Abstract
It has long been suspected that the hypothalamic pituitary adrenal (HPA) axis plays a role in the pathophysiology of depression. Whether this association exists or not, and if it does, the degree of its significance, remain highly disputed. The issue is further complicated as no consensus currently exists on cortisol sampling timepoints or methods. Our study aimed to evaluate HPA functionality by evaluating plasma cortisol levels in a cohort of patients diagnosed with Major Depressive Disorder (MDD). We enrolled 96 subjects admitted for a major depressive episode and tested serum cortisol levels for 80 of them. We found that only 15 (12%) had values that were outside the normal reference range, with 14 of these being below the normal threshold. We also interviewed the patients and obtained self-reported information regarding previous depressive episodes, treatment administration, anxiety, suicidal ideas and suicidal gestures. Our study did not find a significant association between cortisol levels and the number of previous depressive episodes, the presence of feelings of anxiety, suicidal ideas or suicidal gestures. While our cohort did not find an association between cortisol levels and depression other authors have reported significantly different results and as such, more research is needed in order to establish or infirm this hypothesis.
Keywords: cortisol, depression, hypercorticism
Introduction
The hypothalamic pituitary adrenal (HPA) axis has been known to play a part in the process of cognitive function as well as pathophysiology of depression.
The axis connects the brain, pituitary and adrenal glands and contains two loops that regulate the secretion of glucocorticoid hormones-one stimulatory loop and one inhibitory feedback loop.
Once secreted into the bloodstream cortisol binds to the mineralocorticoid receptor (MR) but also to the glucocorticoid receptor (GR), although with a lower affinity.
The GRs are widely distributed throughout the primitive brain while MRs are more densely concentrated in the hippocampus with cortisol exerting its tonic effects at a hippocampal level via MRs and its feedback and inhibition effects on the pituitary via GRs [1,2,3].
Depression is characterized by symptoms of anhedonia, feelings of helplessness and hopelessness, difficulties in concentration and an overall persistent despondent state [4,5].
Its current burden from a macroscopic perspective is quite significant.
According to the WHO depression is currently the fourth major cause of disability with estimates that it might rise to second leading cause of disability by 2020 [5, 6].
Some studies show that a dysregulation of the HPA axis has been associated with major depressive disorder (MDD) in adult individuals [7,8,9] with between 40 to 60% of patients with depression experiencing hypercortisolemia or other alterations to the normal circadian rhythm of the HPA axis [1,10,11].
Other studies however have shown some inconsistencies in this hypothesis with a number of them demonstrating no meaningful association between depressive symptoms and cortisol levels [12,13,14,15].
Multiple factors could play a part in explaining this as the HPA axis is highly complex and can be affected by numerous variables.
One such variable could be the difference in the exact time of cortisol measurement during the day as its levels fluctuate within a certain range per day [12,16].
Another issue could be related to a wide range of methods used to sample cortisol including: salivary, serum and urinary with no clear consensus on which of them could be more valid [12].
Sample characteristics such as physical activity, smoker status, age, race, body mass index (BMI), sleep and administered medication could affect cortisol levels and a failure to account for these variables could affect the observed associations [17,18].
Still, studies that included subjects with underlying mental health issues [19,20] tended to report HPA axis disorders more often than those that included subjects without these issues [21,22].
Our study aimed to evaluate HPA functionality by evaluating serum cortisol levels in a cohort of patients diagnosed with Major Depressive Disorder (MDD).
Materials and Methods
Our study obtained ethical approval from the University of Medicine and Pharmacy of Craiova’s Medical Ethics Committee and approval from the committee of the involved hospital.
All those enrolled had been admitted in the hospital due to a major depressive episode which was quantified using the Hamilton Rating Scale for Depression (HAM-D).
A total of 96 subjects were enrolled, 34 men and 62 women, with a mean age of 54.07±8.67 (range: 28 to 74) from the Clinical Neuropsychiatry Hospital in Craiova’s number I and number II clinics.
A written consent was obtained from each individual only after the aim, methods and purpose of our study was explained to them.
