Abstract
Objective:
To compare the actual health-system cost of elective labor induction at 39 weeks to expectant management.
Methods:
This was an economic analysis of patients enrolled in the five Utah hospitals participating in a multicenter randomized trial of elective labor induction at 39 weeks versus expectant management in low-risk nulliparous women. The entire trial enrolled over 6,000 patients. For this subset, 1,201 had cost data available. The primary outcome was relative direct health care costs of maternal and neonatal care from a health system perspective. Secondary outcomes included the costs of each phase of maternal and neonatal care. Direct health system costs of maternal and neonatal care were measured using advanced costing analytics from the time of randomization at 38 weeks until exit from the study up to 8 weeks postpartum. Costs in each randomization arm were compared using generalized linear models and reported as the relative cost of induction compared to expectant management. With a fixed sample size, we had adequate power to detect a 7.3% or greater difference in overall costs.
Results:
The total cost of elective induction was no different than expectant management (mean difference +4.7%; 95% CI −2.1% to +12.0%; p=0.18). Maternal outpatient antenatal care costs were 47.0% lower in the induction arm (95% CI −58.3% to −32.6%; p<0.001). Maternal inpatient intrapartum and delivery care costs, conversely, were 16.9% higher among women undergoing labor induction (95% CI +5.5% to +29.5%; p=0.003). Maternal inpatient postpartum care, maternal outpatient care after discharge, neonatal hospital care, and neonatal care after discharge did not differ between arms.
Conclusions:
Total costs of elective labor induction and expectant management did not differ significantly. These results challenge the assumption that elective induction of labor leads to significant cost escalation.
Precis:
Total actual costs of elective labor induction do not differ significantly from expectant management among term, nulliparous women enrolled in a randomized controlled trial.
INTRODUCTION
A Randomized Trial of Induction Versus Expectant Management (ARRIVE) was a landmark multicenter trial of over 6,000 low-risk nulliparous women comparing non-obstetrically indicated induction of labor (elective induction) at 39 weeks gestation with expectant management.1 Although there was no difference between groups in the primary perinatal composite outcome, women assigned to labor induction were less likely to have cesarean delivery, hypertensive disorders of pregnancy, and neonates who received respiratory support. The Society for Maternal-Fetal Medicine (SMFM) and American College of Obstetricians and Gynecologists (ACOG) subsequently concluded that it is “reasonable” to offer induction to low-risk nulliparous women at 39 weeks.2,3
The cost consequences of elective induction are unknown, but there is concern that induction may increase the cost of care. Labor induction in the United States is already common, accounting for 20–25% of all deliveries.4 Childbirth is expensive, accounting for the single largest share of hospital-based expenditures.5 Valid, generalizable data on the relative costs of induction versus expectant management are therefore needed.
Past observational studies that concluded elective induction is associated with increased use of resources and costs have used an inappropriate comparison group of women in spontaneous labor.6–8 Moreover, these studies only focused on intrapartum differences, used suboptimal methodologies to determine actual costs9–11, or derived costs that were not measured but modeled12.
In contrast with the results of prior studies, an analysis of healthcare resources used in women enrolled in the ARRIVE trial showed several potential sources of cost savings with elective induction, in the form of decreased antenatal outpatient and inpatient visits, and shorter maternal and neonatal postpartum hospital stays, but also showed that labor induction resulted in a longer duration on labor and delivery.13 This prior study did not present cost estimates, as costs were not available from all institutions that participated in the trial. This limited the ability to quantify the tradeoffs for decreased antenatal resources saved with increased time spent on labor and delivery in those randomized to induction. Thus, the objective of this study was to measure and compare the actual health care costs of induction and expectant management in women enrolled in the ARRIVE trial at Utah sites where detailed cost data were measured.
METHODS
We performed an economic analysis of Utah participants in the ARRIVE trial. The ARRIVE trial was performed from 2014 to 2017 at 41 hospitals in the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units (MFMU) Network. Full details of this trial are described elsewhere.1 Low-risk nulliparous women with vertex, singleton gestations were eligible for the study. Participants were randomized from 38 weeks 0 days gestation to 38 weeks 6 days gestation to induction of labor or expectant management. Induction was planned to occur from 39 weeks 0 days to 39 weeks 4 days. Women in the expectant management arm planned to continue pregnancy until a medical indication for delivery occurred, or until at least 40 weeks 5 days, but no later than 42 weeks 2 days.
