Abstract
Coccidioides species are fungal pathogens that can cause a widely varied clinical manifestation from mild pulmonary symptom to disseminated, life-threatening disease. We have previously created a subunit vaccine by encapsulating a recombinant coccidioidal antigen (rCpa1) in glucan-chitin particles (GCPs) as an adjuvant-delivery system. The GCP-rCpa1 vaccine has shown to elicit a mixed Th1 and Th17 response and confers protection against pulmonary coccidioidomycosis in mice. In this study, we further delineated the vaccine-induced protective mechanisms. Depletion of IL-17A in vaccinated C57BL/6 mice prior to challenge abrogated the protective efficacy of GCP-rCpa1 vaccine. Global transcriptome and ingenuity pathway analysis (IPA) of murine bone marrow derived macrophages after exposure to this vaccine revealed the upregulation of proinflammatory cytokines (TNF-α, IL-6, IL-1β) that are associated with activation of C-type lectin receptors (CLR) Dectin-1- and Dectin-2-mediated CARD9 signaling pathway. The GCP formulation of rCpa1 bound soluble Dectin-1 and Dectin-2 and triggered ITAM signaling of corresponding CLR reporter cells. Furthermore, macrophages that were isolated from Dectin-1−/−, Dectin-2−/− and CARD9−/− mice significantly reduced production of inflammatory cytokines in response to the GCP-rCpa1 vaccine compared to those of WT mice. The GCP-rCpa1 vaccine had significantly reduced protective efficacy in Dectin-1−/−, Dectin-2−/− and CARD9−/− mice that showed decreased acquisition of Th cells in Coccidioides-infected lungs compared to vaccinated wild type mice, especially Th17 cells. Collectively, we conclude that the GCP-rCpa1 vaccine stimulates a robust Th17 immunity against Coccidioides infection through activation of the CARD9-associated Dectin-1 and Dectin-2 signal pathways.
Introduction
Coccidioidomycosis, or San Joaquin Valley Fever, is caused by inhalation of spores produced by Coccidioides immitis and Coccidioides posadasii (1). Coccidioidomycosis displays a broad spectrum of disorders from self-limited flu-like symptoms to progressive pulmonary destruction and life-threatening dissemination (2). Approximately 17-29% of community-acquired pneumonia in endemic areas are caused by Coccidioides infection. These fungal pathogens infect both immunocompromised and immunocompetent individuals, posing a great risk to over 30 million people (approximately 10% of the US population) who live in the endemic regions (3). Historically, the endemic areas of coccidioidomycosis are located on the western hemisphere including southwest US, Central America and certain parts of the South America. An estimated 150,000 new infections occur each year in the US. Recent reports show the geographic distribution of Coccidioides species has expanded as far north as Washington State (4–6). Due to the significant cost of health care and impact in livelihood, Coccidioides poses a major health threat to the public.
Evidence of acquiring long-term memory immunity against coccidioidomycosis has been observed in individuals who recover from symptomatic Coccidioides infections and yet remain skin-test positive (7, 8). Therefore, it is possible to develop an effective vaccine with long-term memory against coccidioidomycosis. Vaccine candidates against Coccidioides infection come in many forms including live attenuated strains, formalin-killed spherules (FKS) and subunit vaccines (3, 9). While the understanding of protective immunity to Coccidioides infection remains incomplete, animal models and clinical data suggest that functional CD4+ T cell responses characterizing the expression of Th1- and Th17-type cytokines are essential for protection against this fungal disease (3, 9–12). Typically, Th1 cells express interferon-γ (IFN-γ), while Th17 cells produce IL-17. Recent data also support the role of IL-17 and Th17 cells in the protective immunity conferred by a live attenuated vaccine against Coccidioides infection in mice (11, 13, 14). Unfortunately, there is no approved adjuvant available for the formulation of subunit vaccines that are able to induce a Th17 response.
Several experimental adjuvants have recently been demonstrated to stimulate IL-17 production and to augment vaccine-mediated immunity against pulmonary Mycobacterium tuberculosis infections (15–17). Glucan particles (GPs) that are primarily composed of β−1,3-D-glucans derived from Saccharomyces cerevisiae cell wall have been shown to elicit a mixed Th1 and Th17 response against fungal infections (18, 19). Our laboratory has created a recombinant Coccidioides polypeptide antigen (rCpa1; GenBank No. KY883768) consisting of previously reported coccidioidal antigens (Ag2/Pra, Cs-Ag, and Pmp1) and 5 peptides with high affinity to human major histocompatibility complex class II (MHC-II) molecules (10). The rCpa1 antigen is encapsulated by Rhodotorula mucilaginosa yeast derived-glucan-chitin particles (GCPs) that allow for the effective delivery of this vaccine to antigen presenting cells (APCs) for the activation of a protective memory response (10, 20). We also show that the GCP adjuvant elicits elevated infiltration of macrophages to the vaccination sites and it activates a superior memory Th17 response in the lungs of C57BL/6 and HLA-DR4 transgenic mice against a potentially lethal challenge with Coccidioides posadasii compared to the GP adjuvant (10). Concurringly, the GCP-rCpa1 vaccine has improved protective efficacy in reduction of CFUs in the lungs and spleen of Coccidioides-infected mice compared to the GP-rCpa1 vaccine.
In this study, we investigated vaccine-induced immune mechanisms of the GCP-rCpa1 vaccine. Depletion of IL-17A in vaccinated mice reduced protective efficacy of GCP-rCpa1 against Coccidioides infection. We applied global transcriptome analysis of murine bone marrow derived macrophages after exposure to the GCP-rCpa1vaccine. Our results showed that both Dectin-1 and Dectin-2 C-type lectin receptors interact with GCPs to activate protective immune response against pulmonary coccidioidomycosis. Furthermore, caspase recruitment domain family member 9 (CARD9), a downstream immune adaptor of Dectin-1 and Dectin-2 was also required for activation of protective immunity. Overall, our data revealed that CLRs-CARD9-mediated Th17 immunity is essential for the GCP-rCpa1 vaccine to confer protection against pulmonary coccidioidomycosis in mice.
Materials and Methods
Fungal culture
Virulent clinical isolate Coccidioides posadasii, C735, was used in this study. The saprobic phase was grown on GYE agar (1% glucose, 0.5% yeast extract, 1.5% agar) to produce spores as previously reported (10). All culturing and preparatory procedures, which involved live cells of C. posadasii, were conducted in a biosafety level 3 (BSL3) laboratory located at the University of Texas at San Antonio (UTSA).
Mice
Dectin-1−/− (21), Dectin-2−/− (22) and CARD9−/− (23) male and female mice (8-10 week-old) on C57BL/6 genetic background were bred in house. Wild type C57BL/6 (H-2b) sex and age-matched mice were purchased from the National Cancer Institute/Charles River Laboratories. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at UTSA according to NIH and ABLS3 laboratory guidelines for housing and care of laboratory animals.
Vaccination protocol, animal challenge, and evaluation of protection
Mice were subcutaneously immunized twice in the abdominal region at a 2-week interval (10). Mice were challenged intranasally with a suspension of 80-100 viable spores of C. posadasii in 35 µl of PBS at 4 weeks after completion of the immunization protocol as previously described (24). Mice were euthanized at 14 days postchallenge for assessing fungal burden as previously described (14, 24, 25).
Neutralization of IL-17A in WT mice
Vaccinated and control C57BL/6 mice were injected with 250 µg of anti-IL-17A monoclonal antibody (clone 17F3, Bio X Cell) or normal rat IgG isotype control (Sigma Chemical So., St. Louis, MO) by the intraperitoneal (i.p.) route as previously described (13, 26). The treatment was conducted at 24 hours prior to challenge and every 3 days after an intranasal challenge with C. posadasii spores. Mice were euthanized at 13 days postchallenge, and fungal burden was analyzed.
Macrophage preparation
Bone marrow-derived macrophages (BMMs) were prepared from femurs and tibiae of mice as previously described (27). Briefly, bone marrow cells were washed, counted and plated at a concentration of 4 × 105 cells/ml in RPMI 1640 medium supplemrented with 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 100 U penicillin/ml, 100 µg of streptomycin/ml, 50 mM 2-mercaptoethanol and 20 ng/ml of murine M-CSF (Peprotech). Cells were incubated at 37°C and 5% CO2, half of the medium replaced every three days, and cells harvested on day 7. BMMs were washed and incubated with macrophage detachment solution DXF (PromoCell) for 40 mins at 4°C. Macrophages were collected off the bottom of the petri dish plate. Purity for BMMs expressing F4/80 (eBioscience, BM8) was checked by flow cytometry and it was routinely >95%.
RNA-sequencing analysis
BMMs (1 × 106 cells/ml) were incubated with GCP-rCpa1 at a ratio of 1:50 (1 macrophage/ 50 particles) or were treated with PBS (the same volume as particles used) as a control for 12 hours with 5% CO2. Total RNA was isolated from BMMs using the Qiagen RNeasy kit. Preparation of sequencing libraries and sequencing analysis using Illumina HiSeq 3000 platform (50 bp single-read module) were conducted at the Genomics Core of UT Health San Antonio. RNA-Seq Analysis module in CLC Genomics workbench was used to align the sequence reads with confidential length (>50bp) to mouse GRCm38 (mm10) reference genome (28). Differential gene expression was analyzed after eliminating lowly expressed gene (counts per million < 1 in more than three samples across the dataset) using edgeR package . Gene Ontology (GO) analysis were performed using DAVID and then visualized by GO plot (29). A heat map showed top 50 differentially expressed genes of the GCP-rCpa1-treated macrophages compared to controls was created with Euclidean distance method using Heatmap.2 function implemented in the “gplots” R package. The causal network analysis was performed using Ingenuity Pathway Analysis (IPA) software (Qiagen) (30, 31).
