Supplemental Digital Content is available in the text.
Key Words: SLC-0111, carbonic anhydrase IX, advanced solid tumor, safety
Abstract
Objectives:
SLC-0111 is an ureido-substituted benzenesulfonamide small molecule inhibitor of carbonic anhydrase IX. The objectives of this first-in-human Phase 1 study were to determine the safety and tolerability of SLC-0111 in patients with advanced solid tumors and to establish the recommended Phase 2 dose for future clinical investigations.
Materials and Methods:
Using a 3+3 design, dose escalation started at 500 mg oral daily dosing of SLC-0111 in cohort 1 and increased to 1000 and 2000 mg in cohorts 2 and 3. Drug-related adverse events (AEs) were monitored to determine safety and tolerability. Pharmacokinetic analyses assessed plasma concentrations of single and repeated doses of SLC-0111. RECIST 1.1 criteria were used to assess disease progression.
Results:
No dose-limiting toxicities were reported and patients dosed at ≤1000 mg exhibited fewer drug-related AEs ≥ grade 3 and fewer AEs such as nausea and vomiting, compared with the 2000-mg cohort. Forty-one percent of patients experienced dose interruptions or discontinuation and the majority (71%) of these occurred in the 2000-mg cohort. Mean Cmax and AUC(0-24) values for single doses were similar at the 1000-mg and 2000-mg dose levels. Mean Tmax and T1/2 values of SLC-0111 were similar after single and repeated dosing. Power-law analysis of Cmax and AUC0-24 showed that exposure to SLC-0111 was generally dose proportional. No objective responses were observed, but stable disease >24 weeks was observed in 2 patients.
Conclusions:
SLC-0111 was safe in patients with previously treated, advanced solid tumors. The safety and pharmacokinetic data support 1000 mg/d as the recommended phase 2 dose for SLC-0111.
Hypoxia occurs in the complex tumor microenvironment of solid cancers and the presence of hypoxic regions within the tumor microenvironment is associated with poor patient prognosis and resistance to anticancer therapies.1,2 Hypoxia is also known to provide an environmental niche for cancer stem cells and to promote invasion and metastasis. Intratumoral hypoxia promotes hypoxia-inducible factor 1 alpha (HIF-1A)-mediated metabolic reprogramming by tumor cells, including a shift toward increased glycolysis and altered oxidative phosphorylation, to meet energy and biosynthetic demands in a low-oxygen environment.3 These processes lead to the accumulation of acidic metabolites, including lactate, protons (H+) and carbon dioxide (CO2), which contribute to the disruption of intracellular pH (pHi) homeostasis, leading to intracellular acidification and impacting cell viability.4,5 To combat hypoxic and acidic cellular stress, cancer cells activate network of enzymes and transporters that function to maintain pHi homeostasis,4 including the exofacial, tumor-associated carbonic anhydrases (CA) IX (CAIX) and CAXII.5,6
CAIX is a cell surface, hypoxia-inducible factor 1 alpha-inducible metalloenzyme that functions to catalyze the reversible hydration of CO2 to bicarbonate (HCO3−) and H+.6 In hypoxic tumors, CAIX activity enables the maintenance of a pHi favorable for cancer cell survival and growth, and simultaneously contributes to extracellular acidification, facilitating tumor cell migration, invasion and metastasis, and therapeutic resistance.7,8 Depletion of CAIX interferes with pH regulation, reduces cancer stem cells, inhibits epithelial mesenchymal transition and ultimately curtails tumor growth and metastasis.9–12 Furthermore, CAIX is highly expressed in many solid tumors, is considered to be an endogenous marker of hypoxia and is well recognized as a prominent biomarker of poor patient prognosis.7,13 Tissue microarray analyses have demonstrated a relatively high frequency of CAIX-positive tumors for many cancers, including 25% of non–small cell lung and ovarian cancers, 50% of basal type breast cancers, 66% of pancreatic ductal adenocarinomas, 71% of bladder cancers, and 95% of clear cell renal cell carcinomas.7,10,12 In contrast, the expression of CAIX in normal human normal tissue is highly restricted, with significant levels being confined to gastric and gall bladder epithelia,8,14 making it an attractive therapeutic target and driving the development of CAIX/CAXII small molecule inhibitors.