Cost data for women in the trial were only available in Utah health systems. The study population for this analysis was therefore derived from five hospitals within two health care systems in the state of Utah: Intermountain Healthcare (Intermountain) and The University of Utah Health. Participants with missing or incomplete cost data (if their prenatal, postpartum or infant care was outside of the two institutions) were excluded.
This economic analysis was approved by the institutional review boards from the University of Utah and Intermountain.
The primary outcome for this analysis was the relative direct health care cost (defined below) for women randomized to elective labor induction compared with those randomized to expectant management. These costs included the total cost of maternal and newborn care from the time of randomization until exit from the trial up to eight weeks after delivery. The analysis was from the perspective of the health care system.
Costs were measured using advanced analytics platforms at each health system. Although each health system has slightly different costing methodology, they both used activity-based costing (ABC) to assign direct episode-based costs to each patient individually. In this regard, they both measure the same outcome. ABC relies on automated and integrated data collection systems that can capture the complexity and fine detail of a health system. Along with itemized costs of supplies, the fixed and semi-fixed costs of overhead are distributed to every episode of care.
Intermountain uses an enhanced ABC methodology called time-derived activity-based costing (TD-ABC) that incorporates clinical process mapping and estimation of time spent in each phase or cycle of care into the advanced cost accounting of ABC. This costing methodology has been used in previous studies.14–16
The University of Utah Health system (UUH) developed and uses the Value-Driven Outcomes (VDO) tool to measure quality and cost metrics in the University health system.17 Data are harvested from the health system’s enterprise data warehouse, incorporating all relevant input from patient encounters in a process similar to methods used by Intermountain. The tool calculates the cost of an encounter by first tallying the number of units of supplies, pharmacy, imaging, and laboratory components of care and then applying a unit cost by cross-referencing to the institution’s complete record of financial transactions. Space, equipment, labor, and ancillary professional time are allocated to a patient depending on the extent of their use of those resources. Supply and contracted costs are derived from the organization’s actual acquisition costs. Physician costs are allocated based on work related value units. VDO data have been used in a number of published studies.18–21
It is the policy of both Intermountain and UUH to limit the granularity of cost data available for publication and dissemination. As such, the actual costs of individual tests and services or the absolute values of overall costs cannot be reported (though they were measured and compared). Instead of absolute overall costs, we report the relative difference in actual measured costs between arms in the trial: elective labor induction and expectant management. The relative cost is presented as percent difference between the cost in the two arms (=100 × [Cost of IOL – Cost of EM] / Cost of EM). For example, a cost difference of +10% signifies actual costs that are 10% higher with induction, whereas a cost difference of −10% signifies actual costs that are 10% lower with induction.
The analysis was by the intention-to-treat principle, with costs compared according to group assignment (i.e., induction vs. expectant management). Descriptive statistics were used to summarize baseline characteristics by group assignment. Continuous variables were compared using the Wilcoxon rank sum test and categorical variables using chi-square or Fisher’s exact tests, as appropriate. Generalized linear models (GLMs) were developed using gamma distributional family and a log link function. Percent differences in cost and 95% confidence intervals were derived from the beta coefficient in the GLM models. For all analyses, we included an indicator for the health care system in which the patient was enrolled as a covariate in our regression models to control for differences in the ABC methodologies, patient care, or the types of patients who obtained care in each health system. This approach allowed us to combine data from two different health systems with different cost estimates. A test of interaction was performed to determine whether the relationship between randomization arm and cost differed by site. No imputation was performed for missing cost data. A p-value <0.05 was used to define statistical significance, and all tests were two-sided. Analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC) and Stata version 14 (StataCorp., College Station, TX).
In order to evaluate the generalizability of this analysis to the overall trial, a Breslow-Day test of homogeneity was used in the entire trial cohort to assess differences in the association between treatment group and the primary perinatal outcome and cesarean delivery between Utah and non-Utah MFMU sites.
