Abstract
In utero exposure to arsenite (iAs) is known to increase disease risks later in life. We investigated the effect of in utero exposure to iAs in the drinking water on metabolic and reproductive parameters in male mouse offspring at postnatal and adult stages. Pregnant CD-1 mice were exposed to iAs (as sodium arsenite) in the drinking water at 0 (control), 10 ppb (EPA standard for drinking water), and 42.5 ppm (tumor-inducing dose in mice) from embryonic day (E) 10 to 18. At birth, pups were fostered to unexposed females. Male offspring exposed to 10 ppb in utero exhibited increase in body weight at birth when compared to controls. Male offspring exposed to 42.5 ppm in utero showed a tendency for increased body weight and a smaller anogenital distance. The body weight in iAs-exposed pups continued to increase significantly compared to control at 3 weeks and 11 weeks of age. At 5 months of age, iAs-exposed males exhibited greater body fat content and glucose intolerance. Male offspring exposed to 10 ppb in utero had higher circulating levels of leptin compared to control. In addition, males exposed to 42.5 ppm in utero exhibited decreased total number of pups born compared to controls and lower average number of litters sired over a six-month period. These results indicate that in utero exposure to iAs at either human relevant concentration or tumor-inducing concentration is a potential cause of developmental origin of metabolic and reproductive dysfunction in adult male mice.
Keywords: Arsenic, Developmental origins of health and diseases, In utero exposure, Metabolic syndromes, Obesity
1. Introduction
Arsenic is a naturally occurring metalloid chemical, known to be a carcinogen and endocrine disruptor, that is present as a contaminant in almost every continent on the globe. Millions of people from certain regions of the world, including Bangladesh and China, are exposed to high concentrations of arsenic [1, 2]. Furthermore, many residents of the United States rely on unregulated private wells for their drinking water, and face a high probability of being exposed to arsenic [3–5]. The effects of arsenic on human health depend on the origin (e.g., air, food, water), time of exposure, and concentration of arsenic. Although arsenic is found in our environment in both inorganic and organic forms, inorganic arsenic (iAs) is more toxic than organic arsenic and is the form mainly found in drinking water. Human exposure to iAs through drinking water became a major world health concern after reports of its adverse effect on health [6, 7]. Indeed, outcomes from human and animal studies highlighted an association between prenatal exposure to iAs and postnatal and adult health [8, 9]. For instance, epidemiological studies performed on cohorts of pregnant women from areas with risk of exposure to high levels of iAs (> 50 ppb) indicated adverse pregnancy outcomes, increased infant mortality [10] and a negative association between iAs exposure and infant body weight at birth [11–13]. In studies using mice as a model, exposure to iAs has been shown to increase the incidences of lung, liver, adrenal, ovarian and testis cancers as well as non-carcinogenic diseases including developmental and metabolic disorders and reproductive failures [14–19]. Exposure to iAs during preconception followed by in utero exposure to low iAs levels (10 ppb) induced a variety of detrimental effects in mouse models. In the collaborative cross mouse strain, in utero exposure to 100 ppb iAs was associated with a significant increase in birth weight in newborns and increases the susceptibility to type 2 diabetes [20]. In addition, Huang et al. demonstrated that mice exposed to iAs (100–1000 ppb) during both preconception and pregnancy developed hyperglycemia, insulin resistance, and increased fat accumulation [21]. These experiments with various dosing schemes and concentrations resulted in a range of phenotypes, therefore rendering the interpretation of data challenging with respect to environmental exposures to iAs in humans [22]. Currently, the maximum limits of iAs in the drinking water for human consumption is set at 10 ppb by the World Health Organization [7]. However, relatively few studies focus on the effect of exposure to low levels of iAs on health. Previously, we discovered that. in utero exposure to iAs (10 ppb) in female mouse offspring led to long-term health defects, even at relatively low-environmental levels [17]. We specifically focused on the second half of the gestation when embryos undergo critical organ development including sex differentiation and reproductive organ formation. In this study, we aimed to evaluate the effects of in utero exposure on metabolic and reproductive parameters of the male offspring with the same exposure window that encompass fetal organ formation (from embryonic day 10 to birth) and two doses of iAs (10 ppb, EPA and WHO standard and 42.5 ppm, the tumor-inducing level).
