Abstract
Background:
Youth whose parents have alcohol use disorder (AUD) are at higher risk for earlier initiation and greater magnitude of alcohol use, and have a higher likelihood of developing an AUD than their peers without parental history of AUD. This increased risk may be partly attributable to altered development of inhibitory control and related neural circuitry. This study examined neural activation during a motor response inhibition Stop Signal Task (SST) in substance-naïve youth aged 9 to 10 years with and without parental family history of AUD.
Methods:
Baseline cross-sectional survey and functional magnetic resonance imaging (fMRI) data was drawn from 6,898 youth in the US-based Adolescent Brain Cognitive Development Study. Generalized additive mixed models were conducted to examine the association between maternal, paternal, and parental (both mother and father) family history of AUD with neural activation during successful and failed response inhibition. Family history interactions with sex and stratification by ethnicity were explored.
Results:
Of 6,898 participants, 951 (14%) were family history positive for parental AUD. Paternal history of AUD was associated with greater activation for successful inhibition in the right medial orbital frontal gyrus, compared to youth with no family history. Maternal history of AUD was associated with greater activation for failed response inhibition among females in the cerebellum, compared to females with no such history. Parental history (both mother and father) of AUD was associated with greater activation during successful inhibition in the left paracentral gyri and left superior parietal lobule. Maternal and parental history of AUD findings were accounted for by a family history of substance use disorder in general. All effect sizes were relatively small.
Conclusions:
Substance-naïve children with a parental family history of AUD exhibit greater neural activation in some regions of the fronto-basal ganglia and cerebellar networks when they successfully or unsuccessfully inhibit a response as compared to children with no such family history. This unique neural response pattern could reflect a compensatory response and may represent an inherent neurobiological vulnerability to risk-related behaviors in these youth which will be examined in future longitudinal analyses of this cohort.
Keywords: response inhibition, functional magnetic resonance imaging, alcohol use disorder, family history, stop signal task
Introduction
Late childhood is a vulnerable developmental period characterized by significant neural and cognitive changes (Crews et al., 2007, Spear, 2013). Important morphometric restructuring and functional neuromaturation continue in parallel throughout this period (Tamnes et al., 2017). The resulting increased neural efficiency (de Graaf-Peters and Hadders-Algra, 2006) is thought to improve cognition, such as executive functions (Casey et al., 2005). Inhibitory control is one component of higher-order executive functions (Giedd et al., 1999, Gogtay et al., 2004). It is subserved by neural circuitry in the fronto-basal ganglia network (Lopez-Caneda et al., 2014, Koyama et al., 2017), which includes the prefrontal (PFC) and inferior frontal cortices (IFC), the pre-supplementary motor area, basal ganglia, and primary motor cortex (Aron, 2011). Typically developing children exhibit progressive reductions in neural network activation across the medial and lateral parts of the PFC and age-related increases in the IFC and insula that are associated with improved inhibitory control performance (Casey et al., 1997, Somerville et al., 2011, Tamm et al., 2002). Therefore, functional differences in fronto-basal ganglia network development may be related to an inherent neurobiological vulnerability in the cognitive control network and thus difficulty suppressing maladaptive behaviors. This in turn may promote risky actions, such as excessive alcohol use (Casey et al., 2008, Steinberg, 2010, Shulman et al., 2016).
The onset of alcohol use typically occurs during adolescence when the brain continues to undergo critical development. In the United States (US), 24% of high school students have consumed alcohol (more than just a few sips) by age 14, and 59% have done so by age 18 (Johnston et al., 2019). Of particular concern, approximately 4% and 14% of US high school students aged 14 and 18 years, respectively, have engaged in binge drinking (i.e., the consumption of 5+ drinks in a row) in the past 2 weeks (Johnston et al., 2019). These statistics are concerning because excessive alcohol use during adolescence is associated with a myriad of negative consequences including alcohol and substance use disorders (AUD, SUD; Dwyer-Lindgren et al., 2018), and other mental health problems (Teesson et al., 2010, Welsh et al., 2017, Pompili et al., 2010). Alcohol use during adolescence has also been associated with alterations in brain structure and function, including aberrant activation patterns during response inhibition tasks (for review, see Squeglia and Gray, 2016, Squeglia and Cservenka, 2017, Lees et al., 2019, Lees et al., in press), as well as poorer test performance across cognitive domains, with executive functions and memory being the most vulnerable (Scott et al., 2018, Gould, 2010, Lees et al., 2019). Recent longitudinal neuroimaging studies have begun investigating, and have shown that, underlying neural vulnerabilities of response inhibition in substance-naïve children appear to contribute to earlier initiation and problematic progression of alcohol use during adolescence (Squeglia and Cservenka, 2017).
Vulnerability for early alcohol initiation is heightened among individuals with a positive family history (FH+) of AUD, particularly among those with a FH+ first degree relative (Dawson et al., 1992). FH+ has been associated with earlier initiation and greater magnitude of alcohol use, a three to five fold increased likelihood of developing an AUD (Cotton, 1979, McCaul et al., 1990), and a heightened likelihood of experiencing alcohol-related problems in adolescence (Lieb et al., 2002), when compared to family history negative (FH−) youth. FH+ risk is thought to be driven at least partially by deficits in motor response inhibition (Sher et al., 2005, Tarter et al., 2003). Emerging research has aimed to uncover the neurobiological markers that may increase risk for early alcohol use and AUD in FH+ youth aged between 8 and 19 years who are largely substance-naïve (Squeglia and Cservenka, 2017). Preliminary functional magnetic resonance imaging (fMRI) research, utilizing the Go/NoGo Task, suggests that FH+ youth may have altered development of the fronto-basal ganglia network. Some studies have reported reduced activation among FH+ youth in the right ventral and lateral parts of the PFC (Koyama et al., 2017), and the fronto-parietal regions (Schweinsburg et al., 2004), while others have reported greater activation among FH+ youth in the ventral caudate (Heitzeg et al., 2010), and frontal regions (Acheson et al., 2014b), when compared to FH− youth. Furthermore, longitudinal research has suggested that alcohol-naïve FH+ youth show increasing anterior cingulate activity over time, while their FH− peers showed the expected reduction in fronto-striatal response to the Go/NoGo Task (Hardee et al., 2014). These fMRI studies highlight that FH+ youth consistently show altered brain activity during response inhibition tasks compared to their FH− peers.
To date, motor response inhibition fMRI studies examining FH+ substance-naïve youth have been restricted to small sample sizes with mostly Caucasian adolescents. This has not allowed for adequate examination of response inhibition as related to: (1) the mother and father’s independent heritable influence of AUD history on their child’s neurobiological development or (2) variability as related to demographic and genetic differences, such as race/ethnicity and sex. Previous neuropsychological research has reported differential effects of paternal and maternal AUD on offspring cognition and impulsivity (Ozkaragoz et al., 1997, Corte and Becherer, 2007), yet how this translates to neural activation during response inhibition remains unknown. Furthermore, differences in alcohol use behaviors by race/ethnicity and sex have been documented (Flewelling et al., 2004, Johnston et al., 2019), however comparisons of these groups on neurobiological predictors of alcohol use remains limited. More nuanced understanding of neural functioning in FH+ (mother, father, both parents) individuals from diverse backgrounds will advance our understanding of the underlying biological vulnerabilities to alcohol-related problems and inform early intervention strategies.
In the present study, we sought to examine neural activation to a motor response inhibition Stop Signal Task in parental FH+/− substance-naïve youth aged 9–10 years enrolled in the Adolescent Brain Cognitive Development (ABCD) study (Garavan et al., 2018, Auchter et al., 2018, Volkow et al., 2018). The Stop Signal paradigm probes neural networks reflective of motor response inhibition, similar to the Go/NoGo Task, but requires greater inhibitory control (Nee et al., 2007). We investigated maternal, paternal, and parental (both mother and father) FH of AUD. By drawing on the large ABCD dataset we were also able to explore differential ethnicity effects and sex interactions, and adjust for in utero exposure to alcohol for the first time. It was hypothesized that FH+ youth would show altered activity in regions known to be involved in SST performance, particularly in fronto-basal ganglia network regions (Cieslik et al., 2015), when compared to FH− youth. We also anticipated that youth with two parents reporting a history of AUD would show greater genetic liability and thus increased vulnerability resulting in greater neural response deviation than youth with one FH+ parent or FH− youth (Khemiri et al., 2019).