Tests of interaction in GLM regressions were also performed using clinical variables defined a priori to see if these variables modified the effect of randomization arm on cost. Clinical variables including maternal age at the time of delivery (< 35, ≥ 35 years), pregnancy body mass index (<30, or ≥30 kg/m2), modified Bishop score (a measure of readiness of the cervix for induction) at the time of randomization (<5, ≥5), and whether cervical ripening agents were used.
To better define sources of cost escalation or cost savings, we measured and compared phases or components of care between arms. Maternal phases of care, in chronological order, were (1) outpatient antenatal care from the time of randomization until delivery admission, (2) inpatient intrapartum and delivery care (including anesthesia) from the time of admission for delivery through time of vaginal or cesarean delivery, (3) inpatient hospital postpartum care from the time of delivery until postpartum hospital discharge, and (4) outpatient care from the time of hospital discharge through eight weeks postpartum. Newborn phases of care included (5) inpatient newborn care from the time of delivery until newborn discharge from the hospital including neonatal intermediate or intensive care unit care, if utilized, and (6) outpatient after discharge care from the time of hospital discharge through eight weeks of life.
RESULTS
Of 6,106 participants in the trial, 1,231 (20%) were enrolled in Utah, with 608 randomized to labor induction and 623 randomized to expectant management. Among Utah participants, 308 (25%) were enrolled in The University system, and the remaining 923 were enrolled in the four Intermountain hospitals. Those four hospitals individually contributed 178 (14%), 256 (21%), 416 (34%), and 73 (6%) of Utah participants. Thirty had incomplete or missing cost data and were thus excluded, leaving 1,201 (98% of Utah) participants available for economic analysis. Demographic and obstetric characteristics did not differ between treatment arms (Table 1).
Table 1.
Characteristics of the Utah study population
| Characteristic | Induction of labor n=596 |
Expectant management n=605 |
P |
|---|---|---|---|
| Age (years) | 24.9 ± 3.8 | 24.8 ± 4.2 | 0.22 |
| BMI (kg/m2) | 30.6 ± 5.7 | 30.3 ± 5.1 | 0.67 |
| Race/ethnicity | 0.46 | ||
| Non-Hispanic black | 1.5 | 1.3 | |
| Non-Hispanic white | 84.9 | 87.6 | |
| Hispanic | 8.4 | 7.8 | |
| Asian | 2.0 | 1.7 | |
| Other | 3.2 | 1.7 | |
| Smoked during pregnancy | 3.0 | 2.5 | 0.57 |
| Modified Bishop score < 5 | 43.0 | 43.1 | 0.95 |
| Admitting specialist | 0.41 | ||
| Midwife | 2.2 | 2.5 | |
| OBGYN or Family Practice | 91.1 | 92.6 | |
| Maternal-Fetal Medicine | 6.7 | 5.0 |
Data presented as mean ± standard deviation or %.
Number of missing values: BMI (n=15).
The association between treatment group (induction vs. expectant management) and the original trial’s primary composite outcome of perinatal death or severe neonatal complications was not significantly different in Utah versus non-Utah MFMU sites (RR 0.87, 95% CI 0.59 to 1.27 and RR 0.77, 95% CI, 0.59 to 1.02, respectively; Breslow-Day p=0.65). Likewise, the association between treatment group and cesarean delivery was not significantly different in Utah versus non-Utah MFMU sites (RR 0.80, 95% CI 0.62 to 1.04 and RR 0.84, 95% CI 0.76 to 0.94, respectively; Breslow-Day p=0.79).
Our primary outcome, the mean total cost of induction was not significantly different than that of expectant management, (adjusted mean difference +4.7%, 95% CI −2.1% to +12.0%, p=0.18; unadjusted mean difference +5.0%, 95% CI −2.1% to +12.5%). The mean total cost of induction compared to expectant management did not differ by health system (interaction p=0.74).
When considering each phase of care, the cost of outpatient antenatal care from the time of enrollment until labor admission was 47% lower in the induction arm (95% CI −58.3% to −32.6%, p <0.001) relative to the expectant management arm. Conversely, the cost of intrapartum and delivery maternal care was 16.9% higher in the induction arm (95% CI +5.5% to +29.5%, p=0.003) relative to the expectant management arm. Inpatient postpartum maternal care, outpatient maternal care after discharge, inpatient neonatal care, and outpatient neonatal care after discharge newborn were not different between arms (Figure 1, Table 2). The relative proportion that each phase of care contributed to the overall costs of the induction and expectant management groups is presented in Figure 2.