2. Materials and Methods
2.1. Animals and treatments
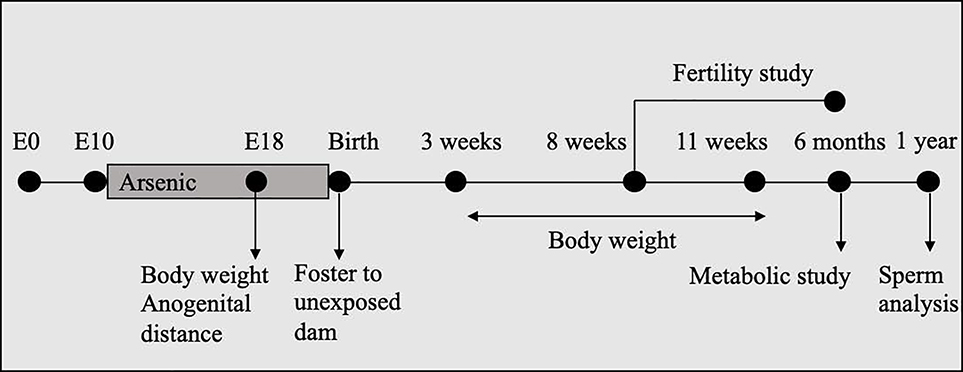
The experimental design was identical to that described by Rodriguez et al. (2016) (Figure 1). Briefly, CD-1 female mice (8–10 weeks-old; Charles River, Wilmington, MA) were timed-mated with CD-1 males. The day that the vaginal plug was detected was considered as embryonic day 0 (E0) and pregnant females were caged individually. Pregnant females were provided at libitum NIH-31 chow and double filtered drinking water (in glass bottles) without any detectable levels of arsenic. The limit of detection for arsenic was 0.005 ppm in the water (Tritest, Raleigh, NC) and <1 ppm in the diet (Microbac, Pittsburgh, PA). At E10, pregnant females were randomly assigned to one of the following treatments: 1) Control: no iAs; 2) 10 ppb iAs (Sodium arsenite or iAs; Spectrum Chemicals, New Brunswick, NJ); or 3) 42.5 ppm iAs. The treatment period lasted from E10 to either E18 or birth. Pregnant females were either euthanized by carbon dioxide inhalation for embryo collection at E18 or allowed to litter (7 dams per treatment group). After birth, pups were fostered to CD-1 females not exposed to iAs and the litter size was normalized to 10 pups (5 male and 5 female pups) per foster mom. Anogenital distance was recorded at birth. All animal procedures were approved by the National Institutes of Health Animals Care and Use Committee and were performed in accordance with an approved National Institute of Environmental Health Sciences animal study proposal.
Figure 1.
Experimental design: Pregnant CD-1 females were exposed to 0 (control), 10 ppb, or 42.5 ppm sodium arsenite in drinking water from E10 to birth. At birth, pups were tossed to females that had not been not exposed to arsenic. Body weight and anogenital distance were measured at E18 the body weight was recorded weekly from 3 to 11 weeks and at 6 months of age. At 8 weeks of age, male pups were included in the fertility study. At 6 months of age, male pups were analyzed for metabolic end points (body composition, glucose test tolerance, serum leptin and insulin levels). At 1 year, sperm samples were collected from the cauda epididymis for quantitative and qualitative analysis.
2.2. Analyses of body weight and fat composition
Mice were weighed either at E18, or every week after birth until week 11 of age and at 6 months. Body composition in 4–5 nonth-old mice was analyzed by using PIXImus® densitometer (GE Lunar Corporation; Waukesha, WI) in 4–5 month-old mice.
2.3. Plasma leptin assay
Plasma leptin assay was performed as described by Rodriguez et al. [17]. Briefly, blood was collected by either cardiac puncture or from the descending vena cava of 6 month-old males (non-fasting). Serum was separated using BD Microtainer™ Plastic Capillary Blood Collectors (BD Diagnostics, Franklin Lakes, NJ) and frozen in −80 °C. Serum levels of leptin was analyzed by Mouse Metabolic Kit (cat # N45124A-1) from MSD (Meso Scale Discovery, Gaithersburg, Maryland, USA) according to manufacturer’s protocols. All samples were assayed in duplicate.
2.4. Glucose tolerance test
Baseline glucose levels in the serum were determined by fasting 5 month-old animals overnight and measuring blood glucose using the novaMaxPlus glucometer (Nova Biomedical, Waltham, MA). Male mice were then given an intraperitoneal injection of D-glucose (2 mg/g body weight) and blood samples were collected for glucose measurement at 20, 40, 60, 120 and 180 min after the injection. The area under the curve (AUC) of plasma glucose concentrations was performed as the sum of linear AUC that was calculated according to the following formula: AUC = x (t1) + y (t2) × 2/(t2 − t1) with x and y as a value of plasma glucose concentration at 2 times (t1 or t2).
2.5. Fertility study
When males reached 8 weeks of age, 2 males per exposed dam, or a total of 14 males per treatment group, were placed individually in a continuous mating scheme with an untreated CD-1 female for 6 months. The parameters analyzed during the 6-months period included: number of litters, total number of pups per litter, and number of days between litters.
2.6. Testis collection and histolopathology
Testes were collected at 6 months of age, then either snap frozen (right testis) or fixed in Bouin’s Fixative overnight at 4 °C, washed three times with PBS, then serially dehydrated in EtOH and stored in 70% EtOH until processed (left testis). Tissues were embedded in paraffin and sectioned at 6 μm thickness. Serial sections from a single testis from 6 control mice, 6 mice exposed to 10 ppb iAs, and 6 mice exposed to 42.5 ppm iAs were stained with hematoxylin and eosin, and evaluated for histopathology by a board-certified veterinary pathologist. The presence of the main testicular lesion observed (6 sections per group), atypical residual bodies, was graded on a five-point scale based on numbers of tubules affected per testis, with 0 being no remarkable lesion, 1 considered as minimal, 2 as mild, 3 as moderate, and 4 as marked.