Quantitative RT-PCR analysis and cytokine ELISAs
BMMs were incubated with GCP-rCpa1 (1:50 ratio) or treated with PBS as a control for 12 and 24 hours for qRT-PCR and cytokine analyses, respectively. Complementary DNA samples were synthesized from 1 µg of total RNA isolated from each sample of BMMs using oligo(dT) primer and SuperScript III™ Reverse Transcriptase (Invitrogen). The cDNA samples were used for qRT-PCR using Sybr-green detection method with gene specific primers. Mouse β-actin gene expression was used as an internal control. Results of real-time PCR data were derived from comparative Ct methods to detect relative gene expression as described (32). For cytokine assays, BMMs were spun down at 3,500 RPM for 3 mins and supernatant samples were stored in −80°C with Halt™ Protease and Phosphatase Inhibitor (Life Technologies). Cytokine concentrations were measured using Bio-Rad’s Bio-Plex Mouse cytokine 23-plex (catalog #:M60009RDPD) as described (27).
CLR reporter assay
Constructs of B3Z/BWZ reporter cells expressing Dectin-1, Dectin-2, Dectin-3 and Mincle have been described previously (33, 34). For B3Z/BWZ cell stimulation, 1 × 105 B3Z/BWZ cells per well in a 96-well plate were incubated for 18 hours with GCPs or plate-coated ligands. β-galactosidase (lacZ) activity was measured in total cell lysates using CPRG (Roche) as a substrate. OD560 was measured using OD620 as a reference.
Dectin-1 and Dectin-2 binding assays
GCPs were incubated at 2 × 106 particles/well with either 20 µg/mL of Fc-Dectin-1, Fc-Dectin-2 or human IgG Fc (negative control) in 100 µl of binding buffer (20 mM Tris-HCl, 150 nM NaCl, 10 mM CaCl2 and 0.05% Tween-20 in pH7.4) as previously described (33). The plate was incubated overnight at 4°C to prevent non-specific binding. The particles were then washed 3 times in binding buffer and incubated with a 1:100 dilution of PE anti-human IgG1 Fc antibody (Jackson ImmunoResearch) for 30 minutes at 4°C. Following incubation, the samples were washed three times in binding buffer and fixed in 500 μl of 2% ultrapure formaldehyde. Data were acquired using a BD LSRII flow cytometer (BD Biosciences) and analyzed with FlowJo software. Aliquots of samples were spun down and resuspended in 30 µL of PBS for imaging analysis using a Leica fluorescent microscope.
FACS analysis
Pulmonary leukocytes were isolated from vaccinated and control Dectin-1−/−(ΔD1), Dectin-2−/− (ΔD2) and CARD9−/− (ΔC9) mice at day 14 postchallenge (4 mice per group) as previously reported (11). A standard flow cytometry methodology was employed for direct monoclonal antibody (mAb) labeling and enumeration of selected pulmonary immune T cell phenotypes using a FACSCalibur cytometer (11). Permeabilized leukocytes were stained with a cocktail of fluorochrome-conjugated antibodies for detecting IFN-γ, IL-17A, CD4 and CD8 molecules. Data were analyzed using FlowJo software version 10.
Statistical analyses
Student t-test was used to analyze results between GCP adjuvant vs GCP-rCpa1 vaccinated groups for cytokine concentrations and calculations of cell numbers of lung-infiltrated immune cells. Student-Newman-Keuls test, a type of ANOVA statistical analysis for multiple comparisons of three or above independently treated groups was used as previously reported (35). The differences in fungal burdens (CFUs) between two groups were analyzed by the Mann-Whitney U ranking test (11). When comparing fungal burden among three and more groups of mice the Kruskal-Wallis test, a non-parametric ranking method was used as previously reported (11). A p value of equal or less than 0.05 was considered statistically significant.
Results
IL-17A is essential for GCP-rCpa1-induced protective immunity
Vaccine toxicity may interfere with immune response and protective efficacy. Our previous histopathological analysis revealed minimal advisory effect on the subcutaneous vaccination sites and each of the recruited antigen presenting cells (i.e., macrophage and dendritic cells) could engulf one GCP particle (10). We further investigated vaccine cytotoxicity by incubation of human hepatocellular carcinoma (Hep2G) cells with the GCP particles loaded with mouse serum albumin (GCP-MSA) and rCpa1 (GCP-rCpa1) at a series of doses from 1:1 to 1:1200 ratios (Supp. Fig. 1S). Viability curves were used to calculate CC50 that was defined as ratio of Hep2G cells to particles leading to 50% cell death. CC50 values were calculated to be 1150 and greater than 1200 for GCP- rCpa1 and GCP-MSA, respectively. In addition, the body weights of the vaccinated mice increased over the course of the study comparable to the non-vaccinated mice (data now shown). These data suggested that both GCP and rCpa1 had minimal cytotoxicity and the GCP-rCpa1 vaccine was suitable for both in vitro and in vivo immunological assays.
We investigated whether IL-17A is essential for the GCP-rCpa1-induced immunity against pulmonary coccidioidomycosis. Depletion of IL-17A in vaccinated and nonvaccinated WT mice was conducted using a monoclonal antibody (Clone 17F3) as described in Materials and Methods. Mice vaccinated with GCP-rCpa1 and treated with anti-IL-17A antibody demonstrated a significant increase in fungal burden in the Coccidioides-infected lungs compared to isotype-treated mice (Fig. 1A; #P < 0.05). Similarly, CFUs were also significantly increased in the spleens of mice that were treated with anti-IL-17 antibody (Fig. 1B). These data suggest that IL-17-mediated immunity is required for GCP-rCpa1-induced immunity against Coccidioides infection.
FIGURE 1.
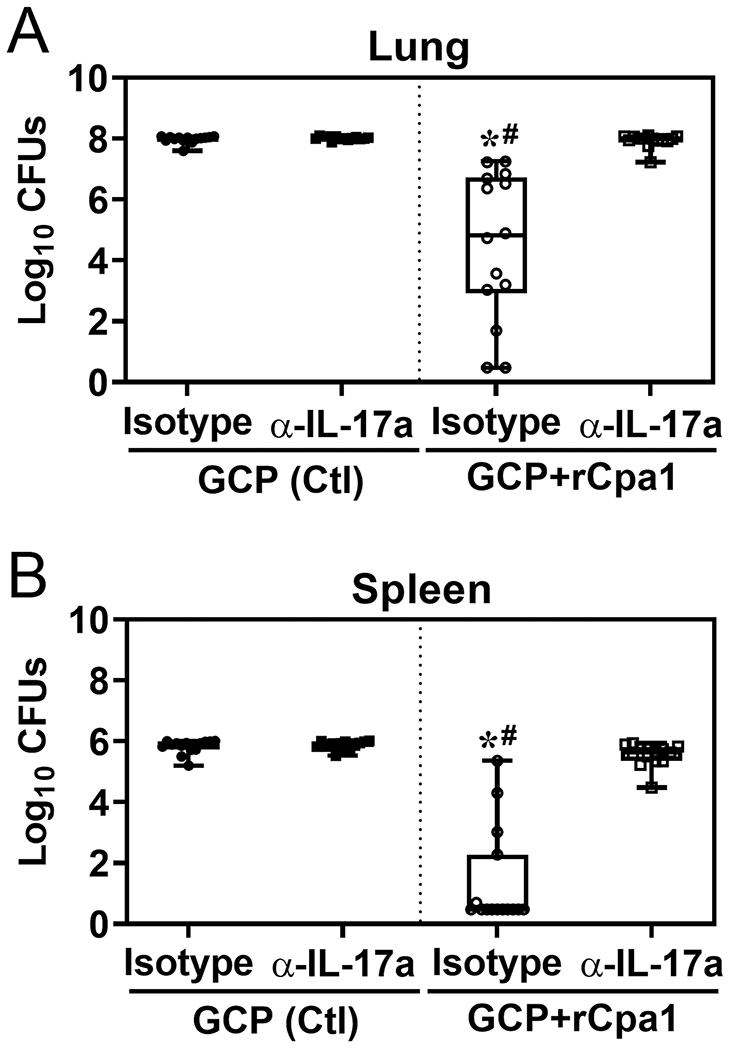
IL-17A is essential for GCP-rCpa1-induced protective immunity. C57BL/6 mice (n= 14 mice/ group) were immunized twice with either GCP-rCpa1vaccine or GCP adjuvant alone as a control. Mice were treated with an anti-IL-17A mAb or isotype IgG by the i.p. route at 24 h prior to challenge and at 3, 6, 9 and 12 dpc to deplete IL-17A. CFUs of C. posadasii were detected in dilution plate cultures of the lung (A) and spleen (B) homogenates of mice at 13 dpc. CFUs are reported in box plots. Asterisks indicate statistically significant differences (Mann-Whitney U test; P< 0.05) between the corresponding vaccinated and nonvaccinated (GCP) mice, while the hashtags indicate significant differences between the IL-17A-depleted and isotype control mice (Kruskal-Wallis ranking test P < 0.05).
Profile transcriptomes of macrophages after exposure to the GCP-rCpa1 vaccine
RNA-seq analysis was conducted in C57BL/6 mouse derived BMMs that were incubated with GCP-rCpa1 vaccine (macrophage: particle ratio = 1: 50) for 12 hours. Aliquots of macrophages without stimulation served as a control. The RNA-seq data have been deposited in NCBI’s GENE Expression Omnibus and is accessible through GEO accession number GSE133140 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE133140). Approximately 37-54 millions of sequences per sample (~80% of total reads) were mapped to the GRCm38(mm10) mouse genome. The global gene expression differences between vaccine-treated and control group were visualized using volcano plot (Fig. 2A). Overall, 1954 genes exhibited significant difference great than 2 folds (Log2 Fold Change >1 or <−1 and False Discovery rate [FDR] < 0.05). Among them 941 genes were down-regulated and 1013 were upregulated genes (Fig. 2A). We focused on the upregulated genes in response to the vaccine stimulation. The gene ontology (GO) terms that were associated with the set of upregulated genes included inflammatory response, innate immune response, cytokine secretion, antigen processing and presentation, cellular response to IFN-γ, IL-1β and IL-6 (Supp. Fig. 2S). Quantitative RT-PCR analysis showed that the expression amounts of IL-1β and IL-6 genes were indeed elevated in the vaccine-treated macrophages (Fig. 2B). Hierarchical cluster from three independent experiments demonstrates the top 50 significantly increased genes in vaccine treated BMMs compared to untreated cells (Fig. 2C).