Enrollment was strictly voluntary.
We proceeded to harvest a 2ml sample of EDTA blood, from which we separated plasma. The blood sample was collected at 8:00 AM.
We also interviewed the patients and obtained self-reported information regarding previous depressive episodes, treatment administration, the presence of feelings of anxiety, suicidal ideas and suicidal gestures.
Cortisol levels were measured using a NovaTec Immundiagnostica ELISA assay. Its sensitivity gave the lowest detectable concentration of cortisol at 2.42ng/ml at a 95% confidence limit.
The normal reference values provided were 60-230ng/ml between 8.00-10.00 AM and 30-150ng/ml at 4.00 PM.
The assay was analyzed using the CLARIOstar® high performance microplate reader in conjunction with BMG Labtech’s proprietary Mars Data Analysis Software.
We performed the statistical analysis using the XLSTAT package.
Results
Out of a total of 96 patients we were able to measure the cortisol levels in only 80 patients for reasons related to sample collection and quality.
Table 1 summarizes the mean and standard deviation of cortisol in the patients with normal values (n=65, 85% of those where values were available).
Table 1.
Cortisol distribution per sex and age group in subjects with normal values.
|
Age group |
Percentage |
Cortisol mean value (µUI/ml) |
|
Females |
61.5% (n=40) |
108.05±38.24 |
|
21-30 |
1.5% (n=1) |
99.05 |
|
31-40 |
4.6% (n=3) |
97.29±19.17 |
|
41-50 |
10.8% (n=7) |
107.85±41.92 |
|
51-60 |
21.5% (n=14) |
110.20±44.32 |
|
61-70 |
21.5% (n=14) |
105.18±35.97 |
|
71-80 |
1.5% (n=1) |
160.84 |
|
Males |
38.5% (n=25) |
122.35±41.13 |
|
41-50 |
4.6% (n=3) |
121.95±53.35 |
|
51-60 |
23.1% (n=15) |
125.34±42.68 |
|
61-70 |
7.7% (n=5) |
106.06±32.46 |
|
71-80 |
3.1% (n=2) |
141.24±54.82 |
|
Total |
100% (n=65) |
113.55±39.68 |
Out of the total 80 subjects evaluated we found that only 15 (12%) had values that were outside the normal reference range, among which only one subject (1.25% of measured values) having a higher than normal cortisol level (399.75ng/ml).
The 14 subjects (17.5%) who had levels below normal range had an average serum cortisol level of 45.67ng/ml as shown in Table 2and illustrated in Figure1.
Table 2.
Cortisol distribution per sex and age group in subjects with below normal range values.
|
Age group |
Percentage |
Cortisol mean value (µUI/ml) |
|
Females |
71.4% (n=10) |
42.83±11.35 |
|
41-50 |
14.3% (n=2) |
30.35±12.53 |
|
51-60 |
35.7% (n=5) |
47.41±11.42 |
|
61-70 |
14.3% (n=2) |
44.64±7.50 |
|
71-80 |
7.1% (n=1) |
41.28 |
|
Males |
28.6% (n=4) |
52.79±6.77 |
|
41-50 |
7.1% (n=1) |
56.54 |
|
51-60 |
21.4% (n=3) |
51.54±7.70 |
|
Total |
100% (n=14) |
45.68±11.03 |
Figure 1.
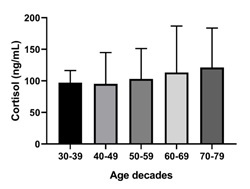
Cortisol mean levels and standard deviation (shown by vertical bars) by age decades. Y axis represents cortisol levels expressed in ng/ml. X axis represents age decades. One-way ANOVA (p=0.8092) shows no significant association despite the apparent trend.
A significant number of those included in our study (83.68%) had suffered at least one previous major depressive episode.
Out of all patients we interviewed, 38 patients (36.48%) also associated strong feelings of anxiety, 59 patients (61.45%) reported suicidal thoughts and 20 patients (20.83%) reported having attempted suicide.