2.7. Steroidogenic gene expression in the testis
Total RNA from testes collected at 6 months of age was extracted using RNeasy Midi Kit (Qiagen, Germantown, USA) and purified using RNase-Free DNase Set (Qiagen, Germantown, USA) according to the manufacturer’s recommendations. Concentration and quality of isolated RNA was determined using a Nanodrop (ThermoFisher, Durham, USA) and stored at −80 °C until use for quantitative real-time polymerase chain reaction (qPCR) analysis. Complementary DNA was generated with the Superscript II cDNA synthesis system (Invitrogen Corp., Carlsbad, CA). The probes used to evaluate the mRNA expression levels of steroidogenic acute regulatory protein (Star), cytochrome P450 cholesterol side-chain cleavage (Cyp11a1), 3β-hydroxysteroid dehydrogenase 1 (Hsd3b1), 17β-hydroxysteroid dehydrogenase 3 (Hsd17b3), cytochrome P450 family 17 subfamily A member 1 (Cyp17a1) and cytochrome P450 family 19 subfamily A member 1 (Cyp19a1) are listed in Table 1. The levels of mRNA expression were standardized to the geometric mean of two reference genes, glyceraldehyde-3-phosphate dehydrogenase (Gapdh) and 18S ribosomal RNA (rn18S), which has been reported as an accurate normalization method [23].
Table 1.
Probe reference numbers used for RT-PCR.
| Gene |
Probe reference number |
|---|---|
| Cypllal | Mm00490735_m1 |
| Cyp17a1 | Mm00484040_m1 |
| Cyp19a1 | Mm00484049_m1 |
| Gapdh | mm99999915_g1 |
| Hsd3b1 | Mm01261921_mH |
| rn18s | mm03928990_g1 |
| Star | Mm00441558_m1 |
| Hsd17b3 | Mm00515131_m1 |
2.8. Sperm quality
Sperm motility parameters were measured by computer-assisted sperm analysis (CASA) using an HTM-IVOS Sperm Analyzer (Hamilton Thorne Inc., Beverly, MA). Sperm from cauda epididymis were collected from 1 year-old mice, incubated in M2 medium (EMD Millipore Corp., Billerica, MA) at room temperature, and loaded into assay chambers. Motility data was captured from a minimum of nine fields per chamber with two chambers per mouse. The concentrations of sperm from the cauda epididymis were determined on samples diluted 1:10 in water and counted using a hemocytometer.
2.9. Statistical Analyis
Body weight and body composition data were analyzed with a mixed model ANOVA using dam as a random effect to take littermate correlation into consideration. One pup per litter (7 litters per group) were used for the other experiments. Hormonal levels and gene expression were compared using ANOVA and Tukey’s multiple comparison tests.
3. Results
3.1. Impacts of in utero exposure to inorganic arsenic on body weight and anogenital distance
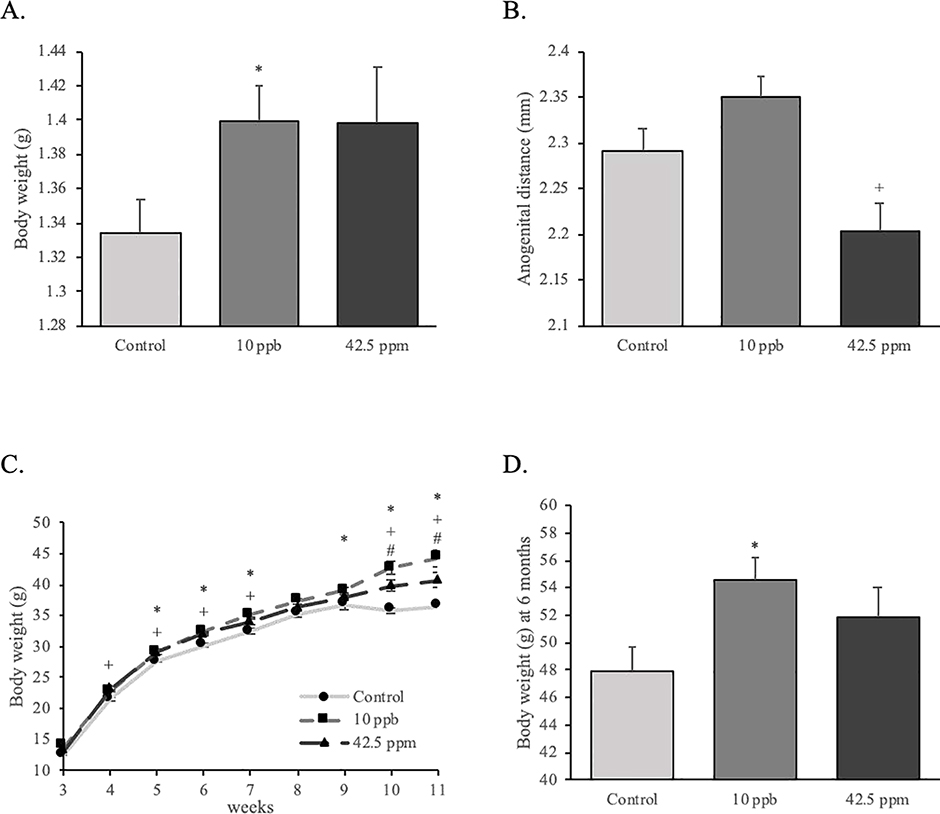
Male mice were weighed at different prenatal and postnatal stages. At E18 (or one day before birth), mean body weight of male fetuses that had been exposed to 10 ppb iAs in utero (10-ppb group hereafter) was increased compared to control group, while there was no effect on anogenital distance (Figure 2A and 2B). Mean body weight of male fetuses exposed to 42.5 ppm iAs in utero (42.5-ppm group hereafter) was higher than the control group, but this was not statistically significantly different. The 42.5-ppm group, but not the 10-ppb group, had smaller anogenital distance when compared to the control group (Figure 2A and 2B). After birth, animals in both the 10-ppb and 42.5-ppm treatment groups had higher mean body weight than the control group. More precisely, we observed that body weights of the male mice in the 10-ppb group were significantly higher compared to the control group from 5 to 7 weeks, then from 9 to 11 weeks and at 6 months of age (Figure 2C and 2D). The body weight of the 42.5-ppm group was significantly higher than the control from 4 to 7 weeks and from 10 to 11 weeks of age (Figure 2C). In addition, mice in the 10-ppb group were significantly heavier than those in the 42.5-ppm group at 10 and 11 weeks of age (Figure 2C).