FIGURE 2.
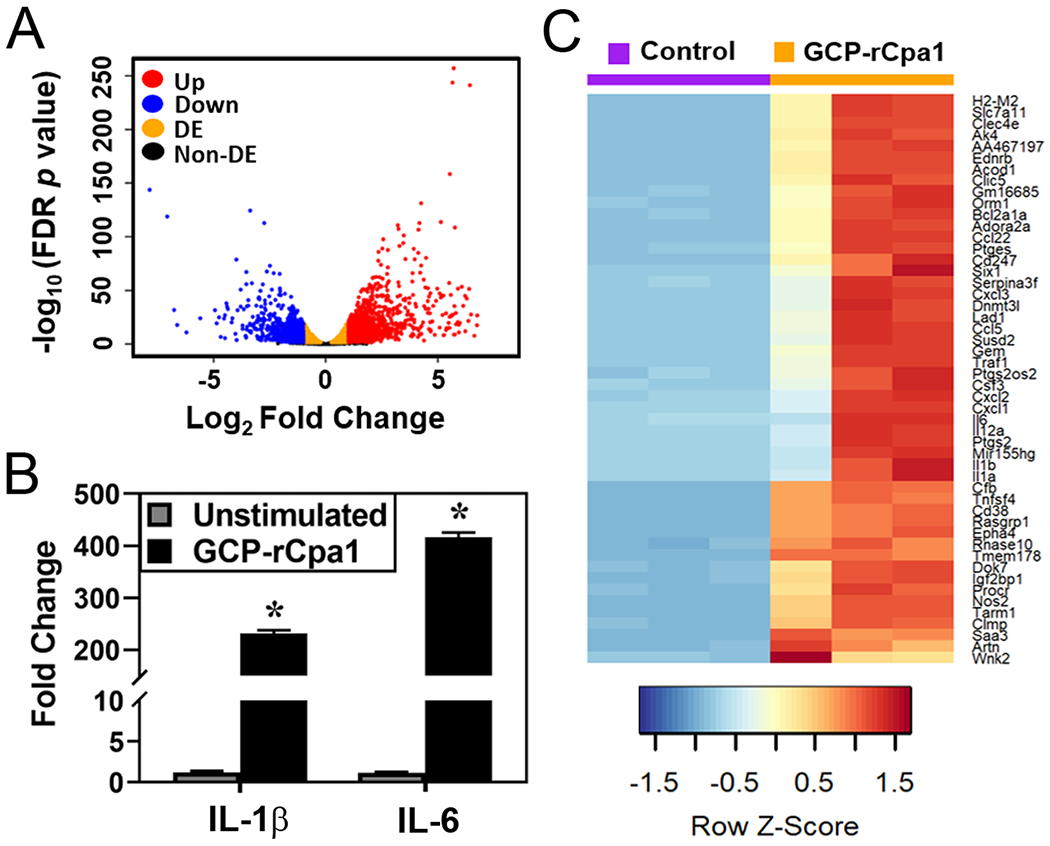
Profiling transcriptomes of macrophages after exposure to the GCP-rCpa1 vaccine. (A) A volcano plot demonstrates up-regulated (red), down-regulated (blue), and non-differentially expressed genes (orange). The non-detected genes were colored in black. This plot was constructed using fold changes and FDR P values for each genes to enable visualization of the relationship between fold changes and statistical significance. The differentially expressed genes were defined for those with changes equal to or greater than 2-fold and FDR P value equal to or lesser than 0.05 (Log2 ≥1 or ≤ −1; FDR p value ≤ 0.05). (B) Quantitative RT-PCR results confirmed that IL-1β and IL-6 gene expressions were significantly increased in the GCP-rCpa1vaccine treated macrophages compared to mock PBS controls. Murine β-actin gene was used as an internal control to normalize the qRT-PCR data. (C) A heat map showed differentially expressed macrophage genes. Hierarchical clustering of top 50 significantly expressed genes of the GCP-rCpa1-treated macrophages compared to controls was performed with Euclidean distance method. Each row corresponds to one gene and each column corresponds to one sample. Red cells indicate high relative expression and blue indicates low relative expression corresponding to a Z score ranging from −2 to 2.
Both Dectin-1 and Dectin-2 recognize GCP-rCpa1 vaccine
We utilized the IPA module of Regulator Effects to identify upstream receptors that could involve in the upregulation of these genes. Results showed that Toll-like receptor 1 (TLR1), TLR2, Dectin-1 and Dectin-2 were upstream of the upregulated genes (z-score > 2). Proinflammatory genes that were downstream of Dectin-1 (Clec7a) including IL-1B, TNF, IL-12A, IL-12B, IL-6, CXCL2, CCL22, PTGS2 (prostaglandin-endoperoxide synthase 2), and CSF3 (colony stimulating factor 3) were upregulated in vaccine-treated macrophages compared to unstimulated controls (Fig. 3A). Dectin-2 (Clec6a or Clec4n) associated downstream genes IL-1B, IL-6, IL-12B, and TNF were also elevated in the treated macrophages (Fig. 3B). Both Dectin-1and Dectin-2-mediated pathways are associated with increased differentiation and polarization of Th17 and Th1 cells. The GCP adjuvant is derived from fungal cell wall containing polysaccharides, which serve as potential ligands for CLRs. Therefore, we focused on CLRs including Dectin-1, Dectin-2, Dectin-3 and Mincle in this study. We employed T-hybridoma cells expressing NFAT-lacZ galactosidase reporter of ITAM signaling (33). In response to GCP-rCpa1 vaccine stimulation, lacZ activity was increased in T-hybridoma cells expressing Dectin-1 and Dectin-2 in a dose-dependent manner (Fig. 3C). In contrast, reporter cells expressing Dectin-3 or Mincle did not increase lacZ activity. T-hybridoma cells expressing the signaling adaptor FcRγ alone did not increase lacZ activity. Thus, Dectin-1 and Dectin-2, but not Dectin-3 or Mincle, recognized the GCP-rCpa1 vaccine and triggered downstream ITAM signaling through FcRγ.
FIGURE 3.
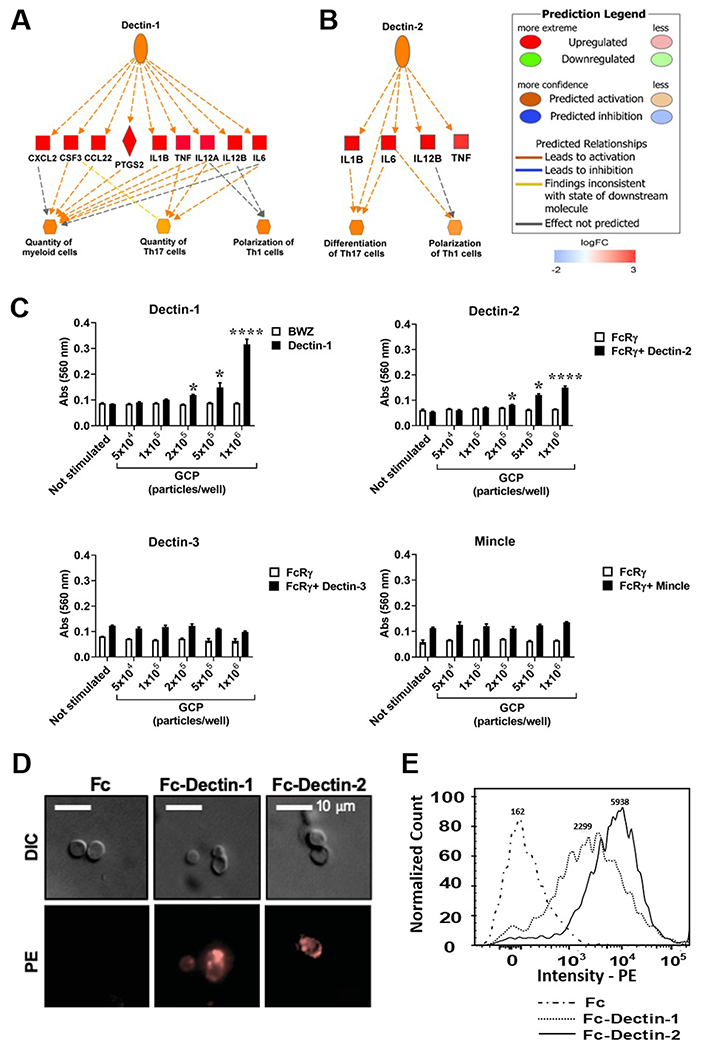
Both Dectin-1 and Dectin-2 recognize GCP-rCpa1 vaccine. (A and B) The differentially expressed gene data were analyzed with regulator effect tools implemented in IPA to predict activation of upstream CLR members, Dectin-1 and Dectin-2, in response to the vaccine. Genes shaded in red are upregulated while green are downregulated. The intensity of the shading demonstrates the degree each gene that was upregulated. Color legend for line representation is illustrated on the upper right panel. (C) T-hybridoma cells expressing Dectin-1-CD3ζ (Dectin-1), as well as B3Z cells expressing Dectin-2 plus FcRγ (Dectin-2), Dectin-3 plus FcRγ (Dectin-3) and Mincle plus FcRγ (Mincle) were stimulated with the indicated numbers of GCPs. T-hybridoma BWZ cells, and B3Z cells only expressing FcRγ were used as controls. After 18 hours, lacZ activity was measured and expressed as OD560 values. Data are the mean ± SEM of triplet wells (Student t -test, *, P < 0.05; ****, P < 0.001). (D) GCPs were incubated with Fc fragment alone, Fc-Dectin-1 or Fc-Dectin-2, followed by staining with PE-conjugated with anti-human Fc antibody. GCPs bound with Dectin-1 and Dectin-2 were visualized with a fluorescent microscope (400x). (E) Overplayed histographs showed the binding intensity of Fc-Dectin-1 and Fc-Dectin 2 to GCPs. Medium fluorescence intensity (MFI) was shown on top of each histographs. GCPs incubated with Fc alone were used as a control.