All patients were undergoing treatment at the point of enrollment with the vast majority (79, 83.68%) having been previously treated for depression.
We applied t-student parametrical testing after checking for normal distribution.
We could not identify any correlations between circulating cortisol levels and sex (p=0.34), history of depression (p=0.19), anxiety (p=0.63), or suicidal thoughts (p=0.41), and/or gestures (p=0.74), as illustrated in Figure 2.
Figure 2.
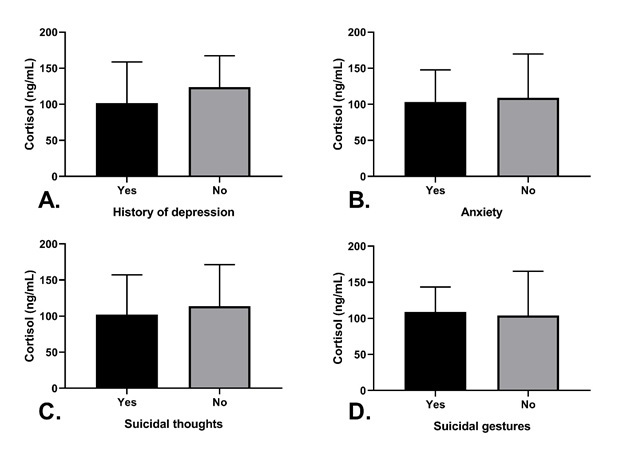
Cortisol breakdown by studied variables. Y axis represents plasma cortisol levels expressed in ng/mL. X axis represent A. history of depression B. self reported anxiety C. presence of suicidal thoughts D. previous manifestation(s) of suicidal gestures. Error bars represent standard deviation. No significant correlations found.
Lastly, we did identify a correlation of cortisol with age (p<0.00010), which is validated by known physiology of aging.
Discussion
Given the inconsistencies briefly described in the introduction, we aimed to assess the association between cortisol levels and depression.
We were expecting, as a well-replicated finding, higher cortisol levels in depressed patients [12,23,24].
Several authors have suggested that low cortisol levels are also associated with psychiatric disease such as depression and bipolar disorder [25].
We found however no correlation between the two as the majority of subjects had cortisol levels within the normal range. In our study 17.5% of them had cortisol levels below normal range, and only 1.25% above normal range.
Further, we attempted to correlate cortisol levels with a variety of factors such as the presence of feelings of anxiety, suicidal ideas or suicidal gestures yet in each case we could not establish any meaningful association.
Below normal cortisol have been described for other mental health disorders such as PTSD and chronic fatigue syndrome. A possible mechanism would be that following the initial hyperactivity of the HPA axis a subsequent period of hypoactivity sets in [26,27].
Given that patients with depression do experience a significant amount of stress it could stand to reason that HPA axis hypoactivity could play a more significant role than previously speculated [25,28].
In order to obtain more robust data, we suggest that multiple sampling points throughout the day would enable a more thorough and comprehensive analysis of cortisol levels in patients suffering from depression.
One limitation for repeated sample collection is the ease of the method used. Using whole blood is less practical and more resource intensive than using alternatives such as measuring salivary cortisol as some authors suggest [7,29].
Although more difficult to realize in a clinical context, obtaining multiple sample points could offer more depth to our understanding of patient’s cortisol levels, allowing an assessment of secretion pattern and not just absolute value [30].
Timing of the sampling may also be key. One study suggests baseline values may not differ between depressive and non-depressive subjects, but depressed patients show higher cortisol during recovery period, pattern that the authors called blunted reactivity-impaired recovery [31].
Determining cortisol levels could be relevant for its effects on cortisol sensitive organs and systems, for instance with cognitive impairment, abdominal obesity, and loss of bone density [32], raising the questions whether action needs to be taken as a preventive measure [33].
Conclusions
Despite conflicting reports in literature, one cannot dispute the fact that the HPA axis is linked through nuanced and incompletely understood mechanisms to depression.
Given the overall burden of disease depression poses globally, future studies must continue to develop more standardized and accurate tools in order to obtain results that can be used to assist clinical practice.
