Abstract
A growing body of evidence indicates that obesity is strongly and independently associated with adverse outcomes of COVID-19, including death. By combining emerging knowledge of the pathological processes involved in COVID-19 with insights into the mechanisms underlying the adverse health consequences of obesity, we present some hypotheses regarding the deleterious impact of obesity on the course of COVID-19. These hypotheses are testable and could guide therapeutic and preventive interventions. As obesity is now almost ubiquitous and no vaccine for COVID-19 is currently available, even a modest reduction in the impact of obesity on mortality and morbidity from this viral infection could have profound consequences for public health.
Obesity is associated with increased COVID-19-related mortality. Lockhart and O’Rahilly review the pathophysiological mechanisms that may underlie this link and converge on a set of testable hypotheses to guide investigation of the effects of obesity on COVID-19.
Introduction
Emerging evidence suggests that people with obesity are at increased risk of mortality from coronavirus disease 2019 (COVID-19), but the mechanisms underlying this are poorly understood. An improved understanding of the pathophysiological intersection of COVID-19 and obesity should help guide preventive and therapeutic strategies for this vulnerable group. Here, we summarize the existing knowledge regarding the pathophysiology of COVID-19 and consider how its various components may be exacerbated by the presence of obesity. We end by suggesting some experiments that could inform public health interventions and/or approaches to therapy.
The Strong Association of Obesity with Adverse Outcomes in COVID-19 Is Real and Relatively Specific to a Subset of Viral Pneumonias
Soon after the emergence of COVID-19, there was a flurry of reports from hospitals around the world, drawing attention to an apparent excess of obese patients among those who were ventilated.1, 2, 3, 4, 5 More recently, preprints have appeared that report much larger and more rigorous epidemiological investigations. OpenSAFELY examined 5,683 COVID-19 deaths in the United Kingdom and related these to preexisting potential risk factors documented in >17 million electronic health records.6 As in all studies to date, age was the most important preexisting risk factor, but the effect of obesity was highly significant and graded according to the severity of the obesity. The hazard ratio (adjusted for ethnicity) for death for those with class III obesity (body mass index [BMI] >40 kg/m2) was as high as 2.28 (1.96–2.65). The International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) study of 16,749 COVID-19-related admissions to intensive care units in the United Kingdom reported a lower hazard ratio of 1.37 (1.16–1.63) associated with clinician-reported obesity.7. It should be noted, however, that BMI was not reported in this study, and reliance on clinical diagnosis is known to seriously underdiagnose obesity.8
In an analysis of COVID-19 mortality in >300,000 patients with diabetes, obesity was associated with mortality in both type 1 (T1D) and type 2 diabetes (T2D).9 Taken together with myriad smaller studies, it seems increasingly clear that obesity does indeed increase the risk of mortality and of requiring admission to intensive care units in people infected with severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2). In contrast to worse outcomes once an obese person is infected, there is no evidence that obesity has a significant impact on the risk of becoming infected by the virus in the first place.
Is there something about infection with the SARS-CoV-2 virus that interacts so adversely with the obese state, or does being obese have a similar impact on other forms of viral pneumonia? Although obesity has been associated with an increased risk of hospitalization in seasonal influenza, a study of almost 10,000 cases of seasonal influenza in the United States did not find any evidence of obesity as a risk factor for requiring mechanical ventilation or death.10 In contrast, it seems clear that during the 2009 H1N1 influenza pandemic, which largely spared the partly immune elderly, obesity was a strong risk factor for adverse outcomes.11 The role of obesity in the severity of SARS-CoV-1 and Middle Eastern respiratory syndrome-coronavirus (MERS-CoV), other pandemic coronavirus infections with poor outcomes, has not been thoroughly examined. The acute respiratory distress syndrome (ARDS) has some pathophysiological similarities to COVID-19 pneumonia. While obesity has been reported to increase the risk of developing ARDS of a variety of etiologies,12 it has been reported to be associated with increased survival rates, something that has come to be known as the ARDS obesity paradox.13 Thus, the association of obesity with worse outcomes in acute lung infection or widespread alveolar damage of other types, appears to be strongest and most consistent with COVID-19 and pandemic H1N1 influenza.
What Are Obese Patients with COVID-19 Dying Of?
The majority of COVID-19 patients die having required artificial ventilation for hypoxemic respiratory failure due to COVID-19 pneumonia.14 Emerging post-mortem histopathology of the COVID-19 lung offers insights into the underlying pathophysiology. Briefly, there is evidence of diffuse alveolar damage, as in other forms of viral pneumonia, but sometimes this is patchy.15 , 16 What is striking and shared to a degree with the pathology of pandemic H1N1 influenza,15 is the extent of pulmonary capillary microangiopathy, which is considerable and nearly universal, at least in some series.17 Complement deposition has also been observed in the endothelium in association with the formation of microthrombi.18 This suggests that COVID-19 may lead to a state of alveolar hypoperfusion due to a microthrombotic pulmonary angiopathy. The frequent finding of elevated levels of fibrin D-dimers in a large proportion of hospitalized patients is consistent with a thrombotic process, as is the frequent occurrence of venous thrombosis and pulmonary emboli during the course of the illness.19 , 20 The clinical characteristics of COVID-19 pneumonia are still being defined, but in early reports from European centers, a substantial proportion of ventilated patients were reported to have preserved pulmonary compliance with well-aerated lungs, suggesting that hypoxia is being driven by microvascular dysfunction.21 , 22 Reports of computed tomography (CT)-based lung perfusion imaging supports this.23 However, a subsequent larger study from the United States described a cohort of patients with respiratory mechanics that are more in keeping with classical ARDS.24 Finally, patients who are seriously ill with COVID-19 show evidence of high levels of inflammation with high C-reactive protein (CRP) and circulating pro-inflammatory cytokines.25 It has been suggested that a hyperinflammatory response, occurring downstream of a vigorous activation of either adaptive or innate immunity or both, may drive the underlying pathophysiological process26 and interleukin-6 (IL-6) antagonists are being trialed in severely ill patients.27
Pathophysiological Mechanisms Mediating the Adverse Effects of Obesity
Obesity is associated with a wide range of adverse health outcomes with diverse underlying pathogenic processes. For some—for example, sleep apnea and reflux esophagitis—the expanded mass of adipose tissue itself is directly and mechanically contributing to the disease. T2D is one of the most common sequelae of obesity. An increase in circulating insulin levels in both fasting and postprandial state is one of the earliest metabolic disturbances associated with obesity, and it is due to impaired insulin action, principally in liver and skeletal muscle.28 This “insulin resistance” clearly predisposes an individual to developing T2D, which ensues when β cell compensation fails.
