Abstract
Background
Alzheimer's disease (AD) is a complex neurodegenerative disease. Due to the complexity of its molecular pathogenesis and the interaction of the numerous factors involved, the etiology and pathogenesis of AD have not been fully elucidated. Therefore, effective treatment for AD remains to be developed. Evodiamine, a quinolone alkaloid, has been found to improve learning and memory ability to in the APPswe/PS1△E9 mouse model of dementia. However, the cytotoxicity and physicochemical properties of evodiamine have limited its use in the treatment of AD.
Methods
Evodiamine and its derivatives were effectively synthesized by EDCI‐mediated condensation at room temperature. These target compounds contained 1 thio‐ and 21 oxo‐evodiamine derivatives with different substituted groups. The cytotoxicity of evodiamine and its derivatives and the neuroprotective effects of the evodiamine derivatives against H2O2‐induced cell loss in SH‐SY5Y cells were investigated using the WST‐8 assay. The Morris water‐maze test was used to detect the effect of evodiamine and its derivatives on improving learning and memory in APPswe/PS1△E9 mice.
Results
In this study, a series of oxo‐ and thio‐evodiamine derivatives was synthesized. Several derivatives showed lower cytotoxicity and stronger neuroprotective effects than evodiamine and elicited enhanced cognitive improvement, especially in the test of spatial memory in APPswe/PS1△E9 mice.
Conclusion
Our study provides insights for developing novel evodiamine derivatives for chemical intervention and treatment of AD.
Keywords: evodiamine derivatives, mouse model, neuroprotective, spatial memory
1. INTRODUCTION
Alzheimer's disease (AD) is a complex neurodegenerative disease that affects more than 35 million people worldwide. Memory loss and cognitive impairment can cause death 3‐9 years after diagnosis. 1 The main pathological features of AD are senile plaques formed by amyloid deposition and tangles formed by tau protein hyperphosphorylation. 2 AD is mainly divided into genetic and non‐genetic forms. Patients with genetic AD inherit a familial autosomal dominant form of the disease, presenting as early‐onset dementia, usually occurring between the ages of 30 and 60. This familial form of AD is caused by mutations in genes such as APP, PSEN1 and PSEN2. 3 These gene mutations lead to the accumulation of amyloid beta protein outside neurons by affecting amyloid beta production, the Aβ‐42/Aβ‐40 ratio, or the structure of amyloid beta. 2 More than 99% of AD is non‐genetic, and sufferers tend to develop the disease later in life, usually after the age of 65 years. Because of the complexity of its molecular pathogenesis and the interaction of the many factors involved in the disease, the etiology and developmental mechanisms of AD have not yet been fully elucidated. Therefore, an effective treatment for AD remains to be developed. 4
APPswe/PS1ΔE9 double transgenic mice express two major mutations in the human APP gene, as well as human PS1 mutations knocked‐in into the mouse PS1 gene. These mice were bred on a C57BL/6J background. 5 The APPswe transgene encodes a mouse–human hybrid with the mouse sequence in the extracellular and intracellular regions and a human sequence within the Aβ domain with Swedish mutations K594N/M595L. The PS1ΔE9 transgene encodes the Δexon9 human presenilin‐1. This mouse shows spatial memory deficits at 3 months of age and senile plaques in brain tissue at 4.5 months of age. 6 Therefore APPswe/PS1ΔE9 double transgenic mice develop behavioral phenotypic and pathological features which make them useful as an AD model.
Chinese herbal medicine, an important element of Chinese medicine, is becoming increasingly popular among physicians and patients. 7 , 8 Many medical plant preparations are marketed to the public for various ailments, including Alzheimer's disease. 9 Evodiamine is a quinazolinone alkaloid isolated from the fruit of fructus evodia, which has been used to treat cancer, cardiovascular disease, headaches, abdominal pain, postpartum bleeding, dysentery and amenorrhea. 10 , 11 , 12 , 13 In addition, some studies have found that evodiamine can reduce overexpression of cytokines IL‐1β, IL‐6, TNF‐α, inhibit the inflammatory response and overactivation of glial cells in the brain, and improve the learning and memory ability of APPswe/PS1ΔE9 dementia model mice. 6
However, the cytotoxicity of evodiamine is relatively high and its physicochemical properties are not suitable for pharmacological use. 14 These disadvantages limited its therapeutic use. In order to discover novel and potent lead compounds for AD treatment, we developed an efficient method for generating quinazolinone alkaloids via carbodiimide‐mediated condensation between carbolines with anthranilic acids. 15 This provided potential for increasing the scaffold diversity of evodiamine. In this study, a series of oxo‐ and thio‐evodiamine derivatives was designed and synthesized using this approach and their potential roles for treatment of AD were investigated.