Acknowledgments
AC, IU, MP, MI developed the study design. AC, IU were responsible for data collection. ALR performed the sample processing, laboratory testing and ALR and MI performed the preliminary data analysis. AC, ALR, MP, MD, MV wrote the first draft of the manuscript. All authors read and approved the final manuscript. The authors would like to thank the departments involved for the openness in collaboration of all personnel.
Conflict of interests
None to declare.
References
- 1.Keller J, Gomez R, Williams G, Lembke A, Lazzeroni L, Murphy GM, Schatzberg AF. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Molecular psychiatry. 2017;22(4):527–536. doi: 10.1038/mp.2016.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2000;23(5):477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 3.Young EA, Lopez JF, Murphy-Weinberg V, Watson SJ, Akil H. Mineralocorticoid receptor function in major depression. Archives of general psychiatry. 2003;60(1):24–28. doi: 10.1001/archpsyc.60.1.24. [DOI] [PubMed] [Google Scholar]
- 4.Gruenberg AM, Goldstein RD, Pincus HA. Classification of depression: research and diagnostic criteria: DSM-IV and ICD-10. Biology of Depression. 2005;11:43–43. [Google Scholar]
- 5.Qin D-d, Rizak J, Feng X-l, Yang S-c, Lü L-b, Pan L, Yin Y, Hu X-t. Prolonged secretion of cortisol as a possible mechanism underlying stress and depressive behaviour. Scientific reports. 2016;6:30187–30187. doi: 10.1038/srep30187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS medicine. 2006;3(11):e442–e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knorr U, Vinberg M, Kessing LV, Wetterslev J. Salivary cortisol in depressed patients versus control persons: a systematic review and meta-analysis. Psychoneuroendocrinology. 2010;35(9):1275–1286. doi: 10.1016/j.psyneuen.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Dedovic K, Ngiam J. The cortisol awakening response and major depression: examining the evidence. Neuropsychiatric disease and treatment. 2015 doi: 10.2147/NDT.S62289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biological psychiatry. 2001;49(5):391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- 10.Murphy BE. Steroids and depression. The Journal of steroid biochemistry and molecular biology. 1991;38(5):537–559. doi: 10.1016/0960-0760(91)90312-s. [DOI] [PubMed] [Google Scholar]
- 11.Deuschle M, Gotthardt U, Schweiger U, Weber B, Korner A, Schmider J, Standhardt H, Lammers CH, Heuser I. With aging in humans the activity of the hypothalamus-pituitary-adrenal system increases and its diurnal amplitude flattens. Life sciences. 1997;61(22):2239–2246. doi: 10.1016/s0024-3205(97)00926-0. [DOI] [PubMed] [Google Scholar]
- 12.Knight JM, Avery EF, Janssen I, Powell LH. Cortisol and depressive symptoms in a population-based cohort of midlife women. Psychosomatic medicine. 2010;72(9):855–855. doi: 10.1097/PSY.0b013e3181f4ab87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penninx BW, Beekman AT, Corsi AM, Bremmer M, Hoogendijk WJ, Guralnik JM, Ferrucci L, Bandinelli S. Late-life depressive symptoms are associated with both hyperactivity and hypoactivity of the hypothalamo-pituitary-adrenal axis. The American journal of geriatric psychiatry. 2007;15(6):522–529. doi: 10.1097/JGP.0b013e318033ed80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Eck M, Berkhof H, Nicolson N, Sulon J. The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosomatic medicine. 1996;58(5):447–458. doi: 10.1097/00006842-199609000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Conrad A, Wilhelm FH, Roth WT, Spiegel D, Taylor CB. Circadian affective, cardiopulmonary, and cortisol variability in depressed and nondepressed individuals at risk for cardiovascular disease. Journal of psychiatric research. 2008;42(9):769–777. doi: 10.1016/j.jpsychires.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Portaluppi F, Bagni B, Montanari L, Cavallini R, Trasforini G, Margutti A, Ferlini M, Zanella M, Parti M. Circadian rhythms of atrial natriuretic peptide, renin, aldosterone, cortisol, blood pressure and heart rate in normal and hypertensive subjects. Journal of hypertension. 