Abstract
Background
Primary gastric diffuse large B-cell lymphoma (PG-DLBCL) is a common subtype of extranodal non-Hodgkin lymphoma (NHL), with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) as the commonly used treatment regimen. However, full cycles of standard R-CHOP present the risk of severe bleeding or perforation, even leading to emergency surgery, especially for those with deep lesions in their first 1–2 cycles of treatment. This study aims to explore the safety and efficacy of fractioned R-CHOP (rituximab d0, 50% dose of CHOP d1 and d5) followed by standard R-CHOP cycles in PG-DLBCL patients guided by endoscopic ultrasonography (EUS).
Patients and Methods
Thirty-one PG-DLBCL patients were analyzed in this retrospective study. All patients had lesions infiltrated to at least the 3rd layer of the stomach under EUS at baseline. Patients switched to standard R-CHOP if they showed the reduced infiltrated layers and restricted lesions after fractioned R-CHOP cycles.
Results
The overall response rate, 5-year progression-free survival (PFS) and overall survival (OS) of patients in our study were 93.5%, 75% and 84%, respectively. No treatment delay or dosage reduction from gastric adverse event was observed. None of the patients in our study suffered from severe bleeding or perforation during the treatment. Kaplan–Meier analyses showed that PG-DLBCL patients characterized by multiple localization, lesions ≥3cm, having B symptoms, lower serum albumin level, and elevated LDH level were associated with worse PFS and OS.
Conclusion
Our data indicate that it might be an effective approach in treating deeply infiltrated PG-DLBCL patients by switching fractioned R-CHOP to standard R-CHOP cycles guided by EUS.
Keywords: primary gastric diffuse large B-cell lymphoma, endoscopic ultrasonography, R-CHOP, prognosis
Background
Primary gastric diffuse large B-cell lymphoma (PG-DLBCL) is one of the most common extranodal non-Hodgkin lymphomas (NHLs).1 Treatment of the disease includes surgery, chemotherapy, and radiotherapy, alone or combined. And the best regimen has not been standardized. The general consensus is that treatment should be given concerning the principal clinical issues of patients, and the biological behavior of the malignancies, which guide the doctors to decide whether to offer local-therapy or systemic chemotherapy.2–4 Six to 8 cycles of rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) are usually used to treat the disease. However, full cycles of standard R-CHOP (Rituximab d0, 100% dose of CHOP d1) present the risk of severe bleeding or perforation, even leading to emergency surgery, especially for those with deep lesions in their first 1–2 cycles of treatment. Fractioned R-CHOP which could increase the safety of treatment has been investigated and applied in clinical practice in recent years.5 However, considering the probability that fractioned R-CHOP might cause resistance to the treatment, the optimal proportion of fractioned dose and applied cycle is unclear at present.
Endoscopic ultrasonography (EUS) has emerged as one of the best tools for loco-regional staging in PG-DLBCL patients. It also has a predictive value in diagnosis, evaluating treatment response and post-treatment follow-up.6,7 EUS could visualize the 5-layer structure of the stomach, provide important information on prognostic features of PG-DLBCL patients and may contribute to the determination of the therapeutic approach.
In the current study, we retrospectively reviewed a cohort of primary gastric diffuse large B-cell lymphoma patients treated with early fractioned R-CHOP (Rituximab d0, 50% dose of CHOP d1 and d5) and were then switched to a standard R-CHOP cycle, guided by EUS. This study aims to provide insights into the safety and efficacy of this treatment strategy.
Patients and Methods
Patients
We retrospectively reviewed patients that were pathologically confirmed as gastric diffuse large B-cell lymphoma and were treated in the Shanghai Cancer Center, China, from October 2011 to October 2018. The inclusion criteria for patients in our study were as follows: 1) age range 18- to 75-years-old; 2) histologically confirmed, previously untreated primary gastric diffuse large B-cell lymphoma; 3) Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2; 4) had EUS examination before treatment, and had lesions infiltrated to at least the 3rd layer of the stomach (5 layers in total) at baseline; and 5) received fractioned R-CHOP as an initial treatment. This study was approved by the Institutional Review Board of the Fudan University Shanghai Cancer Center. And all patients signed informed consent forms for reviewing their medical records and research. Baseline clinicopathological features, laboratory assessments were reviewed and obtained from the hospital database and are summarized in Table 1. Survival data were available with a median follow-up of 513 days (range 105~2607 days).