2. MATERIAL AND METHODS
2.1. Synthesis
Substituted o‐hydroxybenzoic acid (1.2 mmol), 4,9‐dihydro‐3H‐pyrido[3,4‐b] indole (1.0 mmol), 1‐(3‐dimethylaminopropyl)‐3‐ethylcarbodiimide hydrochloride (EDCI, 1.4 mmol), and CH2Cl2 (3.0 mL) were combined and the reaction mixture was stirred at room temperature. After 4 hours, the reaction mixture was diluted with 30 mL of CH2Cl2 and then washed with saturated aqueous NaHCO3 (20 mL), H2O (20 mL), and brine (20 mL). The organic phase was dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (CH2Cl2: MeOH = 100:1) to yield the target compounds. The structure of the target compounds was determined by 1H NMR and HRMS.
2.2. Cell culture
Human neuroblastoma cells (SH‐SY5Y) and human hepatocellular carcinoma (HepG2) cells were cultured in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal bovine serum (FBS), 2 mM l‐glutamine and 0.1 mg/mL antibiotic penicillin streptomycin solution. All cells were cultured in a humidified atmosphere of 5% CO2 at 37°C.
2.3. Animals
APPswe/PS1ΔE9 double‐transgenic mice were maintained on a C57BL/6J genetic background. The mice developed spatial memory deficits at 3 months of age and senile plaques in brain tissue at 4.5 months of age, as previously reported. 16 All mice were bred in an AAALAC‐accredited facility, and the procedures were approved by the Animal Care and Use Committee at the Institute of Laboratory Animal Science, Peking Union Medical College (ILAS‐GC‐2015‐002).
2.4. Groups and treatment
Four‐month‐old APPswe/PS1ΔE9 double‐transgenic mice and non‐transgenic littermates were randomly assigned to seven treatment groups identified as: non‐transgenic (NTG, n = 7), APPswe/PS1ΔE9 double‐transgenic (n = 7), evodiamine (n = 7), evodiamine derivative 49 (n = 7), evodiamine derivative 51 (n = 7), evodiamine derivative 58 (n = 7) and evodiamine derivative 60 treatment (n = 7). Evodiamine and its derivatives were diluted in 20% PEG/PBS solution. In the evodiamine and evodiamine derivatives treatment groups, APPswe/PS1ΔE9 double‐transgenic mice were administered evodiamine and derivatives 49, 51, 58 and 60 at a dose of 1.6 mg/kg via intraperitoneal injection 5 times a week for 4 weeks. The APPswe/PS1ΔE9 double‐transgenic and NTG groups were treated with 20% PEG/PBS solution as the placebo control and wild‐type normal control, respectively.
2.5. Cell viability assays
Cell proliferation was measured by the WST‐8 assay (Cell Counting Kit‐8, Dojindo). Cells were seeded in 96‐well plates in sextuplicate at a density of 104 cells/well with 100 μL culture medium and cultured for 24 hours. The cells were then treated for 24 hours with evodiamine and its derivatives at a dose of 5 μg/mL. At specific time points 10 μL of CCK‐8 solution was added to the cells which were then incubated for 1 hour at 37°C. The absorbance reading was then measured at 450 nm, and the relative cell viability was calculated by normalizing to the vehicle control.
To determine the neuroprotective ability of the treatment compounds, the SH‐SY5Y cells were seeded in 96‐well plates (104 cells/well) and cultured for 24 hours. The cells were then cultured for 3 hours with H2O2 added to the wells to final concentration of 150 μmol/L. Evodiamine and its derivatives were then added to the wells at doses of 0.5, 0.05 and 0.005 μg/mL and the cells were cultured for 24 hours. Finally, cell viability was then evaluated by CCK8 assay.