Materials and Methods
This study used baseline cross-sectional data from the ABCD Data Release 2.0.1. The ABCD Study is a 10-year longitudinal study across twenty-one US-based sites, recruiting 11,878 participants, and funded by the National Institutes of Health (Volkow et al., 2018). A total of 6,898 participants had valid parental history, demographic, and fMRI SST data following quality control processing (Figure 1). The Institutional Review Board at the University of California, San Diego, approved all aspects of this study for the ABCD consortium.
Figure 1:
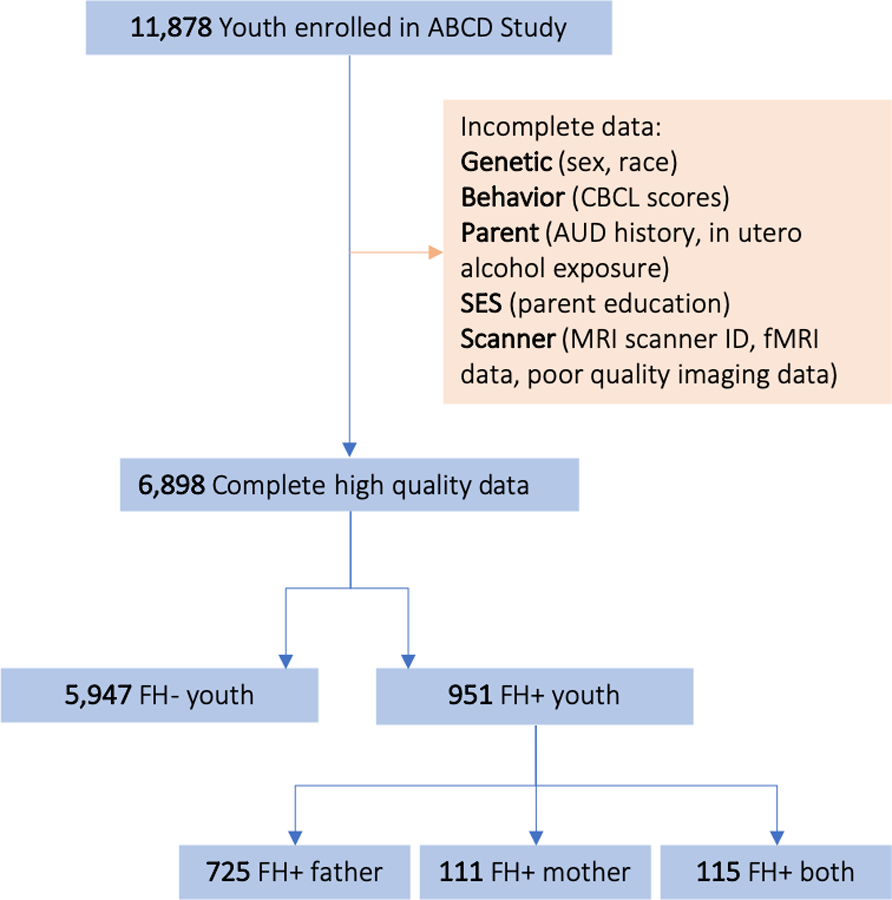
Selection of the ABCD cohort for analyses.
Recruitment.
A detailed account of the recruitment strategy has been previously published (Garavan et al., 2018). The ABCD study primarily utilized a probability sample recruited through schools, where school selection was based on sex, race and ethnicity, socioeconomic status, and urbanicity. Interested participants and their families completed a brief eligibility interview over the phone to ensure interested youth were 9–10 years old and had no MRI contraindications (i.e., irremovable metal in body).
Procedure.
Youth and their parent/guardian presented for study session(s) at their local research site to complete the baseline visit. Parents/guardians provided written consent while the child provided written assent. Youth and their parent/guardian were in separate, private rooms during study participation to maintain confidentiality of their survey responses. The baseline measures included questionnaires, neurocognitive testing, biological samples and an MRI scan (Luciana et al., 2018, Lisdahl et al., 2018, Uban et al., 2018). Study assessment was completed over an 8-hour research session (or two 4-hour sessions). Parents and youth were compensated financially and with prizes for their time.
Family history of alcohol use disorders.
Full descriptions of ABCD environmental, health, and mental health questionnaires are described elsewhere (Barch et al., 2018, Zucker et al., 2018, Lisdahl et al., 2018). The parent/guardian completed a 15-minute modified version of the Family History Assessment Module Screener (FHAM-S; Rice et al., 1995). Parents/guardians reported on the presence or absence of a range of mental health symptoms including those associated with AUD in all first and second degree blood relatives of the youth, including biological siblings, parents, grandparents, aunts, and uncles. Only parental history of alcohol and drug use problems were of interest for the current study (Cservenka, 2016).
Covariates.
Covariates were chosen based on prior evidence of an association with the outcomes (Table 1). Standard ABCD demographic covariates included race, age, sex, and parental education. Substance-related covariates included parental retrospective report of maternal alcohol use during pregnancy (yes/no), as reported in the modified Developmental History Questionnaire (Kessler et al., 2009a, Kessler et al., 2009b, Merikangas et al., 2009). Youth emotional and behavioral covariates included internalizing, externalizing, and total behavioral problems from the Child Behavior Checklist (CBCL; Achenbach and Rescorla, 2013).
Table 1:
Demographic characteristics of sample at baseline, N=6,898.
| FH− n=5947 |
FH+ n=951 |
|||
|---|---|---|---|---|
| Father n=725 |
Mother n=111 |
Both n=115 |
||
| Age (mean [SD]) | 9.9 (0.6) | 9.9 (0.6) | 9.9 (0.6) | 9.9 (0.6) |
| Race (%)acd | ||||
| White | 55.7 | 53.2 | 54.1 | 50.4 |
| Hispanic | 19.6 | 23.2 | 16.2 | 11.3 |
| Black | 13.3 | 11.2 | 17.1 | 13.0 |
| Asian | 2.0 | 0.4 | 0.0 | 0.9 |
| Other | 9.4 | 12.0 | 12.6 | 24.4 |
| Sex (%) | ||||
| Female | 48.4 | 50.3 | 50.5 | 45.2 |
| Male | 51.6 | 49.7 | 49.6 | 54.8 |
| Highest Parent Education (%)abcdf | ||||
| <HS Diploma | 5.0 | 5.2 | 9.0 | 7.0 |
| HS Dip/GED | 10.0 | 11.5 | 9.9 | 18.3 |
| Some College | 27.8 | 40.0 | 40.5 | 43.5 |
| Bachelor | 30.8 | 25.0 | 24.3 | 16.5 |
| Post Grad | 26.4 | 17.9 | 16.2 | 14.8 |
| In utero alcohol exposure (%)abcdef | ||||
| Not exposed | 76.0 | 67.6 | 61.3 | 39.1 |
| Exposed | 24.0 | 32.4 | 38.7 | 60.9 |
| FH of SUD (%)abcdef | ||||
| FH− | 95.3 | 63.5 | 64.0 | 41.7 |
| FH+ Father | 3.5 | 31.7 | 8.1 | 11.3 |
| FH+ Mother | 0.7 | 1.2 | 21.6 | 5.2 |
| FH+ Both | 0.5 | 3.4 | 6.3 | 41.7 |
| Internalizing (T-score [SD])acde | 47.8 (10.4) | 50.7 (10.8) | 48.8 (11.7) | 52.6 (11.6) |
| Externalizing (T-score [SD])abcdef | 44.8 (9.8) | 47.4 (10.4) | 46.4 (10.8) | 50.7 (11.4) |
| Total problems (T-score [SD])acdef | 44.9 (10.9) | 47.8 (11.4) | 46.3 (11.7) | 51.2 (12.8) |
No significant group differences between youth of FH+ mothers vs FH+ fathers.