The mechanism whereby chronic overnutrition leads to insulin resistance appears to primarily involve not the expanded adipose tissue itself, but the additional excess nutrient that is stored ectopically in the major insulin-responsive tissues, muscle, and fat.29 An alternative hypothesis suggests that adipose tissue inflammation contributes directly to insulin resistance in obesity. Inflammation undoubtedly occurs in obesity; however, it has less compelling underpinning support from human genetics or human pharmacology.30
How Might the Metabolic State of Obesity Intersect with and Exacerbate Pathological Mechanisms in COVID-19?
Enhanced Production of Cytokines
A corollary of storing excess fat in non-adipose tissue is that the adipose tissue has reached or is reaching the limits of its ability to store fat safely. Thus, in adipose tissue biopsies from obese, insulin-resistant people, one frequently sees an excess of dead and dying adipocytes, often accompanied by an excess of infiltrating macrophages, usually arranged in crown-like structures.31 These macrophages are activated and contribute to the production of a systemic pro-inflammatory state, characterized by increases in circulating levels of cytokines such as tumor necrosis factor α (TNF-α), IL-6, and IL-1β.32 , 33 Lipotoxic damage to other cells such as hepatocytes can also contribute to the enhanced inflammatory state. If increased inflammation contributes to alveolar damage, then this provides an obvious potential route whereby the metabolic risk factors could drive increased mortality.
Altered Adipose Tissue Hormones
Adipose tissue expansion not only results in the elaboration of inflammatory cytokines but it also changes the profile of secreted hormones. A key signature of insulin resistance is an increase in the ratio of circulating leptin and adiponectin.24 Obesity is associated with higher circulating leptin and lower circulating adiponectin. There is some evidence in the literature associating high leptin levels with pulmonary inflammation but this is not as yet compelling.24 , 34 There is, however, a growing body of evidence more securely implicating adiponectin as an anti-inflammatory agent.35 Notably, adiponectin-deficient mice develop inflammation of the pulmonary vasculature36 and are predisposed to experimental acute lung injury,37 suggesting that the hypoadiponectinemia frequently seen in obesity could facilitate an exaggerated inflammatory response directed to pulmonary capillaries. In addition to being lower in obesity and most insulin-resistant states, it is worth noting that adiponectin levels have been reported to be significantly lower in many of the COVID-19 “at risk” groups—for example, males < females38 and South Asians < white Europeans.38 , 39 Perhaps most interesting is the finding that at equivalent levels of body fat, Black people also tend to have lower levels of adiponectin than white people, despite having no more insulin resistance and a lower propensity to store fat ectopically.40 However, it should be noted that adiponectin levels tend to rise after the age of 70,41 , 42 and old age is by far the biggest risk factor for COVID-19 mortality. However, it is possible that different causal pathways may mediate the risk of age versus obesity on COVID-19 severity.