Table 1.
Baseline Characteristics of 31 PG-DLBCL Patients
| Characteristic | PG-DLBCL | % |
|---|---|---|
| Gender | ||
| Male | 15 | 48.4 |
| Female | 16 | 51.6 |
| Age at diagnosis | ||
| > 60 | 15 | 48.4 |
| ≤ 60 | 16 | 51.6 |
| Lugano stage | ||
| I | 4 | 12.9 |
| II 1 | 6 | 19.4 |
| II 2 | 11 | 35.5 |
| II E | 1 | 3.2 |
| IV | 9 | 29.0 |
| Subtypes (n = 28) | ||
| GCB | 14 | 50.0 |
| Non-GCB | 14 | 50.0 |
| B symptoms | ||
| Yes | 5 | 16.1 |
| No | 26 | 83.9 |
| IPI score | ||
| 0–1 | 21 | 67.7 |
| ≥ 2 | 10 | 32.3 |
| ALB | ||
| ≤35 | 3 | 9.7 |
| > 35 | 28 | 90.3 |
| LDH | ||
| Normal(≤ 250) | 25 | 80.6 |
| Elevated | 6 | 19.4 |
| HBsAg positive | ||
| Yes | 5 | 16.1 |
| No | 26 | 83.9 |
| Family history | ||
| Yes | 4 | 12.9 |
| No | 27 | 87.1 |
| SUVmax before treatment | ||
| Mean±SD | 17.92 ± 10.49 |
Abbreviations: ALB, albumin; LDH, lactic dehydrogenase; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone.
Treatment
Patients received full-dose-intensity R-CHOP with fractioned infusion of cyclophosphamide, doxorubicin and vincristine on days one and five, respectively (rituximab 375mg/m2 d0, cyclophosphamide 375mg/m2 d1 and d5, doxorubicin 25mg/m2 d1 and d5, vincristine 1mg d1 and d5, prednisone 100mg d1-5). The cycles were repeated every 3 weeks. Abdominal symptoms and signs, and fecal occult blood tests were carefully monitored during treatment. The response evaluation was performed by an enhanced CT as well as EUS every 2 or 3 cycles. Patients switched to standard R-CHOP cycles (rituximab 375mg/m2 d0, cyclophosphamide 750mg/m2 d1, doxorubicin 50mg/ m2 d1, vincristine 1.4mg/m2 with a maximal dose of 2mg, d1, and prednisone 100mg d1-5) if they got complete response (CR) or partial response (PR) at the evaluation and showed reduced infiltrated layers (≤2nd layer of stomach) with restricted lesions. All patients were treated with 6 or 8 cycles, if tolerated. A whole-body PET-CT scan was given at the end of the treatment. Granulocyte colony-stimulating factor (G-CSF) could be administered by subcutaneous injection during myelosuppression. Prophylaxis usage of G-CSF is also recommended.
Assessment of Response and Adverse Event
All patients received a whole-body PET-CT scan, enhanced abdominal or gastric CT, as well as EUS at baseline before treatment. The response evaluation was performed by an enhanced CT as well as EUS every 2 or 3 cycles. PET-CT was applied in 4–6 weeks after the last chemotherapy course of R-CHOP regimen. Response criteria were adapted from the international consensus reported by Cheson et al.8 After the completion of therapy, follow-ups were carried out on patients every 3 months for the first two years, and every 6 months for the next 3 years, and annually thereafter. The follow-up visits included a physical examination, routine blood tests, enhanced CT of the involved area, and a gastroscopy.
Adverse events during treatment were evaluated according to the National Cancer Institute common toxicity criteria scale (version 4.0).
Statistical Analysis
All analyses were performed using PASW Statistics 18 (SPSS Inc., Chicago). Kaplan–Meier survival curves were constructed for survival analyses, and differences were tested by the Log rank test. OS was defined as the time between the date of diagnosis and the date of death or the date of last contact. PFS refers to the period from the diagnosis to the observed progression of the disease or the occurrence of death for any reason. The data of patients alive at the end of the study were censored. All P values were two-sided, and the results were considered significant if P < 0.05.