Figure 2.
Effects of in utero exposure to inorganic arsenic on body weight and anogenital distance of male offspring. (A) Body weight and (B) anogenital distance were measured on male fetuses at E18 (control: n = 46; 10 ppb: n = 29; 42.5 ppm: n = 40). (C) Body weight of male offspring was measured every week from 3 to 11 weeks (n = 30; 10 ppb: n = 39; 42.5 ppm: n = 35) and (D) at 6 months (control: n = 6; 10 ppb: n = 8; 42.5 ppm: n = 6). Data are presented as mean ± SEM. Significant differences are represented by different symbols (*: P < 0.05 between control and 10 ppb; +: P < 0.05 between control and 42.5 ppm; #: P < 0.05 between 10 ppb and 42.5 ppm).
3.2. Impacts of in utero exposure to inorganic arsenic on body fat composition, plasma leptin levels and glucose metabolism
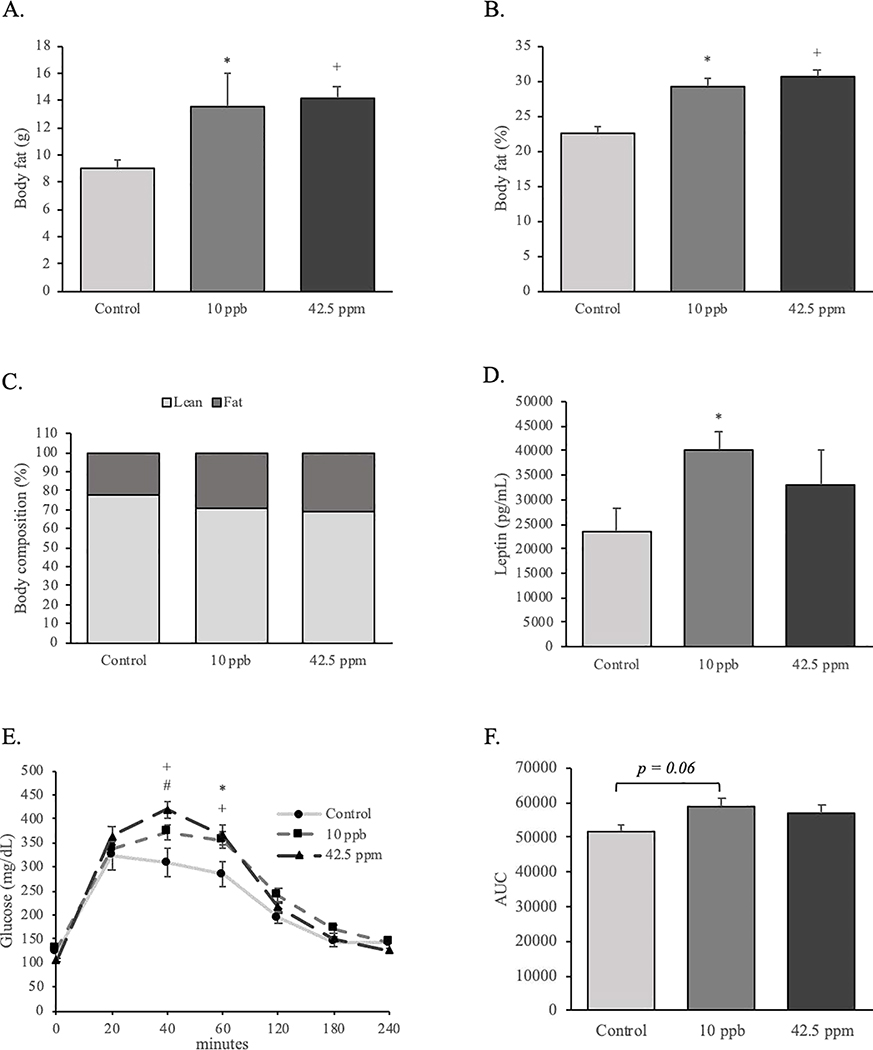
The body fat composition of male mice was evaluated by PIXImus® scans at 5 months of age. The absolute body weight and the percentage of body fat in both treatment groups was 20% higher than those of the control group (Figure 3A and 3B). Animals in both treatment groups showed a higher percentage of fat versus lean mass compared to control mice (Figure 3C). In addition, the plasma leptin levels in the 10-ppb group were significantly higher than that in the control mice (Figure 3D). To determine if the effect of iAs exposure in utero on postnatal body fat was associated with disturbances in glucose metabolism, we performed glucose tolerance tests in control and iAs-exposed mice. After fasting, the levels of circulating glucose were not different among groups. Following the intraperitoneal injection of glucose, the plasma glucose levels of control mice started to decrease after 20 min. On the other hand, serum glucose level in both iAs-exposed groups continued to increase until 40 minutes after injection. These glucose levels continued to be elevated significantly in the iAs-exposed groups at 60 min compared to control. Circulating glucose levels of the exposed groups finally came down to control levels after 120 minutes (Figure 3E). In addition, the area under the curve of serum glucose concentrations tended (P = 0.06) to be higher in the 10 ppb group as compare to the control group. However, no significant difference was found between the 42.5 ppm group and the control or the 10 ppb group (Figure 3F).