Complement Components
Gralinski et al.43 recently reported that mice lacking C3, the central component of the complement system, were protected against severe disease when infected with a mouse-adapted SARS-CoV-1 virus. The role of complement in human COVID-19 has not yet been well studied, but immunohistological examination of lungs and skin lesions from affected patients show the deposition of components of the alternative and lectin complement pathways.18 Moreover, the N-protein of SARS-CoV-2 can activate the lectin pathway,44 and the aberrant activation of complement is clearly implicated in a subgroup of thrombotic microangiopathies, suggesting that complement could play a causal role in the microthrombosis observed in COVID-19.45
Adipocytes are a major source of several of the components of the complement system complement proteins.46 Levels of some of these (e.g., C3, C3a, CFD, properdin) are increased with increasing adiposity.47 Circulating levels of C3 are positively associated with insulin resistance, independent of adiposity.48 Given the existence of amplification loops in the complement pathway, it is conceivable that modest elevations of complement components in obesity could serve as a nidus for microthrombosis and/or pathological inflammation and mediate poor outcomes in obesity, as has been suggested by others.49
Thrombosis
Venous thromboembolism rates are much higher in patients with severe COVID-19 than historically critically ill controls, and there is growing evidence of high rates of thrombotic microangiopathy in severe COVID-19.19 , 20 Obesity is an established risk factor for arterial and venous thrombosis, and dysfunction of the endothelium, platelets, fibrinolytic system, and the clotting cascade have been implicated.50 For example, plasminogen activation inhibitor-1 (PAI-1) is secreted from adipose tissue, associated with insulin resistance, and likely contributes to thrombotic risk in obesity by impairing fibrinolysis.51 In addition, obesity is associated with increased thromboxane metabolites and mean platelet volume (both validated indices of platelet activation) that normalize with weight loss.52 , 53 Notably, obesity is a robust risk factor for the development of thrombocytopenic thrombogenic purpura,54 with one group suggesting increased circulating antibodies to ADAMTS13 in the obese.55 , 56
Vasculature
The role of the vasculature, particularly the endothelium, in the pathogenesis of COVID-19 has recently been highlighted.57 , 58 In a comprehensive analysis of ACE2 (the SARS-CoV-2 receptor) expression in the human vasculature, the highest expression was found in the pericytes of the heart and brain (but not the lung), with little in endothelial cells.57 It was proposed that microvascular dysfunction associated with obesity or T2D could permit viral passage across the endothelium to infect pericytes, with their dysfunction promoting subsequent endothelial activation and microthrombosis.57 The effects of diabetes on endothelial barrier function is well established,59 and there is evidence from studies of large animals that endothelial permeability is increased in obesity.60
Dysfunction of the systemic microcirculation is well described in obesity and the metabolic syndrome.61 While the effects of obesity on the pulmonary circulation are studied less, there is emerging evidence of a pulmonary vascular dysfunction associated with obesity. In a rodent model of obesity, pulmonary resistance vessels were resistant to agonist- and hypoxia-induced vasoconstriction ex vivo compared to lean controls.62 If the vasoconstrictive response to hypoxia is impaired in the human pulmonary vasculature, then this could exacerbate shunting in COVID-19 pneumonia, thus contributing to hypoxia.
Alveolus
The key functional unit of the lung is the alveolar-capillary unit. Key cells include type 1 pneumocytes (AT1) separated from capillary endothelial cells by a fused basement membrane and the less numerous type 2 pneumocytes (AT2) that produce surfactant and serve as alveolar progenitors. ACE2 is the proposed receptor for SARS-CoV-2, and in the alveolus, it is expressed predominantly (if not solely) by AT2.57 Critical to gas exchange and pulmonary function, the alveolar capillary unit is the primary site of injury in COVID-19. Understanding how obesity interacts with pre-morbid alveolar function and injury may guide preemptive therapeutic intervention.
Circulating surfactant proteins A and D have been shown to be increased in patients with obesity and T2D,63 , 64 assuming that these proteins are expressed only in the lung and secreted to the apical membrane, and this suggests that obesity may affect the integrity of the alveolus. The science of ectopic fat has largely focused on the liver, muscle, and heart, and a large body of evidence clearly describes the adverse consequences to these tissues of a chronic excess of intracellular lipid. More recently, however, work is emerging suggesting that in states of overnutrition, ectopic lipid can appear in cells of the pulmonary alveolus, resulting in ultrastructural abnormalities and altered surfactant production.34 Genetic enhancement of endogenous lipid synthesis, specifically in mouse AT2 cells, results in alveolar inflammation.65 AT2 cells of aged mice were noted to demonstrate similar gene expression changes to these mice and also exhibited increased lipid content,66 suggesting that “fatty lung” could be a common causal pathway whereby both obesity and age worsen COVID-19 pathology. Similarly, genetic deletion of the lipid sensor liver X receptor (LXR) resulted in the accumulation of lipid in type 2 pneumocytes and, subsequently, pulmonary inflammation and foam cell accumulation.67
Some Testable Hypotheses and Their Potential Implications for Interventions
Insulin Resistance, Not Fat Mass, Is Key to the Link between Obesity and Poor COVID-19 Outcomes
If it is true that insulin resistance, not fat mass, is the key to the link between obesity and poor COVID-19 outcomes, then this is important, as even short-term low-calorie diets can improve insulin sensitivity within days.68 Human genetics should ultimately come to our aid here as meta-analyses of genome-wide SNP data from COVID victims throughout the world can be undertaken to examine whether the genetic risk scores for insulin resistance are better predictors of outcome than those for obesity per se. In the meantime, animal models of SARS infection may be able to provide some early information through the examination of the effects of insulin-lowering and insulin-sensitizing medications. Some commentators have argued that as it is difficult for obese patients to attain normal weight, there is not much that can be done given the rapid spread of the COVID-19 pandemic. However, if improving insulin sensitivity reduces risk, then even a modest amount of caloric restriction, combined with physical activity and perhaps an insulin-sensitizing or -lowering drug such as metformin, may provide a way of reducing the risk of death for the large number of at-risk obese individuals.
Low Circulating Levels of Adiponectin Predispose to Aggressive Pulmonary Inflammation and Explain Why Obese People Fare Worse with COVID-19
Again, human genetics will be able to help us test this hypothesis, as there are genetic instruments that explain quite a high proportion of the variance in serum adiponectin.69 Agonists of the nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ), such as the thiazolidinedione class of drugs rapidly and markedly increase circulating levels of adiponectin. Examination of the effects of PPARγ agonists on disease outcomes in obese animal models of COVID-19 could provide helpful insights. Pioglitazone is licensed for use in T2D worldwide, and cheap generic formulations are now available for large-scale clinical trials.