Figure 1.
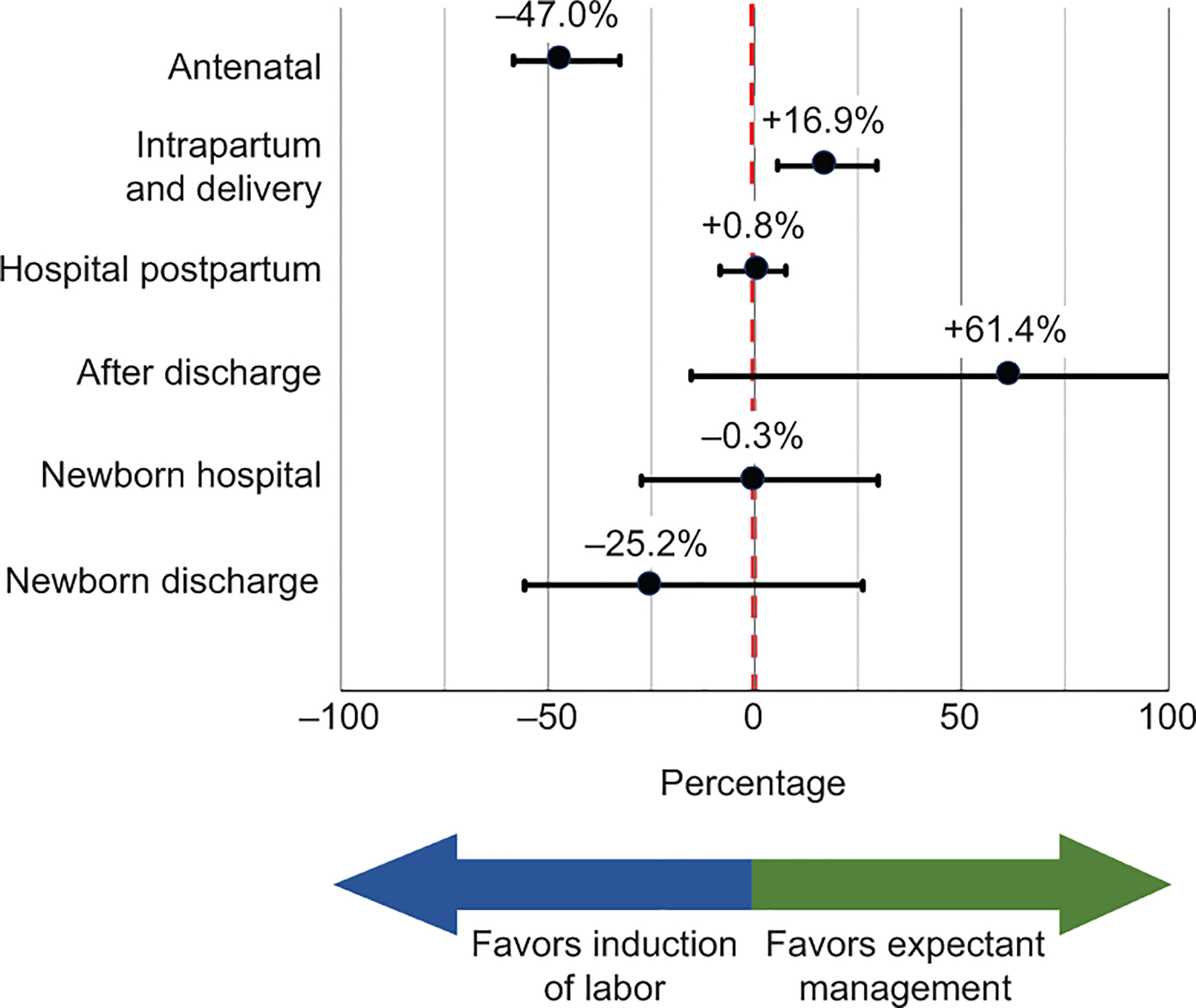
Relative cost of induction of labor compared with expectant management in each phase of care, displayed on the left axis, controlling for differences in health systems. Horizontal whiskers in black represent the 95% CI surrounding the point estimate black dot. Negative values to the left of the red dotted line suggest cost savings with induction of labor. Positive values to the right of the red dotted line suggest cost escalation with induction of labor.
