Highlights
-
•
Neurosensory dysfunction.
-
•
Chronological analysis.
-
•
Viral load.
Keywords: Neurosensory dysfunction, COVID-19, Hyposmia, Hypogeusia, Diagnosis
Abstract
Objective
To describe neurosensory dysfunctions, including hyposmia, hypogeusia, and tinnitus, in patients with COVID-19.
Methods
Clinical characteristics and oropharyngeal swabs were obtained from 86 patients with COVID-19 hospitalized in Guangzhou Eighth People’s Hospital. The chronological analysis method was used to detail neurosensory dysfunction. The cycle threshold (Ct) values were used to approximately indicate viral load.
Results
Forty-four (51.2%) patients had neurosensory dysfunction: hyposmia (34, 39.5%), hypogeusia (33, 38.4%), and tinnitus (three, 3.5%). Neurosensory dysfunction was significantly more common in patients under 40 years old (p = 0.001) and women (p = 0.006). Hyposmia and hypogeusia coexisted in 23 (26.7%) patients. The interval between onset of hyposmia and hypogeusia was 0.7 ± 1.46 days. The interval from onset of hyposmia and hypogeusia to typical COVID-19 symptoms was 0.22 ± 4.57 and 0.75 ± 6.77 days; the interval from onset of hyposmia and hypogeusia to admission was 6.06 ± 6.68 and 5.76 ± 7.68 days; and the duration of hyposmia and hypogeusia was 9.09 ± 5.74 and 7.12 ± 4.66 days, respectively. The viral load was high following symptoms onset, peaked within the first week, and gradually declined.
Conclusions
Neurosensory dysfunction tends to occur in the early stage of COVID-19, and it could be used as a marker for the early diagnosis of COVID-19.
Introduction
A global pandemic named Coronavirus Disease 2019 (COVID-19), caused by SARS-CoV-2 infection, has been wreaking havoc with much of human civilization's health. By April 30, 2020, more than three million patients with COVID-19 had been confirmed worldwide, including over 217 thousand deaths (World Health Organization, 2020).
Early diagnosis is key to the management of the COVID-19 pandemic. Recently, some researchers have reported that patients with COVID-19 suffer from neurosensory dysfunction, including loss of smell (hyposmia) and taste (hypogeusia), with a prevalence of 5.1%–98% (Lee et al., 2020, Mao et al., 2020, Moein et al., 2020) for hyposmia, and 5.6%–90.3% (Lechien et al., 2020a, Lechien et al., 2020b, Lee et al., 2020, Mao et al., 2020) for hypogeusia. However, the exact onset time and the duration of hyposmia and hypogeusia are rarely reported.
Neurosensory dysfunction of patients with COVID-19 might be considered less harmful than typical symptoms (fever, cough, or shortness of breath) (Arons et al., 2020). However, that does not mean they should be neglected. Clarifying the onset time and duration of these symptoms will offer help for early diagnosis and accurate management of COVID-19.
In this study, we report characteristic neurosensory dysfunctions in 44 of 86 patients with COVID-19. We detailed the exact time of onset and duration of neurosensory dysfunction, using the chronological analysis method. The viral load of oropharyngeal swab tests was analyzed.
Materials and methods
Patients
Eighty-six confirmed cases of COVID-19 (admission date from March 16 to April 12, 2020) at Guangzhou Eighth People’s Hospital in Guangdong, China, which was the designated hospital exclusively for COVID-19 in Guangzhou, were included in this study. The confirmed criteria followed the latest Diagnosis and Treatment Guidelines for COVID-19 issued by the National Health Committee of the People’s Republic of China (National Health Commission of the People’s Republic of China, 2020). This study was performed according to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Guangzhou Eighth People’s Hospital. Verbal consent was obtained from patients before enrollment.
Data collection
Demographic information, clinical characteristics (included medical history, comorbidities, signs, and symptoms), and laboratory findings were obtained from the electronic medical record system of Guangzhou Eighth People's Hospital and analyzed by three independent researchers. Neurosensory symptoms were obtained on the day of discharge using a self-made questionnaire. The onset date was defined as the day when any symptoms were noticed by the patients. A chronological method (a record of the times and the order in which a series of past events took place) was used for analysis.