Figure 3.
Effects of in utero exposure to inorganic arsenic on adult body fat composition, plasma leptin levels and glucose tolerance. (A, B & C) Body composition analysis was performed using a PIXImus scan on 4–5 month-old male offspring (control: n = 8; 10 ppb: n = 10; 42.5 ppm: n = 16). (D) Serum leptin concentrations were measured at 6 months (control: n = 6; 10 ppb: n = 6; 42.5 ppm: n = 6). (E) Serum glucose concentrations of 5 month-old male offspring were measure following overnight fasting and glucose challenge (control: n = 11; 10 ppb: n = 19; 42.5 ppm: n = 16). (F) Area under the curve of serum glucose concentration of 5 month-old male offspring. Data are presented as mean ± SEM. Significant differences are represented by different symbols (*: P < 0.05 between control and 10 ppb; +: P < 0.05 between control and 42.5 ppm; #: P < 0.05 between 10 ppb and 42.5 ppm).
3.3. Impacts of in utero exposure to inorganic arsenic on reproductive parameters
To assess the effects of in utero exposure to iAs on fertility, 8-week-old control or exposed male mice were mated with unexposed females for 6 months. Number of days to first litter, average days between litters, average number of pups per litter, average number of litters per female, and total number of pups of female were measured (Table 2). These parameters were not different between the control and exposed groups with the exception of the total number of pups per female in the 42.5 ppm group, which was significantly lower when compared to the control group (Table 2). Since some defects in fertility were observed, we looked at testis histology and the expression levels of steroidogenic enzymes in testis.
Table 2.
Effects of in utero exposure to arsenic on fertility of adult males.
| Treatment | Days to 1st litter | Average days between litters | Average number of pups per litter | Average number of litters per female | Total number of pups per female |
|---|---|---|---|---|---|
| Control | 21 ± 0.3 | 27 ± 1.6 | 13 ± 0.4 | 6 ± 0 | 76 ± 3.9 |
| 10 ppb | 21 ± 0.4 | 29 ± 1.6 | 14 ± 0.4 | 5 ± 0.4 | 70 ± 6.0 |
| 42.5 ppm | 22 ± 0.4 | 31 ± 1.8 | 12 ± 0.6 | 5 ± 0.5 | 55 ± 6.3 * |
Exposed males were mated with normal females that were not exposed to arsenic. Breeding began at 8 weeks of age and continued for 6 months. Numbers represent mean ± SEM. Significant differences from control are indicated by an asterisk (P < 0.05, control: n = 6; 10 ppb: n = 6; 42.5 ppm: n = 6).
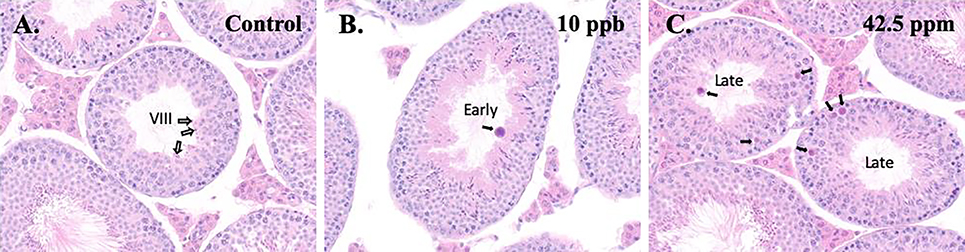
We examined testis histology at 6 months of age. In general, there were no significant differences between the control and exposed groups in terms of spermatogenesis, with the exception of increased formation of atypical residual bodies in males exposed in utero to iAs. Atypical residual bodies were seen microscopically as dense basophilic, globular bodies of redundant organelles and excess cytoplasm that are abnormally large and/or misshapen. Their significance is unclear, but they are presumed to result from impaired maturation of elongating spermatids and/or processing of residual bodies by the Sertoli cell [24]. Residual bodies, left at the luminal surface of the germinal epithelium after spermiation (stage VIII; see control animal in Figure 4A), are normally transported to the basal Sertoli cell cytoplasm during the subsequent stages (IX-XI) where they are phagocytized and disappear. Minimal atypical residual bodies were found in the lumen of stages X and XI seminiferous tubules in 1 out of 6 control male testis. However, mild to marked atypical residual bodies were found in 2 out of 6 testes from males of both exposed groups. In the iAs exposed males with atypical residual bodies, they were sometimes seen in early-stage tubules (e.g. I-VI), which are stages that should never contain residual bodies (Figure 4B). Atypical residual bodies in the lumen or within the germinal epithelium were more typically seen in the iAs-exposed males in late stage tubules (e.g., X-XII) (Figure 4C).
Figure 4.