Table 2.
Relative costs of induction of labor compared with expectant management in each phase of care.
| Phase of Care | Relative Cost | 95% CI | P |
|---|---|---|---|
| Maternal Care | |||
| Outpatient Antenatal | −47.0% | −58.3% to −32.6% | <0.001 |
| Inpatient Intrapartum / Delivery | +16.9% | +5.5% to +29.5% | 0.003 |
| Inpatient Hospital Postpartum | +0.8% | −8.4% to +7.5% | 0.85 |
| Outpatient After Discharge | +61.4% | −15.4% to +207.9% | 0.15 |
| Newborn Care | |||
| Inpatient and Delivery Care | −0.3% | −27.4% to +29.8% | 0.84 |
| Outpatient After Discharge | −25.2% | −55.6% to +26.1% | 0.29 |
CI, confidence interval.
Referent group is expectant management.
Relative cost based on a generalized linear model with system of enrollment as a covariate and using a gamma distributional family and log link.
Figure 2.

The unadjusted proportion of cost contributed by each phase of care in the induction and expectant management arms, including outpatient antenatal maternal care (pink), inpatient intrapartum and delivery maternal care (green), inpatient hospital postpartum maternal care (blue), outpatient after discharge maternal care (grey), inpatient newborn care (yellow), and outpatient after discharge newborn care (orange).
The effect of induction versus expectant management on cost was not statistically different according to maternal age (interaction p=0.10), pregnancy BMI (interaction p=0.16), modified Bishop score at randomization (interaction p=0.30), or need for cervical ripening (interaction p=0.33).
A post-hoc power analysis demonstrated that with 1,201 patients and a two-sided alpha of 0.05 we had a power of 85% to detect a 7.3% difference in mean cost between randomization arms.
DISCUSSION
In this economic analysis of five hospitals that participated in a randomized controlled trial of nulliparous women at full term, we have demonstrated the total cost of care for elective induction at 39 weeks is not statistically different than that of expectant management. Of note, the cost of inpatient intrapartum maternal care was higher with elective induction, but this increase was offset by cost savings during antenatal care.
These results fill an important evidence gap, given that it has been unclear whether elective induction at full term increases health care costs. Indeed, past studies that compared women undergoing induction with those in spontaneous labor found that the former were more likely to experience outcomes that increase cost, including cesarean delivery, operative vaginal delivery, and postpartum hemorrhage.6,22–25 Seyb and colleagues estimated elective induction was associated with costs that were 17% higher than spontaneous labor.6 Additionally, women undergoing induction spent on average 3–4 more hours in labor compared with those in spontaneous labor and were more likely to receive epidural analgesia.7,26
Our study stands in contrast to a recent cost-effectiveness analysis by Hersh and colleagues concluding that elective induction is likely to add considerable cost ($2 billion annually) to the U.S. health system.12 Differing conclusions can be explained by several differences in study design. First, cost-effectiveness modeling is not the same thing as cost measurement. We measured and compared the actual costs of health care for each Utah patient enrolled in the trial. Cost-effectiveness modeling as performed in the analysis by Hersh and colleagues does not measure cost, but uses simulation modeling to compare the cost and clinical effectiveness of different approaches for patient care. These simulation models are parametrized using estimates derived from the published literature or other data sources. As a result, Hersh and colleagues included costs associated with induction27 ($1,979.87, range $1000-$3000) that were higher than actual costs we measured. Second, Hersh modeled costs of rare events not found to be different in the ARRIVE trial (e.g. neonatal death and brachial plexus injury) over an entire life span for both the newborn and mother, whereas our study was limited to the costs produced during the study period from 38 weeks to 6–8 weeks postpartum. Third, Hersh evaluated cost from a societal perspective, which is appropriate for cost-effectiveness modeling, but would not be appropriate or possible for our study’s main objective of measuring actual costs in a health system.”