4-[(4-fluorophenyl)carbamoyl]amino-benzene sulfonamide, designated SLC-0111, is a selective small molecule inhibitor of CAIX.15 Several preclinical studies have demonstrated that targeting CAIX activity with SLC-0111, alone and in combination with chemotherapy or immune checkpoint blockade, results in antitumor efficacy in multiple solid tumor models, including triple negative breast cancer,10,11,16–18 pancreatic cancer,12 glioblastoma,19 and melanoma.20
Although anti-CAIX monoclonal antibodies, such as girentuximab (aka cG250), have been evaluated clinically in the setting of clear cell renal cell carcinoma,21 the current study is the first clinical trial of a highly selective small molecule inhibitor of CAIX/CAXII. The objective of this multicenter, open-label, first-in-human Phase 1 dose escalation study was to determine the safety and tolerability of SLC-0111 in patients with previously treated, advanced solid tumors and to establish the recommended dose for future clinical investigations.
MATERIALS AND METHODS
Eligibility
Male and female patients aged 18 years or older who had a histologically or cytologically confirmed solid malignancy that was metastatic or unresectable, and for which standard curative measures did not exist, were eligible for the study. All eligible patients were required to have recovered to ≤ Grade 1 from the effects of any prior therapy for their malignancy, and to have an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1, a life expectancy of >3 months, adequate organ function and coagulation parameters, and the ability to swallow oral medication. All patients gave written, informed consent before enrolling in the study. The study was registered with ClinicalTrials.gov (identifier NCT02215850).
Exclusion criteria included pregnancy or lactation, refractory nausea or vomiting, chronic gastrointestinal disorders, history of a cardiovascular event within 6 months before initiation of therapy, poorly controlled hypertension, known symptomatic central nervous system metastases requiring treatment, known hypersensitivity to sulfonamides, SLC-0111, or excipients, or prior therapy with drugs known to inhibit hypoxia.
Drug Supply
SLC-0111 oral liquid (Lot number MP0002) was provided by Welichem Biotech Inc. (WBI) at a concentration of 100 mg/g (equivalent to 100 mg/mL) and was stored refrigerated at 2 to 8°C. The active ingredient in SLC-0111 was 4-[(4-fluorophenyl)carbamoyl] amino-benzene sulfonamide (C13H12FN3O3S).15 The drug product also contained soy lecithin, propylene glycol, polyethylene glycol 200, polyethylene glycol 400, and vitamin E tocopherol polyethylene glycol succinate as excipients.
Study Objectives and Ethics
The primary objective of this multicenter, open-label, first-in-human Phase 1 dose escalation study was to determine the safety of SLC-0111, administered orally once daily, in patients with previously treated, advanced solid tumors by evaluating toxicity and tolerability during the first cycle of therapy. The secondary objectives were to determine the maximum tolerated dose by assessment of dose-limiting toxicities (DLTs), obtain the pharmacokinetic (PK) of single and multiple doses of SLC-0111 by monitoring the blood exposure levels and establish the recommended dose for future clinical investigations. Tumor response was assessed as an exploratory objective. The study protocol, amendments and informed consent were approved by the investigational center ethics committee or institutional review board (IRB) at BC Cancer (Vancouver, BC, Canada) Cross Cancer Institute (Edmonton, AB, Canada) and Princess Margaret Cancer Centre (Toronto, ON, Canada). This study was performed in full compliance with the International Conference on Harmonization (ICH) Technical Requirements for Registration of Pharmaceuticals for Human Use and all applicable local Good Clinical Practice (GCP) regulations.
Study Design, Dose Selection, and Dose Escalation
The study used a conventional 3+3 design and 3 dose levels of SLC-0111 were evaluated over the course of the study. SLC-0111 liquid formulation administered orally, once a day, at least 2 hours before or after a meal for 28 consecutive days constituted one treatment cycle. A starting clinical dose of 500 mg was selected based on data from GLP 28-day toxicology studies in rats and dogs. The highest dose tested in rats, 500 mg/kg/d (3000 mg/m2), was selected as severe toxic dose in 10% (STD10) of treated rodents, whereas the highest nonsevere toxic dose (HNSTD) in dogs was 150 mg/kg (3000 mg/m2). Using these values and a body surface area conversion factor (37×60 kg), the proposed starting clinical dose was calculated to be 486 mg/d (1/10th the STD10/37×60 kg) based on the rodent data and 810 mg/d (one sixth the HNSTD/37×60 kg) based on the dog data. Thus, a starting dose of 500 mg was considered acceptable for the current study and was administered to patient cohort 1, followed by 1000 mg in cohort 2 and 2000 mg in cohort 3. A cohort (same dose level) of at least 3 evaluable patients was required to complete 1 cycle of treatment before dose escalation in the next cohort. The decision to proceed to the next dose level was made only after the safety of the previous dose level had been established. Patients who did not meet the discontinuation criteria continued to receive SLC-0111 for multiple cycles. Criteria for withdrawal from the study included disease progression, unacceptable toxicity, need for anti-cancer therapy not specified in the protocol, noncompliance, or patient-initiated request for withdrawal.