Results
Patient Characteristics
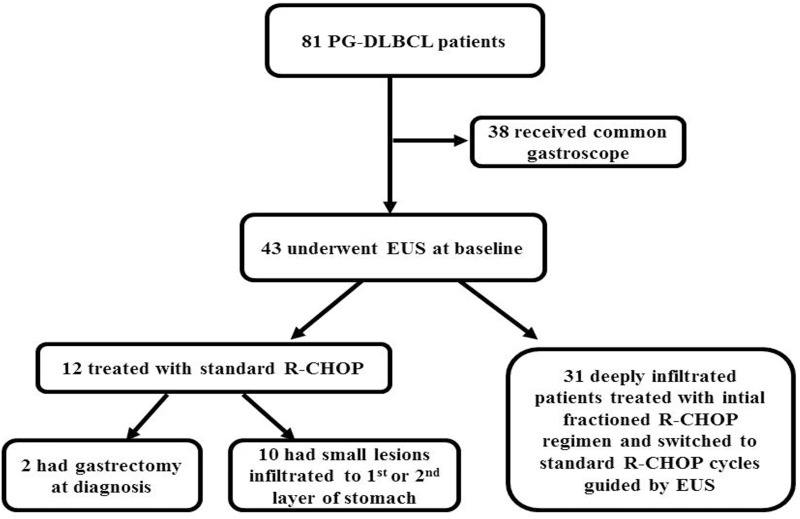
Flow-charts of all patients with primary gastric diffuse large B-cell lymphoma at our hospital treated during the period was described in Figure 1. During the period, 81 PG-DLBCL patients were treated. Forty-three patients underwent EUS at baseline, among whom 31 had deeply infiltrated lesions and accorded with the inclusion criteria of this study and retrospectively enrolled. All 31 cases were from hospitalized patients, of whom 15 (48.4%) were men and 16 (51.6%) were women. The median age was 54 years (range 18 to 75 years). According to the 1994 Lugano modifications of the Musshoff staging classification,9 21 patients (67.8%) were diagnosed with stage I and II disease, 1 (3.2%) and 9 (29.0%) patients were diagnosed with stage IIE and IV disease, respectively. Symptoms of the patients with this disease were unspecific. The most common were abdominal pain or discomfort, gastric hemorrhage, lack of appetite and weight loss. Basic features of the patients are recorded concerning the clinical/pathological parameters of tumors (Table 1). We found that 13 (41.9%) patients had lesions more than one site of stomach. And the most frequent site of PG-DLBCL in our study was the body of the stomach (Table 2).
Figure 1.
Flow-charts of patients with primary gastric diffuse large B-cell lymphoma (PT-DLBCL) who were treated at our department between October 2011 and October 2018. Eighty-one PG-DLBCL patients were treated during the period. Forty-three patients underwent endoscopic ultrasonography (EUS) at baseline, among whom 12 were treated with standard R-CHOP cycles, and 31 had deeply infiltrated lesions and were initially treated with fractioned R-CHOP regime and were then switched to standard R-CHOP cycles guided by EUS.
Table 2.