Oropharyngeal swabs were collected and placed into a sterile tube containing an RNA preservation solution. The swabs were sent for SARS-CoV-2 RNA extraction and detection within one hour by a real-time reverse transcriptional polymerase chain reaction (RT-PCR)system, following the commercial test kit instructions (Da’an Gene cooperation, Cat DA0930) as previously described (Chen et al., 2020). Briefly, two PCR primer and probe sets targeting ORF1a/b and nCoV-N genes were separately added into the same reaction tube. Positive and negative controls were involved in the detection. Cycle threshold (Ct) values were used to quantify the viral load, with lower values indicating higher viral load. The samples were defined as viral-positive when either or both genes had a Ct value <41.
Statistical analysis
Continuous variables were described as median and range values. The analyses were carried out using GraphPad Prism 9 or IBM SPSS Statistics 25. Categorical variables were compared using Fisher's exact test and continuous variables with the Mann–Whitney U test. Spearman’s correlation test was performed to analyze the relationship between age and viral load and between days after symptom onset and test values. The significance level was set as 0.05.
Results
A total of 86 hospitalized patients (44 male and 42 female) with confirmed COVID-19 were included in this study. The demographic and clinical characteristics are shown in Table 1 . The median age of patients was 25.5 years (range 6–57). 85 patients had mild COVID-19, and one was a severe case. 18 (20.9%) patients had at least one comorbidity: chronic liver diseases (eight, 9.3%), hyperlipidemia (three, 3.5%), cardio-cerebrovascular disease (three, 3.5%), followed by hypertension, anemia, and hyperthyroidism (two, 2.3%). The most common typical symptom was cough (41, 47.7%), followed by fever (26, 30.2%), fatigue and pharyngalgia (16, 18.6%), anorexia (15, 17.4%), headache (12, 14.0%), myalgia (eight, 9.3%), diarrhea (six, 7.0%), and vomiting (four, 4.7%); and eleven (12.8%) patients showed no typical symptoms.
Table 1.
Clinical characteristics of patients with COVID-19.
| Cases (n = 86) | ||
|---|---|---|
| Age, year–median (range) | 25.5 (6–57) | |
| Gender, n (%) | ||
| Male | 44 (51.2) | |
| Female | 42 (48.8) | |
| Severity, n (%) | ||
| Mild | 85 (98.8) | |
| Severe | 1 (0.2) | |
| Comorbidity, n (%) | ||
| Any | 18 (20.9) | |
| Chronic liver disease | 8 (9.3) | |
| Hyperlipidemia | 3 (3.5) | |
| Cardio cerebrovascular disease | 3 (3.5) | |
| Hypertension | 2 (2.3) | |
| Anemia | 2 (2.3) | |
| Hyperthyroidism | 2 (2.3) | |
| Typical symptoms, n (%) | ||
| Any | 75 (87.2) | |
| Cough | 41 (47.7) | |
| Fever | 26 (30.2) | |
| Fatigue | 16 (18.6) | |
| Pharyngalgia | 16 (18.6) | |
| Anorexia | 15 (17.4) | |
| Headache | 12 (14.0) | |
| Myalgia | 8 (9.3) | |
| Diarrhea | 6 (7.0) | |
| Vomiting | 4 (4.7) | |
| Neurosensory dysfunction, n (%) | ||
| Any | 44 (51.2) | |
| Hyposmia | 34 (39.5) | |
| Hypogeusia | 33 (38.4) | |
| Tinnitus | 3 (3.5) | |
Forty-four (51.2%) patients had neurosensory dysfunction: hyposmia (34, 39.5%), hypogeusia (33, 38.4%), and tinnitus (three, 3.5%). Table 2 shows the demographic characteristics and laboratory findings of 44 cases with neurosensory dysfunction. Patients with neurosensory dysfunction were noticed to be of a younger age (median 23.5 years vs. 31.5 years, p = 0.024). Of the 44 patients, 42 (95.5%) were under 40 years old (6–39 years old). Neurosensory dysfunction was significantly more common in patients under 40 years old (p = 0.001). Women develop neurosensory dysfunction more commonly than men (p = 0.006). There was no significant correlation between comorbidity and neurosensory dysfunction. No obvious differences in laboratory tests were noticed between patients with and without neurosensory dysfunction.
Table 2.