Response Evaluation
Before enrollment in the study, all patients received a detailed medical history and physical examination. Clinical laboratory evaluations included clinical chemistry, complete blood count, urinalysis, coagulation factors, and a lipid panel. Other safety assessments included vital signs, physical examination, electrocardiogram, and echocardiogram with multigated acquisition (ECHO/MUGA). Safety assessments were performed on days 1, 8, 15, 22 and 28 of cycle 1, days 1, 8, 15 and 22 of cycle 2, and days 1 and 15 of cycles 3 and 4.
A DLT was defined as 1 or more drug-related adverse events (AE) occurring during cycle 1 at any dose level: (1) grade 4 hematologic toxicity of any duration; (2) grade 3 or 4 febrile neutropenia; (3) grade ≥3 neutropenia persisting for >7 days; (4) grade ≥3 thrombocytopenia associated with bleeding; (5) grade 3 or 4 clinical nonhematologic toxicity (excluding nausea, or vomiting controlled by optimal antidiarrheal or antiemetic therapy and grade 3 fatigue ≤7 d); (6) grade ≥3 rash for >7 days; (7) grade ≥3 hypersensitivity reaction; (8) any continuous dose interruption for >7 days due to intolerable drug related toxicities considered as potentially related to SLC-0111. Toxicity and AEs were classified according to the NCI Common Terminology Criteria for Adverse Events V4.03 (CTCAE). Diarrhea, fatigue, and vomiting were considered AEs of special interest because they were either known class effects or because they were expected based on nonclinical safety studies.
PK Analyses
Blood samples for determination of SLC-0111 plasma concentrations were collected predose and at 1, 2, 4, 6, 8 and 24 hours after SLC-0111 administration on days 1 and 28 of cycle 1. After collection, blood samples were centrifuged, and plasma separated and stored at −80°C.
Plasma concentrations of SLC-0111 were measured by a validated analytical method involving liquid-liquid extraction followed by high performance liquid chromatography-mass spectroscopy assay using a high performance liquid chromatography system and a Quattro Ultima mass spectroscopy (Waters Corp, Milford, MA) with MassLynx v. 4.1 software. Plasma analysis was carried out by Microconstants (San Diego, CA) in compliance with the US FDA GLP regulations. The calibration range of the assay was from 1.00 to 1000 ng/mL. Quality control samples prepared at 3 different analyte concentrations were analyzed with each batch of samples against separately prepared calibration standard.
PK parameters were collected following single (Day 1) and repeat dose (Day 28) administration. SLC-0111 concentration-time data were analyzed by noncompartmental methods using WinNonlin Professional 6.3 software (Pharsight Corp., Mountain View, CA). The analysis provided estimates of the area under the SLC-0111 plasma concentration time curve from time of dose to 24 hours after dose (AUC0-24), maximum observed SLC-0111 plasma concentration (Cmax) for the 24 dosing window, time to achieve maximum SLC-0111 plasma concentration (Tmax), and the time taken for half of the initial SLC-0111 dose to be eliminated (T1/2). The relationship between SLC-0111 PK exposure (Cmax and AUC0-24) and SLC-0111 dose was examined using a power-law model using GraphPad Prism v 5.01 (GraphPad Inc., San Diego, CA).
Tumor Response
Tumor responses were recorded using the Response Evaluation Criteria of Solid Tumors (RESIST v1.1). Disease assessment included imaging (CT/MRI) and was recorded as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). Assessments were repeated every 8 weeks (±7 d), relative to Day 1 of cycle 1, until objective disease progression, study drug discontinuation or withdrawal of informed consent.
