina H et al. Effects of continuous dexamethasone treatment on differentiation capabilities of bone marrow-derived mesenchymal cells Bone 41, 575–583 (2007). [DOI] [PubMed] [Google Scholar]
- [42].Ding S et al. Synergistic effect of released dexamethasone and surface nanoroughness on mesenchymal stem cell differentiation. Biomater. Sci. 1, 1091–1100 (2013). [DOI] [PubMed] [Google Scholar]
- [43].Yuasa M et al. Dexamethasone Enhances Osteogenic Differentiation of Bone Marrow- and Muscle-Derived Stromal Cells and Augments Ectopic Bone Formation Induced by Bone Morphogenetic Protein-2. PLoS One 10, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]