Ectopic Lipid in Alveolar Type 2 Cells Influences the Extent of Alveolar Damage Due to COVID-19
SARS-CoV-2 causes pneumonia by first entering the AT2 through ACE2, which is abundantly expressed on its surface. These cells are lipid rich, storing polar lipids in lamellar bodies, and their structure and possibly function are influenced by diet and obesity, at least in animal models.34 , 70 , 71 Experiments should be undertaken to examine the effects of the lipid content of cells on ACE2 expression, viral uptake, replication, and release. Some viruses (e.g., hepatitis C) seem entirely reliant on intracellular fat droplets to facilitate their movement around a cell.72 Viral infections of cells frequently lead to a rapid switch from oxidative phosphorylation to aerobic glycolysis, the so-called Warburg effect.73 Ectopic lipid in cells elsewhere is known to be associated with metabolic inflexibility,74 the inability to shift rapidly between fat and carbohydrate metabolism. Could AT2 cells that have excess lipid be less able to switch to aerobic glycolysis and thus be more prone to cell death during viral infection? In mice, diet-induced obesity is associated with the downregulation of fatty acid synthase (Fasn) in the lung, and the genetic deletion of Fasn in AT2 cells impairs the induction of glycolysis in response to hyperoxic stress in vitro and predisposes to acute lung injury in mice.70 Although unproven, it is likely that ectopic lipid in the lung will begin to reduce quickly after people enter negative energy balance, so that modest changes in diet and exercise may have benefit.
Conclusions
In summary, we have applied insights into the pathophysiology of the adverse consequences of obesity and emerging evidence regarding the pathological mechanisms in COVID-19 to suggest possible routes whereby obesity can exacerbate the tissue damage associated with infection by the SARS-CoV-2 virus (Figure 1 ). These hypotheses suggest several tractable experiments in cells, animals, and humans, some of which we are undertaking and which we encourage others to pursue. Obesity is a notoriously difficult condition to “cure,” and this may explain why widespread public health messaging about weight loss in the obese as a preventive measure to reduce COVID-19 mortality has not been vigorously pursued. If obesity is exerting its effects on COVID-19 outcomes through its metabolic sequelae, such as insulin resistance, then those abnormalities begin to improve very rapidly when energy intake drops below energy expenditure. In addition to its effects on energy expenditure, regular physical activity, even of moderate intensity and duration, can also improve insulin sensitivity and lower circulating insulin levels.75 The potential implications for unintended adverse consequences of intense COVID-19 “lockdown” strategies that limit opportunities for exercise are obvious.
Figure 1.
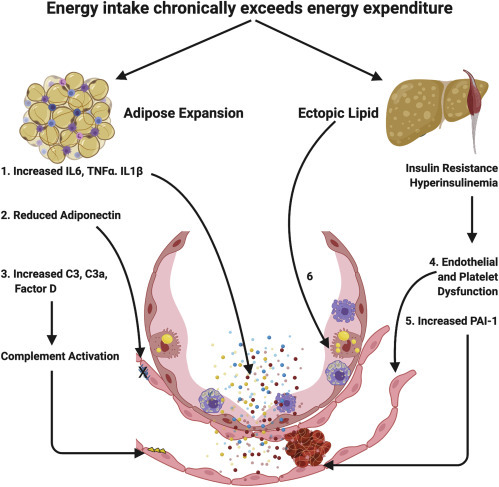
Obesity Exacerbates COVID-19: Potential Mechanisms
Obesity is a disorder of energy balance that ensues when energy intake exceeds expenditure. Adipose tissue expansion occurs to safely store excess energy safely in triglyceride-rich lipid droplets. This process is associated with adipose tissue inflammation and elaboration of pro-inflammatory cytokines, increased components of the complement system, and altered adipose tissue hormones.
(1) Increased inflammatory cytokines are secreted into the systemic circulation and can act on the alveolar capillary unit to potentiate the inflammatory response to SARS-CoV-2 infection.
(2) Adipose tissue expansion is associated with a reduction in adiponectin secretion from the adipose tissue that is at least partly driven by systemic insulin resistance. Mouse studies suggest that adiponectin is abundant in the pulmonary endothelium in the healthy lung and that adiponectin deficiency results in pulmonary vascular inflammation and predisposes to experimental lung injury.
(3) Increases in circulating complement components elaborated from adipose tissue occur in expanded adipose tissue and in association with insulin resistance and could predispose to complement activation and subsequent thrombotic microangiopathy. When the capacity for adipose tissue to expand is exceeded, lipid is deposited in other organs. Lipid deposition in the skeletal muscle and liver likely plays a causal role in the development of insulin resistance and hyperinsulinemia.
(4) Systemic insulin resistance is associated with endothelial and platelet dysfunction that may predispose to thrombosis and contribute to lung injury via vascular inflammation and enhanced endothelial permeability.
(5) Insulin resistance is robustly associated with increased plasminogen activator inhibitor-1 (PAI-1), which impairs fibrinolysis and may contribute to the risk of thrombosis in COVID-19.
(6) Finally, ectopic lipid may actually be directly deposited in type 2 pneumocytes pre-disposing to lung injury in SARS-CoV-2 infection.