RESULTS
Patient Characteristics
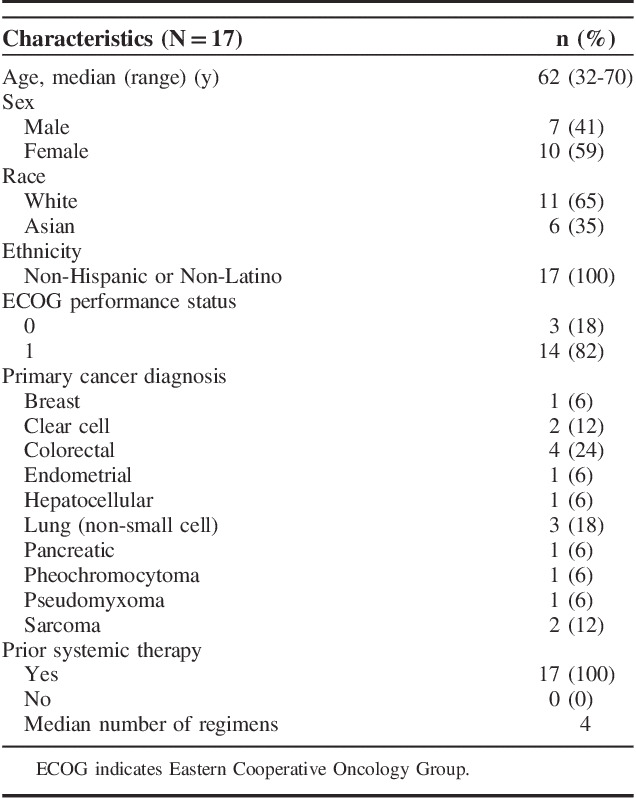
Seventeen patients were enrolled in the study and received treatment between October 2014 and July 2016 (Table 1). The median age was 62 years (range, 32 to 70 y). Three patients (18%) had an ECOG PS score of 0 and 14 patients (82%) had a PS of 1 (Table 1). The most common diagnoses of cancer were reported as colorectal (24%), lung (18%), clear cell carcinoma of the kidney (12%), and sarcoma (12%) (Table 1). Other tumor types included breast carcinoma, endometrial carcinoma, hepatocellular carcinoma, pancreatic cancer, pheochromocytoma, and pseudomyxoma (Table 1). All patients had received prior chemotherapy for treatment of their disease with a median of 4 prior regimens administered to each patient (Table 1).
TABLE 1.
Baseline Demographics and Clinical Characteristics
Drug Safety, DLTs, and AEs
Of the 17 patients who received at least one dose of SLC-0111, 4 were assigned to each of cohorts 1 (500 mg) and 2 (1000 mg), and 9 were assigned to cohort 3 (2000 mg) (Table 2). The mean duration of exposure to SLC-0111 across all cohorts was 62 days with a range of 4 to 336 days (Table 2).
TABLE 2.
Summary of Dosing Duration, DLTs, and Reasons for Treatment Discontinuation
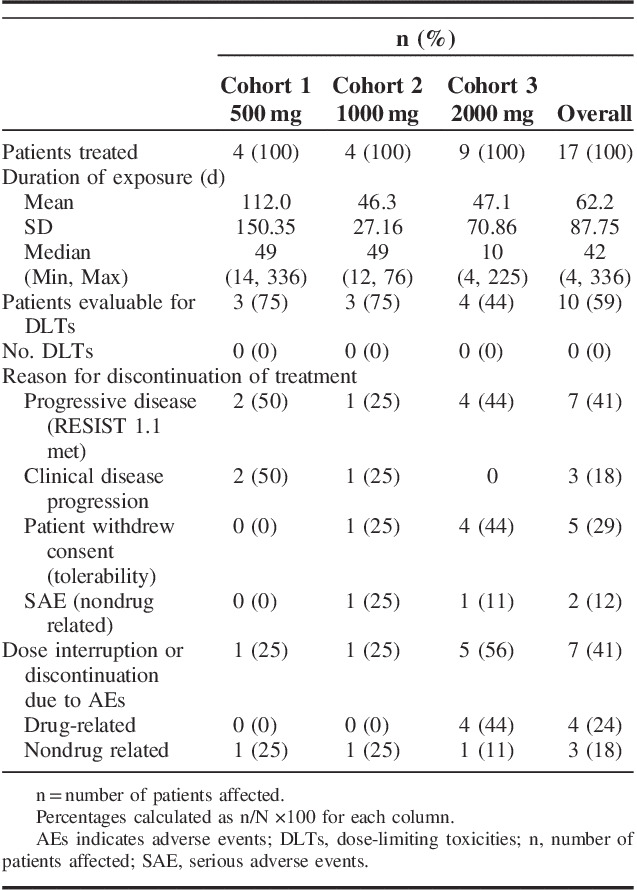
Of the 17 patients given SLC-0111, 10 patients (59%) were evaluable for DLTs, including 3 patients (75%) in cohort 1, 3 patients (75%) in cohort 2 and 4 patients (44%) in cohort 3 (Table 2). The remaining 7 patients (41%) were not evaluable for DLTs because they did not complete cycle 1 of dosing. No protocol defined DLTs were observed at any of the doses examined (Table 2).