FH− youth ≠ FH+ youth, p < .05
FH− youth ≠ youth of FH+ mothers, p < .05
FH− youth ≠ youth of FH+ fathers, p < .05
FH− youth ≠ youth of FH+ both parents, p < .05
Youth of FH+ mothers ≠ youth of FH+ both parents, p < .05
Youth of FH+ fathers ≠ youth of FH+ both parents, p < .05
fMRI Stop Signal Task.
Full task information has been described previously (Casey et al., 2018). The SST measures domains of motor response inhibition and impulsivity, showing child/adolescent-specific and substance use effects (Whelan et al., 2012, Smith et al., 2014). The Stop Signal Task (SST) requires participants to withhold or interrupt a motor response to a “Go” stimulus when it is followed unpredictably by a signal to stop. Participants completed two sets, each containing 180 trials. Each trial began with the presentation of a leftward or rightward pointing arrow and participants were instructed to indicate the direction, responding quickly and accurately via a two-button response panel. Thirty of the trials were “Stop” trials where the leftward or rightward facing arrow was followed by an up-right arrow, indicating to participants to stop their prepotent “Go” response. To ensure that there were approximately 50% successful and 50% unsuccessful inhibition trials for Stop trials, a tracking algorithm varied the interval of trials (see Casey et al., 2018 for further details). Mean beta weights for correct stop contrasts (correct stop contrasted with correct go) and failed stop contrasts (incorrect stop contrasted with correct go) were used in all analyses to compare neural response differences to both successful and unsuccessful inhibition trials.
Imaging Data Acquisition and Processing.
Full MRI and fMRI acquisition and scanning parameters are described elsewhere (Casey et al., 2018). All scans were uploaded to a shared server that were processed by the Data Analytics and Information Core (DAIC) of ABCD, to maintain consistency across methodology and ensure quality, with details on processing described by Hagler et al. (2018). All parcellations based on the Desikan-Killiany Atlas were examined (68 cortical and 30 subcortical regions). Only participants whose SST task scans met all quality checks by DAIC were used in analyses. There was no significant difference in the number of FH− (81%) and FH+ youth (80%) who provided useable, high quality SST fMRI data.
Data Analysis.
Differences in demographic and behavioral variables between the FH+/− for AUD (none, father, mother, both parents) were determined using χ2-tests for categorical data and ANOVAs for continuous variables. First, a series of generalized additive mixed models (GAMM) were conducted to examine the association between parental FH+/− and neural activation during correct stop and failed stop contrasts in the SST task, using the GAMM4 package in R, version 3.5.3. Covariates included race/ethnicity, age, sex, parental education, in utero alcohol exposure, total emotional/behavioral problems, externalizing symptoms, and internalizing symptoms, as well as nesting of subjects by scanner. Participants with missing data for any of these variables were excluded from analyses. This series of models were then repeated to examine family history x sex interactions. Next, a series of main effect and sex interaction GAMMs were conducted for significant regions and contrasts, with participants grouped by race/ethnicity to account for the heterogeneity of socioeconomic and social covariates (e.g., income, youth education) within ethnic groups across FH+/− (i.e., FH− vs. FH+ for Asian, Black, Hispanic, White). All covariates, besides race/ethnicity, were included in this pass. Finally, sensitivity analyses were conducted to determine whether results were specific to parental FH of AUD or whether they were influenced by other substance use problems. Analyses described above were repeated for significant brain regions with parental FH of SUD included as an additional covariate. In all analyses, the false discovery rate (FDR) was used to correct for multiple comparisons and the adjusted p-values are reported (Benjamini and Hochberg, 1995, Benjamini and Yekutieli, 2001).
Results
Participant characteristics
Demographic characteristics of FH− and FH+ youth are provided in Table 1. Of 6,898 participants, 951 youth (13.8%) were FH+ for parental AUD; 725 youth had a FH+ father, 111 had a FH+ mother, and 115 had two FH+ parents. In terms of parent-reported in utero alcohol exposure, 24.0% of FH−, and 32.4%, 38.7% and 60.9% of youth with a FH+ father, mother, and both parents were exposed, respectively. There were relatively high rates of co-occurring alcohol and substance use problems among parents: 31.7%, 21.6% and 41.7% of FH+ AUD father-only, mother-only, and both parents also reported a SUD history, respectively. Youth with two FH+ parents had the highest internalizing, externalizing, and total problems on the CBCL, compared to youth with one FH+ parent or FH− youth. Participants in all groups scored similarly on SST performance, besides youth with two FH+ parents who had a lower correct go rate than other groups.
Between-group family history findings
Table 3 and Figure 3 presents brain regions exhibiting significantly greater activation for correct stop and failed stop contrasts among FH+ compared to FH− youth, when controlling for relevant covariates. Children with FH+ mothers had greater activation for failed stop contrasts in the right cerebellum compared to FH− youth (R2 = .003). FH+ fathers had greater neural activation for correct stop contrasts in the right medial orbital frontal cortex compared to FH− youth (R2 = .001). For children with two FH+ parents, greater neural activation for correct stop contrasts were observed in the left paracentral lobule (R2 = .002) and left superior parietal lobule (R2 = .048; Figure 2). No significant between-group differences were observed for any other ROI. A family history x sex interaction was observed in the right cerebellum (B = 0.288, S.E. = 0.055, p < .001, R2 = .003). Female youth of FH+ mothers exhibited significantly greater activation than FH− female youth for the failed stop contrast (p<.001), while no significant difference was observed between male youth of FH+ mothers compared to FH− parents in the cerebellum. No other significant interactions were observed. The results for FH+ fathers remained significant during sensitivity analyses when FH of SUD was included as an additional covariate, however the results for FH+ mothers (main effect and family history x sex interaction) and two FH+ parents were no longer significant (Supplement Table 1).
Table 3:
Brain regions exhibiting greater activation in FH+ compared to FH− youth for the correct stop vs. correct go contrast, after controlling for relevant covariates. There were no significant differences between groups for the incorrect stop vs. correct go contrast. N=6,898.
| FH+ Parent | Contrast | Region | B (SE) | p | R2 | AIC | BIC |
|---|---|---|---|---|---|---|---|
| Mother | Failed stop vs correct go | Cerebellum, R | 0.288 (.055) | <.001 | .0033 | 7234.6 | 7371.4 |
| Father | Correct stop vs correct go | Medial orbital frontal, R | 0.083 (.025) | .034 | .0013 | 13007.9 | 13144.7 |
| Both | Correct stop vs correct go | Paracentral, L | 0.058 (.017) | .029 | .0016 | −4262.3 | −4125.6 |
| Superior parietal, L | 0.063 (.019) | .048 | .0019 | −2518.8 | −2382.0 |
L: left; R: right. Only regions where the model passed the FDR correction are presented.
Figure 3:
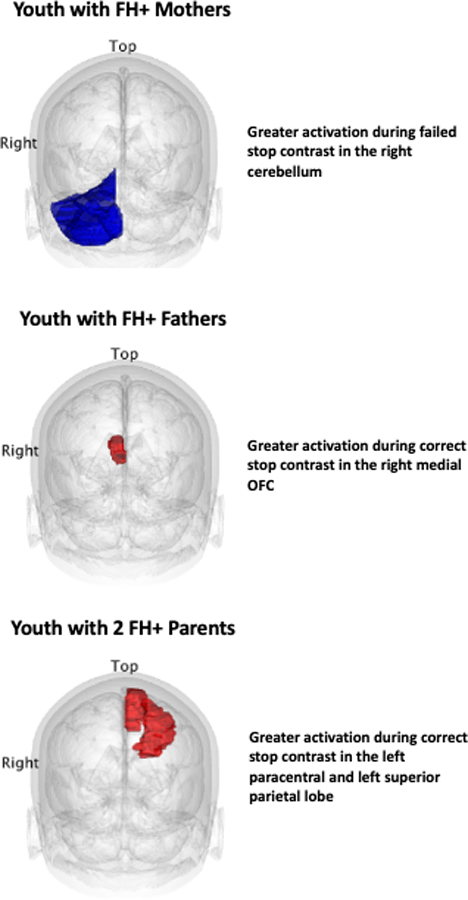
Summary of brain parcellations exhibiting significantly greater activation in FH+ vs. FH− youth. Blue = greater activation among FH+ vs. FH− youth during failed stop contrast. Red = greater activation among FH+ vs. FH− youth during correct stop contrast. OFC = orbitofrontal cortex.