Clinical characteristics and laboratory findings of patients with neurosensory dysfunction.
| Neurosensory dysfunction |
||||
|---|---|---|---|---|
| No (n = 42) | Any (n = 44) | p Value | ||
| Age, year–median (range) | 31.5 (6–57) | 23.5 (14–51) | 0.024 | |
| Age group, n | 0.001 | |||
| 6–39 | 28 | 42 | ||
| 40–57 | 14 | 2 | ||
| Gender, n | 0.006 | |||
| Male | 28 | 16 | ||
| Female | 14 | 28 | ||
| Comorbidities, n | 0.601 | |||
| Any | 10 | 8 | ||
| Laboratory findings, median (range) | ||||
| Leukocytes, ×109/L | 5.27 (2.57–11.68) | 5.54 (2.87–8.82) | 0.863 | |
| Neutrophils, ×109/L | 3.29 (1.35–9.07) | 3.38 (0.98–6.45) | 0.9 | |
| Lymphocytes, ×109/L | 1.47 (0.61–3.51) | 1.54 (0.84–2.67) | 0.739 | |
| D-dimer, mg/L | 0.78 (0.1–1.68) | 0.82 (0.23–3.24) | 0.547 | |
| Procalcitonin, ng/mL | 0.047 (0.01–0.13) | 0.039 (0.001–0.087) | 0.121 | |
| Lactate dehydrogenase, U/L | 171 (112–283) | 168.5 (99–359) | 0.982 | |
| Urea, mmol/L | 3.39 (1.97–7.22) | 3.24 (1.83–6.84) | 0.26 | |
| Creatinine, μmol/L | 69.7 (41.5–98.5) | 67.0 (35.7–88) | 0.351 | |
| Alanine aminotransferase, U/L | 21 (8.3–138.2) | 18.1 (8.6–225.2) | 0.262 | |
| Aspartate aminotransferase, U/L | 17.9 (11.5–69.6) | 16.9 (12.5–57) | 0.19 | |
Note: Data was presented as median (range) and n (%). P values denote the comparison between patients with and without neurosensory dysfunction. Categorical variables were compared using the Fisher’s exact test and continuous variables with the Mann–Whitney U test.
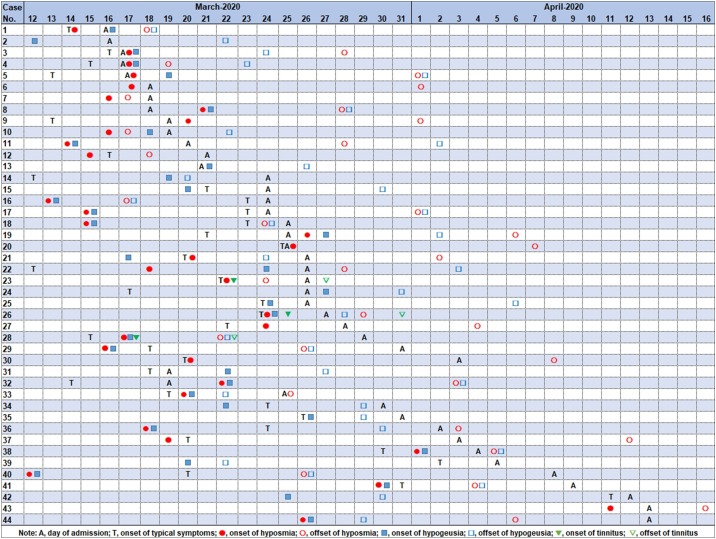
We followed the developmental pattern of neurosensory dysfunction in these 44 patients by conducting a chronological analysis (Figure 1 ). Hyposmia and hypogeusia coexisted in 23 (26.7%) patients, and the interval between onset of hyposmia and hypogeusia was 0.7 ± 1.46 days. Tinnitus existed in Cases 23, 26, and 28. Cases 2, 6, 7, 8, 10, 11, 13, 43, and 44 did not have typical COVID-19 symptoms. The average interval from onset of hyposmia, hypogeusia, and tinnitus to typical symptoms was 0.22 ± 4.57, 0.75 ± 6.77 and 1 ± 1 days, respectively; while the average interval from onset of hyposmia, hypogeusia, and tinnitus to admission was 6.06 ± 6.68, 5.76 ± 7.68 and 6 ± 5.29 days, respectively; the average duration of hyposmia, hypogeusia and tinnitus was 9.09 ± 5.74, 7.12 ± 4.66 and 5 ± 0 days, respectively.
Figure 1.
The chronology of neurosensory dysfunction of 44 patients.
Note: A, days of admission; T, onset of typical symptoms; Red solid circle, onset of hyposmia; Red hollow circle, offset of hyposmia; Blue solid square, onset of hypogeusia; Blue hollow square, offset of hypogeusia; Green solid triangle, onset of tinnitus; Green hollow triangle, offset of tinnitus.
