Highlights
-
•
COVID-19 was first detected in Uganda in the third week of March 2020.
-
•
Although the number of COVID-19 cases has grown steadily, data have not been analyzed to determine whether a pattern in the nature of cases has emerged.
-
•
The first cases were detected among international arrivals and after that among their local contacts. However, in mid-April, a number of cases were detected among long-distance truck drivers arriving from the neighboring countries.
-
•
Among the 442 cases that have tested positive, a majority, 317 (71.8%) were truck drivers, 75 (16.9%) were community cases, and 50 (11.3%) were international arrivals.
-
•
A majority of the community cases have been linked to contact with long-distance truck drivers; interventions are urgently needed to protect long-distance truck drivers.
Abstract
Objective
To examine the patterns of COVID-19 transmission in Uganda.
Methods
We reviewed ten weeks of press releases from the Uganda Ministry of Health from the day when the first case was announced, March 22, through May 29, 2020. We obtained the press releases from the MoH website and the Twitter handle (@MinofHealthUG). Data include the number of persons tested and the categories were classified as international arrivals, community members, and long-distance truck drivers.
Results
The first cases were international arrivals from Asia and Europe, and after that, community cases emerged. However, in the middle of April 2020, COVID-19 cases were detected among long-distance truck drivers. By May 29, 2020, 89, 224 persons had been tested; overall, 442 tested positive. Of those that tested positive, the majority, or 317 (71.8%) were truck drivers, 75 (16.9%) were community cases, and 50 (11.3%) were international arrivals. The majority of community cases have been linked to contact with truck drivers.
Conclusions
Truck drivers were the most frequently diagnosed category, and have become a core group for COVID-19 in Uganda. They have generated significant local transmission, which now threatens a full-blown epidemic unless strict controls are put in place.
Introduction
Subgroups of the population with a higher prevalence or incidence of an infectious disease are often called core groups and can serve as a source of infection to the general population. The term has commonly been applied to sexually transmitted infections such as HIV, syphilis, and gonorrhea (Watts et al., 2010, Gesink et al., 2011, Lewis, 2013) but can apply to non-sexually transmitted infections (Lietman et al., 2018). Core groups may spread an infection to the general population directly or through bridge populations. They may sustain infection levels at endemic or even epidemic levels unless control measures are instituted to lower the prevalence of disease among them or restrict their contact with the general population.
In December 2019, cases of a rare pneumonia connected to a seafood market were reported in Wuhan, China; the causative agent was identified as a viral agent that was named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Zhu et al., 2020), the cause of what we now know as Coronavirus disease (COVID-2019). Starting in China, the infection spread very rapidly and has now reached nearly all continents. Given the scale and rate of spread, it is clear that SARS-CoV-2 is a highly infectious agent, with a relatively high basic reproductive rate estimated to be at least three (D’Arienzo and Coniglio, 2020, Liu et al., 2020, Zhuang et al., 2020). Also, given its origin from a single country, all new cases in other countries were introduced by travelers from China and eventually to new hotspots in Asia and Europe.
In Africa, the first case was reported in Egypt in mid-February; in Uganda, the first case was reported by the Ministry of Health (MoH) on March 22, 2020, of a traveler arriving from Dubai. The Ministry initiated steps to screen travelers from hotspot countries, and presidential directives were issued to close international borders. However, long-distance trucks were allowed access to bring essential goods into the country or to transit to neighboring countries of Rwanda, the Democratic Republic of Congo, and South Sudan. In this paper, we track the reports of new COVID-19 cases reported by the MoH, trace the new cases’ emerging patterns, and identify the categories of persons at high risk for COVID-19 in Uganda.
Methods
On a regular basis, we reviewed MoH reports and press releases for new COVID-19 cases detected in Uganda as they were made public. The reports are posted on the Twitter page for the Ministry of Health (@MinofHealthUG); all entries were verified based on posts using this handle. Data were entered into Excel and analyzed using STATA version 12. We plotted an epidemic curve using a 7-day interval as the unit on the time scale. We classify the cases into three categories: international travelers, local/community cases, and long-distance truck drivers. International travelers are those passengers who arrived from a foreign country regardless of their citizenship. Local cases are those diagnosed among local residents that had no evidence of recent foreign travel; these were assumed to be a result of local transmission, or what the MoH refers to as community cases. Long-distance truck drivers arrived at Uganda’s borders as drivers of trucks carrying merchandise or goods, regardless of their nationality. We report a combination of both Ugandan and foreign national cases detected in Uganda and do not specify the nationalities of the cases.