Figure 2:
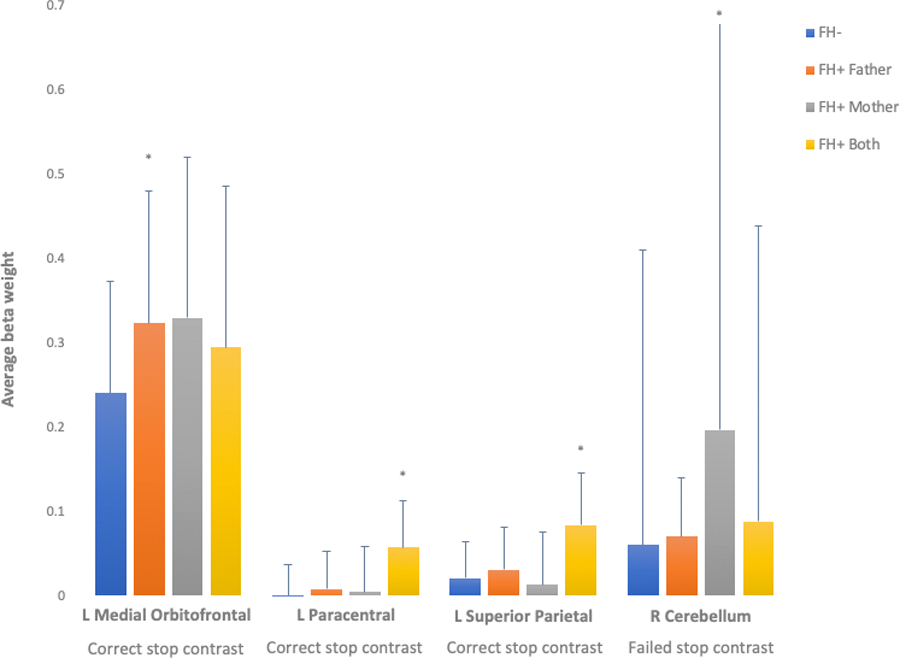
Significantly greater neural activation for correct stop contrast (correct stop vs. correct go) observed in youth with fathers or both parents exhibiting a history of alcohol use problems, compared to FH− youth. Significantly greater neural activation for failed stop contrast (failed stop vs. correct go) observed in youth with mothers exhibiting a history of alcohol use problems, compared to FH− youth. n=6,898. * = p(FDR) < .05.
Ethnicity findings
Demographic characteristics for each ethnic group are provided in Supplement Table 2. Between-group analyses for all regions that showed significant group differences for correct stop and failed stop contrasts were rerun separately for each ethnic group, as summarized in Table 4. When controlling for relevant covariates, Hispanic youth with FH+ mothers exhibited significantly greater activation for failed stop contrasts in the right cerebellum compared to FH− Hispanic youth (R2 = .034). Hispanic youth with FH+ fathers exhibited significantly greater activation for correct stop contrasts in the right medial orbital frontal lobe compared to FH− Hispanic youth (R2 = .009). White youth with two FH+ parents exhibited greater activation in the left paracentral lobule (R2 = .001) and left superior parietal lobule (R2 = .003), when compared to White FH− youth for correct stop contrasts.
Table 4:
Brain regions exhibiting greater activation in FH+ compared to FH− youth for the correct stop vs. correct go contrast when ethnicity groups were analysed separately, after controlling for relevant covariates. N=6,898.
| Asian N=122 |
Black N=908 |
Hispanic N=1363 |
White N=3819 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FH+ Parent | Contrast | Region | B (SE) | p | B (SE) | p | B (SE) | p | B (SE) | p |
| Mother | Failed stop vs correct go | Cerebellum, R | NA | −0.108 (.109) | 1.000 | 0.928 (.132) | <.001 | −0.003 (.042) | 1.000 | |
| Father | Correct stop vs correct go | Medial orbital frontal, R | −0.074 (.232) | 1.000 | 0.187 (.118) | .339 | 0.181 (.048) | <.001 | 0.056 (.024) | .083 |
| Both | Correct stop vs correct go | Paracentral, L | −0.111 (.142) | 1.000 | 0.061 (.060) | .937 | 0.064 (.057) | .810 | 0.052 (.020) | .023 |
| Superior parietal, L | −0.094 (.172) | 1.000 | 0.086 (.070) | .674 | 0.078 (.064) | .665 | 0.063 (.023) | .018 | ||
L: left; R: right. Reference group for analyses was FH− youth who identified as one of the four ethnicities. Youth identifying as ‘other’ race/ethnicity were not included in these analyses. FDR-corrected p-values are presented. NA= fixed-effect model matrix is rank deficient. N=total number of participants for each racial category.
FH+ mother x sex interactions were driven by Hispanic families: female Hispanic youth with FH+ mothers exhibited significantly greater activation than FH− female Hispanic youth in the right cerebellum (p <.001) for failed stop contrasts. No significant differences were observed among FH+ and FH− male Hispanic youth in the cerebellum, or for any other ethnicity. During sensitivity analyses when FH of SUD was included as an additional covariate, the results remained significant for Hispanic families (main effects and FH x sex interaction), while the results did not remain significant for White families (Supplement Table 3). No significant between-group differences were observed for Asian or Black youth.
Discussion
Leveraging a large multi-site US sample, this study compared neural response in substance-naïve youth aged 9 to 10 years with and without parental FH of AUD during a response inhibition task. Overall, substance-naïve youth with a FH+ mother had significantly greater neural activation for the failed stop contrast in the right cerebellum compared to FH− youth. Exploration of sex interactions demonstrated that this effect was driven by activation differences in young females. Youth with a FH+ father had significantly greater neural response as compared to FH− youth for the successful stop contrast in the right medial orbital frontal gyrus. Youth with two FH+ parents demonstrated greater neural response for the successful stop contrast in the left paracentral and left superior parietal lobule compared to FH− youth. Sensitivity analyses demonstrated activation differences observed among youth with FH+ mothers or two FH+ parents were accounted for by FH of SUD effects. Neural activation profiles differed for each ethnic group; greater response for female youth with FH+ mothers and greater response for youth with FH+ fathers were driven by Hispanic families while greater response for youth with two FH+ parents was driven by White families. Only effects observed among Hispanic families were robust to inclusion of FH of SUD as an additional covariate. Between-group effect sizes were very small.
Our findings align with previous research and suggest that greater activation in FH+ youth aged 9 to 10 years occur in some regions of the fronto-basal ganglia network (i.e., PFC, supplementary motor area) during successful response inhibition (Silveri et al., 2011, Acheson et al., 2014a, Acheson et al., 2014b, DeVito et al., 2013). Greater activation was also observed in parts of the default network (left superior parietal lobule), which is involved in diverse cognitive operations, including aspects of attention, visuospatial processing and executive functioning (Koenigs et al., 2009, Johns, 2014). Greater neural activation in the cerebellum during failed response inhibition have been previously reported among FH+ youth (Acheson et al., 2014b). Observed effects were small which is consistent with previous research in this age group (Acheson et al., 2014b). Other studies have reported larger effect sizes (Acheson et al., 2014a) and have also reported increased fronto-basal ganglia network activation during failed response inhibition contrasts for FH+ individuals (Heitzeg et al., 2010, Jamadar et al., 2012). However, these smaller, less diverse study samples included adolescents and adults who had initiated substance use. Therefore, these findings may reflect an altered neural response pattern that is more characteristic of a later stage of neurodevelopment among FH+ individuals.