Features of the Lesion in PG-DLBCL Patients Receiving EUS Before Treatment
| Characteristics | Case Number | Proportion (%) |
|---|---|---|
| Site | ||
| Body | 10 | 32.3 |
| Antrum | 8 | 25.8 |
| Multiple locations | 13 | 41.9 |
| Size | ||
| <3cm | 18 | 58.1 |
| ≥3cm | 13 | 41.9 |
| Depth (n = 30) | ||
| < 11mm | 18 | 60.0 |
| ≥ 11mm | 12 | 40.0 |
| Depth of infiltration (n = 30) | ||
| Submucosa | 2 | 6.6 |
| Muscular | 17 | 56.7 |
| Serosa | 11 | 36.7 |
Treatment Effect and Response of the Patients
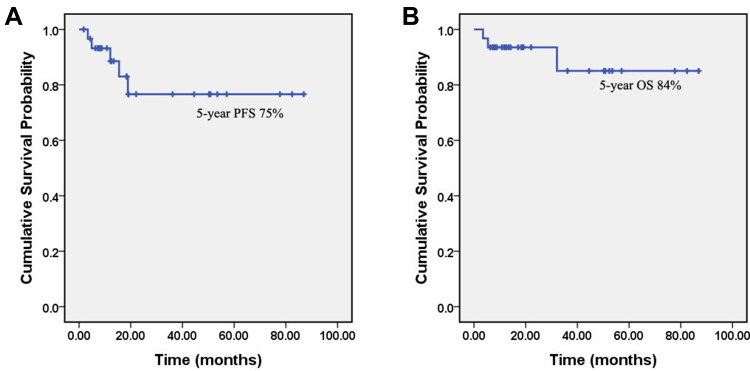
None of the patients in our study underwent surgery at the onset of disease. All 31 patients received initial fractioned R-CHOP (Rituximab d0, 50% dose of CHOP d1 and d5) for at least 2 cycles. Patients switched to standard R-CHOP if they showed the reduced infiltrated layers and restricted lesions under EUS after fractioned R-CHOP cycles. Twenty-three (74.2%) patients successfully switched to standard R-CHOP after 2 cycles of fractioned R-CHOP regimen. Three (9.7%) patients received 3 cycles of fractioned R-CHOP and then switched to standard R-CHOP course. Another 4 (12.9%) patients had 4 cycles of fractioned R-CHOP and then switched. Only 1 (3.2%) patient got progression of disease (PD) after two cycles of fractioned R-CHOP treatment. All together, 29 patients received R-CHOP*6, and 2 patients had R-CHOP*8. The overall response rate of the patients was 93.5% (29/31). The CR and PR rate of patients were 80.6% (25/31) and 12.9% (4/31), respectively. No treatment delay or dosage reduction from gastric adverse event was observed. More importantly, no patients in our study suffered from severe bleeding or perforation during the treatment. Five-year PFS and 5-year OS were 75% and 84%, respectively. Three (9.7%) patients had died of the disease at the date of last follow-up. OS and PFS of all patients in our study were showed in Figure 2.
Figure 2.
Progression-free survival (PFS) and overall survival (OS) of primary gastric diffuse large B-cell lymphoma (PG-DLBCL) patients in our study. Kaplan–Meier curves show the PFS (A) and OS (B) of the PG-DLBCL patients in our study.
The adverse events during treatment are presented in Table 3. Haematological toxicity was the predominant adverse event. Grade 3/4 neutropenia occurred in 35.5% of the patients and febrile neutropenia in one patient (3.2%). Pneumonia and nausea were the top two non-hematologic toxicities. We observed no treatment-related deaths in our study.
Table 3.
Adverse Event from Chemotherapy and Event Grade
| Toxicity | No. of Events | % Grade1+2 | No. of Events | % Grade 3+4 | ||
|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |||
| Hematologic | ||||||
| Neutropenia | 7 | 5 | 38.7 | 7 | 4 | 35.5 |
| Anemia | 3 | 4 | 22.6 | 0 | 0 | 0 |
| Thrombocytopenia | 4 | 1 | 16.1 | 1 | 0 | 3.2 |