Effect of in utero exposure to inorganic arsenic on testis histopathology. Testis from control (A), 10 ppb (B), or 42.5 ppm (C) groups were collected at 6 months of age, sectioned and stained with hematoxylin and eosin (original magnification 40×). The white arrows in the control testis in (A) indicate typical residual bodies at the lumen in a stage VIII tubule shortly after spermiation. After spermiation, the residual bodies normally rapidly descend within the seminiferous epithelium to the basal region in stage IX and X, where they are phagocytized and then disappear, typically by stage XI. In the testis from a 10 ppb male (B), residual bodies were atypical, appearing much larger than normal. Also, the atypical residual body in B (black arrow) is present in an early stage tubule, in which residual bodies are normally never present. In the testis from a 42.5 ppm male (C), two late-stage tubules contain atypical residual bodies (black arrows).
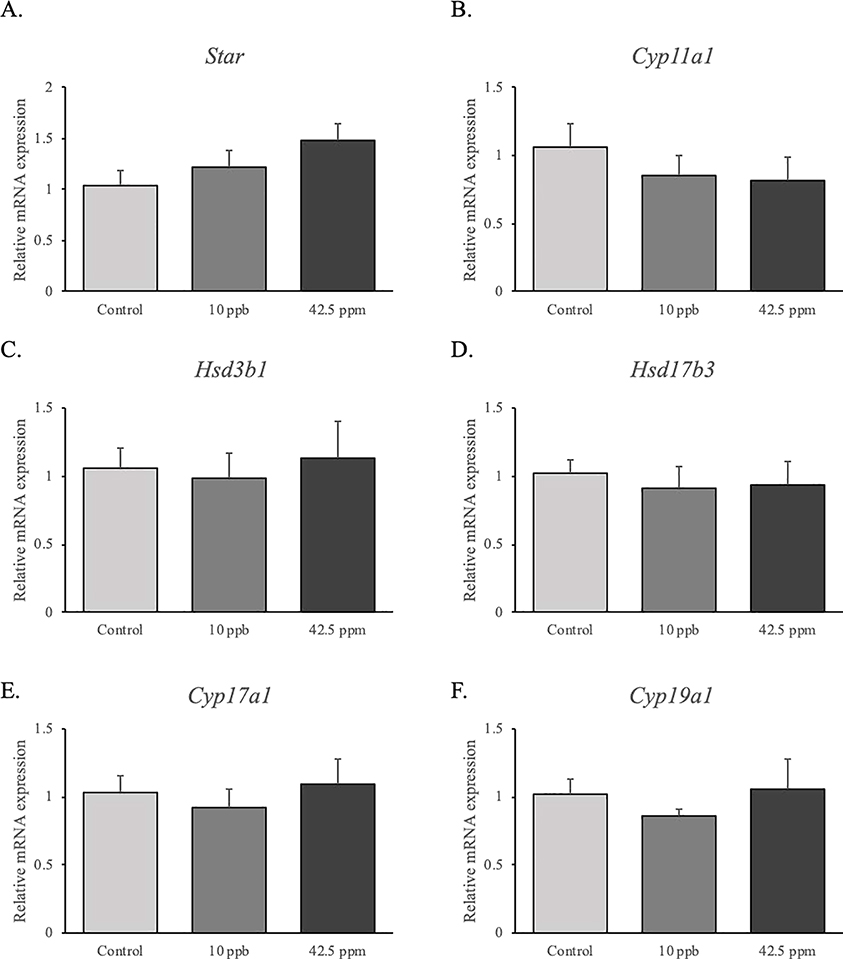
The mRNA expression levels of genes that are involved in testosterone synthesis were measured by qPCR at 6 months of age. In utero exposure to iAs had no effects on StAR, Cyp11a1, Hsd3b1, Hsd17b3, Cyp17a1 and Cyp19a1 mRNA expression compared to control group (Figure 5).
Figure 5.
Effects of in utero exposure to inorganic arsenic on the expression of steroidogenic genes in the testis. Real time-PCR analysis of Star, Cyp11a1, Hsd3b1, Hsd17b3, Cyp17a1 and Cyp19a1 (mRNA) expression in the testes were performed at 6 months of age (control: n = 6; 10 ppb: n = 6; 42.5 ppm: n = 6). Data are presented as mean ± SEM. Differences were considerate significant when P < 0.05.
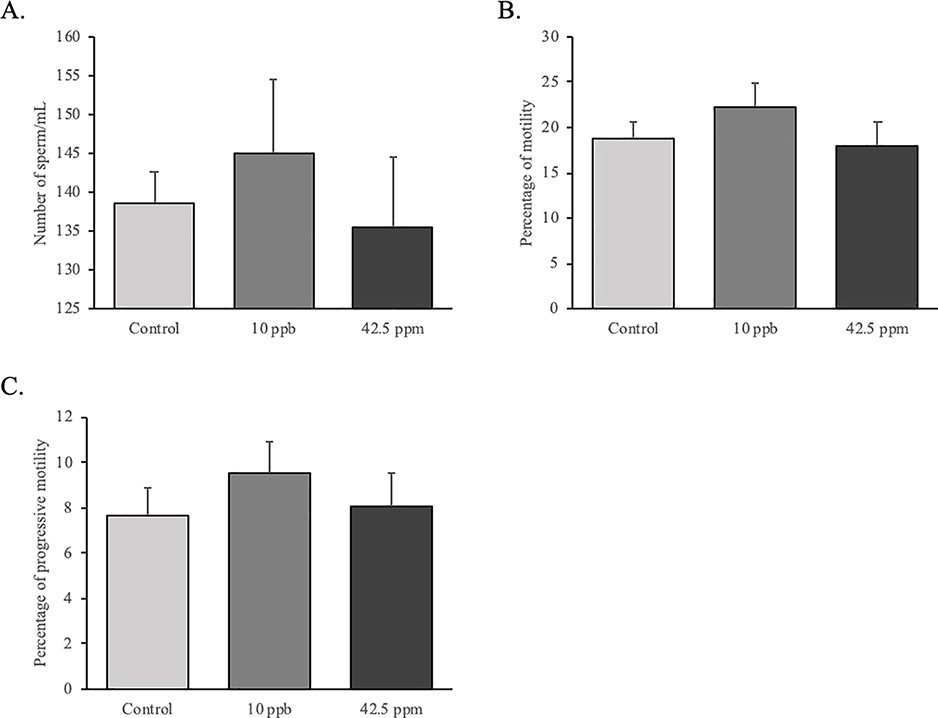
We also analyzed sperm quality using computer-assisted sperm analysis (CASA) at 1 year of age and we found that in utero exposure to iAs does not alter either the sperm number nor sperm motility (Figure 6).