Consequently, we sought to determine if Dectin-1 and Dectin-2 receptors bind to GCPs using soluble fusion proteins, Fc-Dectin-1 and Fc-Dectin-2, which were created by fusing carbohydrate recognition domain (CRD) of C-type lectin to the Fc fragment of human IgG1 (33, 36). Binding of GCP particles with Fc-Dectin-1 and Fc-Dectin-2, but not Fc fragment alone, was detected by fluorescent microscopy (Fig. 3D). Binding affinity was also validated by FACS analysis, where Dectin-1 and Dectin-2 had greater affinity compared to Fc alone (Fig. 3E).
Dectin-1, Dectin-2 and CARD9 are essential for macrophages to produce inflammatory cytokines after exposure to the GCP-rCpa1 vaccine
BMMs were prepared from C57BL/6 (WT) and deficient mice that lack expression of Dectin-1 (ΔD1) and Dectin-2 (ΔD2). CARD9 is an intracellular immune adaptor downstream of Dectin-1 and Dectin-2 receptors. Thus, BMMs were also prepared from CARD9 deficient mice (ΔC9) for comparison. BMMs from each strains of mice were separately incubated with GCP-rCpa1 or PBS. Amounts of Th1-type (TNF-α, IL-12/p40, IL-12/p70), Th2-type (IL-4, IL-5, IL-13) as well as Th17-type (IL-1α, IL-1β, and IL-6) cytokines were determined and compared among BMMs prepared from these 4 strains of mice (Fig. 4). Wild type BMMs that were incubated with the vaccine elicited a mixed Th1, Th2 and Th17 type response as evident by increased production of TNF-α, IL-12, IL-13, IL-1α, IL-1β, and IL-6 compared to the unstimulated macrophages (*P < 0.05). Notably, BMMs prepared from ΔD1, ΔD2 and ΔC9 mice produced reduced amounts of TNF-α, IL-12, IL-13, and IL-6 compared to those of WT mice (*P < 0.05). Interestingly, the vaccine-treated BMMs isolated from ΔC9 mice significantly increased IL-5 production compared to BMMs of WT mice. Taken together, these data suggest that Dectin-1, Dectin-2 and CARD9 molecules are essential for macrophages to response to the GCP-rCpa1 vaccine in a mixed Th1- and Th17 manner.
FIGURE 4.
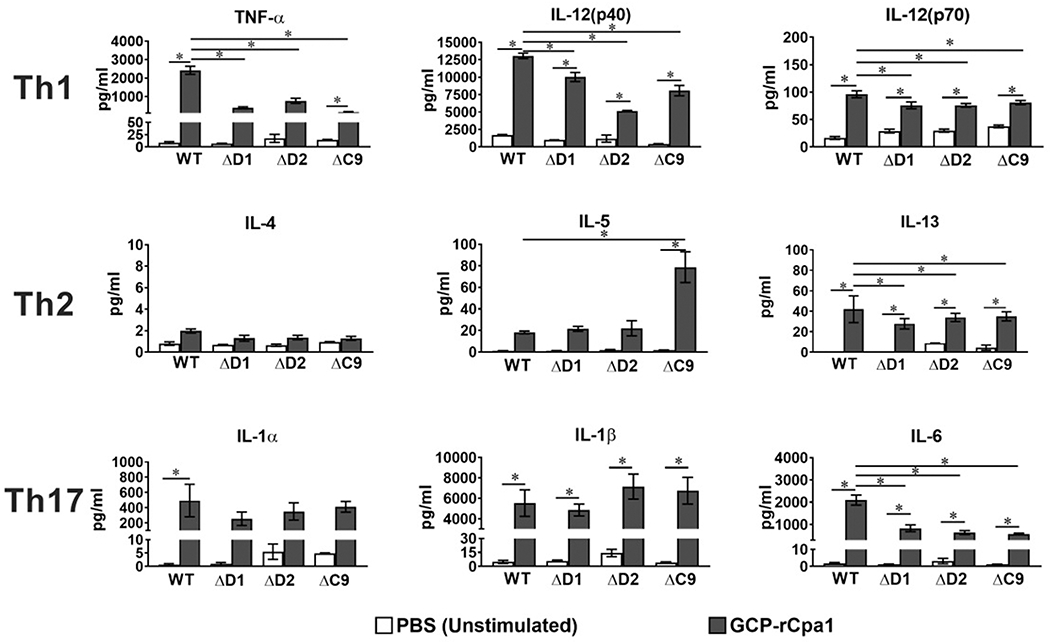
Dectin-1, Dectin-2 and CARD9 are essential for macrophages to produce inflammatory cytokines in response to GCP-rCpa1 vaccine. Selected Th1-, Th2- and Th17-type cytokines including TNF-α, IL-12(p40), IL-12(p70), IL-4, IL-5, IL-13, IL-1α, IL-1β and IL-6 were assayed for bone marrow derived macrophages isolated from WT, Dectin-1−/− (ΔD1), Dectin-2−/− (ΔD2), or CARD9−/− (ΔC9) mice. BMMs were incubated with GCP-rCpa1 at a 1:50 ratio (black bars) or left unstimulated with PBS (white bars) for 24 hours and then cytokine levels were measured from supernatants. GCP-rCpa1 vaccine elicited a strong Th1 and Th17 proinflammatory cytokine response. In the absence of Dectin-1, Dectin-2 or adaptor molecule CARD9, macrophages produced reduced amount of TNF-α, IL-12 and IL-6 (Student t-test; *, P < 0.05). Studies were conducted in triplicate wells and data were the mean ± SEM.
CARD9 deficient mice showed increased fungal burden and reduced Th17 activation in response to GCP-rCpa1 vaccination
We evaluated whether CARD9 molecule was required for the GCP-rCpa1 vaccine to induce protective immunity against Coccidioides infection. Card9−/− mice and wild type littermates (n=10 per group) were subcutaneously vaccinated twice with the GCP-rCpa1 vaccine. Mice immunized with GCP adjuvant alone served as controls. All mice were intranasally challenged with ~80 viable C. posadasii spores followed by the assessment of fungal burden in the lungs and spleen at 14 dpc. As expected, ΔC9 mice that were vaccinated with the GCP-rCpa1 vaccine had significantly elevated CFUs in their lungs and spleen compared to vaccinated WT mice (Fig. 5A; #P < 0.05). We further evaluated T cells that were activated and recruited to the Coccidioides-infected lungs of vaccinated and control mice at 7 and 14 dpc. Gating strategies for subsets of CD4+ Th cells (Th1 and Th17) and CD8+ Tc cells (Tc1 and Tc17) were based on their differentiation expression of CD45, CD4, CD8, IFN-γ and IL-17A as shown in Supp. Fig. 3S. Concurringly, percentages and total numbers of Th17 cells in the lungs of vaccinated ΔC9 mice were significantly reduced compared to vaccinated WT mice (Fig. 5B and 5C; #P < 0.05). In contrast, percentages and total numbers of Th1 cells in the lungs of vaccinated ΔC9 were not significantly reduced compared to wild type mice. These results demonstrate that CARD9 is necessary for the GCP-rCpa1-mediated activation of Th17 cells and protective immunity against pulmonary coccidioidomycosis in mice.
FIGURE 5.
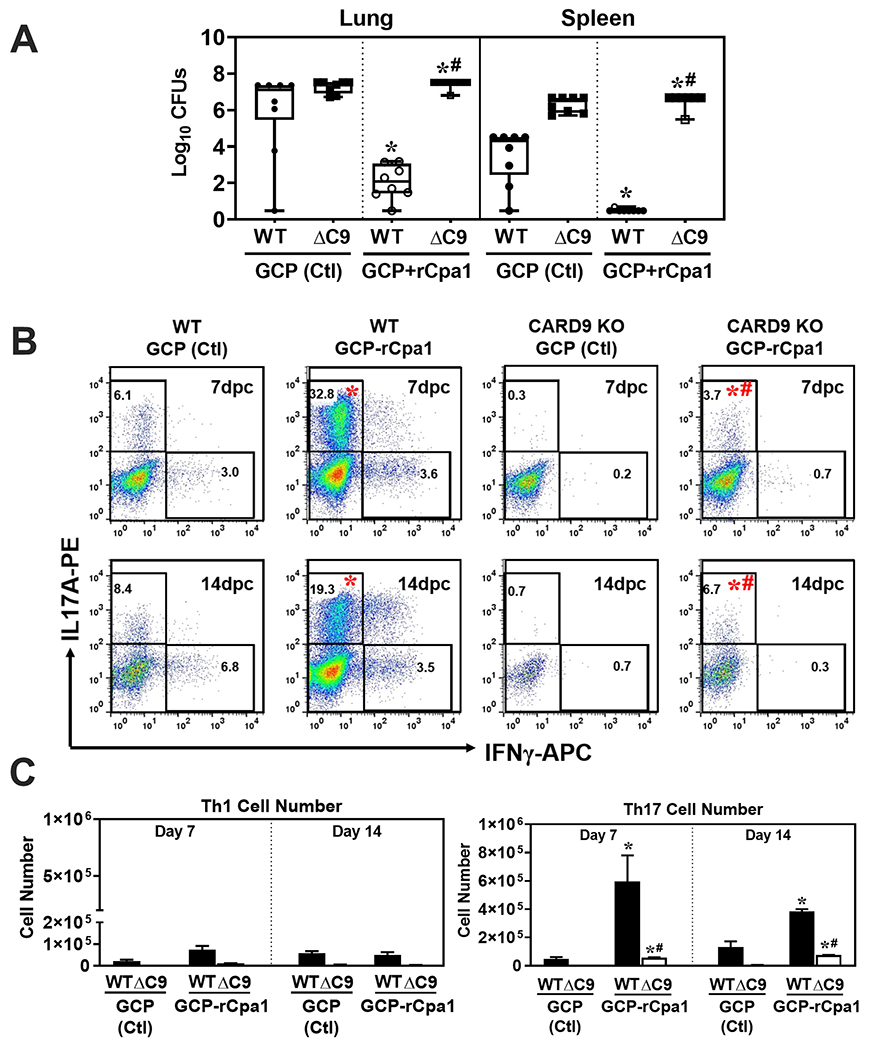
CARD9 deficient mice have a reduced protective efficacy concurring with reduced Th17 response to the GCP-rCpa1 vaccine. WT and Card9−/− (ΔC9) mice (n=10) were subcutaneously immunized with GCP adjuvant alone or GCP-rCpa1 vaccine twice and then intranasally challenged with a lethal dose of Coccidioides spores. (A) Fungal CFUs were detected by dilution plate culture of lung and spleen homogenates at 14 dpc. CFUs are reported in box plots and asterisks indicate statistically significant differences (Mann-Whitney U test; p <0.05) between the vaccinated and nonvaccinated mice of the same strain, while the hashtags indicate significant differences between these 2 strains of mice (Mann-Whitney U test, P< 0.05). (B) Representation plots of gated IL-17a- and IFN-γ-expressing Th17 and Th1 cells derived from the lungs of WT and ΔC9 mice were analyzed by the intracellular cytokine staining method. (C) Data were processed using FlowJo X software. Total numbers of Th1 and Th17 cells were determined for both vaccinated WT and ΔC9 mice at 7 and 14 dpc and compared to those of nonvaccinated mice. Data are mean values ± SEM of 3-4 mice per group. For statistical analysis of cell population, asterisks indicate significantly higher percentage (B) and cell numbers (C) of the responsive T-cell phenotypes in lungs of vaccinated compared to nonvaccinated mice of the same strain, while hashtags represent significant differences in the vaccinated ΔC9 mice compared to WT mice (Mann-Whitney U test, P< 0.05).