| Febrile neutropenia | – | – | – | 1 | 0 | 3.2 |
| Nonhematologic | ||||||
| Nausea | 3 | 1 | 12.9 | 0 | 0 | 0 |
| Increased ALT/AST | 2 | 0 | 6.5 | 0 | 0 | 0 |
| Pneumonia | 0 | 5 | 16.1 | 0 | 0 | 0 |
Effect of Clinicopathological Parameters on PFS and OS of PG-DLBCL Patients
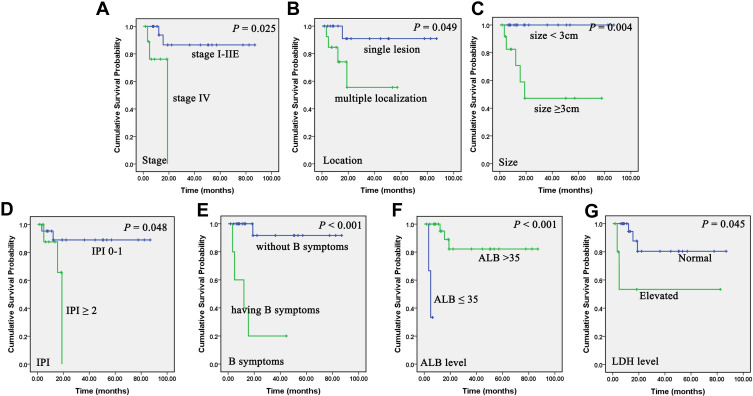
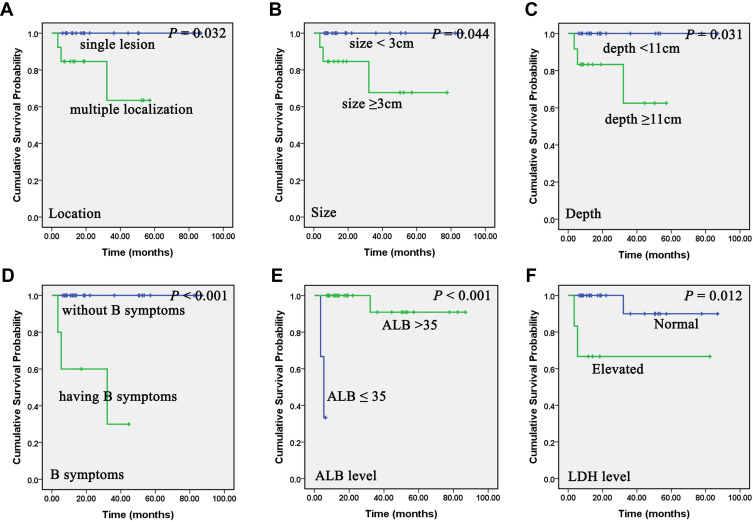
Kaplan–Meier analyses showed that PG-DLBCL patients characterized by late stage (stage IV) (P = 0.025), multiple localization (sites > 1) (P = 0.049), large size (P = 0.004), higher international prognostic index (IPI) score (P= 0.048), having B symptoms (P < 0.001), lower serum albumin (ALB) level (P < 0.001), and elevated lactate dehydrogenase (LDH) level (P = 0.045) had shorter PFS (Figure 3). And patients with multiple localization (P= 0.032), large size (≥ 3cm) (P = 0.044), and deep lesions (≥ 11mm) (P = 0.031) under EUS, having B symptoms (P < 0.001), lower ALB level (≤ 35g/L) (P < 0.001), and elevated LDH level (> 250U/L) (P = 0.012) associated with worse OS (Figure 4).
Figure 3.
Correlation of clinical and laboratory parameters on progression-free survival (PFS) of primary gastric diffuse large B-cell lymphoma (PG-DLBCL) patients. Kaplan–Meier curves show the association between stage (A), location (B), size (C), international prognostic index (IPI) (D), B symptoms (E), serum albumin (ALB) level (F), lactate dehydrogenase (LDH) level (G) and PFS of PG-DLBCL patients in our study. All the P values are shown in the graph, by Log rank test.
Figure 4.
Association of clinical and laboratory parameters on overall survival (OS) of primary gastric diffuse large B-cell lymphoma (PG-DLBCL) patients. Kaplan–Meier curves showing the association between location (A), size (B), depth under endoscopic ultrasonography (EUS) (C), B symptoms (D), serum albumin (ALB) level (E), lactate dehydrogenase (LDH) level (F) and OS of PG-DLBCL patients in our study. All the P values are shown in the graph, by Log rank test.
Discussion
DLBCL is a common subtype of primary gastric lymphoma. PG-DLBCL has been treated with various modalities, such as surgery, chemotherapy and radiotherapy, alone or in combination. Traditionally, radical gastrectomy was regarded as the front-line treatment for PG-DLBCL patients. In recent years, surgery is given only to patients with a massive bleed or perforation at presentation.4 Besides, surgery could lower the quality of life and postpone the beginning of chemotherapy. R-CHOP is now considered to be the standard regimen to treat the disease.10–13 However, management of deeply infiltrated PG-DLBCL patients is still challenging as it has high incidence of bleeding as well as perforation during treatment, which might be fatal. In our study, we evaluated the feasibility and efficacy of switching fractioned R-CHOP to standard R-CHOP to treat this subset of patients.