Results
We tracked new cases of COVID-19 detected between March 22, 2020, and May 29, 2020, a period of 10 weeks. The first case was reported on March 21, 2020. Out of a total of 89, 224 persons, 442 tested positive. Of those that tested positive, a majority, 317 (71.8%) were truck drivers, 75 (16.9%) were community cases, and 50 (11.3%) were international arrivals, as shown in Table 1 below.
Table 1.
Number of positive COVID-19 tests reported by the Ministry of Health Uganda.
| Number of positive COVID-19 cases |
||||||
|---|---|---|---|---|---|---|
| Date | Week | Number tested | Total | International arrivals | Community | Truck drivers |
| March 21–27 | 1 | 724 | 23 | 23 | 0 | 0 |
| March 28–April 3 | 2 | 1 473 | 25 | 22 | 3 | 0 |
| April 4–10 | 3 | 2 070 | 5 | 5 | 0 | 0 |
| April 11–17 | 4 | 5 302 | 3 | 0 | 1 | 2 |
| April 18–24 | 5 | 7 609 | 17 | 0 | 2 | 15 |
| April 25-May 1 | 6 | 12 427 | 8 | 0 | 2 | 6 |
| May 2–8 | 7 | 20 771 | 29 | 0 | 3 | 26 |
| May 9–15 | 8 | 15 527 | 89 | 0 | 0 | 89 |
| May 16–22 | 9 | 11 928 | 89 | 0 | 11 | 78 |
| May 23–29 | 10 | 11,393 | 154 | 0 | 53 | 101 |
| Total | 89, 224 | 442 | 50 | 75 | 317 | |
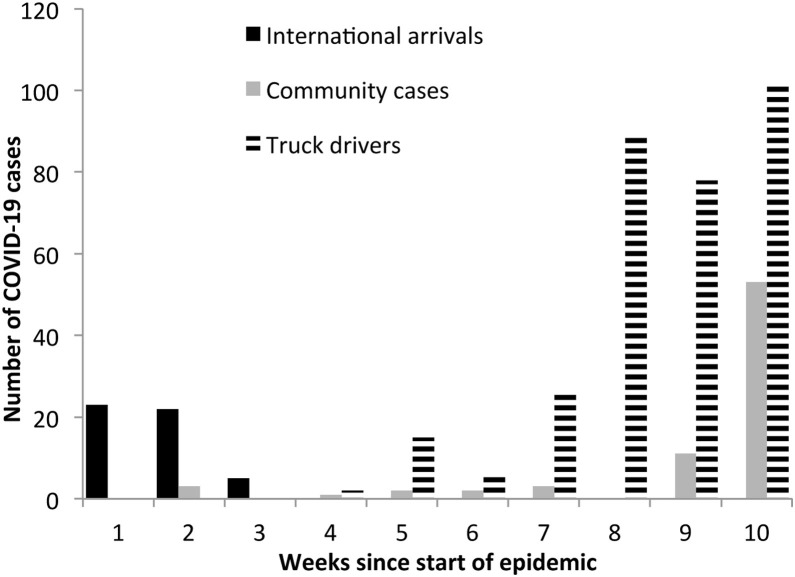
The data are also shown in Figure 1 , indicating that the cases were all international arrivals at the beginning of the Ugandan epidemic. These quickly shifted to community cases but since mid-April have been dominated by truck drivers, followed by a surge in community cases starting in mid-May. The steady rise in COVID-19 cases among truck drivers has been accompanied by a rise in community cases. At the beginning of the epidemic, the first five community cases identified were all linked to contact with international travelers. However, subsequent community cases have all been linked to probable contact with truck drivers.
Figure 1.
Epidemic curve showing the distribution of COVID-19 cases in Uganda by category of international arrivals, community cases and truck drivers, March to May 2020.
Discussion
We present original data that describe the dynamics of COVID transmission in a setting where local transmission has been mostly contained, but current evidence suggests that the number of new cases could escalate. The first cases were all international travelers, as described before (Olum and Bongomin, 2020). Community cases arising from contact with international travelers were then detected and, currently, long-distance truck drivers and their local contacts now dominate the epidemic, threatening an escalation in the epidemic. These events occurred during a national lockdown that was announced on March 19, 2020, and only eased on May 25, 2020.