2.6. Morris water‐maze test
The protocol of the Morris water‐maze test was modified from the reported methods. 17 , 18 Briefly, the water maze is a 100 cm diameter pool with a 15 cm diameter escape platform placed 0.5 cm below the water surface. Since C57BL/6J mice are black, we poured an appropriate amount of white paint into the water to make the water white and opaque, and maintained the water temperature at 22‐24°C. The mice were placed in the water near the side of the pool. East, west, south, and north positions were randomly selected and each mouse was tested once. We recorded the times taken by the animals to find the underwater platform. In previous training sessions, if this time exceeded 60 seconds, we guided the animal to the platform and let the animal stay on the platform for 10 seconds. The mice were then removed and dried with a towel and placed in a cage. Each mouse was trained 4 times a day, with a 2 hour interval between two training sessions, for 5 consecutive days.
The platform was removed on the sixth day and exploration training lasted for 60 seconds. The animals were placed in the water at the side opposite the original platform quadrant. The duration that animals spent in the quadrant where the platform was originally placed and the number of times they entered the quadrant were recorded, and were used as detection indicators of spatial memory. A video tracking system (Noldus Ltd, Ethovision XT, Holland) was used for detection and analysis. The results were expressed as SD ± SEM.
2.7. Statistical analysis
The data were analysed by two‐tailed unpaired t‐tests and one‐way ANOVA followed by Tukey's post hoc analysis. Data shown are means ± S.D, with P < .05 considered statistically significant.
3. RESULTS
3.1. Synthesis of evodiamine and its derivatives
As shown in Figure 1, evodiamine and its derivatives were effectively synthesized by EDCI‐mediated condensation at room temperature. These target compounds contained 1 thio‐ and 21 oxo‐evodiamine derivatives with different substituted groups.
Figure 1.
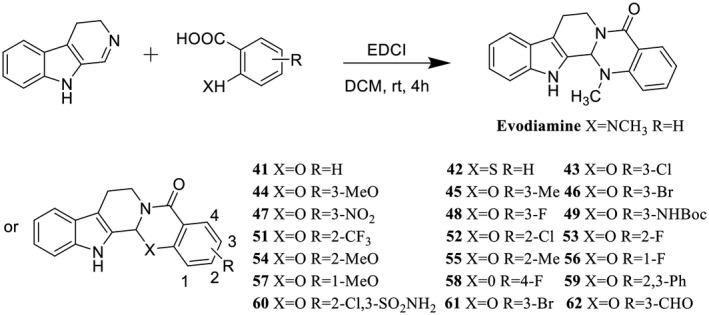
The structure and synthesis of evodiamine and its derivatives. Evodiamine and its derivatives were effectively synthesized by EDCI‐mediated condensation at room temperature
3.2. The cytotoxicity of the evodiamine derivatives
The cytotoxicity of evodiamine and its derivatives (41‐49 and 51‐62) was first investigated. Compared with the vehicle group, the cell viability of SH‐SY5Y cells treated with evodiamine decreased about 50%. However, the cell viability of SH‐SY5Y cells treated with evodiamine decreased about 50%. However, the cell viability of SH‐SY5Y cells treated with the evodiamine derivatives (47, 49, 51, 54, 58, 59, 60 and 61) increased 91.7%, 68.8%, 52.1%, 58.3%, 109.4%, 131.3%, 170.8%, 110.4% (Figure 2A, n = 6, P < .001), respectively. A similar increase in cell viability was also observed in HepG2 cells (Figure 2B, n = 6, P < .001). These results indicated that the cytotoxicity of evodiamine derivatives 47, 49, 51, 54, 58, 59, 60 and 61 was obviously decreased compared to evodiamine in both of SH‐SY5Y and HepG2 cells.
Figure 2.