Given how rapidly large trials of a wide variety of pharmacological agents in COVID-19 are being undertaken (some with a rather tenuous rationale76), it should be possible to consider undertaking trials of simple interventions in people with obesity either before or immediately after the onset of COVID-19 symptoms. These could involve diet and exercise intentions that do not aim for unrealistic amounts of weight loss but would be designed to ameliorate insulin resistance. These interventions could be supplemented by drugs that assist in modest weight loss and lower circulating insulin, such as metformin or sodium glucose co-transporter 2 (SGLT2) inhibitors, or agents that improve insulin sensitivity, reduce ectopic lipid, and increase circulating adiponectin, such as pioglitazone. Such approaches would also be applicable to T2D, another condition that predisposes to increased mortality from COVID-19.6 , 7 , 9 In the majority of T2D cases, obesity precedes and contributes to the development of diabetes by inducing compensatory hyperinsulinemia, necessitated by insulin resistance, which eventually exhausts the ability of genetically vulnerable pancreatic β cells to maintain insulin production. There is evidence that in both T1D and T2D, the level of glycemia is related to COVID-19 outcomes.9 , 77 We urgently need to know whether the intensification of glycemic control using an approach that sensitizes patients to insulin would provide benefits to the COVID-19-infected T2D patient that are greater than those achieved by approaches that increase the levels of circulating insulin, either through exogenous injection or the stimulation of endogenous secretion.
Obesity affects a very large proportion of the population of most developed and developing countries. Understanding the nature of the link between chronic positive caloric imbalance and COVID-19 pathology could provide novel avenues to reduce the death toll produced by this dangerous new viral infection. Funding agencies will need to foster the interdisciplinary approaches that will be required to respond to this new biomedical challenge that lies at the intersection between traditional disciplines.
Acknowledgments
S.M.L. is supported by an academic clinical fellowship from the National Institute for Health Research (NIHR). S.O. is supported by the Wellcome Trust (WT 095515/Z/11/Z), the Medical Research Council (MRC) Metabolic Disease Unit (MC_UU_00014/1), and the NIHR Cambridge Biomedical Research Centre and NIHR Rare Disease Translational Research Collaboration.
Declaration of Interests
S.O. is an employee of the University of Cambridge and has provided remunerated consultancy services to the following pharmaceutical and biotechnology companies: AstraZeneca, eRx Network, Glaxo-Smith-Kline, Novo Nordisk, and Pfizer.
References
- 1.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., Greninger A.L., Pipavath S., Wurfel M.M., Evans L. Covid-19 in Critically Ill Patients in the Seattle Region - Case Series. N. Engl. J. Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caussy C., Wallet F., Laville M., Disse E. Obesity is Associated with Severe Forms of COVID-19. Obesity (Silver Spring) 2020;28:1175. doi: 10.1002/oby.22842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Q., Zheng Z., Zhang C., Zhang X., Wu H., Wang J., Wang S., Zheng C. Clinical characteristics of 145 patients with corona virus disease 2019 (COVID-19) in Taizhou, Zhejiang, China. Infection. 2020 doi: 10.1007/s15010-020-01432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahase E. Covid-19: most patients require mechanical ventilation in first 24 hours of critical care. BMJ. 2020;368:m1201. doi: 10.1136/bmj.m1201. [DOI] [PubMed] [Google Scholar]
- 5.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., Labreuche J., Mathieu D., Pattou F., Jourdain M. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williamson E., Walker A.J., Bhaskaran K.J., Bacon S., Bates C., Morton C.E., Curtis H.J., Mehrkar A., Evans D., Inglesby P., The OpenSAFELY Collaborative OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. medRxiv. 2020 doi: 10.1101/2020.05.06.20092999. [DOI] [Google Scholar]
- 7.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., Holden K.A., Read J.M., Dondelinger F., Carson G. Features of 16,749 hospitalised UK patients with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol. medRxiv. 2020 doi: 10.1101/2020.04.23.20076042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pantalone K.M., Hobbs T.M., Chagin K.M., Kong S.X., Wells B.J., Kattan M.W., Bouchard J., Sakurada B., Milinovich A., Weng W. Prevalence and recognition of obesity and its associated comorbidities: cross-sectional analysis of electronic health record data from a large US integrated health system. BMJ Open. 2017;7:e017583. doi: 10.1136/bmjopen-2017-017583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barron, E., Bakhai, C., Kar, P., Weaver, A., Bradley, D., Ismail, H., Knighton, P., Holman, N., Khunti, K., Sattar, N., et al. (2020). Type 1 and type 2 diabetes and COVID-19 related mortality in England. https://www.england.nhs.uk/publication/type-1-and-type-2-diabetes-and-covid-19-related-mortality-in-england/. [DOI] [PMC free article] [PubMed]
- 10.Braun E.S., Crawford F.W., Desai M.M., Meek J., Kirley P.D., Miller L., Anderson E.J., Oni O., Ryan P., Lynfield R. Obesity not associated with severity among hospitalized adults with seasonal influenza virus infection. Infection. 2015;43:569–575. doi: 10.1007/s15010-015-0802-x. [DOI] [PubMed] [Google Scholar]