The median time to treatment termination was 49 days in the 500 and 1000-mg cohorts, and was 10 days in the 2000-mg cohort (Table 2). The primary reason for discontinuation from treatment was PD determined as per RECIST 1.1 criteria, which was reported for 7 patients (41%) (Table 2). A further 3 patients (18%) discontinued treatment due to clinical disease progression, although RECIST 1.1 criteria were not met. In addition, 5 patients (29%) withdrew their consent, including 1 patient in the 1000-mg cohort and 4 patients in the 2000-mg cohort, and discontinued treatment due to issues with tolerability of the drug (Table 2). The reasons for discontinuation by the patients in the 2000-mg group included nausea and vomiting, diarrhea, anorexia and fatigue, and taste alterations. The remaining 2 patients discontinued treatment due to serious adverse events (SAE) that were not related to the study drug (Table 2). Specifically, 1 patient discontinued the study due to hospitalization for a pulmonary embolism (see SAE below) that was considered to be related to a history of atrial tachycardia. A second patient discontinued the study during in-patient hospitalization for an infection. The patient was diagnosed with Clostridium enterocolitis (see SAE below), likely due to therapy with antibiotics related to replacement of a stent before entering the study.
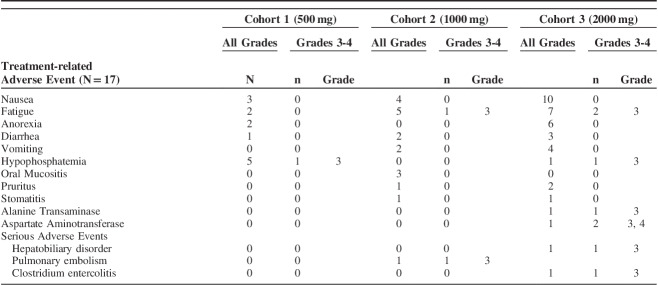
A total of 184 treatment emergent AEs were reported over the course of the study, 91 of which were considered to be related to SLC-0111 (see Table S1, Supplemental Digital Content 1, http://links.lww.com/AJCO/A329, which summarizes the number of drug-related AEs). The majority of these drug related AEs occurred in the 1000-mg and 2000-mg cohorts. Seven patients (41%) experienced dose interruptions or discontinuation of dosing resulting from AEs, the majority of which were in the 2000-mg cohort where 5 patients (56%) experienced a dose interruption, 4 of which were related to the study drug (Table 2). The most frequently reported drug related AEs, occurring in ≥10% of patients, were nausea, fatigue, anorexia, diarrhea, vomiting, hypophosphatemia, oral mucositis, pruritus, and stomatitis (Table 3). Most of the drug-related AEs were grades 1 or 2, but there were 8 grade 3 drug-related AEs reported by 5 patients, including fatigue, increases in alanine transaminase and aspartate aminotransferase, and hypophosphatemia (Table 3). The single grade 4 drug related AE was an increase in aspartate aminotransferase reported the day following the final dose of SLC-0111. This particular AE was associated with hepatobiliary disorder (see below) and was attributable to the study drug. Since this event occurred after the patient had stopped the study drug, it was not considered a DLT.
TABLE 3.
Treatment-related Adverse Events, All Grades (Occurring in ≥10% of Patients) and Grades 3-4 (Occurring in ≥1 Patient)
Three SAEs were recorded across the entire study group. These SAEs included grade 3 pulmonary embolism in one patient in cohort 2, grade 3 entercolitis secondary to clostridium difficile in 1 patient in cohort 3 and grade 3 hepatobiliary disorder with other elevated liver enzymes in one patient in cohort 3 (Table 3). Only the hepatobiliary disorder was considered to be an SAE related to the study drug and none of the SAEs was considered a DLT.
PK Analyses
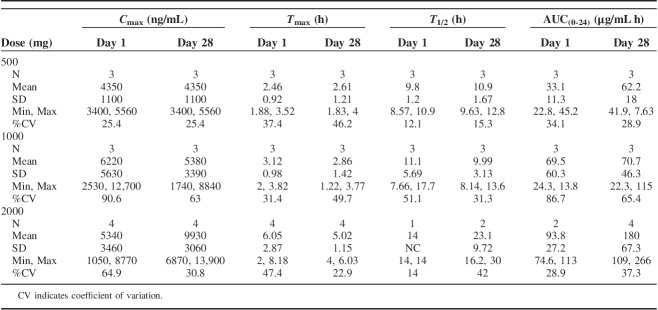
PK analyses were carried out on 10 DLT evaluable patients who were administered SLC-0111 daily for 28 days. Following a single dose of SLC-0111, mean Cmax values were 4350, 6220, and 5340 ng/mL for the 500, 1000, and 2000 mg cohorts, respectively (Fig. 1, Table 4). The corresponding mean AUC(0-24) values at each dose were 33, 70, and 94 μg/mL h, suggesting that, while interindividual variability in PK parameters was evident, similar levels of drug exposure were observed at the 1000 and 2000 mg doses. Mean Tmax and T1/2 values of SLC-0111 were similar after single and repeated dosing, with Tmax values ranging from 2.46 to 6.05 hours on Day 1 and 2.61 to 5.02 hours on Day 28 (Fig. 1 and Table 4). Day 28/Day 1 Cmax±SD ratios were 1.32±0.298, 1.17±1.02 and 3.70±4.30 and AUC(0-24) ratios were 2.01±0.879, 1.47±1.42, and 2.18±0.247 following doses of 500, 1000, and 2000 mg/d, respectively, indicating accumulation of SLC-0111 with repeated dosing (Fig. 1, Table 4).