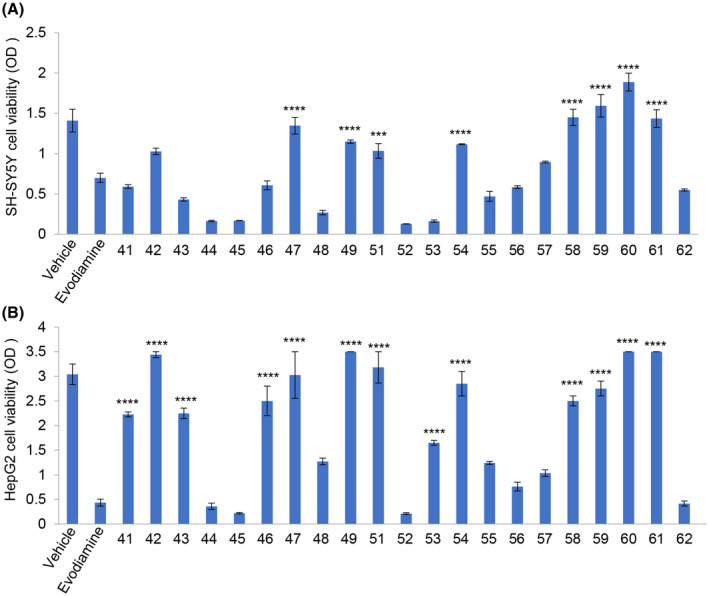
The SH‐SY5Y and HepG2 cells viability was assessed by the CCK‐8 assay. A, Effect of 5 μg/mL evodiamine and its derivatives on SH‐SY5Y cell viability (n = 6, ****P < .0001, ***P < .001, evodiamine derivatives treatment groups vs evodiamine treatment group). B, Effect of 5 μg/mL evodiamine and its derivatives on HepG2 cell viability (n = 6, ****P < .0001, ***P < .001, evodiamine derivatives treatment groups vs evodiamine treatment group)
3.3. The neuroprotective effects of the evodiamine derivatives against H2O2‐induced cell loss in SH‐SY5Y cells
Based on the results of the cell cytotoxicity experiments, evodiamine derivatives 41, 47, 49, 51, 54, 58, 59, 60 and 61 were selected to further evaluate their neuroprotective effects. Compared with the vehicle group, the cell viability of SH‐SY5Y cells treated with 150 μM H2O2 decreased by about 50%. Evodiamine and its derivatives (49, 51, 54, 58, 59 and 60) significantly improved the viability of the H2O2‐treated SH‐SY5Y cells (Figure 3, n = 3), indicating a significant neuroprotective effect. However, there was no significant difference in cell viability between the treatments with evodiamine and its derivatives.
Figure 3.
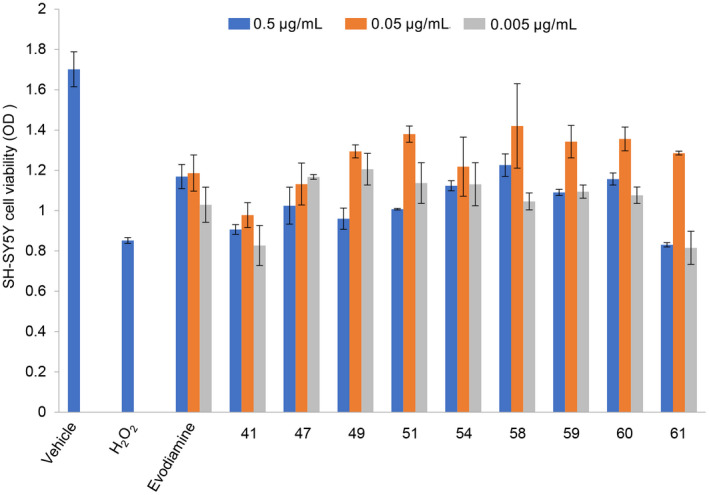
Effects of evodiamine and its derivatives on H2O2‐induced cytotoxicity in SH‐SY5Y cells determined by CCK‐8 assay. The SH‐SY5Y cells were seeded in 96‐well plates (10 000 cells/well) and cultured for 24 h. Evodiamine and its derivatives (41, 47, 49, 51, 54, 58, 59, 60 and 61) were then added to the wells at doses of 0.5 µg/mL, 0.05 µg/mL and 0.005 µg/mL and the cells were cultured for 3 h. H2O2 was then added to the wells at a final concentration of 150 μmol/L and the cells were cultured for 24 h. Cell viability was then evaluated by CCK8 assay. The values shown are means ± SE (n = 3)
3.4. Treatment with evodiamine derivatives increased spatial learning and memory in APPswe/PS1ΔE9 transgenic mice
We used APPswe/PS1ΔE9 transgenic mice to explore the effect of evodiamine and its derivatives on learning and memory, using the Morris water‐maze test. After 4 weeks of treatment, the residence durations in the target quadrant of the mice treated with evodiamine and its derivatives (49, 51, 58 and 60) was significantly higher than that of the untreated APPswe/PS1ΔE9 transgenic mice group (Figure 4A,B, n = 7, P < .001), and the effect of the evodiamine derivatives (49, 51, 58 and 60) was stronger than that of evodiamine. Compared with the APPswe/PS1ΔE9 group, the number of times that mice crossed the target quadrant increased significantly in the evodiamine and derivatives groups. Among the derivatives groups, the improvements recorded for groups 49 and 60 were more obvious than that of the evodiamine group.