- 11.Morgan O.W., Bramley A., Fowlkes A., Freedman D.S., Taylor T.H., Gargiullo P., Belay B., Jain S., Cox C., Kamimoto L. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A(H1N1) disease. PLOS ONE. 2010;5:e9694. doi: 10.1371/journal.pone.0009694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong M.N., Bajwa E.K., Thompson B.T., Christiani D.C. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax. 2010;65:44–50. doi: 10.1136/thx.2009.117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umbrello M., Fumagalli J., Pesenti A., Chiumello D. Pathophysiology and Management of Acute Respiratory Distress Syndrome in Obese Patients. Semin. Respir. Crit. Care Med. 2019;40:40–56. doi: 10.1055/s-0039-1685179. [DOI] [PubMed] [Google Scholar]
- 14.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., Barnaby D.P., Becker L.B., Chelico J.D., Cohen S.L., Northwell COVID-19 Research Consortium Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menter T., Haslbauer J.D., Nienhold R., Savic S., Hopfer H., Deigendesch N., Frank S., Turek D., Willi N., Pargger H. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. 2020 doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carsana L., Sonzogni A., Nasr A., Rossi R., Pellegrinelli A., Zerbi P., Rech R., Colombo R., Antinori S., Corbellino M. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet. 2020 doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., Baxter-Stoltzfus A., Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., Merdji H., Clere-Jehl R., Schenck M., Fagot Gandet F., CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis) High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gattinoni L., Chiumello D., Caironi P., Busana M., Romitti F., Brazzi L., Camporota L. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. COVID-19 Does Not Lead to a “Typical” Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang M., Som A., Mendoza D.P., Flores E.J., Reid N., Carey D., Li M.D., Witkin A., Rodriguez-Lopez J.M., Shepard J.O. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finucane F.M., Luan J., Wareham N.J., Sharp S.J., O’Rahilly S., Balkau B., Flyvbjerg A., Walker M., Højlund K., Nolan J.J., Savage D.B., on behalf of the European Group for the Study of Insulin Resistance: Relationship between Insulin Sensitivity and Cardiovascular Disease Risk Study Group Correlation of the leptin:adiponectin ratio with measures of insulin resistance in non-diabetic individuals. Diabetologia. 2009;52:2345–2349. doi: 10.1007/s00125-009-1508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., HLH Across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toniati P., Piva S., Cattalini M., Garrafa E., Regola F., Castelli F., Franceschini F., Airò P., Bazzani C., Beindorf E.A. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun. Rev. 2020;19:102568. doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reaven G.M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 29.Langenberg C., Lotta L.A. Genomic insights into the causes of type 2 diabetes. Lancet. 2018;391:2463–2474. doi: 10.1016/S0140-6736(18)31132-2. [DOI] [PubMed] [Google Scholar]
- 30.O’Rahilly S. Harveian Oration 2016: some observations on the causes and consequences of obesity. Clin. Med. (Lond.) 2016;16:551–564. doi: 10.7861/clinmedicine.16-6-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cinti S., Mitchell G., Barbatelli G., Murano I., Ceresi E., Faloia E., Wang S., Fortier M., Greenberg A.S., Obin M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Marques-Vidal P., Bastardot F., von Känel R., Paccaud F., Preisig M., Waeber G., Vollenweider P. Association between circulating cytokine levels, diabetes and insulin resistance in a population-based sample (CoLaus study) Clin. Endocrinol. (Oxf.) 2013;78:232–241. doi: 10.1111/j.1365-2265.2012.04384.x. [DOI] [PubMed] [Google Scholar]
- 33.Um J.Y., Chung H.S., Song M.Y., Shin H.D., Kim H.M. Association of interleukin-1beta gene polymorphism with body mass index in women. Clin. Chem. 2004;50:647–650. doi: 10.1373/clinchem.2003.025858. [DOI] [PubMed] [Google Scholar]
- 34.Foster D.J., Ravikumar P., Bellotto D.J., Unger R.H., Hsia C.C. Fatty diabetic lung: altered alveolar structure and surfactant protein expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010;298:L392–L403. doi: 10.1152/ajplung.00041.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scherer P.E. The many secret lives of adipocytes: implications for diabetes. Diabetologia. 2019;62:223–232. doi: 10.1007/s00125-018-4777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Summer R., Fiack C.A., Ikeda Y., Sato K., Dwyer D., Ouchi N., Fine A., Farber H.W., Walsh K. Adiponectin deficiency: a model of pulmonary hypertension associated with pulmonary vascular disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;297:L432–L438. doi: 10.1152/ajplung.90599.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konter J.M., Parker J.L., Baez E., Li S.Z., Ranscht B., Denzel M., Little F.F., Nakamura K., Ouchi N., Fine A. Adiponectin attenuates lipopolysaccharide-induced acute lung injury through suppression of endothelial cell activation. J. Immunol. 2012;188:854–863. doi: 10.4049/jimmunol.1100426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duncan B.B., Schmidt M.I., Pankow J.S., Bang H., Couper D., Ballantyne C.M., Hoogeveen R.C., Heiss G. Adiponectin and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2004;53:2473–2478. doi: 10.2337/diabetes.53.9.2473. [DOI] [PubMed] [Google Scholar]