PG-DLBCL lesions, large in size and deep under the EUS, had a greater risk of bleeding or perforation during treatment, and patients often suffered worse PFS and OS. Full cycles of standard R-CHOP (Rituximab d0, 100% dose of CHOP d2) had the risk of severe bleeding or perforation, potentially leading to emergency surgery, especially for those with large and deep lesions in their first 1–2 cycles of treatment.14–17 It is known that dose intensity of chemotherapy is crucial in the treatment of lymphoma. As it is defined as the average weekly dose of chemotherapy drugs per square meter of body surface area in the whole treatment course, we fractioned conventional R-CHOP to day one and day five in order not to reduce patients' dose-intensity. There were also studies of splitting R-CHOP to day one and day two in older patients.5 We believe it is more rational to split on day one and day five, as doctors can closely monitor routine blood tests and fecal blood tests after the 50% CHOP on the first day of treatment. All patients enrolled in our study had a high possibility of perforation and bleeding during treatment. However, after applying the initial treatment of fractioned R-CHOP, no adverse effects, such as bleeding or perforation, were observed. However, full cycles of fractioned R-CHOP might cause resistance to the treatment. Switching fractioned R-CHOP to standard R-CHOP could provide a balance between treatment effects and the avoidance of severe side effects. In our study, patients switched in good time to the standard R-CHOP cycle when lesions were restricted under EUS evaluation.
EUS is a valuable tool in the diagnosis of PG-DLBCL patients, as it could diagnose and utilize the loco-regional staging of the disease. Furthermore, EUS could measure the invasive depth of lesions, this could not be accomplished by an ordinary gastroscopy. Our previous work provided new insights about evaluation of PG-DLBCLs based on EUS results. PG-DLBCL patients having lesions with large size (≥3cm), or deep depth (≥11mm) were more inclined to have a worse OS.18 This study further extends its role in the guidance of treatment when switching to standard R-CHOP regimens.
In our previous work, we illustrated some prognostic factors of PG-DLBCL patients. In this study, we also confirmed that a tumor located at multiple sites of the stomach, having B symptoms, lower ALB levels and an elevated LDH level could act as adverse prognostic biomarkers. Besides, the tumor size and depth as examined by endoscopic methodology were valuable parameters to predict the outcomes of PG-DLBCL patients.
To our knowledge, this is the first study including only deeply infiltrated PG-DLBCL patients (infiltrated ≥ 3rd layer of the stomach under EUS). The ORR, 5-year PFS and 5-year OS of patients in our study were 93.5%, 75% and 84%, respectively. Concerning safety parameters, the most common adverse event was neutropenia. It could successfully be addressed through using growth factors or dose modifications. Other common AEs were grade 1 or grade 2 anemia, thrombocytopenia, and nausea. All the toxicities, but pneumonia, were tolerable without interruption of treatment.
Our data indicate that an effective and tolerable approach in treating deeply infiltrated PG-DLBCL patients is by switching fractioned R-CHOP cycles to standard R-CHOP cycles, guided by EUS. We cannot deny some limitations of this study, such as the relatively small number of patients, the retrospective analysis, the single institution study, as well as a relatively short follow-up period. However, our work might shed light on novel treatment strategies in treating the disease. More prospective, multi-center clinical trials are still needed.
Funding Statement
This work was supported by the National Natural Science Foundation of China (81702259), and Shanghai Municipal Commission of Health and Family Planning Fund (20174Y0075).
Disclosure
The authors declare that they have no competing interests.