Figure 4.
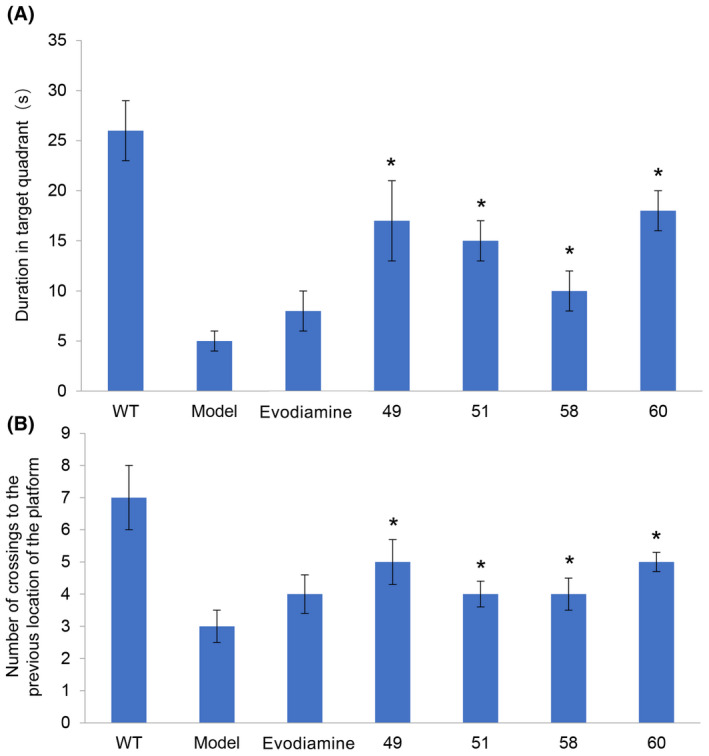
Behavioral performance of animals in the Morris water maze. A, Time spent in the quadrant that previously contained the platform (n = 7, *P < .05, evodiamine derivatives 49, 51, 58 and 60 vs APPswe/PS1ΔE9). B, Number of crossings to the previous location of the platform (n = 7, *P < .05, evodiamine derivatives 49, 51, 58 and 60 vs APPswe/PS1ΔE9)
4. DISCUSSION
Scaffold diversity has proved to be a powerful strategy for the discovery of novel lead compounds. In this study, a series of oxo‐ and thio‐evodiamine derivatives was synthesized using our reported protocol. In contrast to simple derivatization, our protocol provided an effective route for enhancing the scaffold of evodiamine to increase chemical diversity (Figure 1).
Increasingly in vitro cell models are used to improve the translation value of in vivo models and provide a deeper understanding of the pathological mechanisms of diseases. 19 The main characteristic of AD is the consumption of acetylcholine, which causes the degeneration of cholinergic neurons in the hippocampus. 20 Neuroblastoma cells come in both undifferentiated and differentiated forms. Immature cholinergic neurons are undifferentiated, while mature cholinergic cells acquire the capcity to differentiate. These similarities to AD make neuroblastoma cells very useful in drug development. 21 , 22 Therefore, we selected SH‐SY5Y cells, a neuroblastoma cell line, and HepG2 cells, 23 which are sensitive to drug toxicity, to analyze the cytotoxicity of evodiamine and its derivatives. Our results indicate that evodiamine and some of its derivatives have high cytotoxicity. However, evodiamine derivatives 49, 51, 54, 58, 59 and 60 have substantially no side effects on neurons and liver cells (Figure 2).