To the best of our knowledge, no previous study has investigated the association between FH of AUD and neural activation during response inhibition using a family-based design, stratified by race/ethnicity. Drawing on the large ABCD dataset meant we were uniquely positioned to explore these effects, allowing us to better understand phenotypic mechanisms of FH of AUD. We found that both paternal and parental AUD when compared to FH− was associated with greater activation across portions of the fronto-basal ganglia network. This was not observed in youth with a maternal FH of AUD. Interestingly, maternal FH of AUD was associated with activation differences in the cerebellum of young females. Previous studies have reported weaker fronto-cerebellar connectivity in FH+ youth (Cservenka, 2016), and in adults with AUDs (Sullivan et al., 2003). Altered cerebellar activation has been associated with reward processing and risky decision-making in FH+ youth (Cservenka, 2016), although whether these neurofunctional differences increase risk for excessive alcohol use remains unknown. Future prospective investigations of this cohort should explore how connectivity within and between the fronto-basal ganglia and cerebellar networks confers risk to uptake, and escalation, of alcohol use. While we hypothesized that youth with two FH+ parents would show greater deviations than youth with one FH+ parent, our findings suggest that maternal and paternal AUD potentially confer differential risk to offspring neurofunction in the fronto-basal ganglia network. Previous research has reported that paternal AUD is associated with poorer response inhibition, impulsivity and externalizing problems, such as alcohol use, in offspring (Grekin et al., 2005, Ozkaragoz et al., 1997, Corte and Becherer, 2007), however the neural mechanisms of this differential risk remain unknown. The null fronto-basal ganglia network findings for maternal AUD may also be partly due to adjustment for in utero alcohol exposure (39% of FH+ mothers reported alcohol use during pregnancy) which is known to impact offspring brain development (Lees et al., Under review). It is important to note that given the lower frequency of FH+ mothers compared to FH+ fathers in the ABCD Study, analyses examining a maternal effect had lower statistical power and this may have yielded less reliable estimates, as observed by similar average beta weights with larger standard errors in the medial orbital frontal gyrus (Figure 2).
Neural activation to response inhibition in FH+ youth also appears to vary across racial/ethnic groups. Race and ethnicity variables may be a proxy for more meaningful factors, such as level of acculturation, quality of education, socioeconomic status and racial socialization, which may be contributing to differences in neurofunction (Manly, 2006). Genetic and other biological variants associated with different racial and ethnic backgrounds (e.g., aldehyde dehydrogenase 2 deficiency) can also induce pronounced effects on alcohol consumption, which may also be contributing to the observed findings (Edenberg, 2007). Further neuroimaging research is required in a diverse ethnic population to investigate mechanisms underlying differential parental risk for altered offspring neurodevelopmental trajectories.
Patterns of greater activation in FH+ youth may reflect heightened processing effort and energy utilization throughout the fronto-basal ganglia network to successfully inhibit prepotent responses, which is more automatic and less effortful for FH− youth. Greater neural response may reflect a developmental lag in functional organization to some extent, given the lack of findings in the ventrolateral PFC, a key region for developmental changes in inhibition-related neural response (Braet et al., 2009, Aron et al., 2007). Previous research suggests that altered white matter integrity may also contribute to neural response differences via decreased neural efficiency and the need for recruitment of more neural resources (thus resulting in greater neural response; Burzynska et al., 2013, Zhu et al., 2015). Greater recruitment of inhibitory control regions in FH+ youth may therefore reflect an altered neurodevelopmental trajectory of the fronto-basal ganglia network, creating an inherent neurobiological vulnerability which affects their ability to suppress behavior. Examining the developmental trajectories of neural responses during cognitive control in this cohort when multiple waves of data are available, and correlating these to risk-related behaviors that change between childhood and adolescence (i.e. uptake of alcohol), may help identify patterns of brain activity that predict the onset of heavy alcohol use in FH+ youth.
There are several limitations to this study. Firstly, information on FH of AUD or SUD, as well as in utero alcohol exposure, may have been underreported or imprecisely recalled. Self-report data on substance use can be influenced by social stigma, desirability bias, and fear of intervention by child protection or social services (Stone, 2015, Johnson and Fendrich, 2005). Effects of reporting influenced by social stigma can also significantly vary by race/ethnicity (Garland and Bumphus, 2012, Kulesza et al., 2016). The effects of underreporting and imprecise recollection of substance use resulting in FH of AUD misclassification would likely attenuate the observed association toward the null. Potentially this means the reported associations are smaller in magnitude than the true effects. Secondly, despite the large sample size, there were relatively few cases of youth with FH+ mother or two FH+ parents. The small sample size of youth with FH+ mothers or two FH+ parents resulted in wider variance in neural responses and may underestimate the true impact. This was also evident when we conducted analyses for separate race/ethnicity. The ABCD cohort has a smaller proportion of Asian and Black families relative to White and Hispanic, and this resulted in very small FH+ samples of Asian and Black youth. The low statistical power may yield less reliable estimates for these cases. The current study was uniquely positioned to separately explore maternal and paternal AUD on youth neurofunction, however the effects of second- and third-degree relatives with AUD (i.e., FH+ density) should be further explored in future studies. Finally, as this study utilized observational and cross-sectional data, it remains unclear how greater forebrain activations may relate to risk for developing substance use disorders. Altered and/or delayed development of regions of the fronto-basal ganglia and cerebellar networks could be a risk factor and potential mechanistic target for intervention. However, we observed very small effect sizes in a small portion of the fronto-basal ganglia network, and this may limit applicability of neural responses to inhibitory control in late childhood as a clinically relevant marker of later alcohol use outcomes. Larger effect sizes in more regions have been observed with older cohorts (Acheson et al., 2014a), and may reflect a more advanced stage of altered neurodevelopment in FH+ individuals. Longitudinal analyses in the ABCD cohort are necessary to address these issues.
In conclusion, substance-naïve children aged 9 to 10 years with parental FH of AUD exhibited greater neural activation in some regions of the fronto-basal ganglia and cerebellar networks when successfully or unsuccessfully inhibiting a response during the SST compared to FH− youth, although effect sizes were very small. These youth are part of the longitudinal ABCD Study, and as they reach adolescence, we will investigate how elevated activation during response inhibition at baseline predicts later uptake of risk-related behaviors.
Supplementary Material
Table 2:
Behavioral data for the Stop Signal Task, N=6,898.
| FH− n=5947 |
FH+ n=951 |
|||
|---|---|---|---|---|
| Father n=725 |
Mother n=111 |
Both n=115 |
||
| Mean Go RT (ms mean [SD]) | 472.5 (82.2) | 468.8 (78.4) | 470.1 (74.7) | 467.5 (88.0) |
| Mean Stop RT (ms mean [SD]) | 301.5 (79.6) | 302.3 (79.3) | 290.3 (73.9) | 298.0 (75.5) |
| Correct Go (%)abcd | 81.4 | 80.7 | 82.8 | 77.0 |
| Correct Stop (%) | 50.8 | 50.7 | 51.7 | 50.9 |
| Failed Stop (%) | 45.4 | 45.3 | 43.7 | 45.2 |
No significant group differences: FH− youth vs youth of FH+ mothers, FH− youth vs youth of FH+ fathers, youth of FH+ mothers vs FH+ fathers.
FH− youth ≠ FH+ youth, p < .05
FH− youth ≠ youth of FH+ both parents, p < .05
Youth of FH+ mothers ≠ youth of FH+ both parents, p < .05
Youth of FH+ fathers ≠ youth of FH+ both parents, p < .05
Acknowledgements:
Work on this paper was supported by the National Institute of Alcohol Abuse and Alcoholism and National Institute of Drug Abuse grant awards R21 DA047953 (Jacobus), U01 DA041089 (Jacobus and Infante); K23 AA025399 (Squeglia), U01 DA041093 (Squeglia), T32AA013525 (P.I.: Riley/Tapert to Wade, Aguinaldo, and Hernandez Mejia); the California Tobacco-Related Disease Research Grants Program Office of the University of California Grant 580264 (Jacobus); and by the National Health and Medical Research Council: GNT1169377 (Lees). The content is solely the view of the authors and does not necessarily represent the official view of the NIH or NHMRC.