Figure 6.
Effects of in utero exposure to inorganic arsenic on sperm quality. Sperms were collected from cauda epididymis and analyzed using computer-assisted sperm analysis (CASA) at 1 year of age (control: n = 7; 10 ppb: n = 8; 42.5 ppm: n = 8. Data are presented as mean ± SEM. Differences were considerate significant when P < 0.05.
4. Discussion
In this study, we investigated the impact of in utero exposure to the EPA mandated maximum concentration (10 ppb) or tumor-inducing concentration (42.5 ppm) of iAs (sodium arsenite) in the drinking water on metabolic and reproductive functions in male mice. We found that in utero exposure to 10 ppb of iAs increased prenatal weight whereas both concentrations caused increased postnatal body weight, adult body fat composition and glucose intolerance. However, the effect on reproductive endpoints was limited to a decrease in the total number of pups in the 42.5-ppb group and the formation of atypical residual bodies in seminiferous tubules in both exposed groups.
Similar to our findings in iAs-exposed female offspring, in utero iAs exposure resulted in an increase in body weight in male offspring in the 10-ppb group that became apparent at the end of fetal development to adulthood (6 months). Although a trend was observed in mice exposed to 42.5 ppm of iAs, body weight was not statistically different in 6 month-old male mice. However, an increase of the percentage of body fat was likely associated with leptin resistance. iAs could act as a metabolic disruptor by dysregulating lipid and glucose homeostasis [22, 25]. For instance, in utero exposure to iAs (10 ppb) disrupted normal metabolic profiling and the expression levels of key genes involved in lipid and glucose homeostasis in male mice [26]. However, non-fostered offspring exposed to 10 ppb of iAs had growth deficits at birth that were reversed after cross-fosterirg pups [27], suggesting an effect of iAs on the quality of the milk from the dam. In addition, prepubertal male mice exposed daily to 50 ppm of iAs in the drinking water for 8 weeks developed impaired glucose tolerance [22]. In our study, although male pups exposed to iAs in utero took longer in controlling the levels of circulating glucose following a challenge, they were able to bring the levels back to normal. This observation suggests that although mice exposed to iAs in utero are not diabetic, they display slight differences in glucose metabolism. Therefore, exposure to iAs elevated the risk of increasing body weight and glucose intolerance as observed in our study under either low (10 ppb) or high (42.5 ppm) iAs levels [17]. Overall, these results strengthened the hypothesis that iAs act as a potential diabetogenic and obesity-inducing agent.
In male mice, chronic exposure to iAs (0.3–3 mg/L of arsenic trioxide or 20–40 mg/L of sodium arsenite) for 5 weeks was associated with degeneration of testicular cells and reduction in sperm count and quality [28, 29]. Male mice exposed to comparable doses of iAs (arsenic trioxide) used in chemotherapy produced fewer normal sperm, and showed degeneration of seminiferous epithelium, germ cell exfoliation of tubular lumen, reduction in volumetric proportion of Leydig cells and ultimately affected fetal viability [29]. Similarly, in 8 week-old male mice, daily exposure to iAs (sodium arsenite) at 20 or 40 mg/L for 5 weeks in drinking water led to a decrease in epididymal sperm counts and testicular weight [28]. Our exposure model, particularly the 42.5 ppm group, developed shorter anogenital distance. Anogenital distance is an indicator of male reproductive health and a reduction of its length increased the risk of hypospadias and cryptorchidism in human and rodents [30, 31]. Other than this defect, few reproductive functions were affected in both levels of iAs in our study. The 42.5 ppm group produced fewer pups after 6 months of breeding while the 10-ppb group produced comparable number of pups as control mice. In addition, in vitro studies on mouse Leydig cell lines have supported the notion that iAs (2 and 4 mg/L of iAs for 48 h) can impair spermatogenesis by inhibiting major enzymes responsible for steroidogenesis, and thus reducing the testosterone levels [32]. However, we did not observe any effects of iAs on the expression of key steroidogenic enzymes at 6 months of age. The reproductive phenotype observed in the 42.5 ppm group could be explained by the increasing formation of atypical residual bodies in seminiferous tubules. Atypical residual bodies found in the lumen of seminiferous tubules are an indication of testosterone depletion and failure of spermiation in the rat [33].