- 39.Abate N., Chandalia M., Snell P.G., Grundy S.M. Adipose tissue metabolites and insulin resistance in nondiabetic Asian Indian men. J. Clin. Endocrinol. Metab. 2004;89:2750–2755. doi: 10.1210/jc.2003-031843. [DOI] [PubMed] [Google Scholar]
- 40.Bush N.C., Darnell B.E., Oster R.A., Goran M.I., Gower B.A. Adiponectin is lower among African Americans and is independently related to insulin sensitivity in children and adolescents. Diabetes. 2005;54:2772–2778. doi: 10.2337/diabetes.54.9.2772. [DOI] [PubMed] [Google Scholar]
- 41.Adamczak M., Rzepka E., Chudek J., Wiecek A. Ageing and plasma adiponectin concentration in apparently healthy males and females. Clin. Endocrinol. (Oxf.) 2005;62:114–118. doi: 10.1111/j.1365-2265.2004.02182.x. [DOI] [PubMed] [Google Scholar]
- 42.Cnop M., Havel P.J., Utzschneider K.M., Carr D.B., Sinha M.K., Boyko E.J., Retzlaff B.M., Knopp R.H., Brunzell J.D., Kahn S.E. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 43.Gralinski L.E., Sheahan T.P., Morrison T.E., Menachery V.D., Jensen K., Leist S.R., Whitmore A., Heise M.T., Baric R.S. Complement Activation Contributes to Severe Acute Respiratory Syndrome Coronavirus Pathogenesis. MBio. 2018;9 doi: 10.1128/mBio.01753-18. e01753–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao T., Hu M., Zhang X., Li H., Zhu L., Liu H., Dong Q., Zhang Z., Wang Z., Hu Y. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. medRxiv. 2020 doi: 10.1101/2020.03.29.20041962. [DOI] [Google Scholar]
- 45.Brocklebank V., Wood K.M., Kavanagh D. Thrombotic Microangiopathy and the Kidney. Clin. J. Am. Soc. Nephrol. 2018;13:300–317. doi: 10.2215/CJN.00620117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vlaicu S.I., Tatomir A., Boodhoo D., Vesa S., Mircea P.A., Rus H. The role of complement system in adipose tissue-related inflammation. Immunol. Res. 2016;64:653–664. doi: 10.1007/s12026-015-8783-5. [DOI] [PubMed] [Google Scholar]
- 47.Xin Y., Hertle E., van der Kallen C.J.H., Schalkwijk C.G., Stehouwer C.D.A., van Greevenbroek M.M.J. Longitudinal associations of the alternative and terminal pathways of complement activation with adiposity: The CODAM study. Obes. Res. Clin. Pract. 2018;12:286–292. doi: 10.1016/j.orcp.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Wlazlo N., van Greevenbroek M.M., Ferreira I., Feskens E.J., van der Kallen C.J., Schalkwijk C.G., Bravenboer B., Stehouwer C.D. Complement factor 3 is associated with insulin resistance and with incident type 2 diabetes over a 7-year follow-up period: the CODAM Study. Diabetes Care. 2014;37:1900–1909. doi: 10.2337/dc13-2804. [DOI] [PubMed] [Google Scholar]
- 49.Gavriilaki E., Brodsky R.A. Severe COVID-19 infection and thrombotic microangiopathy: success does not come easily. Br. J. Haematol. 2020;189:e227–e230. doi: 10.1111/bjh.16783. [DOI] [PubMed] [Google Scholar]
- 50.Vilahur G., Ben-Aicha S., Badimon L. New insights into the role of adipose tissue in thrombosis. Cardiovasc. Res. 2017;113:1046–1054. doi: 10.1093/cvr/cvx086. [DOI] [PubMed] [Google Scholar]
- 51.Festa A., D’Agostino R., Jr., Mykkänen L., Tracy R.P., Zaccaro D.J., Hales C.N., Haffner S.M., The Insulin Resistance Atherosclerosis Study (IRAS) Relative contribution of insulin and its precursors to fibrinogen and PAI-1 in a large population with different states of glucose tolerance. Arterioscler. Thromb. Vasc. Biol. 1999;19:562–568. doi: 10.1161/01.atv.19.3.562. [DOI] [PubMed] [Google Scholar]
- 52.Coban E., Yilmaz A., Sari R. The effect of weight loss on the mean platelet volume in obese patients. Platelets. 2007;18:212–216. doi: 10.1080/09537100600975362. [DOI] [PubMed] [Google Scholar]
- 53.Davì G., Guagnano M.T., Ciabattoni G., Basili S., Falco A., Marinopiccoli M., Nutini M., Sensi S., Patrono C. Platelet activation in obese women: role of inflammation and oxidant stress. JAMA. 2002;288:2008–2014. doi: 10.1001/jama.288.16.2008. [DOI] [PubMed] [Google Scholar]
- 54.Vesely S.K., George J.N., Lämmle B., Studt J.D., Alberio L., El-Harake M.A., Raskob G.E. ADAMTS13 activity in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients. Blood. 2003;102:60–68. doi: 10.1182/blood-2003-01-0193. [DOI] [PubMed] [Google Scholar]
- 55.Lombardi A.M., Fabris R., Scarda A., Zanato V., Dal Prà C., Scarparo P., Vettore S., Granzotto M., Berti De Marinis G., Foletto M. Presence of anti-ADAMTS13 antibodies in obesity. Eur. J. Clin. Invest. 2012;42:1197–1204. doi: 10.1111/j.1365-2362.2012.02710.x. [DOI] [PubMed] [Google Scholar]
- 56.Zanato V., Lombardi A.M., Busetto L., Prà C.D., Foletto M., Prevedello L., De Marinis G.B., Fabris F., Vettor R., Fabris R. Weight loss reduces anti-ADAMTS13 autoantibodies and improves inflammatory and coagulative parameters in obese patients. Endocrine. 2017;56:521–527. doi: 10.1007/s12020-016-1059-6. [DOI] [PubMed] [Google Scholar]
- 57.He L., Mäe M.A., Sun Y., Muhl L., Nahar K., Liébanas E.V., Fagerlund M.J., Oldner A., Liu J., Genové G. Pericyte-specific vascular expression of SARS-CoV-2 receptor ACE2 – implications for microvascular inflammation and hypercoagulopathy in COVID-19 patients. bioRxiv. 2020 doi: 10.1101/2020.05.11.088500. [DOI] [Google Scholar]