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9–10 and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, and U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/Consortium_Members.pdf. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from Annual Release 2.0.1, doi: 10.15154/1504041. DOIs can be found at https://ndar.nih.gov/study.html?id=721
Footnotes
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- ACHENBACH T & RESCORLA L 2013. Achenbach system of empirically based assessment, Springer. [Google Scholar]
- ACHESON A, FRANKLIN C, COHOON AJ, GLAHN DC, FOX PT & LOVALLO WR 2014a. Anomalous temporoparietal activity in individuals with a family history of alcoholism: studies from the Oklahoma Family Health Patterns Project. Alcohol Clin Exp Res, 38, 1639–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACHESON A, TAGAMETS MA, ROWLAND LM, MATHIAS CW, WRIGHT SN, HONG LE, KOCHUNOV P & DOUGHERTY DM 2014b. Increased forebrain activations in youths with family histories of alcohol and other substance use disorders performing a Go/NoGo task. Alcohol Clin Exp Res, 38, 2944–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARON AR 2011. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry, 69, e55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARON AR, DURSTON S, EAGLE DM, LOGAN GD, STINEAR CM & STUPHORN V 2007. Converging Evidence for a Fronto-Basal-Ganglia Network for Inhibitory Control of Action and Cognition. J Neurosci, 27, 11860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AUCHTER AM, HERNANDEZ MEJIA M, HEYSER CJ, SHILLING PD, JERNIGAN TL, BROWN SA, TAPERT SF & DOWLING GJ 2018. A description of the ABCD organizational structure and communication framework. Dev Cogn Neurosci, 32, 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARCH DM, ALBAUGH MD, AVENEVOLI S, CHANG L, CLARK DB, GLANTZ MD, HUDZIAK JJ, JERNIGAN TL, TAPERT SF, YURGELUN-TODD D, ALIA-KLEIN N, POTTER AS, PAULUS MP, PROUTY D, ZUCKER RA & SHER KJ 2018. Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: Rationale and description. Dev Cogn Neurosci, 32, 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENJAMINI Y & HOCHBERG Y 1995. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society, 57, 289–300. [Google Scholar]
- BENJAMINI Y & YEKUTIELI D 2001. The Control of the False Discovery Rate in Multiple Testing under Dependency. The Annals of Statistics, 29, 1165–1188. [Google Scholar]
- BRAET W, JOHNSON KA, TOBIN CT, ACHESON R, BELLGROVE MA, ROBERTSON IH & GARAVAN H 2009. Functional developmental changes underlying response inhibition and error-detection processes. Neuropsychologia, 47, 3143–3151. [DOI] [PubMed] [Google Scholar]
- BURZYNSKA AZ, GARRETT DD, PREUSCHHOF C, NAGEL IE, LI SC, BACKMAN L, HEEKEREN HR & LINDENBERGER U 2013. A scaffold for efficiency in the human brain. J Neurosci, 33, 17150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASEY BJ, CANNONIER T, CONLEY MI, COHEN AO, BARCH DM, HEITZEG MM, SOULES ME, TESLOVICH T, DELLARCO DV, GARAVAN H, ORR CA, WAGER TD, BANICH MT, SPEER NK, SUTHERLAND MT, RIEDEL MC, DICK AS, BJORK JM, THOMAS KM, CHAARANI B, MEJIA MH, HAGLER DJ JR., DANIELA CORNEJO M, SICAT CS, HARMS MP, DOSENBACH NUF, ROSENBERG M, EARL E, BARTSCH H, WATTS R, POLIMENI JR, KUPERMAN JM, FAIR DA, DALE AM & WORKGROUP AIA 2018. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev Cogn Neurosci, 32, 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASEY BJ, GALVAN A & HARE TA 2005. Changes in cerebral functional organization during cognitive development. Curr Opin Neurobiol, 15, 239–44. [DOI] [PubMed] [Google Scholar]
- CASEY BJ, GETZ S & GALVAN A 2008. The adolescent brain. Dev Rev, 28, 62–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASEY BJ, TRAINOR RJ, ORENDI JL, SCHUBERT AB, NYSTROM LE, GIEDD JN, CASTELLANOS FX, HAXBY JV, NOLL DC, COHEN JD, FORMAN SD, DAHL RE & RAPOPORT JL 1997. A Developmental Functional MRI Study of Prefrontal Activation during Performance of a Go-No-Go Task. J Cogn Neurosci, 9, 835–47. [DOI] [PubMed] [Google Scholar]
- CIESLIK EC, MUELLER VI, EICKHOFF CR, LANGNER R & EICKHOFF SB 2015. Three key regions for supervisory attentional control: evidence from neuroimaging meta-analyses. Neurosci Biobehav Rev, 48, 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORTE C & BECHERER M 2007. Differential Effects of Maternal and Paternal Alcoholism and Gender on Drinking, Alcohol-Related Self-Cognition, and Psychopathology. Journal of Addictions Nursing, 18, 175–185. [Google Scholar]
- COTTON NS 1979. The familial incidence of alcoholism: a review. J Stud Alcohol, 40, 89–116. [DOI] [PubMed] [Google Scholar]
- CREWS F, HE J & HODGE C 2007. Adolescent cortical development: A critical period of vulnerability for addiction. Pharmacology, Biochemistry and Behaviour, 86, 189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSERVENKA A 2016. Neurobiological phenotypes associated with a family history of alcoholism. Drug and alcohol dependence, 158, 8–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON DA, HARFORD TC & GRANT BF 1992. Family history as a predictor of alcohol dependence. Alcohol Clin Exp Res, 16, 572–5. [DOI] [PubMed] [Google Scholar]
- DE GRAAF-PETERS VB & HADDERS-ALGRA M 2006. Ontogeny of the human central nervous system: what is happening when? Early Hum Dev, 82, 257–66. [DOI] [PubMed] [Google Scholar]
- DEVITO EE, MEDA SA, JIANTONIO R, POTENZA MN, KRYSTAL JH & PEARLSON GD 2013. Neural correlates of impulsivity in healthy males and females with family histories of alcoholism. Neuropsychopharmacology, 38, 1854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DWYER-LINDGREN L, BERTOZZI-VILLA A, STUBBS RW & et al. 2018. Trends and patterns of geographic variation in mortality from substance use disorders and intentional injuries among us counties, 1980–2014. JAMA, 319, 1013–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDENBERG HJ 2007. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol research & health, 30, 5–13. [PMC free article] [PubMed] [Google Scholar]
- FLEWELLING RL, PASCHALL MJ, RINGWALT C, BONNIE RJ & O’CONNELL ME, EDITORS 2004. Reducing Underage Drinking: A Collective Responsibility The epidemiology of underage drinking in the United States: An overview. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- GARAVAN H, BARTSCH H, CONWAY K, DECASTRO A, GOLDSTEIN RZ, HEERINGA S, JERNIGAN T, POTTER A, THOMPSON W & ZAHS D 2018. Recruiting the ABCD sample: Design considerations and procedures. Dev Cogn Neurosci, 32, 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARLAND TS & BUMPHUS VW 2012. Race, Bias, and Attitudes Toward Drug Control Policy. Journal of Ethnicity in Criminal Justice, 10, 148–161. [Google Scholar]
- GIEDD JN, BLUMENTHAL J, JEFFRIES NO, CASTELLANOS FX, LIU H, ZIJDENBOS A, PAUS T, EVANS AC & RAPOPORT JL 1999. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci, 2, 861–3. [DOI] [PubMed] [Google Scholar]