Dectin-1 and Dectin-2 deficient mice also displayed increased fungal burden and reduced Th17 response after vaccination with the GCP-rCpa1 vaccine
We further investigated the impact of Dectin-1 and Dectin-2 in the development of protective immune response to GCP-rCpa1 vaccination using Dectin-1−/− (ΔD1) and Dectin-2−/− (ΔD2) mice. WT mice, which were immunized with GCP-rCpa1 or GCP alone served as controls. These 3 strains of mice (WT, ΔD1 and ΔD2) that were vaccinated with the GCP-rCpa1 vaccine displayed significantly reduced CFUs in their lungs and spleens compared to their corresponding control mice of the same strain (Fig. 6A, Mann Whitney U test; * p < 0.05). Importantly, the vaccinated ΔD1 and ΔD2 mice showed elevated CFUs in their lungs and spleens compared to the vaccinated WT mice (Fig. 6A; #p < 0.05). These results indicate that Dectin-1 and Dectin-2 receptors are required for the GCP-rCpa1 vaccine to induce protective immune responses against pulmonary C. posadasii infection. We further detected that all three strains of vaccinated mice (WT, ΔD1 and ΔD2) had significantly elevated percentages and total numbers of Th17 cells in the gated CD4+ T cell populations at 7 and 14 dpc compared to nonvaccinated mice (Fig. 6B and 6C). Importantly, the total numbers of Th17 cells of vaccinated ΔD1and ΔD2 mice were significantly reduced compared to vaccinated WT mice (FIG. 6B and 6C). Contrarily, the total numbers of Th1 cells in the lungs were comparable between vaccinated and control mice of all three strains (Fig. 6C). Assessment of percentages and total numbers of CD8+ T cells (Tc1 and Tc17) subsets in the lungs of vaccinated and control mice showed comparable results among these three strains of mice (data not shown). These results suggested that both Dectin-1 and Dectin-2 were required for the activation of Th17 cells that were associated with GCP-rCpa1-induced protection against pulmonary coccidioidomycosis.
FIGURE 6.
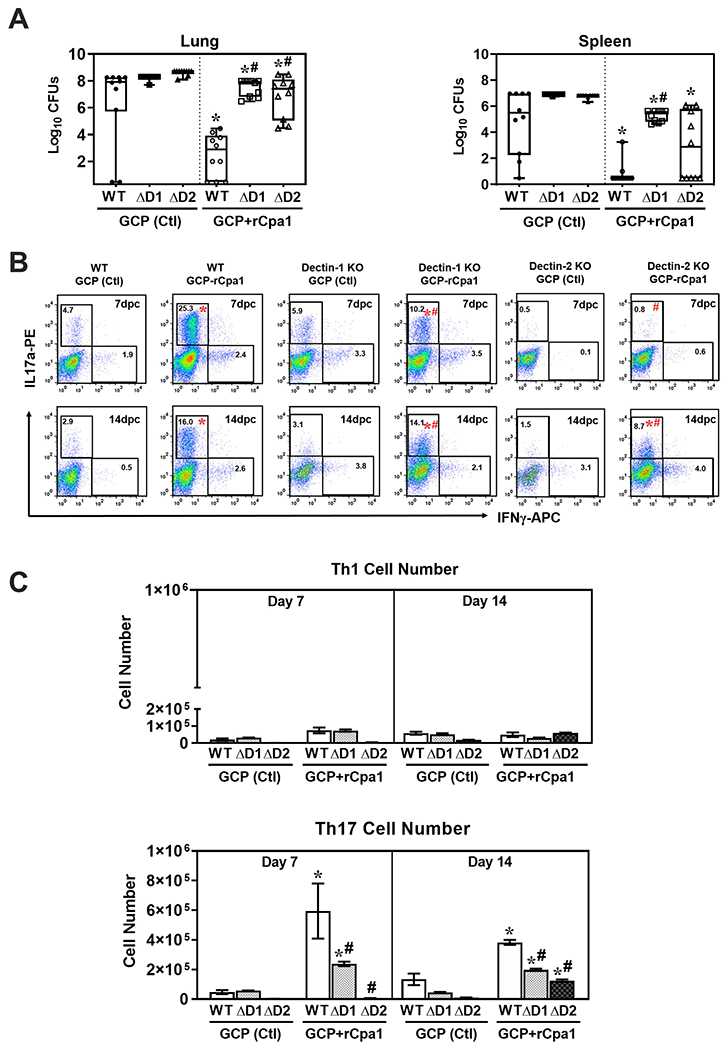
Protective efficacy of the GCP-rCpa1 vaccine was reduced in both Dectin-1 and Dectin-2 deficient mice. Groups of 10 mice (WT, ΔD1 and ΔD2) were immunized with GCP adjuvant alone or GCP-rCpa1 vaccine twice. (A) Coccidioides CFUs were detected by dilution plate culture of lung and spleen homogenates at 14 dpc. Asterisks indicate statistically significant differences (Mann-Whitney U test; p <0.05) between the vaccinated and nonvaccinated mice of the same strains, while the hashtags indicate significant differences among these 3 strains of mice (Kruskal-Wallis ranking test; P< 0.05). (B) Flow cytometry analysis of IL-17a- and IFN-γ-expressing Th17 and Th1 cells, respectively, were recorded and compared for lung homogenates derived from vaccinated and control WT, ΔD1 and ΔD2 mice at 7 and 14 dpc. Representative plots of percentages gated on CD4+ T cells positive for IFN-γ IL-17a at 7 and 14 dpc. (C) The numbers of Th1 and Th17 cells in the lungs were plotted for vaccinated and nonvaccinated mice at 7 and 14 dpc. For statistical analysis of cell population, asterisks indicate significantly higher percentage (B) and absolute numbers (C) of the responsive T-cell phenotypes in lungs of vaccinated compared to nonvaccinated mice of the same strain, while hashtags represent significant differences in the ΔD1 and ΔD2 mice compared to WT mice (Mann-Whitney U test, P< 0.05 test).
Discussion
Vaccination has been shown historically to be one of the most effective methods for controlling infectious diseases (37). While the majority of clinical vaccines today contain whole cells of attenuated, live or killed organisms, subunit vaccines designed on a defined antigen or antigens are attractive prospect for a number of reasons (38). First, subunit antigens offer enhanced safety profiles and they eliminate the need of attenuated live organisms or killed whole cells. Second, subunit antigens tend to have lower reactogenicity than whole-cell vaccines, which may lead to severe inflammation at the vaccination sites or systemic symptoms (25). Third, subunit antigens can be fully characterized to ensure vaccine reproducibility and quality. The overall purity and simplicity of subunit antigens, however, comes at a price. Subunit antigens require an adjuvant to enhance immunoreactivity. The adjuvant components constitute a diverse group of compounds, which are typically categorized as either immunostimulators or delivery systems (39). We have previously created a subunit vaccine that is composed of a recombinant multivalent antigen (rCpa1) and yeast-derived glucan-chitin particles (GCPs) against pulmonary coccidioidomycosis (10). GCPs serve as a combination adjuvant and vaccine delivery system similar to the previously reported pure glucan particles (GPs) (18, 19, 40). Both GPs and GCPs can stimulate a mixed Th1 and Th17 immunity, while GCPs elicit an augmented Th17 response compared to GPs (10). The GCP-rCpa1 vaccine has low cytotoxicity (Supp. Fig. 1S) and shows minimal advisory effect upon subcutaneous immunization in our previous report (10). Vaccination of mice with this subunit vaccine achieves comparable protection generated by an attenuated live vaccine ΔT against coccidioidomycosis (10). Combination of low cytotoxicity with defined antigen composition, a Th17 boosting adjuvant, and a marked protective efficacy provide the advantage of the GCp-rCpa1 subunit vaccine over currently available experimental coccidioidal vaccines. Accumulated evidences have demonstrated the importance of Th17 immunity in protection against various fungal infections (41, 42). Furthermore, with the increase of clinical anti-IL-17 treatments against autoimmune diseases (e.g. psoriasis) more individuals may become susceptible to fungal infections (43, 44). These studies shed light on the need to elucidate the role of T helper cells in vaccine designs against fungal infections. In this study, we have extended our previous findings to show that GCP-rCpa1-induced protection and enhancement of Th17 responses are mediated through CARD9-associated Dectin-1 and Dectin-2 molecules as illustrated in Fig. 7. This conclusion is supported by both in vitro and in vivo experiments using bone-marrow-derived macrophages and T-hybridoma reporter cells and a murine model of pulmonary coccidioidomycosis, respectively. Macrophages that are isolated from Dectin-1, Dectin-2 or CARD9 knockout mice produced less IL-12, IL-1 and IL-6 cytokines in response to GCP-rCpa1 stimulation. These macrophage cytokines are required for shaping the differentiation and development of Th1 and Th17 cells. Furthermore, protective efficacy and the activation of Th17 cells responding to vaccination with GCP-rCpa1 are also significantly reduced in Dectin-1, Dectin-2 (Fig. 6) or CARD9 (Fig. 5) knockout mice. We have previously reported that GCP-rCpa1 vaccination stimulated a mixed Th17 and Th1 responses by immune CD4+ cell recall with rCpa1 via the assessment of pulmonary Th cells in vaccinated mice following pulmonary challenge with Coccidioides spores (10). Although there is a much stronger and sustained Th17 response compared to Th1, our RNA-seq analysis did reveal cellular response to TNF and IL-12 in BMMs stimulated by GCP-rCpa1 implying subsequent Th1 immunity could be generated. Investigation of the role of Th1 immunity in GCP-rCpa1 mediated protection against coccidioidal infection is currently underway.