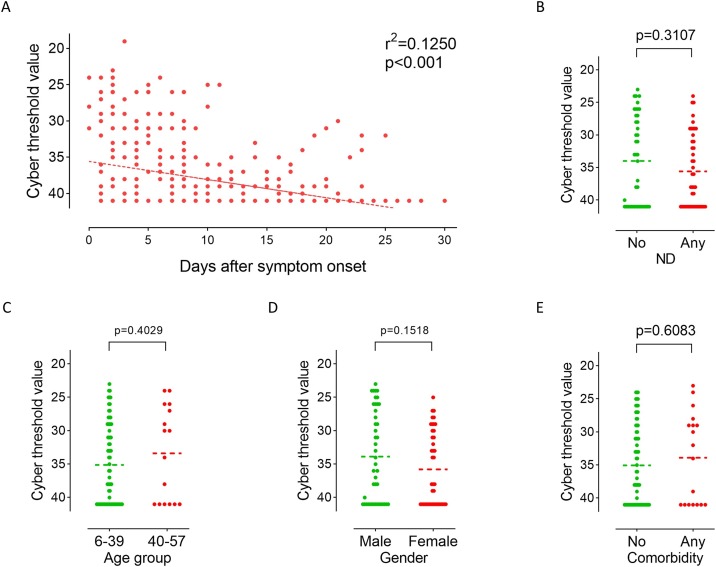
A total of 407 oropharyngeal swabs were obtained from 86 hospitalized patients (mean 4.7 specimens per patient). SARS-CoV-2 RNA was undetectable in oropharyngeal swabs from 24 patients after admission. The results showed that the viral load peaked within the first week after symptoms onset and then gradually declined; a significant negative correlation was noticed between viral load and days after symptom onset (r2 = 0.1250, p < 0.001; Figure 2 A). The first positive results (Ct value <41) of oropharyngeal swabs after admission were used to evaluate the initial viral load. There was no significant difference in initial Ct values between patients with and without neurosensory dysfunction (Figure 2B). Age group (Figure 2C) and gender (Figure 2D) had no significant effect on initial Ct values.
Figure 2.
The Cycle threshold values of patients with COVID-19.
(A): Chronological changes in Ct values detected in 407 oropharyngeal swabs obtained from 86 patients after hospital admission. The Ct value is considered to be inversely correlated to viral RNA copy number and a value below 40 means the viral RNA is undetectable.
(B): Comparison of initial viral load (the first positive Ct value after admission) between patients with and without neurosensory dysfunction. ND, neurosensory dysfunction.
(C): Comparison of initial viral load between age groups (6–39 vs. 40–57 years old).
(D): Comparison of initial viral load between male and female patients.
(E): Comparison of initial viral load between patients with and without comorbidity.
Statistical tests: Spearman’s correlation test (A) and Mann–Whitney U test (B–E).
Discussion
In this study, we detailed the exact time of onset and duration of neurosensory dysfunction, including hyposmia, hypogeusia, and tinnitus, of patients with COVID-19. Patients under 40 years old and women seem to be more susceptible to neurosensory dysfunction. Hyposmia tends to cooccur with hypogeusia in the early stage of COVID-19, even before the onset of typical symptoms.
Most of the reports about loss of smell and taste appear in countries outside East Asia, with the incidence rate of 54.2%–90.3% (Moein et al., 2020, Arons et al., 2020, Beltran-Corbellini et al., 2020, Yan et al., 2020, Giacomelli et al., 2020). There are only two reports on neurosensory dysfunction of patients with COVID-19 in China and Korea. Specifically, Mao et al. (2020) reported that hyposmia and hypogeusia accounted for 5.1% and 5.8% of hospitalized patients in Wuhan, China. In the study via telephone interview by Lee et al. (2020), anosmia or ageusia was observed in 15.3% of patients in the early stage of COVID-19. In our cohort, 44 (51.2%) showed neurosensory dysfunction, a percentage much higher than that in these two studies. The reason for this inconsistency may be that most of the patients in our cohort were imported cases who were infected with the coronavirus abroad; as Forster et al. (2020) reported, the genotyping of the coronavirus may be different (potential mutations).