Long-distance truck drivers have established themselves currently as the highest risk group for COVID-19 in Uganda. This population has historically been at risk and formed a core group for other infectious diseases such as HIV and other STDs (Pickering et al., 1997, Gysels et al., 2001). The reasons why long-distance truck drivers are likely to test positive for COVID are not clear, although there are some possible explanations. First, they drive long distances, exposing themselves to a more extensive social network, primarily urban and likely crowded places such as trading centers or ports of goods sheds where the probability of contact with infected persons may be increased. The second is that they may represent the general prevalence of the communities where they come from, signifying high prevalence in neighboring countries. There is limited screening data from the neighboring countries to corroborate this hypothesis.
The data suggest that interventions for drivers to limit social contact along their journey are urgently required. Transport companies will need to test their drivers and ensure those who are positive do not continue with their journey. Uganda embarked on testing truck drivers in mid-April but initially allowed them to continue their journey before results were available. Samples were then transported to a hub in Entebbe, near the capital, Kampala. This may have cost the country an increase in community transmission, evidenced by the growth in the number of cases in weeks nine and ten. With evidence of this increased community transmission, the MoH changed its approach to now test truck drivers at border points; truck drivers who test positive are not allowed to continue their journey.
This report’s strength is that we present new data, comprehensively including all confirmed cases to-date as reported by MoH, and collected using innovative means. The limitation of this analysis is that new data are added daily, the epidemic is dynamic. Data analysis should be done on a continuous basis to obtain a more complete and up-to-date picture of the epidemic's trajectory.
In conclusion, the COVID-19 epidemic in Uganda was sparked by travelers from Europe and Asia. The country is now facing threats of new transmission from regional long-distance truck drivers arriving from neighboring countries. The epidemic is now literally being driven by truck drivers who have emerged as a core group for COVID-19 in Uganda. Public health prevention measures that take into account regional integration of efforts are required to ensure success for the COVID-19 programs in Uganda and its neighbors.
Conflict of interest
We declare no conflict of interest.
Funding source
None.
Ethics approval
Our work did not require ethics approval as we used data in the public domain and carried no human subject issues.
Authors’ contributions
FB conceived the idea, all authors collected data, JI did the analysis, FB created the first draft, all authors revised and contributed to the final version.
References
- D’Arienzo M., Coniglio A. Assessment of the SARS-CoV-2 basic reproduction number, R0, based on the early phase of COVID-19 outbreak in Italy. Biosafety and Health. 2020;2(2):57–59. doi: 10.1016/j.bsheal.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesink D.C., Sullivan A.B., Miller W.C., Bernstein K.T. Sexually transmitted disease core theory: roles of person, place, and time. Am J Epidemiol. 2011;174:81–89. doi: 10.1093/aje/kwr035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gysels M., Pool R., Bwanika K. Truck drivers, middlemen and commercial sex workers: AIDS and the mediation of sex in southwest Uganda. AIDS Care. 2001;13:373–385. doi: 10.1080/09540120120044026. [DOI] [PubMed] [Google Scholar]
- Lewis D. The role of core groups in the emergence and dissemination of antimicrobial-resistant N gonorrhea. Sex Transm Infect. 2013;89:iv47–iv51. doi: 10.1136/sextrans-2013-051020. [DOI] [PubMed] [Google Scholar]
- Lietman T.M., Deiner M.S., Oldenburg C.E., Nash S.D., Keenan J.D., Porco T.C. Identifying a sufficient core group for trachoma transmission. PLoS Negl Trop Dis. 2018;12 doi: 10.1371/journal.pntd.0006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Gayle A.A., Wilder-Smith A., Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020 doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olum R., Bongomin F. Uganda’s first 100 COVID-19 cases: trends and lessons. Int J Infect Dis. 2020;96:517–518. doi: 10.1016/j.ijid.2020.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering H., Okongo M., Nnalusiba B., Bwanika K., Whitworth J. Sexual networks in Uganda: casual and commercial sex in a trading town. AIDS Care. 1997;9:199–207. doi: 10.1080/09540129750125217. [DOI] [PubMed] [Google Scholar]
- Watts C., Zimmerman C., Foss A.M., Hossain M., Cox A., Vickerman P. Remodelling core group theory: the role of sustaining populations in HIV transmission. Sex Transm Infect. 2010;86:iii85–iii92. doi: 10.1136/sti.2010.044602. [DOI] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Z., Zhao S., Lin Q., Cao P., Lou Y., Yang L. Preliminary estimating the reproduction number of the coronavirus disease (COVID-19) outbreak in Republic of Korea and Italy by March 5, 2020. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]