FIGURE 7.
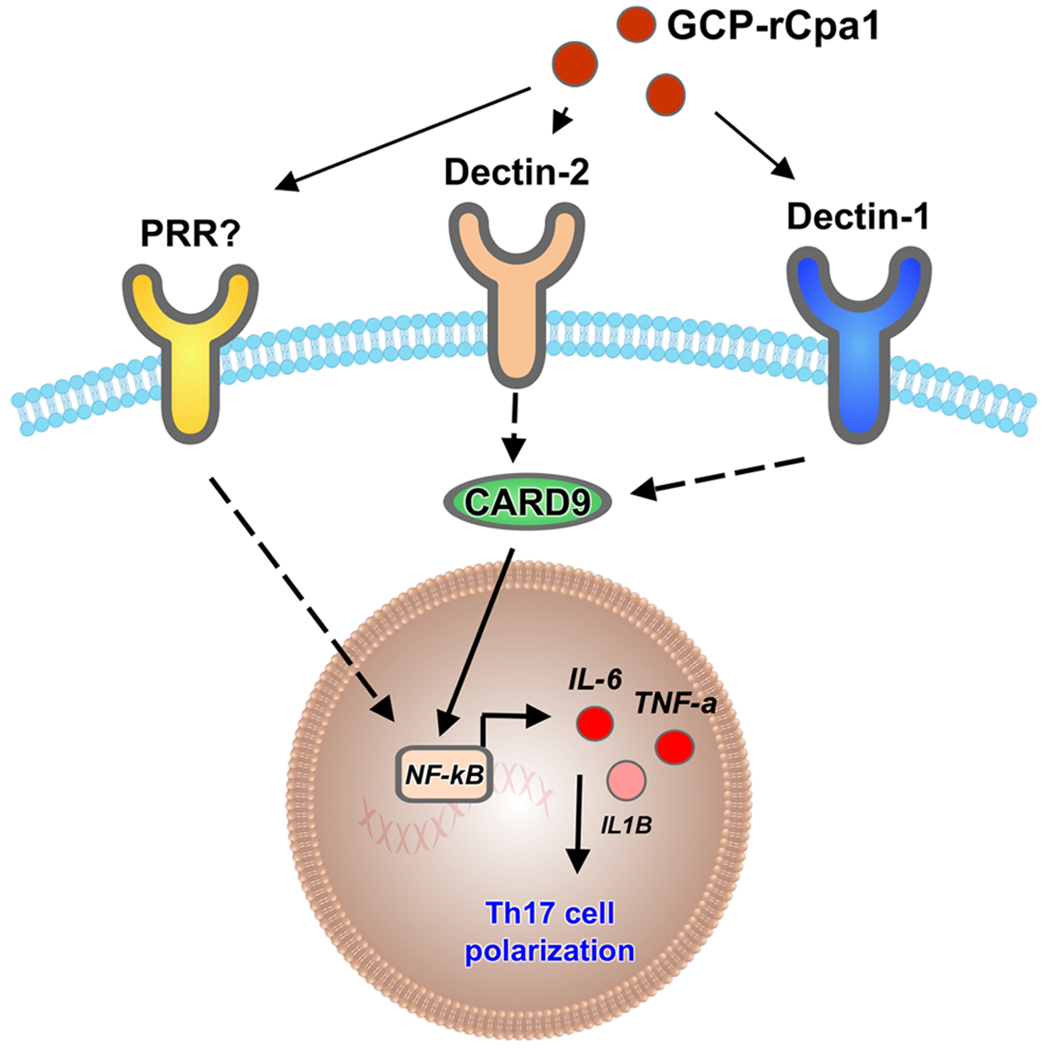
Schematic illustration of GCP-rCPa1 vaccine-induced protective immune response. Dectin-1, Dectin-2 and other yet unidentified pattern recognition receptors on antigen presenting cells interact with the GCP-rCpa1 vaccine to facilitate antigen presentation to T cells. Interaction of Dectin-1 and Dectin 2 trigger the activation of intracellular CARD9 adaptor molecule. CARD9 and other signal molecules form a complex to regulate the activation of downstream transcription factors such as NF-κB that induces the expression of cytokines TFN-α, IL-12, IL-6, IL-1α and IL-1β. These pro-inflammatory cytokines lead to a robust Th17 response against Coccidioides infection.
Various T cell subsets with different cytokine production properties and functionalities have been identified as protective immune responses against fungal infections (45). IFN-γ-producing Th1 cells promotes clearance of Histoplasma capsulatum and Cryptococcus neoformans infections in the diseased lungs (46, 47). Likewise, IL-17- producing Th17 cells are an essential arm of protective immunity against Blastomyces dermatitidis infection (13, 33). Finding the optimal combination of T-cell subpopulations to be induced by vaccines against Coccidioides infection remains elusive (9). We have previously reported that subcutaneous vaccination of IFN-γ and IL-4 receptor knockout mice with an attenuated, live vaccine (ΔT) derived from Coccidioides posadasii C735 isolate still induced protective immunity against pulmonary challenge with an autologous virulent isolate (11). In contrast, fungal burden, clearance and survival are significantly compromised in mice defective in IL-17A or IL-17 receptor (11). Similarly, we demonstrated in this study that depletion of IL-17A in mice reduces protective efficacy of the GCP-rCpa1 vaccine against a pulmonary challenge with C. posadasii C735 isolate. Interestingly, an attenuated live vaccine (ΔCps1) created from C. posadasii Silveira isolate elicits a Th1-biased response that confers protection against a pulmonary challenge with the autologous isolate, or C. immitis RS isolate (48, 49). The genetic background of the attenuated, live vaccines and the virulent isolates used for challenge in those experiments may attribute to the differences in immune responses.
The identification and development of novel adjuvants capable of inducing a Th17 or Th1/Th17 adaptive responses for enhancing protective efficacy of recombinant protein antigens will provide new opportunities to advance vaccines against bacterial and fungal pathogens. Several infectious diseases of significant public health concerns could benefit from a Th17-inducing adjuvant system including Mycobacterium tuberculosis, Staphylococcus aureus, Pseudomonas aeruginosa, Streptococcus pneumonia, Candida albicans, Aspergillus fumigatus (17, 45, 50). Cationic adjuvant formulation 1 (CAF01) and derivatives of trehalose diesters that can bind to Mincle receptor on macrophages were used with subunit vaccines against Mycobacterium tuberculosis (16, 51). A nanoemulsification adjuvant made of soybean oil with cetyl pyridinium chloride, Tween 80 and ethanol in water has been shown to induce a Th1 and Th17 mixed immune response against mucosal viral infection (15). Yeast cell-wall derived particles (GPs and GCPs) are among various adjuvants currently under evaluation with subunit vaccines to stimulate a mixed Th1 and Th17 response against fungal infections (10, 19, 40). The use of GCPs as an adjuvant and vaccine delivery vehicle are ideal for fungal infections, which typically rely on IL-17 and IFN-γ for clearance (10, 13, 40, 45, 52). These particles can be packaged with multiple antigens as well as other immunomodulatory molecules such as TLR agonists, DNA, RNA among others. GCPs will be mainly recognized and internalized by antigen presenting cells since they typically bare pattern recognition receptors.
Recent reviews have highlighted the importance of C-type lectin receptors in shaping immune responses to fungal infections (53, 54). Interaction of CLRs with fungal carbohydrates induces intracellular activation of signals that trigger responses ranging from cytokine production to induction of adaptive immunity. Our data show that immune responses to GCPs are dependent on Dectin-1 and Dectin-2, but not Dectin-3 and Mincle. GCP particles are comprised of ~50–60% β-glucans, 20-30% chitin/chitosan and <1% of mannans (20). The major GCP component, β-glucan is mainly recognized by Dectin-1, a member of C-type lectin receptors expressed on the surface of macrophages and dendritic cells. Dectin-1 is essential for recognition and activation of protective immunity against Coccidioides (33, 55–57). Dectin-1 deficient mice reduced expression of Th1 and Th17 cytokines required for vaccine immunity against pulmonary Coccidioides infection (26, 33). Similarly, human Dectin-1 deficiency predisposes patients to vulvovaginal candidiasis and onychomycosis. Mannans in GCPs are likely recognized by Dectin-2 in order to stimulate an adaptive immunity against fungal infections (53). Our data are in agreement with the role of Dectin-2 in activation of adaptive Th1 and Th17 immunity against pulmonary coccidioidomycosis as shown for the live-attenuated ∆T vaccine (33). While Dectin-2 is required for development of adaptive immune response to Coccidioides infection, it is not essential for inducing innate immunity against this disease (58).