The present study is the first to use a chronological analysis method to clarify the neurosensory dysfunction of patients with COVID-19. Neurosensory dysfunction tends to occur in the early stage of COVID-19, even before the onset of typical symptoms. The first evidence was, that out of the eleven patients with no typical symptoms, nine reported neurosensory dysfunction. Secondly, the onset time of neurosensory dysfunction is close to or even earlier than that of typical symptoms. Thirdly, the average duration of hyposmia and hypogeusia in this cohort was 9.09 ± 5.74 days and 7.12 ± 4.66 days, which was nearer to that of 7.5 ± 3.2 days reported in Spain (Beltran-Corbellini et al., 2020). Currently, the epidemic in Guangzhou has entered the final stage; all patients were admitted to our hospital for treatment on the day of confirmation. These facts indicate that the neurosensory dysfunction may be present before SARS-CoV-2 is detected by oropharyngeal swab. All the above evidence fully shows that neurosensory dysfunction can be used as a diagnostic marker of early COVID-19. Thus, our findings suggest adding neurosensory symptoms to the routine screening list for COVID-19. When encountering patients with hyposmia or hypogeusia, doctors should pay attention to the possibility of a SARS-CoV-2 infection, and consider virus detection and isolation of these patients.
The reasons why neurosensory dysfunction often occurs early are still unclear. The following two factors could be taken into consideration. Firstly, a high viral load at the beginning of infection may be caused by the development of neurosensory dysfunction. Our data revealed that the viral load remained at a high level for a week after symptom onset, which coincided with the duration of neurosensory dysfunction. However, no difference was noted in viral load between patients with and without neurosensory dysfunction, suggesting that the effect of viral load on the development of neurosensory dysfunction varies. Secondly, the oral cavity and nasal cavity are the main routes for the SARS-CoV-2 invasion. Studies show that ACE2 could be expressed in tongue epithelial cells (Xu et al., 2020a, Xu et al., 2020b) and olfactory epithelial cells (Bilinska et al., 2020). These facts might lead to the early occurrence of neurosensory dysfunction.
Interestingly, neurosensory dysfunction seems to affect young patients more than the elderly, which is consistent with a study by Lechien et al., 2020a, Lechien et al., 2020b in Europe. This finding may corroborate Yan et al. (Yan et al., 2020), demonstrating that smell loss in Covid-19 may associate with a milder clinical course. We also noticed a correlation between gender and the development of neurosensory dysfunction. There are many differences between men and women in the immune response to SARS-CoV-2 infection and inflammatory diseases (Conti and Younes, 2020); women are less susceptible to viral infections based on a different innate immunity, steroid hormones, and factors related to sex chromosomes (Conti and Younes, 2020). A study by Suzuki et al. (2007) found that women are more likely to suffer from postviral olfactory dysfunction in infections caused by parainfluenza, Epstein-Barr virus, or human rhinovirus. Similar findings in SARS-CoV-2 infection were obtained from our data.
Consistent with previous reports (To et al., 2020, Huang et al., 2020), a negative correlation, although weak (r2 = 0.1250, Figure 2A), was noticed between viral load and days after symptom onset. The tendency suggests that the viral load is high at the initial stage of SARS-CoV-2 infection, and then gradually decreases after admission. SARS-CoV took nearly ten days after symptom onset until peak virus load (Peiris et al., 2003). High initial virus load in COVID-19 patients suggested that SARS-CoV-2 can be transmitted earlier and easier than SARS-CoV. The viral load was reduced rapidly after admission, but could rebound within 2-4 weeks (i.e., days 11, 20, 21, 23, 25 after symptom onset) (Figure 2A); a similar rebound pattern was noticed by Huang et al. (2020) and by Xu et al., 2020a, Xu et al., 2020b. Antivirals can act effectively on the upper respiratory tract and most of the lower respiratory tract, but bronchioli terminals could hardly be affected. Coronavirus particles in bronchioli terminals and virus resistance may result in the viral rebound in the later course of treatment.
The present study noted no significant difference in Ct values between patients with and without neurosensory dysfunction (Figure 2B). Lechien et al., 2020a, Lechien et al., 2020b reported that the viral load was significantly higher in patients with an olfactory dysfunction duration <12 days compared to those with a duration >12 days. They suggest that it is beneficial to perform diagnostic swabs in the first 12 days of olfactory dysfunction to avoid the risk of a false-negative result. Our data may support these findings, with the fact that the viral load is gradually reduced under treatment after admission. Neither gender (Figure 2D) nor comorbidity (Figure 2E) was noticed to significantly affect viral load. These findings are consistent with the report by Huang et al. (2020) and To et al. (2020). To et al. (2020) reported a positive correlation between age and peak viral load. However, in this study, no difference in Ct values was noticed between age groups (Figure 2C). This inconsistency may be because patients in our cohort are much younger (median 25.5 vs. 62 years old), with only one severe COVID-19 case.