FIGURE 1.
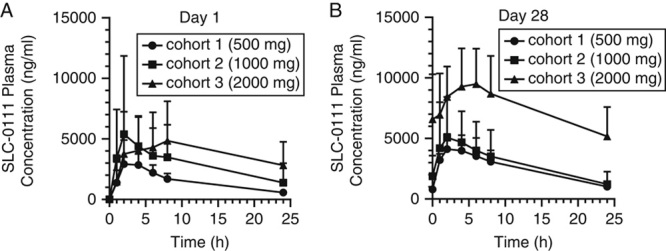
Plasma concentrations of SLC-0111 in patients after a single dose (A) and after repeated dosing for 1 cycle of 28 days (B). Data show the mean±SD for each dose cohort.
TABLE 4.
Summary of Plasma SLC-0111 Pharmacokinetic Parameters on Cycle 1, Days 1 and 28
Power-law model analysis of Cmax and AUC(0-24) on days 1 and 28 resulted in slopes that varied significantly from 1.0 (see Table S2, Supplemental Digital Content 1, http://links.lww.com/AJCO/A329, which summarizes data showing dose proportionality and PK linearity of SLC-0111), indicating that exposure to SLC-0111 in these patients was generally dose proportional.
Tumor Response
Although no objective responses were observed during the course of the study, prolonged SD of >24 weeks was observed in 2 patients (Fig. 2). In cohort 1, one patient diagnosed with sarcoma of spine and who had PD following 2 prior lines of chemotherapy (first line, cyclophosphamide, adriamycin, vincristine; second line, ifofosfamide, etoposide) maintained SD for 10 cycles of SLC-0111 administration. In cohort 3, one patient diagnosed with colorectal cancer and who had PD following 4 lines of chemotherapy (first line, folinic acid, irinotecan, fluororacil, bevacizumab; second line, folinic acid, irinotecan, fluororacil; third line, folinic acid, irinotecan, oxaliplatin; fourth line, regorafenib) before enrollment in the study, exhibited SD up to cycle 7 of SLC-0111 administration.
FIGURE 2.
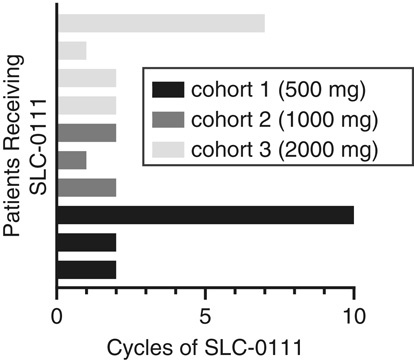
Period of stable disease observed in DLT evaluable patients administered SLC-0111. Each bar represents 1 patient and the period is measured as the number of 28 day cycles during which each patient maintained stable disease. DLT indicates dose-limiting toxicities.
DISCUSSION
The data presented in this study demonstrate that SLC-0111 was generally well tolerated at doses of 1000 mg daily or below, however, taste alterations at 2000 mg led to frequent early discontinuation. Most drug-related AEs were grade 1 or 2 in severity and the most frequently reported AEs were fatigue, nausea and anorexia, all of which were reversible. Although the maximum tolerated dose of SLC-0111 was not established, the poor tolerability of the 2000 mg dosing level was reflected in the severity of AEs experienced by subjects in this dose cohort. The cohort dosed at 1000 mg exhibited a more favorable safety profile than the 2000 mg cohort with respect to incidence of drug-related AEs ≥ grade 3, the incidence of select AEs such as vomiting, and the incidence of AEs leading to patient withdrawal from the study. Furthermore, the majority of patients enrolled in the 2000-mg dose cohort withdrew consent due to issues related to taste alterations of SLC-0111, indicating that the current formulation of the drug will need to be improved to enhance taste and convenience for patients.