Proteomic analysis has shown that Rhodotorula mucilaginosa yeasts used to prepare GCP adjuvant express chitin deacetylase. The enzyme converts chitin to chitosan, the deacetylated form of chitin (59). Binding assays using a chitin binding dye, calcofluor white, suggest that chitin may be more accessible in fungal cell wall of Rhodotorula mucilaginosa than that of Cryptococcus species (60). The receptors on myeloid cells that bind chitin or chitosan have yet definitively identified while a study shows that fungal chitin can induce pro-inflammatory cytokines such as IL-6 and TNF-α via TLR2 signaling in murine and human monocytes (61). In contrast, a prior study showed that chitosan, but not chitin, can stimulate the NLRP3 inflammasomes in activated BMMs to enhance IL-1β release (62). IL-1β-mediated response is essential for vaccine immunity against pulmometry coccidioidomycosis (35, 63). Our data have also revealed that GCPs stimulate murine BMMs to produce and secret IL-1β (Fig. 2 and Fig. 4) although the ratio of chitosan to chitin in GCPs is still under investigation.
CARD9 is a critical intracellular adaptor protein that operates downstream of several ITAM-associated CLRs, including Dectin-1, Dectin-2, Dectin-3 and Mincle receptors (64). CARD9 complex regulates the activation of NF-кB pathway that triggers the transcription of IL-6, IL-1α and IL-1β, which further drive the activation of Th17 response as illustrated in Fig. 7. CARD9 is mainly expressed in myeloid and epithelial cells (23). Mice deficient in CARD9 expression have increased susceptibility to fungal infections. CARD9 is also necessary for resistance to Coccidioides infection (26). CARD9−/− mice tend to be more susceptible to Coccidioides infection compared to Dectin-1−/− and Dectin-2−/− mice, suggesting that these two CLRs have additive or synergistic effect in recognition of GCPs. It is also possible that other CLRs upstream of CARD9 are involved in the response to GCP stimulation. Furthermore, TLR2 and TLR6 upstream of MyD88 are also predicted to respond to GCP stimulation in our global gene analysis. These receptors may recognize GCPs by interacting with specific linages of β-glucan and chitin/chitosan that have not been identified yet. Further studies are underway to delineate the roles of TLRs in response to this novel Coccidioides subunit vaccine.
Supplementary Material
Key point:
IL-17A is critical for GCP-rCpa1 vaccine mediated protection against Coccidioides.
GCP-rCpa1 is recognized by macrophages expressing Dectin-1- and Dectin-2.
GCP-rCpa1 activates CLR-CARD9 signaling to induce protective Th17 immunity.
ACKNOWLEDGEMENTS
We thank Dr. Sandra Cardona, Manager of the Cell Analysis Core at UTSA for excellent technical assistance in flow cytometry analysis.
This work was supported by National Institutes of Health Grants R01AI135005 (to C.Y.H), AI093553 (to M.W.), AI035681 (to B.K.), and AI040996 (to B.K. and M.W.).
Abbreviations used in this article:
- Ag2/Pra
Antigen 2/Proline-rich protein
- BMM
bone marrow derived macrophage
- CARD9
caspase recruitment domain containing protein 9
- CFU
colony-forming unit
- CLR
C-type lectin receptor
- Cs-Ag
Coccidioides-specific antigen
- ΔC9
CARD9 deficient mice
- ΔD1
Dectin-1 deficient mice
- ΔD2
Dectin-2 deficient mice
- dpc
days postchallenge
- FcRγ
Fc receptor gamma chain
- GCP
glucan-chitin-particle
- ITAM
immunoreceptor tyrosine-based activation motif
- Mincle
macrophage inducible Ca2+-dependent lectin receptor
- NFAT
nuclear factor of activated T-cell transcription factor
- Pmp1
peroxisomal membrane protein 1
- rCpa1
recombinant Coccidioides polypeptide antigen
- WT
wild type
Footnotes
The online version of this article contains supplemental materials.
REFERENCES
- 1.Brown J, Benedict K, Park BJ, and Thompson GR 3rd. 2013. Coccidioidomycosis: epidemiology. Clin Epidemiol 5: 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Geertsma F, Hoover SE, Johnson RH, Kusne S, Lisse J, MacDonald JD, Meyerson SL, Raksin PB, Siever J, Stevens DA, Sunenshine R, and Theodore N. 2016. 2016 Infectious Diseases Society of America (IDSA) clinical practice guideline for the treatment of coccidioidomycosis. Clin. Infect. Dis 63: e112–146. [DOI] [PubMed] [Google Scholar]
- 3.Kirkland TN 2016. The quest for a vaccine against coccidioidomycosis: a neglected disease of the Americas. J Fungi (Basel) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marsden-Haug N, Goldoft M, Ralston C, Limaye AP, Chua J, Hill H, Jecha L, Thompson GR 3rd, and Chiller T. 2013. Coccidioidomycosis acquired in Washington State. Clin. Infect. Dis 56: 847–850. [DOI] [PubMed] [Google Scholar]
- 5.Litvintseva AP, Marsden-Haug N, Hurst S, Hill H, Gade L, Driebe EM, Ralston C, Roe C, Barker BM, Goldoft M, Keim P, Wohrle R, Thompson GR 3rd, Engelthaler DM, Brandt ME, and Chiller T. 2015. Valley fever: finding new places for an old disease: Coccidioides immitis found in Washington State soil associated with recent human infection. Clin. Infect. Dis 60: e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oltean HN, Etienne KA, Roe CC, Gade L, McCotter OZ, Engelthaler DM, and Litvintseva AP. 2019. Utility of whole-genome sequencing to ascertain locally acquired cases of coccidioidomycosis, Washington, USA. Emerg. Infect. Dis 25: 501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole GT, Hurtgen BJ, and Hung CY. 2012. Progress toward a human vaccine against coccidioidomycosis. Curr Fungal Infect Rep 6: 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox RA, and Magee DM. 2004. Coccidioidomycosis: host response and vaccine development. Clin. Microbiol. Rev 17: 804–839, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hung CY, Hsu AP, Holland SM, and Fierer J. 2019. A review of innate and adaptive immunity to coccidioidomycosis. Medical mycology 57: S85–s92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hung CY, Zhang H, Castro-Lopez N, Ostroff GR, Khoshlenar P, Abraham A, Cole GT, Negron A, Forsthuber T, Peng T, Galgiani JN, Ampel NM, and Yu JJ. 2018. Glucan-chitin particles enhance Th17 response and improve protective efficacy of a multivalent antigen (rcpa1) against pulmonary Coccidioides posadasii infection. Infect. Immun 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hung CY, Gonzalez A, Wuthrich M, Klein BS, and Cole GT. 2011. Vaccine immunity to coccidioidomycosis occurs by early activation of three signal pathways of T helper cell response (Th1, Th2, and Th17). Infect. Immun 79: 4511–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ampel NM, Robey I, Nguyen CT, Roller B, August J, Knox KS, and Pappagianis D. 2018. Ex vivo cytokine release, determined by a multiplex cytokine assay, in response to coccidioidal antigen stimulation of whole blood among subjects with recently diagnosed primary pulmonary coccidioidomycosis. mSphere 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wuthrich M, Gern B, Hung CY, Ersland K, Rocco N, Pick-Jacobs J, Galles K, Filutowicz H, Warner T, Evans M, Cole G, and Klein B. 2011. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J. Clin. Invest 121: 554–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurtgen BJ, Castro-Lopez N, Jimenez-Alzate MDP, Cole GT, and Hung CY. 2016. Preclinical identification of vaccine induced protective correlates in human leukocyte antigen expressing transgenic mice infected with Coccidioides posadasii. Vaccine 34: 5336–5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bielinska AU, Gerber M, Blanco LP, Makidon PE, Janczak KW, Beer M, Swanson B, and Baker JR Jr. 2010. Induction of Th17 cellular immunity with a novel nanoemulsion adjuvant. Crit. Rev. Immunol 30: 189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith AJ, Miller SM, Buhl C, Child R, Whitacre M, Schoener R, Ettenger G, Burkhart D, Ryter K, and Evans JT. 2019. Species-specific structural requirements of alpha-branched trehalose diester mincle agonists. Front Immunol 10: 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodworth JS, Christensen D, Cassidy JP, Agger EM, Mortensen R, and Andersen P. 2019. Mucosal boosting of H56:CAF01 immunization promotes lung-localized T cells and an accelerated pulmonary response to Mycobacterium tuberculosis infection without enhancing vaccine protection. Mucosal Immunol. 12: 816–826. [DOI] [PubMed] [Google Scholar]
- 18.Mirza Z, Soto ER, Dikengil F, Levitz SM, and Ostroff GR. 2017. Beta-glucan particles as vaccine adjuvant carriers. Methods Mol. Biol 1625: 143–157. [DOI] [PubMed] [Google Scholar]
- 19.Specht CA, Lee CK, Huang H, Hester MM, Liu J, Luckie BA, Torres Santana MA, Mirza Z, Khoshkenar P, Abraham A, Shen ZT, Lodge JK, Akalin A, Homan J, Ostroff GR, and Levitz SM. 2017. Vaccination with recombinant Cryptococcus proteins in glucan particles protects mice against cryptococcosis in a manner dependent upon mouse strain and cryptococcal species. MBio 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young SH, Ostroff GR, Zeidler-Erdely PC, Roberts JR, Antonini JM, and Castranova V. 2007. A comparison of the pulmonary inflammatory potential of different components of yeast cell wall. J. Toxicol. Environ. Health A 70: 1116–1124. [DOI] [PubMed] [Google Scholar]
- 21.Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, and Brown GD. 2007. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat. Immunol 8: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, Fujikado N, Kusaka T, Kubo S, Chung SH, Komatsu R, Miura N, Adachi Y, Ohno N, Shibuya K, Yamamoto N, Kawakami K, Yamasaki S, Saito T, Akira S, and Iwakura Y. 2010. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32: 681–691. [DOI] [PubMed] [Google Scholar]
- 23.Hsu YM, Zhang Y, You Y, Wang D, Li H, Duramad O, Qin XF, Dong C, and Lin X. 2007. The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nat. Immunol 8: 198–205. [DOI] [PubMed] [Google Scholar]