From randomized studies comparing induction versus expectant management in various obstetric conditions, the data are mixed with regard to cost. Induction after 36 weeks in women with hypertensive disease in pregnancy was associated with a cost savings of 831 Euro.28 In a United Kingdom trial of elective induction among women age 35 and older, Walker et al29 found mean cost savings of 263 British pounds. In contrast, cost escalation was noted with induction in pregnancies affected by growth restriction30 and preterm prelabor rupture of membranes.31 Some of these studies did not include outpatient costs or neonatal costs, or used a cost perspective other than the health system perspective.
The results of the ARRIVE trial demonstrate several reasons why labor induction, despite leading to longer duration in labor, is not associated with increased costs overall. Electively induced women had fewer cesarean deliveries, fewer cases of hypertensive disorders of pregnancy, and neonates who less frequently required neonatal respiratory support.1 Moreover, in a planned secondary analysis of data from the trial, Grobman et al quantified individual resources and found that women in the induction arm utilized significantly fewer resources (e.g., ambulatory visits and tests such as sonograms) in the antenatal period, utilized some types of intrapartum medications less frequently, and had shorter postpartum stays.13 That analysis, however, could not compare actual costs, since costs were not provided by the non-Utah hospitals in the trial.
Our analysis has several strengths. First, we utilized time-derived ABC technology that allowed for accurate measurement of actual patient costs from a health system perspective. This approach represents a substantial improvement on cost estimates reported elsewhere that are derived from surrogate markers (e.g. tallies of tests or time-spent in the hospital), modeled costs, or generalized cost-charge ratios.9–11,32 Second, the results were obtained from several different hospitals (including academic and community centers) that care for patients with different demographic characteristics, have different payor mixes, have different staff providing obstetric care, had different mean costs (not reported), and have different obstetric volumes. These consistent results lend support to the generalizability of these findings. Third, our costs were measured among actual women participating in a randomized trial, reducing the potential for bias inherent in observational and modeling studies.
Our analysis also has several limitations. First, our cost estimates were derived from a single geographic area, which may limit generalizability. Yet, as noted, the health outcomes among this population were similar to the outcomes overall among participants in the 36 other geographically disparate hospitals in the trial. Second, although our analysis measured the actual cost of care in the setting of a randomized trial, we cannot know whether these costs will be similar among women undergoing care outside of a trial setting, and in particular with volume or patient flow differences that hospitals may experience after implementing a policy allowing elective induction. Third, we were unable to report actual dollar amounts in each arm due to proprietary concerns. Nevertheless, the relative costs based on actual measured dollar amounts still provide important comparative insight for health systems in which costs of maternity and newborn care may differ from those in Utah. Fourth, there is the possibility that our study is underpowered to detect meaningful differences in cost. Although we found no statistical difference in costs in this study, real cost differences below the threshold our study was powered to detect (+/− 7.3%) may still be relevant. If, in contrast to our findings, real cost differences exist, the population cost or savings of elective labor induction could be substantial. Until this time, elective labor induction has been thought to be clearly more expensive than expectant management (by 17–50%).6–8,12 Our analysis of actual cost suggests this difference is not likely.
Finally, from a philosophical standpoint, it is important to acknowledge that this analysis is from just one economic perspective – the health system. Patients facing out-of-pocket copays may prefer an approach that minimizes additional visits and testing. Hospitals with critical staffing shortages may prioritize a strategy that limits patient time on the labor ward.
In this economic analysis of a randomized study of low-risk nulliparous women, we found that the total costs of elective labor induction at 39 weeks and expectant management were similar. This analysis challenges the longstanding assumption that elective induction of labor at term leads to significant cost escalation.
Supplementary Material
Acknowledgements:
The authors thank the following for their contributions to the original trial: Gail Mallett, R.N., M.S., C.C.R.C. and Kim Hill, R.N., B.S.N. for protocol development and coordination between clinical research centers; Lindsay Doherty, M.S. for protocol and data management; and Elizabeth A. Thom, Ph.D., Madeline M. Rice, Ph.D., Alan T.N. Tita, M.D., Ph.D., and Yasser Y. El-Sayed, M.D for protocol development and oversight.