On the basis of PK analyses both the 1000 and 2000 mg doses achieved similar levels of drug exposure following single doses of SLC-0111. Furthermore, these doses of SLC-0111 reached similar Tmax and T1/2 values after both single (Day 1) and multiple doses (Day 28) and exhibited modest accumulation following repeated, consecutive daily doses for 28 days. Preclinical studies have demonstrated efficacy at doses ranging from 25 to 100 mg/kg,10,11,18 which, based on a conversion factor for mice of 12.3×60, is approximately equivalent on a body surface area basis to 120 to 480 mg in human patients, which is below the doses tested in the current study. Collectively, the data showing that SLC-0111 was better tolerated at the 1000-mg dose compared with the 2000-mg dose, together with data showing that the PK parameters are similar at these doses, support 1000 mg/d as the recommended phase II dose (RP2D) for SLC-0111.
It is noteworthy 2 patients who exhibited PD after treatment with several prior chemotherapy regimens showed a period of SD >24 weeks following administration of SLC-0111. The presence of SD for a prolonged period of time in at least some patients given SLC-0111, especially as the patient population was heavily pretreated and refractory to multiple therapies, is encouraging and suggests that further clinical development of SLC-0111 is warranted.
As a result of the small sample size of the study, including the presence of only 3 and 4 patients evaluable for DLTs at the 1000 and 2000 mg doses, respectively, the RP2D of 1000 mg should be approached with some caution and it will be important to evaluate safety in a larger cohort of patients treated in future studies. Another limitation is that the pharmacodynamic effects of SLC-0111 were not assessed in serial tumor biopsies. Furthermore, expression of CAIX was not assessed in archival tumor samples. As only patients expressing CAIX in their tumors would be expected to benefit from treatment with SLC-0111, selection of CAIX-positive patients for enrollment in future studies is critical. Along these lines, a phase 1b trial to evaluate SLC-0111 in a defined population of CAIX-positive pancreatic cancer patients is currently ongoing (NCT03450018) and future clinical development will include evaluation of SLC-0111 in combination with chemotherapy in patients with glioblastoma. In addition, the recent demonstration that the combination of SLC-0111 with immune checkpoint blockade resulted in improved efficacy of immune therapy in preclinical models of melanoma and breast cancer20 warrants future investigation of CAIX inhibitors in combination with immune checkpoint inhibitors in the clinical setting.
In conclusion, this is the first clinical trial of a specific CAIX inhibitor in human patients with cancer. SLC-0111 was found to be safe in patients with previously treated, advanced solid tumors at doses up to 1000 mg. Collectively, data on the tolerability and PK of SLC-0111 support 1000 mg/d as the RP2D for the current formulation of SLC-0111.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.amjclinicaloncology.com.
ACKNOWLEDGMENTS
The authors thank the patients, their families and caregivers for participating in the study.
Footnotes
Support for the trial was provided by SignalChem Lifesciences Inc and Welichem Biotechnology Inc. This work was supported by grants to SD from the Canadian Cancer Society Research Institute (CCSRI grant # 703191) and the Canadian Institutes of Health Research (CIHR grant # FDN-143318).
S.C., P.L.B., Q.C., M.L., P.C.M., D.J.R, and S.D.: conception and design. P.C.M., M.L., L.T., M.S., Z.Z., C.T.S., and S.D.: development of materials and methodology. S.C., P.L.B., Q.C., and D.J.R.: acquisition of data. P.C.M., S.C., P.L.B., Q.C., M.S., D.J.R., and S.D.: analysis and interpretation of data. P.C.M., P.L.B, Q.C., M.L., D.J.R., and S.D.: writing, and/or revision of the manuscript. M.S.: administrative, technical, or material support (ie, reporting or organizing data). S.C., P.L.B., Q.C., M.L., M.S., D.J.R., and S.D.: study supervision.
Disclosures: P.C.M., C.T.S., and S.D. are inventors of SLC-0111. M.L and L.T. are currently employees of Welichem Biotechnology Inc. Z.Z. and M.S. were employees of SignalChem Lifesciences Inc. during the study. Z.Z. is currently an employee of SignalChem Lifesciences Inc. P.L.B. reports grants from Seattle Genetics during the conduct of the study; grants and other from BristolMyersSquibb, grants and other from Sanofi, grants and other from Genentech/Roche, grants from Novartis, grants from GlaxoSmithKline, grants from Nektar Therapeutics, grants from Merck, grants from Lilly, grants from Servier, grants from PTC Therapeutics, other from Pfizer, outside the submitted work; and Past Chair, Investigational New Drug Committee, Canadian Clinical Trials Group; Executive Board Member, Breast International Group; Steering Committee Member, American Association for Cancer Research Project GENIE; Member, NCI-BIO Breast Cancer Immunotherapy Task Force. The other authors declare no conflicts of interest.