References
- 1.Koch P, Probst A, Berdel WE, et al. Treatment results in localized primary gastric lymphoma: data of patients registered within the German multicenter study (GIT NHL 02/96). J clin oncol. 2005;23(28):7050–7059. doi: 10.1200/JCO.2005.04.031 [DOI] [PubMed] [Google Scholar]
- 2.Mehmet K, Sener C, Uyeturk U, et al. Treatment modalities in primary gastric lymphoma: the effect of rituximab and surgical treatment. A study by the anatolian society of medical oncology. Contemp Oncol. 2014;18:273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang HW, Jiang YB, Fu TW, et al. [Efficacy of surgery and rituximab in primary gastric diffuse large B-cell lymphoma].. Zhonghua Xue Ye Xue Za Zhi. 2016;37(7):602–606. doi: 10.3760/cma.j.issn.0253-2727.2016.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikoma N, Badgwell BD, Mansfield PF. Multimodality treatment of gastric lymphoma. Surg Clin North Am. 2017;97(2):405–420. doi: 10.1016/j.suc.2016.11.012 [DOI] [PubMed] [Google Scholar]
- 5.Kreher S, Lammer F, Augustin D, Pezzutto A, Baldus CD. R-split-CHOP chemotherapy for elderly patients with diffuse large B-cell lymphoma. Eur J Haematol. 2014;93(1):70–76. doi: 10.1111/ejh.12304 [DOI] [PubMed] [Google Scholar]
- 6.Vanis N, Mesihovic R, Ibricevic L, Dobrila-Dintinjana R. Predictive value of endoscopic ultrasound in diagnosis and staging of primary gastric lymphoma. Coll Antropol. 2013;37(Suppl 1):291–297. [PubMed] [Google Scholar]
- 7.Vetro C, Chiarenza A, Romano A, et al. Prognostic assessment and treatment of primary gastric lymphomas: how endoscopic ultrasonography can help in tailoring patient management. Clin Lymphoma Myeloma Leuk. 2014;14(3):179–185. doi: 10.1016/j.clml.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 8.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J clin oncol. 2014;32(27):3059–3068. doi: 10.1200/JCO.2013.54.8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohatiner A, d’Amore F, Coiffier B, et al. Report on a workshop convened to discuss the pathological and staging classifications of gastrointestinal tract lymphoma. Ann Oncol. 1994;5(5):397–400. doi: 10.1093/oxfordjournals.annonc.a058869 [DOI] [PubMed] [Google Scholar]
- 10.Coiffier B. State-of-the-art therapeutics: diffuse large B-cell lymphoma. J Clin Oncol. 2005;23(26):6387–6393. doi: 10.1200/JCO.2005.05.015 [DOI] [PubMed] [Google Scholar]
- 11.Zhang HY, Guan ZZ, Wang B, Huang HQ, Xia ZJ, Lin TY. [Relationship between clinopathological features and outcome of rituximab treatment for diffuse large B-cell lymphoma]. Zhonghua Zhong Liu Za Zhi [Chinese Journal of Oncology]. 2008;30(5):381–384. Chinese. [PubMed] [Google Scholar]
- 12.Zhang J, Li G, Yang H, Liu X, Cao J. Rituximab in treatment of primary gastric diffuse large B-cell lymphoma. Leuk Lymphoma. 2012;53(11):2175–2181. doi: 10.3109/10428194.2012.680451 [DOI] [PubMed] [Google Scholar]
- 13.Zhao W, Gao Y, Bai B, et al. Safety and efficacy of non-initial rapid infusion of rituximab plus chemotherapy in Chinese patients with CD20+ non-Hodgkin’s lymphoma. Expert Opin Drug Saf. 2015;14(1):21–29. doi: 10.1517/14740338.2015.988138 [DOI] [PubMed] [Google Scholar]
- 14.Spectre G, Libster D, Grisariu S, et al. Bleeding, obstruction, and perforation in a series of patients with aggressive gastric lymphoma treated with primary chemotherapy. Ann Surg Oncol. 2006;13(11):1372–1378. doi: 10.1245/s10434-006-9069-x [DOI] [PubMed] [Google Scholar]
- 15.Kadota T, Seo S, Fuse H, et al. Complications and outcomes in diffuse large B-cell lymphoma with gastric lesions treated with R-CHOP. Cancer Med. 2019;8(3):982–989. doi: 10.1002/cam4.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wada M, Onda M, Tokunaga A, et al. Spontaneous gastrointestinal perforation in patients with lymphoma receiving chemotherapy and steroids. Report of three cases. Nihon Ika Daigaku Zasshi. 1999;66(1):37–40. doi: 10.1272/jnms.66.37 [DOI] [PubMed] [Google Scholar]
- 17.Yoshino S, Nakamura S, Matsumoto T, et al. A case of primary gastric malignant lymphoma perforated immediately after administration of chemotherapy. Nihon Shokakibyo Gakkai Zasshi. 2006;103(2):162–167. [PubMed] [Google Scholar]
- 18.Liu YZ, Xue K, Wang BS, et al. The size and depth of lesions measured by endoscopic ultrasonography are novel prognostic factors of primary gastric diffuse large B-cell lymphoma. Leuk Lymphoma. 2019;60(4):934–939. doi: 10.1080/10428194.2018.1515942 [DOI] [PubMed] [Google Scholar]