- GOGTAY N, GIEDD JN, LUSK L, HAYASHI KM, GREENSTEIN D, VAITUZIS AC, NUGENT TF 3RD, HERMAN DH, CLASEN LS, TOGA AW, RAPOPORT JL & THOMPSON PM 2004. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A, 101, 8174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOULD TJ 2010. Addiction and cognition. Addiction science & clinical practice, 5, 4–14. [PMC free article] [PubMed] [Google Scholar]
- GREKIN ER, BRENNAN PA & HAMMEN C 2005. Parental alcohol use disorders and child delinquency: the mediating effects of executive functioning and chronic family stress. J Stud Alcohol, 66, 14–22. [DOI] [PubMed] [Google Scholar]
- HAGLER DJ, HATTON SN, MAKOWSKI C, CORNEJO MD, FAIR DA, DICK AS, SUTHERLAND MT, CASEY BJ, BARCH DM, HARMS MP, WATTS R, BJORK JM, GARAVAN HP, HILMER L, PUNG CJ, SICAT CS, KUPERMAN J, BARTSCH H, XUE F, HEITZEG MM, LAIRD AR, TRINH TT, GONZALEZ R, TAPERT SF, RIEDEL MC, SQUEGLIA LM, HYDE LW, ROSENBERG MD, EARL EA, HOWLETT KD, BAKER FC, SOULES M, DIAZ J, RUIZ DE LEON O, THOMPSON WK, NEALE MC, HERTING M, SOWELL ER, ALVAREZ RP, HAWES SW, SANCHEZ M, BODURKA J, BRESLIN FJ, MORRIS AS, PAULUS MP, SIMMONS WK, POLIMENI JR, VAN DER KOUWE A, NENCKA AS, GRAY KM, PIERPAOLI C, MATOCHIK JA, NORONHA A, AKLIN WM, CONWAY K, GLANTZ M, HOFFMAN E, LITTLE R, LOPEZ M, PARIYADATH V, WEISS SRB, WOLFF-HUGHES DL, DELCARMEN-WIGGINS R, FELDSTEIN EWING SW, MIRANDA-DOMINGUEZ O, NAGEL BJ, PERRONE AJ, STURGEON DT, GOLDSTONE A, PFEFFERBAUM A, POHL KM, PROUTY D, UBAN K, BOOKHEIMER SY, DAPRETTO M, GALVAN A, BAGOT K, GIEDD J, INFANTE MA, JACOBUS J, PATRICK K, SHILLING PD, DESIKAN R, LI Y, SUGRUE L, BANICH MT, FRIEDMAN N, HEWITT JK, HOPFER C, SAKAI J, TANABE J, COTTLER LB, NIXON SJ, CHANG L, CLOAK C, ERNST T, REEVES G, KENNEDY DN, HEERINGA S, PELTIER S, et al. 2018. Imaging processing and analysis methods for the Adolescent Brain Cognitive Development Study. Neuroimage, 202:116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDEE JE, WEILAND BJ, NICHOLS TE, WELSH RC, SOULES ME, STEINBERG DB, ZUBIETA J-K, ZUCKER RA & HEITZEG MM 2014. Development of Impulse Control Circuitry in Children of Alcoholics. Biological Psychiatry, 76, 708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEITZEG MM, NIGG JT, YAU W-YW, ZUCKER RA & ZUBIETA J-K 2010. Striatal Dysfunction Marks Preexisting Risk and Medial Prefrontal Dysfunction Is Related to Problem Drinking in Children of Alcoholics. Biological Psychiatry, 68, 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAMADAR S, DEVITO EE, JIANTONIO RE, MEDA SA, STEVENS MC, POTENZA MN, KRYSTAL JH & PEARLSON GD 2012. Memantine, an NMDA receptor antagonist, differentially influences Go/No-Go performance and fMRI activity in individuals with and without a family history of alcoholism. Psychopharmacology, 222, 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNS P 2014. Chapter 3 - Functional neuroanatomy In: JOHNS P (ed.) Clinical Neuroscience. Churchill Livingstone. [Google Scholar]
- JOHNSON T & FENDRICH M 2005. Modeling sources of self-report bias in a survey of drug use epidemiology. Ann Epidemiol, 15, 381–9. [DOI] [PubMed] [Google Scholar]
- JOHNSTON LD, MIECH RA, O’MALLEY PM, BACHMAN JG, SCHULENBERG JE & PATRICK ME 2019. Monitoring the Future national survey results on drug use, 1975–2018: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan. [Google Scholar]
- KESSLER RC, AVENEVOLI S, COSTELLO EJ, GREEN JG, GRUBER MJ, HEERINGA S, MERIKANGAS KR, PENNELL B-E, SAMPSON NA & ZASLAVSKY AM 2009a. Design and field procedures in the US National Comorbidity Survey Replication Adolescent Supplement (NCS-A). Int J Methods Psychiatr Res, 18, 69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESSLER RC, AVENEVOLI S, COSTELLO EJ, GREEN JG, GRUBER MJ, HEERINGA S, MERIKANGAS KR, PENNELL B-E, SAMPSON NA & ZASLAVSKY AM 2009b. National comorbidity survey replication adolescent supplement (NCS-A): II. Overview and design. J Am Acad Child Adolesc Psychiatry, 48, 380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHEMIRI L, LARSSON H, KUJA-HALKOLA R, D’ONOFRIO BM, LICHTENSTEIN P, JAYARAM-LINDSTRÖM N & LATVALA A 2020. Association of parental substance use disorder with offspring cognition: a population family-based study. Addiction, 115, 326–336. [DOI] [PubMed] [Google Scholar]
- KOENIGS M, BARBEY AK, POSTLE BR & GRAFMAN J 2009. Superior parietal cortex is critical for the manipulation of information in working memory. J Neurosci, 29, 14980–14986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOYAMA MS, PARVAZ MA & GOLDSTEIN RZ 2017. The adolescent brain at risk for substance use disorders: a review of functional MRI research on motor response inhibition. Curr Opin Behav Sci, 13, 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KULESZA M, MATSUDA M, RAMIREZ JJ, WERNTZ AJ, TEACHMAN BA & LINDGREN KP 2016. Towards greater understanding of addiction stigma: Intersectionality with race/ethnicity and gender. Drug Alcohol Depend, 169, 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEES B, MEREDITH LR, KIRKLAND AE, BRYANT BE, SQUEGLIA LM In press, 2020. Effect of alcohol use on the adolescent brain and behavior. Pharmacology, Biochemistry and Behavior. [DOI] [PMC free article] [PubMed]
- LEES B, MEWTON L, JACOBUS J, VALADEZ E, STAPINSKI LA, TEESSON M, TAPERT SF & SQUEGLIA LM Under review. Association of prenatal alcohol exposure with psychological, behavioral, and neurodevelopmental outcomes in children from the adolescent brain cognitive development study. [DOI] [PMC free article] [PubMed]
- LEES B, MEWTON L, STAPINSKI LA, SQUEGLIA LM, RAE CD & TEESSON M 2019. Neurobiological and cognitive profile of young binge drinkers: A systematic review and meta-analysis. Neuropsychology Review, 29, 357–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIEB R, MERIKANGAS KR, HOFLER M, PFISTER H, ISENSEE B & WITTCHEN HU 2002. Parental alcohol use disorders and alcohol use and disorders in offspring: a community study. Psychol Med, 32, 63–78. [DOI] [PubMed] [Google Scholar]
- LISDAHL KM, SHER KJ, CONWAY KP, GONZALEZ R, FELDSTEIN EWING SW, NIXON SJ, TAPERT S, BARTSCH H, GOLDSTEIN RZ & HEITZEG M 2018. Adolescent brain cognitive development (ABCD) study: Overview of substance use assessment methods. Dev Cogn Neurosci, 32, 80–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOPEZ-CANEDA E, RODRIGUEZ HOLGUIN S, CADAVEIRA F, CORRAL M & DOALLO S 2014. Impact of alcohol use on inhibitory control (and vice versa) during adolescence and young adulthood: a review. Alcohol Alcohol, 49, 173–81. [DOI] [PubMed] [Google Scholar]
- LUCIANA M, BJORK JM, NAGEL BJ, BARCH DM, GONZALEZ R, NIXON SJ & BANICH MT 2018. Adolescent neurocognitive development and impacts of substance use: Overview of the adolescent brain cognitive development (ABCD) baseline neurocognition battery. Dev Cogn Neurosci, 32, 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANLY JJ 2006. Deconstructing race and ethnicity: implications for measurement of health outcomes. Med Care, 44, S10–6. [DOI] [PubMed] [Google Scholar]