Funding: Supported by grants HD36801, HD34208 and HD034208-23S1 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Financial Disclosure
Torri D. Metz reports money was paid to her institution as an NICHD supplement to the Maternal-Fetal Medicine Units Network grant to the Utah center to support Dr. Einerson’s work on this project. She received royalties from UptoDate for two topics on VBAC. Money was paid to her institution from Pfizer (site PI for RSV vaccine trial; received institutional startup but trial stopped prior to enrolling any patients). Money was paid to her institution from GestVision (site PI for preeclampsia POC test). She has an additional financial relationship with Novartis and GSK (site PI for GBS vaccination trial, institution received money to conduct study). Money was paid to her institution from Novavax (site PI for RSV vaccination trial). Robert M. Silver reports receiving funds from GestVision as a consultant. The other authors did not report any potential conflicts of interest.
Presented in part at the Annual Clinical Meeting of the American College of Obstetricians and Gynecologists in Nashville, Tennessee on May 4, 2019.
REFERENCES
- 1.Grobman WA, Rice MM, Reddy UM et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The American College of Obstetricians and Gynecologists. Practice Advisory: Clinical guidance for integration of the findings of The ARRIVE Trial: Labor Induction versus Expectant Management in Low-Risk Nulliparous Women. <https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Clinical-guidance-for-integration-of-the-findings-of-The-ARRIVE-Trial.> August 8, 2018. Accessed Oct 16, 2019.
- 3.SMFM Statement on Elective Induction of Labor in Low-Risk Nulliparous Women at Term: the ARRIVE Trial. Am J Obstet Gynecol. 2019;221:B2–B4. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton BE, Martin JA, Osterman MJK, Curtin SC, Matthews TJ. Births: Final Data for 2014. Natl Vital Stat Rep 2015;64:1–64. [PubMed] [Google Scholar]
- 5.Sudhof L, Shah NT. In pursuit of value-based maternity care. Obstet Gynecol. 2019;133:541–51. [DOI] [PubMed] [Google Scholar]
- 6.Seyb ST, Berka RJ, Socol ML, Dooley SL. Risk of cesarean delivery with elective induction of labor at term in nulliparous women. Obstet Gynecol 1999;94:600–607. [DOI] [PubMed] [Google Scholar]
- 7.Maslow AS, Sweeney AL. Elective induction of labor as a risk factor for cesarean delivery among low-risk women at term. Obstet Gynecol. 2000;95:917–922. [DOI] [PubMed] [Google Scholar]
- 8.Allen VM, O’Connell CM, Farrell SA, Baskett TF. Economic implications of method of delivery. Am J Obstet Gynecol. 2005;193:192–197. [DOI] [PubMed] [Google Scholar]
- 9.Barnett PG. An improved set of standards for finding cost for cost-effectiveness analysis. Med Care. 2009;47:S82–88. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan RS, Witkowski M, Abbott M, et al. Using time-driven activity-based costing to identify value improvement opportunities in healthcare. J Healthc Manag. 2014;59(6):399–412. [PubMed] [Google Scholar]
- 11.Kaplan RS, Porter ME. How to solve the cost crisis in health care. Harv Bus Rev. 2011;89(9):46–61. [PubMed] [Google Scholar]
- 12.Hersh AR, Skeith AE, Sargent JA, Caughey AB. Induction of labor at 39 weeks of gestation versus expectant management for low-risk nulliparous women: a cost-effectiveness analysis. Am J Obstet Gynecol. 2019;220(6):590.e1–590.e10. [DOI] [PubMed] [Google Scholar]
- 13.Grobman WA, Sandoval G, Reddy UM et al. Health resource utilization of labor induction versus expectant management: resource utilization at induction of labor. Am J Obstet Gynecol. 2020. [ePub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Padia R, Thomas A, Alt J, Gale C, Meier JD. Hospital cost of pediatric patients with complicated acute sinusitis. Int J Pediatr Otorhinolaryngol. 2016;80:17–20. [DOI] [PubMed] [Google Scholar]