- 58.Teuwen L.-A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rask-Madsen C., King G.L. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab. 2013;17:20–33. doi: 10.1016/j.cmet.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galili O., Versari D., Sattler K.J., Olson M.L., Mannheim D., McConnell J.P., Chade A.R., Lerman L.O., Lerman A. Early experimental obesity is associated with coronary endothelial dysfunction and oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H904–H911. doi: 10.1152/ajpheart.00628.2006. [DOI] [PubMed] [Google Scholar]
- 61.van der Heijden D.J., van Leeuwen M.A.H., Janssens G.N., Lenzen M.J., van de Ven P.M., Eringa E.C., van Royen N. Body Mass Index Is Associated With Microvascular Endothelial Dysfunction in Patients With Treated Metabolic Risk Factors and Suspected Coronary Artery Disease. J. Am. Heart Assoc. 2017;6:e006082. doi: 10.1161/JAHA.117.006082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moral-Sanz J., Menendez C., Moreno L., Moreno E., Cogolludo A., Perez-Vizcaino F. Pulmonary arterial dysfunction in insulin resistant obese Zucker rats. Respir. Res. 2011;12:51. doi: 10.1186/1465-9921-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fernández-Real J.M., Chico B., Shiratori M., Nara Y., Takahashi H., Ricart W. Circulating surfactant protein A (SP-A), a marker of lung injury, is associated with insulin resistance. Diabetes Care. 2008;31:958–963. doi: 10.2337/dc07-2173. [DOI] [PubMed] [Google Scholar]
- 64.López-Cano C., Lecube A., García-Ramírez M., Muñoz X., Sánchez E., Seminario A., Hernández M., Ciudin A., Gutiérrez L., Hernández C., Simó R. Serum Surfactant Protein D as a Biomarker for Measuring Lung Involvement in Obese Patients With Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2017;102:4109–4116. doi: 10.1210/jc.2017-00913. [DOI] [PubMed] [Google Scholar]
- 65.Plantier L., Besnard V., Xu Y., Ikegami M., Wert S.E., Hunt A.N., Postle A.D., Whitsett J.A. Activation of sterol-response element-binding proteins (SREBP) in alveolar type II cells enhances lipogenesis causing pulmonary lipotoxicity. J. Biol. Chem. 2012;287:10099–10114. doi: 10.1074/jbc.M111.303669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Angelidis I., Simon L.M., Fernandez I.E., Strunz M., Mayr C.H., Greiffo F.R., Tsitsiridis G., Ansari M., Graf E., Strom T.M. An atlas of the aging lung mapped by single cell transcriptomics and deep tissue proteomics. Nat. Commun. 2019;10:963. doi: 10.1038/s41467-019-08831-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dai Y.B., Miao Y.F., Wu W.F., Li Y., D’Errico F., Su W., Burns A.R., Huang B., Maneix L., Warner M., Gustafsson J.Å. Ablation of Liver X receptors α and β leads to spontaneous peripheral squamous cell lung cancer in mice. Proc. Natl. Acad. Sci. USA. 2016;113:7614–7619. doi: 10.1073/pnas.1607590113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kirk E., Reeds D.N., Finck B.N., Mayurranjan S.M., Patterson B.W., Klein S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology. 2009;136:1552–1560. doi: 10.1053/j.gastro.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Richards J.B., Waterworth D., O’Rahilly S., Hivert M.F., Loos R.J., Perry J.R., Tanaka T., Timpson N.J., Semple R.K., Soranzo N., GIANT Consortium A genome-wide association study reveals variants in ARL15 that influence adiponectin levels. PLOS Genet. 2009;5:e1000768. doi: 10.1371/journal.pgen.1000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Plataki M., Fan L., Sanchez E., Huang Z., Torres L.K., Imamura M., Zhu Y., Cohen D.E., Cloonan S.M., Choi A.M. Fatty acid synthase downregulation contributes to acute lung injury in murine diet-induced obesity. JCI Insight. 2019;5:e127823. doi: 10.1172/jci.insight.127823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yilmaz C., Ravikumar P., Gyawali D., Iyer R., Unger R.H., Hsia C.C. Alveolar-capillary adaptation to chronic hypoxia in the fatty lung. Acta Physiol. (Oxf.) 2015;213:933–946. doi: 10.1111/apha.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miyanari Y., Atsuzawa K., Usuda N., Watashi K., Hishiki T., Zayas M., Bartenschlager R., Wakita T., Hijikata M., Shimotohno K. The lipid droplet is an important organelle for hepatitis C virus production. Nat. Cell Biol. 2007;9:1089–1097. doi: 10.1038/ncb1631. [DOI] [PubMed] [Google Scholar]
- 73.Sanchez E.L., Lagunoff M. Viral activation of cellular metabolism. Virology. 2015;479-480:609–618. doi: 10.1016/j.virol.2015.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Galgani J.E., Moro C., Ravussin E. Metabolic flexibility and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2008;295:E1009–E1017. doi: 10.1152/ajpendo.90558.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Houmard J.A., Tanner C.J., Slentz C.A., Duscha B.D., McCartney J.S., Kraus W.E. Effect of the volume and intensity of exercise training on insulin sensitivity. J. Appl. Physiol. (1985) 2004;96:101–106. doi: 10.1152/japplphysiol.00707.2003. [DOI] [PubMed] [Google Scholar]
- 76.Ferner R.E., Aronson J.K. Chloroquine and hydroxychloroquine in covid-19. BMJ. 2020;369:m1432. doi: 10.1136/bmj.m1432. [DOI] [PubMed] [Google Scholar]
- 77.Zhu L., She Z.G., Cheng X., Qin J.J., Zhang X.J., Cai J., Lei F., Wang H., Xie J., Wang W. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020;31:1068–1077.e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]