- 24.Hurtgen BJ, Hung CY, Ostroff GR, Levitz SM, and Cole GT. 2012. Construction and evaluation of a novel recombinant T cell epitope-based vaccine against Coccidioidomycosis. Infect. Immun 80: 3960–3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xue J, Chen X, Selby D, Hung CY, Yu JJ, and Cole GT. 2009. A genetically engineered live attenuated vaccine of Coccidioides posadasii protects BALB/c mice against coccidioidomycosis. Infect. Immun 77: 3196–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hung CY, Castro-Lopez N, and Cole GT. 2016. Card9- and MyD88-mediated gamma interferon and nitric oxide production is essential for resistance to subcutaneous Coccidioides posadasii infection. Infect. Immun 84: 1166–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campuzano A, Castro-Lopez N, Wozniak KL, Leopold Wager CM, and Wormley FL Jr. 2017. Dectin-3 is not required for protection against Cryptococcus neoformans infection. PLoS ONE 12: e0169347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frankish A, Diekhans M, Ferreira AM, Johnson R, Jungreis I, Loveland J, Mudge JM, Sisu C, Wright J, Armstrong J, Barnes I, Berry A, Bignell A, Carbonell Sala S, Chrast J, Cunningham F, Di Domenico T, Donaldson S, Fiddes IT, Garcia Giron C, Gonzalez JM, Grego T, Hardy M, Hourlier T, Hunt T, Izuogu OG, Lagarde J, Martin FJ, Martinez L, Mohanan S, Muir P, Navarro FCP, Parker A, Pei B, Pozo F, Ruffier M, Schmitt BM, Stapleton E, Suner MM, Sycheva I, Uszczynska-Ratajczak B, Xu J, Yates A, Zerbino D, Zhang Y, Aken B, Choudhary JS, Gerstein M, Guigo R, Hubbard TJP, Kellis M, Paten B, Reymond A, Tress ML, and Flicek P. 2019. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res 47: D766–d773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walter W, Sanchez-Cabo F, and Ricote M. 2015. GOplot: an R package for visually combining expression data with functional analysis. Bioinformatics 31: 2912–2914. [DOI] [PubMed] [Google Scholar]
- 30.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, and Sherlock G. 2000. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet 25: 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conesa A, Madrigal P, Tarazona S, Gomez-Cabrero D, Cervera A, McPherson A, Szczesniak MW, Gaffney DJ, Elo LL, Zhang X, and Mortazavi A. 2016. A survey of best practices for RNA-seq data analysis. Genome Biol. 17: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hung CY, Castro-Lopez N, and Cole GT. 2014. Vaccinated C57BL/6 mice develop protective and memory T cell responses to Coccidioides posadasii infection in the absence of interleukin-10. Infect. Immun 82: 903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H, LeBert V, Hung CY, Galles K, Saijo S, Lin X, Cole GT, Klein BS, and Wuthrich M. 2014. C-type lectin receptors differentially induce Th17 cells and vaccine immunity to the endemic mycosis of North America. J. Immunol 192: 1107–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wuthrich M, Wang H, Li M, Lerksuthirat T, Hardison SE, Brown GD, and Klein B. 2015. Fonsecaea pedrosoi-induced Th17-cell differentiation in mice is fostered by Dectin-2 and suppressed by Mincle recognition. Eur. J. Immunol 45: 2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hung CY, Jimenez-Alzate Mdel P, Gonzalez A, Wuthrich M, Klein BS, and Cole GT. 2014. Interleukin-1 receptor but not Toll-like receptor 2 is essential for MyD88-dependent Th17 immunity to Coccidioides infection. Infect. Immun 82: 2106–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steele C, Rapaka RR, Metz A, Pop SM, Williams DL, Gordon S, Kolls JK, and Brown GD. 2005. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 1: e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Panhuis WG, Grefenstette J, Jung SY, Chok NS, Cross A, Eng H, Lee BY, Zadorozhny V, Brown S, Cummings D, and Burke DS. 2013. Contagious diseases in the United States from 1888 to the present. N. Engl. J. Med 369: 2152–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plotkin SA 2015. Increasing Complexity of Vaccine Development. The Journal of Infectious Diseases 212: S12–S16. [DOI] [PubMed] [Google Scholar]
- 39.Christensen D 2016. Vaccine adjuvants: Why and how. Human vaccines & immunotherapeutics 12: 2709–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deepe GS Jr., Buesing WR, Ostroff GR, Abraham A, Specht CA, Huang H, and Levitz SM. 2018. Vaccination with an alkaline extract of Histoplasma capsulatum packaged in glucan particles confers protective immunity in mice. Vaccine 36: 3359–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sparber F, and LeibundGut-Landmann S. 2019. Interleukin-17 in antifungal immunity. Pathogens 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Speakman EA, Dambuza IM, Salazar F, and Brown GD. 2020. T cell antifungal immunity and the role of C-type lectin receptors. Trends Immunol. 41: 61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark C, and Drummond RA. 2019. The hidden cost of modern medical interventions: how medical advances have shaped the prevalence of human fungal disease. Pathogens 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farahnik B, Beroukhim K, Zhu TH, Abrouk M, Nakamura M, Singh R, Lee K, Bhutani T, and Koo J. 2016. Ixekizumab for the treatment of psoriasis: A review of phase III trials. Dermatol Ther (Heidelb) 6: 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDermott AJ, and Klein BS. 2018. Helper T-cell responses and pulmonary fungal infections. Immunology 155: 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kroetz DN, and Deepe GS. 2012. The role of cytokines and chemokines in Histoplasma capsulatum infection. Cytokine 58: 112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wormley FL Jr., Perfect JR, Steele C, and Cox GM. 2007. Protection against cryptococcosis by using a murine gamma interferon-producing Cryptococcus neoformans strain. Infect. Immun 75: 1453–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shubitz LF, Powell DA, Trinh HT, Lewis ML, Orbach MJ, Frelinger JA, and Galgiani JN. 2018. Viable spores of Coccidioides posadasii Deltacps1 are required for vaccination and provide long lasting immunity. Vaccine 36: 3375–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Narra HP, Shubitz LF, Mandel MA, Trinh HT, Griffin K, Buntzman AS, Frelinger JA, Galgiani JN, and Orbach MJ. 2016. A Coccidioides posadasii CPS1 deletion mutant is avirulent and protects mice from lethal infection. Infect. Immun 84: 3007–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li W, Zhang X, Yang Y, Yin Q, Wang Y, Li Y, Wang C, Wong SM, Wang Y, Goldfine H, Akerley BJ, and Shen H. 2018. Recognition of conserved antigens by Th17 cells provides broad protection against pulmonary Haemophilus influenzae infection. Proc. Natl. Acad. Sci. U. S. A 115: E7149–e7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pedersen GK, Andersen P, and Christensen D. 2018. Immunocorrelates of CAF family adjuvants. Semin. Immunol 39: 4–13. [DOI] [PubMed] [Google Scholar]
- 52.Cole GT, Hung CY, Sanderson SD, Hurtgen BJ, Wuthrich M, Klein BS, Deepe GS, Ostroff GR, and Levitz SM. 2013. Novel strategies to enhance vaccine immunity against coccidioidomycosis. PLoS Pathog. 9: e1003768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drummond RA, and Lionakis MS. 2016. Mechanistic insights into the role of C-type lectin receptor/CARD9 signaling in human antifungal immunity. Front Cell Infect Microbiol 6: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goyal S, Castrillon-Betancur JC, Klaile E, and Slevogt H. 2018. The interaction of human pathogenic fungi with C-type lectin receptors. Front Immunol 9: 1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Viriyakosol S, Fierer J, Brown GD, and Kirkland TN. 2005. Innate immunity to the pathogenic fungus Coccidioides posadasii is dependent on Toll-like receptor 2 and Dectin-1. Infect. Immun 73: 1553–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.del Pilar Jimenez AM, Viriyakosol S, Walls L, Datta SK, Kirkland T, Heinsbroek SE, Brown G, and Fierer J. 2008. Susceptibility to Coccidioides species in C57BL/6 mice is associated with expression of a truncated splice variant of Dectin-1 (Clec7a). Genes Immun. 9: 338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Viriyakosol S, Jimenez Mdel P, Gurney MA, Ashbaugh ME, and Fierer J. 2013. Dectin-1 is required for resistance to coccidioidomycosis in mice. MBio 4: e00597–00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Viriyakosol S, Jimenez Mdel P, Saijo S, and Fierer J. 2014. Neither dectin-2 nor the mannose receptor is required for resistance to Coccidioides immitis in mice. Infect. Immun 82: 1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ilyas S, Rehman A, Coelho AV, and Sheehan D. 2016. Proteomic analysis of an environmental isolate of Rhodotorula mucilaginosa after arsenic and cadmium challenge: Identification of a protein expression signature for heavy metal exposure. J Proteomics 141: 47–56. [DOI] [PubMed] [Google Scholar]
- 60.Yockey J, Andres L, Carson M, Ory JJ, and Reese AJ. 2019. Cell envelope integrity and capsule characterization of Rhodotorula mucilaginosa strains from clinical and environmental sources. mSphere 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fuchs K, Cardona Gloria Y, Wolz OO, Herster F, Sharma L, Dillen CA, Taumer C, Dickhofer S, Bittner Z, Dang TM, Singh A, Haischer D, Schloffel MA, Koymans KJ, Sanmuganantham T, Krach M, Roger T, Le Roy D, Schilling NA, Frauhammer F, Miller LS, Nurnberger T, LeibundGut-Landmann S, Gust AA, Macek B, Frank M, Gouttefangeas C, Dela Cruz CS, Hartl D, and Weber AN. 2018. The fungal ligand chitin directly binds TLR2 and triggers inflammation dependent on oligomer size. EMBO Rep. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bueter CL, Lee CK, Wang JP, Ostroff GR, Specht CA, and Levitz SM. 2014. Spectrum and mechanisms of inflammasome activation by chitosan. J. Immunol 192: 5943–5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Viriyakosol S, Walls L, Okamoto S, Raz E, Williams DL, and Fierer J. 2018. Myeloid differentiation factor 88 and interleukin-1R1 signaling contribute to resistance to Coccidioides immitis. Infect. Immun 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Campuzano A, and Wormley JFL. 2018. Innate immunity against Cryptococcus, from recognition to elimination. Journal of Fungi 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