This study has both strengths and limitations. Its major strength is using the chronological analysis method to detail the exact time of onset and duration of neurosensory dysfunction. This study proves that neurosensory dysfunction could be used as a biomarker for early diagnosis of COVID-19. There are two limitations. First, only 86 patients were included. It would be better to conduct multicenter research with a larger sample size. Second, for patients’ comfort, we did not use nasopharyngeal swabs, which could have been better for assessing viral load on olfactory mucosa.
In conclusion, the present study detailed the exact time of onset and duration of neurosensory dysfunction and reported the viral load of hospitalized patients with COVID-19. Our findings suggest that neurosensory dysfunction can be used as a diagnostic marker of early COVID-19, and should be added to the routine screening list for COVID-19.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding source
This work is supported by the Guangdong Financial Fundfor High-Caliber Hospital Construction.
Ethical approval
This study was performed following the principles of the Declaration of Helsinki and has been approved by the Ethics Committee of Guangzhou Eighth People’s Hospital.
References
- Arons M.M., Hatfield K.M., Reddy S.C., Kimball A., James A., Jacobs J.R. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382(22):2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran-Corbellini A., Chico-Garcia J.L., Martinez-Poles J., Rodríguez-Jorge F., Natera-Villalba E., Gómez-Corral J. Acute-onset smell and taste disorders in the context of Covid-19: a pilot multicenter PCR-based case-control study. Eur J Neurol. 2020 doi: 10.1111/ene.14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilinska K., Jakubowska P., VON Bartheld C.S., Butowt R. Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: identification of cell types and trends with age. ACS Chem Neurosci. 2020;11(11):1555–1562. doi: 10.1021/acschemneuro.0c00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Lan Y., Yuan X., Deng X., Li Y., Cai X. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg Microbes Infect. 2020;9(1):469–473. doi: 10.1080/22221751.2020.1732837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti P., Younes A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J Biol Regul Homeost Agents. 2020;34(2) doi: 10.23812/Editorial-Conti-3. [DOI] [PubMed] [Google Scholar]
- Forster P., Forster L., Renfrew C., Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc Natl Acad Sci U S A. 2020;117(17):9241–9243. doi: 10.1073/pnas.2004999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomelli A., Pezzati L., Conti F., Bernacchia D., Siano M., Oreni L. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis. 2020;71(15):889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Ran R., Lv Z., Feng L., Ran C., Tong Y. Chronological changes of viral shedding in adult inpatients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien J.R., Cabaraux P., Chiesa-Estomba C.M., Khalife M., Plzak J., Hans S. Psychophysical olfactory tests and detection of COVID-19 in patients with sudden onset olfactory dysfunction: a prospective study. Ear Nose Throat J. 2020 doi: 10.1177/0145561320929169. [DOI] [PubMed] [Google Scholar]
- Lechien J.R., Chiesa-Estomba C.M., Place S., Van Laethem Y., Cabaraux P., Mat Q. Clinical and epidemiological characteristics of 1,420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020 doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Min P., Lee S., Kim S.W. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J Korean Med Sci. 2020;35(18):e174. doi: 10.3346/jkms.2020.35.e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q. Neurological Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moein S.T., Hashemian S.M.R., Mansourafshar B., Khorram-Tousi A., Tabarsi p., Doty R.L. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 2020 doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health Commission of the People’s Republic of China . 7th ed. 2020. The guidelines for the diagnosis and treatment of new coronavirus pneumonia. Available from: https://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml. [Cited 9 May 2020] [Google Scholar]
- Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Huang I.F.N., Poon L.L.M. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Saito K., Min W.P., Vladau C., Toida K., Itoh H. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117(2):272–277. doi: 10.1097/01.mlg.0000249922.37381.1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K.K.-W., Tsang O.T.-Y., Leung W.-S., Tam A.R., Wu T.C., Lung D.C. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2020. Coronavirus disease 2019 (COVID-19) situation report — 101. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. [Cited 3 May 2020]. [Google Scholar]
- Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.H., Faraji F., Prajapati D.P., Ostrander B.T., DeConde A.S. Self-reported olfactory loss associates with outpatient clinical course in Covid-19. Int Forum Allergy Rhinol. 2020;10(7):821–831. doi: 10.1002/alr.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]