During the brain aging process in female AD patients, mitochondrial respiration decreases and mitochondrial hydrogen peroxide production increases. 24 Over the past few decades, the neuroprotective activity of many natural compounds has been evaluated for its protective effect on H2O2‐induced cell damage in SH‐SY5Y and other related neuronal cell lines. 25 , 26 , 27 , 28 Our results showed that evodiamine and its derivatives 49, 51, 54, 58, 59 and 60 displayed increased neuroprotective effects (Figure 3).
Furthermore, using the Morris water‐maze test, we showed that treatment with evodiamine and its derivatives for 4 weeks can improve spatial learning and memory in APPswe/PS1ΔE9 transgenic mice with AD symptoms at 5 months of age (Figure 4). Compared with evodiamine, our derivatives numbers 49, 51, 58 and 60 significantly improved the spatial memory ability of APPswe/PS1ΔE9 transgenic mice.
In this study, a series of oxo‐ and thio‐evodiamine derivatives was designed and synthesized using the strategy of scaffold diversification. Some of the derivatives showed lower cytotoxicity, and stronger neuroprotective effects, and elicited significant improvements in spatial memory in the APPswe/PS1ΔE9 AD mouse model. This study further explores the use of evodiamine as a treatment for AD, and also provides insights into developing novel and potent evodiamine derivatives for chemical intervention in AD.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
YY, XP and LZ designed the experiment. YY, XP, SP, CS and SG performed the experiment. SP and YY worked on the statistical analysis of the data. YY, XP and LZ prepared the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This research was funded by the Drug Innovation Major Project (2018ZX09711‐001‐005), Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CAMS‐I2M, 2016‐I2M‐1‐004), and National Natural Science Foundation (31970508).
Pang S, Sun C, Gao S, Yang Y, Pan X, Zhang L. Evodiamine derivatives improve cognitive abilities in APPswe/PS1ΔE9 transgenic mouse models of Alzheimer's disease. Anim Models Exp Med. 2020;3:193–199. 10.1002/ame2.12126
REFERENCES
- 1. Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362(4):329‐344. [DOI] [PubMed] [Google Scholar]
- 2. Shi Y, Holtzman DM. Interplay between innate immunity and Alzheimer disease: APOE and TREM2 in the spotlight. Nat Rev Immunol. 2018;18(12):759‐772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Holtzman DM, Morris JC, Goate AM. Alzheimer's disease: the challenge of the second century. Sci Transl Med. 2011;3(77):77sr1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pimenova AA, Raj T, Goate AM. Untangling genetic risk for Alzheimer's disease. Biol Psychiatry. 2018;83(4):300‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yu W, An S, Shao T, et al. Active compounds of herbs ameliorate impaired cognition in APP/PS1 mouse model of Alzheimer's disease. Aging (Albany NY). 2019;11(23):11186‐11201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yuan SM, Gao K, Wang DM, et al. Evodiamine improves congnitive abilities in SAMP8 and APP(swe)/PS1(ΔE9) transgenic mouse models of Alzheimer's disease. Acta Pharmacol Sin. 2011;32(3):295‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu Q, Wang SC, Ding K. Research advances in the treatment of Alzheimer's disease with polysaccharides from traditional Chinese medicine. Chin J Nat Med. 2017;15(9):641‐652. [DOI] [PubMed] [Google Scholar]
- 8. Jarrell JT, Gao L, Cohen DS, Huang X. Network medicine for Alzheimer's disease and traditional Chinese medicine. Molecules. 2018;23(5):1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Howes MR, Fang R, Houghton PJ. Effect of Chinese herbal medicine on Alzheimer's disease. Int Rev Neurobiol. 2017;135:29‐56. [DOI] [PubMed] [Google Scholar]
- 10. Jiang J, Hu C. Evodiamine: a novel anti‐cancer alkaloid from Evodia rutaecarpa. Molecules. 2009;14(5):1852‐1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tian KM, Li JJ, Xu SW. Rutaecarpine: a promising cardiovascular protective alkaloid from Evodia rutaecarpa (Wu Zhu Yu). Pharmacol Res. 2019;141:541‐550. [DOI] [PubMed] [Google Scholar]