REFERENCES
- 1.Nakazawa MS, Keith B, Simon MC. Oxygen availability and metabolic adaptations. Nat Rev Cancer. 2016;16:663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillies RJ, Brown JS, Anderson ARA, et al. Eco-evolutionary causes and consequences of temporal changes in intratumoural blood flow. Nat Rev Cancer. 2018;18:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie H, Simon MC. Oxygen availability and metabolic reprogramming in cancer. J Biol Chem. 2017;292:16825–16832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parks SK, Chiche J, Pouyssegur J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat Rev Cancer. 2013;13:611–623. [DOI] [PubMed] [Google Scholar]
- 5.Corbet C, Feron O. Tumour acidosis: from the passenger to the driver's seat. Nat Rev Cancer. 2017;17:577–593. [DOI] [PubMed] [Google Scholar]
- 6.Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov. 2011;10:767–777. [DOI] [PubMed] [Google Scholar]
- 7.McDonald PC, Winum JY, Supuran CT, et al. Recent developments in targeting carbonic anhydrase IX for cancer therapeutics. Oncotarget. 2012;3:84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pastorekova S, Gillies RJ. The role of carbonic anhydrase IX in cancer development: links to hypoxia, acidosis, and beyond. Cancer Metastasis Rev. 2019;38:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiche J, Ilc K, Laferriere J, et al. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 2009;69:358–368. [DOI] [PubMed] [Google Scholar]
- 10.Lou Y, McDonald PC, Oloumi A, et al. Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res. 2011;71:3364–3376. [DOI] [PubMed] [Google Scholar]
- 11.Lock FE, McDonald PC, Lou Y, et al. Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene. 2013;32:5210–5219. [DOI] [PubMed] [Google Scholar]
- 12.McDonald PC, Chafe SC, Brown WS, et al. Regulation of pH by carbonic anhydrase 9 mediates survival of pancreatic cancer cells with activated KRAS in response to hypoxia. Gastroenterology. 2019;157:823–837. [DOI] [PubMed] [Google Scholar]
- 13.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393–410. [DOI] [PubMed] [Google Scholar]
- 14.Supuran CT, Alterio V, Di Fiore A, et al. Inhibition of carbonic anhydrase IX targets primary tumors, metastases, and cancer stem cells: Three for the price of one. Med Res Rev. 2018;38:1799–1836. [DOI] [PubMed] [Google Scholar]
- 15.Pacchiano F, Carta F, McDonald PC, et al. Ureido-substituted benzenesulfonamides potently inhibit carbonic anhydrase IX and show antimetastatic activity in a model of breast cancer metastasis. J Med Chem. 2011;54:1896–1902. [DOI] [PubMed] [Google Scholar]
- 16.Hedlund EE, McDonald PC, Nemirovsky O, et al. Harnessing induced essentiality: targeting carbonic anhydrase IX and angiogenesis reduces lung metastasis of triple negative breast cancer xenografts. Cancers (Basel). 2019;11:1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chafe SC, Lou Y, Sceneay J, et al. Carbonic anhydrase IX promotes myeloid-derived suppressor cell mobilization and establishment of a metastatic niche by stimulating G-CSF production. Cancer Res. 2015;75:996–1008. [DOI] [PubMed] [Google Scholar]
- 18.Bozdag M, Carta F, Ceruso M, et al. Discovery of 4-Hydroxy-3-(3-(phenylureido)benzenesulfonamides as SLC-0111 analogues for the treatment of hypoxic tumors overexpressing carbonic anhydrase IX. J Med Chem. 2018;61:6328–6338. [DOI] [PubMed] [Google Scholar]
- 19.Boyd NH, Walker K, Fried J, et al. Addition of carbonic anhydrase 9 inhibitor SLC-0111 to temozolomide treatment delays glioblastoma growth in vivo. JCI Insight. 2017;2:e92928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chafe SC, McDonald PC, Saberi S, et al. Targeting hypoxia-induced carbonic anhydrase IX enhances immune-checkpoint blockade locally and systemically. Cancer Immunol Res. 2019;7:1064–1078. [DOI] [PubMed] [Google Scholar]
- 21.Chamie K, Donin NM, Klopfer P, et al. Adjuvant weekly girentuximab following nephrectomy for high-risk renal cell carcinoma: the ARISER randomized clinical trial. JAMA Oncol. 2017;3:913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.amjclinicaloncology.com.