- MCCAUL ME, TURKKAN JS, SVIKIS DS, BIGELOW GE & CROMWELL CC 1990. Alcohol and Drug Use by College Males as a Function of Family Alcoholism History. Alcohol Clin Exp Res, 14, 467–471. [DOI] [PubMed] [Google Scholar]
- MERIKANGAS K, AVENEVOLI S, COSTELLO J, KORETZ D & KESSLER RC 2009. National comorbidity survey replication adolescent supplement (NCS-A): I. Background and measures. J Am Acad Child Adolesc Psychiatry, 48, 367–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEE DE, WAGER TD & JONIDES J 2007. Interference resolution: Insights from a meta-analysis of neuroimaging tasks. Cognitive, Affective, & Behavioral Neuroscience, 7, 1–17. [DOI] [PubMed] [Google Scholar]
- OZKARAGOZ T, SATZ P & NOBLE EP 1997. Neuropsychological functioning in sons of active alcoholic, recovering alcoholic, and social drinking fathers. Alcohol, 14, 31–37. [DOI] [PubMed] [Google Scholar]
- POMPILI M, SERAFINI G, INNAMORATI M, DOMINICI G, FERRACUTI S, KOTZALIDIS GD, SERRA G, GIRARDI P, JANIRI L, TATARELLI R, SHER L & LESTER D 2010. Suicidal behavior and alcohol abuse. Int J Environ Res Public Health, 7, 1392–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICE JP, REICH T, BUCHOLZ KK, NEUMAN RJ, FISHMAN R, ROCHBERG N, HESSELBROCK VM, NURNBERGER JI JR., SCHUCKIT MA & BEGLEITER H 1995. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res, 19, 1018–23. [DOI] [PubMed] [Google Scholar]
- SCHWEINSBURG AD, PAULUS MP, BARLETT VC, KILLEEN LA, CALDWELL LC, PULIDO C, BROWN SA & TAPERT SF 2004. An FMRI study of response inhibition in youths with a family history of alcoholism. Ann N Y Acad Sci, 1021, 391–4. [DOI] [PubMed] [Google Scholar]
- SCOTT J, SLOMIAK ST, JONES JD, ROSEN AG, MOORE TM & GUR RC 2018. Association of cannabis with cognitive functioning in adolescents and young adults: A systematic review and meta-analysis. JAMA Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHER KJ, GREKIN ER & WILLIAMS NA 2005. The development of alcohol use disorders. Annu Rev Clin Psychol, 1, 493–523. [DOI] [PubMed] [Google Scholar]
- SHULMAN EP, SMITH AR, SILVA K, ICENOGLE G, DUELL N, CHEIN J & STEINBERG L 2016. The dual systems model: Review, reappraisal, and reaffirmation. Dev Cogn Neurosci, 17, 103–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVERI MM, ROGOWSKA J, MCCAFFREY A & YURGELUN-TODD DA 2011. Adolescents at risk for alcohol abuse demonstrate altered frontal lobe activation during Stroop performance. Alcohol Clin Exp Res, 35, 218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH JL, MATTICK RP, JAMADAR SD & IREDALE JM 2014. Deficits in behavioural inhibition in substance abuse and addiction: a meta-analysis. Drug Alcohol Depend, 145, 1–33. [DOI] [PubMed] [Google Scholar]
- SOMERVILLE LH, HARE T & CASEY BJ 2011. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J Cogn Neurosci, 23, 2123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPEAR LP 2013. Adolescent neurodevelopment. J Adolesc Health, 52, S7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SQUEGLIA LM & CSERVENKA A 2017. Adolescence and Drug Use Vulnerability: Findings from Neuroimaging. Curr Opin Behav Sci, 13, 164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SQUEGLIA LM & GRAY KM 2016. Alcohol and drug use and the developing brain. Current Psychiatry Reports, 18, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINBERG L 2010. A dual systems model of adolescent risk-taking. Dev Psychobiol, 52, 216–224. [DOI] [PubMed] [Google Scholar]
- STONE R 2015. Pregnant women and substance use: fear, stigma, and barriers to care. Health & Justice, 3, 2. [Google Scholar]
- SULLIVAN EV, HARDING AJ, PENTNEY R, DLUGOS C, MARTIN PR, PARKS MH, DESMOND JE, CHEN SH, PRYOR MR, DE ROSA E & PFEFFERBAUM A 2003. Disruption of frontocerebellar circuitry and function in alcoholism. Alcohol Clin Exp Res, 27, 301–9. [DOI] [PubMed] [Google Scholar]
- TAMM L, MENON V & REISS AL 2002. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry, 41, 1231–8. [DOI] [PubMed] [Google Scholar]
- TAMNES CK, HERTING MM, GODDINGS AL, MEUWESE R, BLAKEMORE SJ, DAHL RE, GUROGLU B, RAZNAHAN A, SOWELL ER, CRONE EA & MILLS KL 2017. Development of the Cerebral Cortex across Adolescence: A Multisample Study of Inter-Related Longitudinal Changes in Cortical Volume, Surface Area, and Thickness. J Neurosci, 37, 3402–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TARTER RE, KIRISCI L, MEZZICH A, CORNELIUS JR, PAJER K, VANYUKOV M, GARDNER W, BLACKSON T & CLARK D 2003. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry, 160, 1078–85. [DOI] [PubMed] [Google Scholar]
- TEESSON M, HALL W, SLADE T, MILLS K, GROVE R, MEWTON L, BAILLIE A & HABER P 2010. Prevalence and correlates of DSM-IV alcohol abuse and dependence in Australia: findings of the 2007 National Survey of Mental Health and Wellbeing. Addiction, 105, 2085–94. [DOI] [PubMed] [Google Scholar]
- UBAN KA, HORTON MK, JACOBUS J, HEYSER C, THOMPSON WK, TAPERT SF, MADDEN PAF & SOWELL ER 2018. Biospecimens and the ABCD study: Rationale, methods of collection, measurement and early data. Dev Cogn Neurosci, 32, 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLKOW ND, KOOB GF, CROYLE RT, BIANCHI DW, GORDON JA, KOROSHETZ WJ, PEREZ-STABLE EJ, RILEY WT, BLOCH MH, CONWAY K, DEEDS BG, DOWLING GJ, GRANT S, HOWLETT KD, MATOCHIK JA, MORGAN GD, MURRAY MM, NORONHA A, SPONG CY, WARGO EM, WARREN KR & WEISS SRB 2018. The conception of the ABCD study: From substance use to a broad NIH collaboration. Dev Cogn Neurosci, 32, 4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELSH JW, KNIGHT JR, HOU SS, MALOWNEY M, SCHRAM P, SHERRITT L & BOYD JW 2017. Association between substance use diagnoses and psychiatric disorders in an adolescent and young adult clinic-based population. J Adolesc Health, 60, 648–652. [DOI] [PubMed] [Google Scholar]
- WHELAN R, CONROD PJ, POLINE J-B, LOURDUSAMY A, BANASCHEWSKI T, BARKER GJ, BELLGROVE MA, BÜCHEL C, BYRNE M, CUMMINS TDR, FAUTH-BÜHLER M, FLOR H, GALLINAT J, HEINZ A, ITTERMANN B, MANN K, MARTINOT J-L, LALOR EC, LATHROP M, LOTH E, NEES F, PAUS T, RIETSCHEL M, SMOLKA MN, SPANAGEL R, STEPHENS DN, STRUVE M, THYREAU B, VOLLSTAEDT-KLEIN S, ROBBINS TW, SCHUMANN G, GARAVAN H & THE IC 2012. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nature Neuroscience, 15, 920–925. [DOI] [PubMed] [Google Scholar]
- ZHU Z, JOHNSON NF, KIM C & GOLD BT 2015. Reduced frontal cortex efficiency is associated with lower white matter integrity in aging. Cereb Cortex, 25, 138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZUCKER RA, GONZALEZ R, FELDSTEIN EWING SW, PAULUS MP, ARROYO J, FULIGNI A, MORRIS AS, SANCHEZ M & WILLS T 2018. Assessment of culture and environment in the Adolescent Brain and Cognitive Development Study: Rationale, description of measures, and early data. Dev Cogn Neurosci, 32, 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