1990;8(1):85–95. doi: 10.1097/00004872-199001000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Kudielka BM, Hellhammer DH, Wüst S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34(1):2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Hansen ÅM, Garde AH, Persson R. Sources of biological and methodological variation in salivary cortisol and their impact on measurement among healthy adults: a review. Scandinavian journal of clinical and laboratory investigation. 2008;68(6):448–458. doi: 10.1080/00365510701819127. [DOI] [PubMed] [Google Scholar]
- 19.Deuschle M, Schweiger U, Weber B, Gotthardt U, Korner A, Schmider J, Standhardt H, Lammers CH, Heuser I. Diurnal activity and pulsatility of the hypothalamus-pituitary-adrenal system in male depressed patients and healthy controls. The Journal of clinical endocrinology and metabolism. 1997;82(1):234–238. doi: 10.1210/jcem.82.1.3689. [DOI] [PubMed] [Google Scholar]
- 20.Inslicht SS, Marmar CR, Neylan TC, Metzler TJ, Hart SL, Otte C, McCaslin SE, Larkin GL, Hyman KB, Baum A. Increased cortisol in women with intimate partner violence-related posttraumatic stress disorder. Annals of the New York Academy of Sciences. 2006;1071:428–429. doi: 10.1196/annals.1364.035. [DOI] [PubMed] [Google Scholar]
- 21.Whitehead DL, Perkins-Porras L, Strike PC, Magid K, Steptoe A. Cortisol awakening response is elevated in acute coronary syndrome patients with type-D personality. Journal of psychosomatic research. 2007;62(4):419–425. doi: 10.1016/j.jpsychores.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 22.O’Donnell K, Badrick E, Kumari M, Steptoe A. Psychological coping styles and cortisol over the day in healthy older adults. Psychoneuroendocrinology. 2008;33(5):601–611. doi: 10.1016/j.psyneuen.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Wolkowitz OM, Reus VI. Treatment of depression with antiglucocorticoid drugs. Psychosomatic medicine. 1999;61(5):698–711. doi: 10.1097/00006842-199909000-00011. [DOI] [PubMed] [Google Scholar]
- 24. Kennis M, Gerritsen L, van Dalen M, Williams A, Cuijpers P, Bockting C. Prospective biomarkers of major depressive disorder: a systematic review and meta-analysis. Molecular psychiatry. 25(2):321–338. doi: 10.1038/s41380-019-0585-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maripuu M, Wikgren M, Karling P, Adolfsson R, Norrback K-F. Relative hypo-and hypercortisolism are both associated with depression and lower quality of life in bipolar disorder: a cross-sectional study. PloS one. 2014;9(6) doi: 10.1371/journal.pone.0098682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological bulletin. 2007;133(1):25–25. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 27.Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30(10):1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25(1):1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 29.Bhagwagar Z, Hafizi S, Cowen PJ. Increased salivary cortisol after waking in depression. Psychopharmacology. 2005;182(1):54–57. doi: 10.1007/s00213-005-0062-z. [DOI] [PubMed] [Google Scholar]
- 30.Sachar EJ, Hellman L, Roffwarg HP, Halpern FS, Fukushima DK, Gallagher T. Disrupted 24-hour patterns of cortisol secretion in psychotic depression. Archives of general psychiatry. 1973;28(1):19–24. doi: 10.1001/archpsyc.1973.01750310011002. [DOI] [PubMed] [Google Scholar]
- 31.Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30(9):846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Brown ES, Varghese FP, McEwen BS. Association of depression with medical illness: does cortisol play a role. Biological psychiatry. 2004;55(1):1–9. doi: 10.1016/s0006-3223(03)00473-6. [DOI] [PubMed] [Google Scholar]
- 33.Herbert J. Cortisol and depression: three questions for psychiatry. Psychological medicine. 2013;43(3):449–469. doi: 10.1017/S0033291712000955. [DOI] [PubMed] [Google Scholar]