- 12. Lee SH, Son JK, Jeong BS, et al. Progress in the studies on rutaecarpine. Molecules. 2008;13(2):272‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang L, Hu CP, Deng PY, et al. The protective effects of rutaecarpine on gastric mucosa injury in rats. Planta Med. 2005;71(5):416‐419. [DOI] [PubMed] [Google Scholar]
- 14. Gavaraskar K, Dhulap S, Hirwani RR. Therapeutic and cosmetic applications of Evodiamine and its derivatives–a patent review. Fitoterapia. 2015;106:22‐35. [DOI] [PubMed] [Google Scholar]
- 15. Yang Y, Zhu C, Zhang M, et al. Condensation of anthranilic acids with pyridines to furnish pyridoquinazolones via pyridine dearomatization. Chem Commun (Camb). 2016;52(87):12869‐12872. [DOI] [PubMed] [Google Scholar]
- 16. Wang X, Liu P, Zhu H, et al. miR‐34a, a microRNA up‐regulated in a double transgenic mouse model of Alzheimer's disease, inhibits bcl2 translation. Brain Res Bull. 2009;80(4‐5):268‐273. [DOI] [PubMed] [Google Scholar]
- 17. Bromley‐Brits K, Deng Y, Song W. Morris water maze test for learning and memory deficits in Alzheimer's disease model mice. J Vis Exp. 2011;53:2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bye CM, Hong NS, Moore K, Deibel SH, McDonald RJ. The effects of pool shape manipulations on rat spatial memory acquired in the Morris water maze. Learn Behav. 2019;47(1):29‐37. [DOI] [PubMed] [Google Scholar]
- 19. Poon A, Zhang Y, Chandrasekaran A, et al. Modeling neurodegenerative diseases with patient‐derived induced pluripotent cells: Possibilities and challenges. N Biotechnol. 2017;39(Pt B):190‐198. [DOI] [PubMed] [Google Scholar]
- 20. Belle SH, Zhang S, Czaja SJ, Burns R, Schulz R. Use of cognitive enhancement medication in persons with Alzheimer disease who have a family caregiver: results from the Resources for Enhancing Alzheimer's Caregiver Health (REACH) project. Am J Geriatr Psychiatry. 2004;12(3):250‐257. [PubMed] [Google Scholar]
- 21. Kadoshima T, Sakaguchi H, Nakano T, et al. Self‐organization of axial polarity, inside‐out layer pattern, and species‐specific progenitor dynamics in human ES cell‐derived neocortex. Proc Natl Acad Sci USA. 2013;110(50):20284‐20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dubey SK, Ram MS, Krishna KV, et al. Recent expansions on cellular models to uncover the scientific barriers towards drug development for Alzheimer's disease. Cell Mol Neurobiol. 2019;39(2):181‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yokoyama Y, Sasaki Y, Terasaki N, et al. Comparison of drug metabolism and its related hepatotoxic effects in HepaRG, cryopreserved human hepatocytes, and HepG2 cell cultures. Biol Pharm Bull. 2018;41(5):722‐732. [DOI] [PubMed] [Google Scholar]
- 24. Klosinski LP, Yao J, Yin F, et al. White matter lipids as a ketogenic fuel supply in aging female brain: Implications for Alzheimer's disease. EBioMedicine. 2015;2(12):1888‐1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Law BN, Ling AP, Koh RY, Chye SM, Wong YP. Neuroprotective effects of orientin on hydrogen peroxide‐induced apoptosis in SH‐SY5Y cells. Mol Med Rep. 2014;9(3):947‐954. [DOI] [PubMed] [Google Scholar]
- 26. Nirmaladevi D, Venkataramana M, Chandranayaka S, Ramesha A, Jameel NM, Srinivas C. Neuroprotective effects of bikaverin on H2O2‐induced oxidative stress mediated neuronal damage in SH‐SY5Y cell line. Cell Mol Neurobiol. 2014;34(7):973‐985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Han SM, Kim JM, Park KK, Chang YC, Pak SC. Neuroprotective effects of melittin on hydrogen peroxide‐induced apoptotic cell death in neuroblastoma SH‐SY5Y cells. BMC Complement Altern Med. 2014;14:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhong L, Zhou J, Chen X, et al. Quantitative proteomics study of the neuroprotective effects of B12 on hydrogen peroxide‐induced apoptosis in SH‐SY5Y cells. Sci Rep. 2016;6:22635. [DOI] [PMC free article] [PubMed] [Google Scholar]