- 15.Richards J, Korgenski EK, Srivastava R, Bonkowsky JL. Costs of the diagnostic odyssey in children with inherited leukodystrophies. Neurology. 2015;85:1167–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meier JD, Duval M, Wilkes J, Andrews S, Korgenski EK, Park AH, Srivastava R. Surgeon dependent variation in adenotonsillectomy costs in children. Otolaryngol Head Neck Surg. 2014;150(5):887–892. [DOI] [PubMed] [Google Scholar]
- 17.Kawamoto K, Martin CJ, Williams K, et al. Value Driven Outcomes (VDO): a pragmatic, modular, and extensible software framework for understanding and improving health care costs and outcomes. J Am Med Inform Assoc. 2015;22:223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee VS, Kawamoto K, Hess R et al. Implementation of a Value-Driven Outcomes Program to Identify High Variability in Clinical Costs and Outcomes and Association With Reduced Cost and Improved Quality. JAMA. 2016;316:1061–1072. [DOI] [PubMed] [Google Scholar]
- 19.Wilde H, Azab MA, Abunimer AM et al. Evaluation or cost and survival in intracranial gliomas using the Value Driven Outcomes database: a retrospective cohort analysis. J Neurosurg. Published online March 29, 2019. [DOI] [PubMed] [Google Scholar]
- 20.Yarbrough PM, Kukhareva PV, Horton D, Edholm K, Kawamoto K. Multifaceted intervention including education, rounding checklist implementation, cost feedback, and financial incentives reduces inpatient laboratory costs. J Hosp Med. 2016;11:348–354. [DOI] [PubMed] [Google Scholar]
- 21.Einerson BD, Stehlikova Z, Nelson RE, Bellows BK, Kawamoto K, Clark EAS. Transfusion preparedness strategies for obstetric hemorrhage: a cost-effectiveness analysis. Obstet Gynecol. 2017;130:1347–1355. [DOI] [PubMed] [Google Scholar]
- 22.Dublin S, Lydon-Rochelle M, Kaplan RC, Watts DH, Critchlow CW. Maternal and neonatal outcomes after induction of labor without an identified indication. Am J Obstet Gynecol. 2000;183:986–994. [DOI] [PubMed] [Google Scholar]
- 23.Johnson DP, Davis NR, Brown AJ. Risk of cesarean delivery after induction at term in nulliparous women with an unfavorable cervix. Am J Obstet Gynecol. 2003;188:1565–1569. [DOI] [PubMed] [Google Scholar]
- 24.Luthy DA, Malmgren JA, Zingheim RW. Cesarean delivery after elective induction in nulliparous women: the physician effect. Am J Obstet Gynecol. 2004;191:1511–1515. [DOI] [PubMed] [Google Scholar]
- 25.Sheiner E, Sarid L, Levy A, Seidman DS, Hallak M. Obstetric risk factors and outcome of pregnancies complicated with early postpartum hemorrhage: a population-based study. J Mat Fet Neonat Med. 2005;18:149–154. [DOI] [PubMed] [Google Scholar]
- 26.Cammu H, Martens G, Ruyssinck G, Amy JJ. Outcome after elective labor induction in nulliparous women: a matched cohort study. Am J Obstet Gynecol. 186;240–244. [DOI] [PubMed] [Google Scholar]
- 27.Bost B Cesarean delivery on demand: what will it cost? Am J Obstet Gynecol. 2003;188:1418–21. [DOI] [PubMed] [Google Scholar]
- 28.Vijgen SMC, Koopmans CM, Opmeer BC et al. An economic analysis of induction of labour and expectant monitoring in women with gestational hypertension or pre-eclampsia at term (HYPITAT trial). BJOG. 2010;117:1577–1585. [DOI] [PubMed] [Google Scholar]
- 29.Walker KF, Kritsaki M, Bugg G et al. Labour induction near term for women aged 35 or over: an economic analysis. BJOG. 2017;124:929–934. [DOI] [PubMed] [Google Scholar]
- 30.Vijgen SM, Boers KE, Opmeer BC et al. Economic analysis comparing induction of labour and expectant management for intrauterine growth restriction at term (DIGITAT trial). Eur J Obstet Gynecol Reprod Biol. 2013;170:358–363. [DOI] [PubMed] [Google Scholar]
- 31.Vijgen SM, van der Ham DP, Bijlenga D et al. Economic analysis comparing induction of labor and expectant management in women with preterm prelaor rupture of membranes between 34 and 37 weeks (PPROMEXIL trial). Acta Obstet Gynecol Scand. 2014;93:374–381. [DOI] [PubMed] [Google Scholar]
- 32.Porter ME. What is value in health care? N Engl J Med. 2010;363:2477–2481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