Interestingly, under our experimental conditions, the effects of iAs on reproductive parameters appear to be different in female versus male mice [17]. Although no differences between the iAs exposed groups and controls were detected, female mice exposed to 10 ppb of iAs during the second half of gestation produced more pups per litter and had a longer fertile period compared to female mice exposed to 42.5 ppm of iAs [17]. Many studies demonstrated that iAs induced a variety of pathological changes in a sex-dependent manner. For instance, in utero exposure to iAs in drinking water (42 or 85 ppm iAs from E8 to E18) led to ovarian and lung tumors in female offspring whereas it induced liver and adrenal tumors in male offspring [19]. In addition, gestational and post-weaning exposure to iAs (100 ppb) in mice exacerbated high fat diet-induced fatty liver disease in males later in life while it had no effects in females [20]. These observations raise the hypothesis that the sex-specific differences may be explained by different biotransformation of iAs in the organism. Inorganic arsenic is metabolized through a series of oxidation and methylation reactions that lead to the production of trivalent methylated metabolites methylarsonic acid (MMA) and dimethylarsinic acid (DMA) [34, 35]. Arsenic (+3 oxidation state) methyltransferase (AS3MT) the main enzyme responsible of iAs methylation [36]. Mice deficient for the As3mt gene showed sex-differences in urinary and plasma metabolite profiles related to lipid, amino acid, and carbohydrate metabolic function [37]. However, little is known about the mechanisms of AS3MT that can explain the sex-specific effects of iAs responsible for obesity or subfertility in metabolic and reproductive tissues.
There is some evidence that iAs could potentially modulate the estradiol pathway. Indeed, Lopez et. al. reported that iAs may interact with estrogen receptor alpha (ER-alpha), leading to modulation of estrogen associated functions [38]. In adult female rats, exposure to iAs (0.4 ppm for seven estrous cycles or 4 ppm for 48 days) decreased circulating levels of estradiol and downregulated the expression of ER-alpha in the uterus along with reduction of reproductive functions [39, 40]. Similar to estrogen treatment, adult male rats exposed to iAs (5 ppm for 4 weeks) produced less androgen and accumulated oxidative stress in the testis associated with spermatogenesis defects [41]. However, under our experimental conditions we found no dramatic effects on male fertility.
5. Conclusion
In utero exposure of CD-1 male offspring to EPA-mandated level and tumor-inducing levels of iAs in drinking water during a critical window of organ development induces significant metabolic changes (obesity and glucose intolerance) with little detrimental effects on reproductive functions when the exposed male offspring reach adulthood. These animals were exposed to iAs only during the second half of gestation and were never exposed to iAs again after birth, indicating that in utero exposure to iAs could be involved in the developmental origin of health and diseases (DOHaD). In addition, the low level of iAs used in this study raise even more questions on the effect of iAs in humans, since mice metabolize iAs faster than humans do.
Highlights.
In utero exposure to arsenite resulted in obesity phenotypes in male mice
In utero exposure to arsenite had minimal impact on reproductive phenotype in male mice
These changes were observed at 10 ppb and 42.5 ppm of arsenite in the drinking water
Funding
This study was funded in part by the National Institute of Environmental Health Sciences Intramural Research Fund ES102965.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of Conflicting Interests
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Smith AH, Lingas EO, Rahman M, Contamination of drinking-water by arsenic in Bangladesh: a public health emergency, Bull World Health Organ 78(9) (2000) 1093–103. [PMC free article] [PubMed] [Google Scholar]
- [2].Amini M, Abbaspour KC, Berg M, Winkel L, Hug SJ, Hoehn E, Yang H, Johnson CA, Statistical modeling of global geogenic arsenic contamination in groundwater, Environ Sci Technol 42(10) (2008) 3669–75. [DOI] [PubMed] [Google Scholar]
- [3].Johnson TD, Belitz K, Lombard MA, Estimating domestic well locations and populations served in the contiguous U.S. for years 2000 and 2010, Sci Total Environ 687 (2019) 1261–1273. [DOI] [PubMed] [Google Scholar]
- [4].Allevi RP, Krometis LA, Hagedorn C, Benham B, Lawrence AH, Ling EJ, Ziegler PE, Quantitative analysis of microbial contamination in private drinking water supply systems, J Water Health 11(2) (2013) 244–55. [DOI] [PubMed] [Google Scholar]
- [5].Sanders AP, Messier KP, Shehee M, Rudo K, Serre ML, Fry RC, Arsenic in North Carolina: public health implications, Environ Int 38(1) (2012) 10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, Suk WA, The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem, Environ Health Perspect 121(3) (2013) 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].WHO, Guidelines for Drinking-Water Quality, Fourth Edition (2011).
- [8].Waalkes MP, Ward JM, Diwan BA, Induction of tumors of the liver, lung, ovary and adrenal in adult mice after brief maternal gestational exposure to inorganic arsenic: promotional effects of postnatal phorbol ester exposure on hepatic and pulmonary, but not dermal cancers, Carcinogenesis 25(1) (2004) 133–41. [DOI] [PubMed] [Google Scholar]
- [9].Smeester L, Fry RC, Long-Term Health Effects and Underlying Biological Mechanisms of Developmental Exposure to Arsenic, Curr Environ Health Rep 5(1) (2018) 134–144. [DOI] [PubMed] [Google Scholar]
- [10].Quansah R, Armah FA, Essumang DK, Luginaah I, Clarke E, Marfoh K, Cobbina SJ, Nketiah-Amponsah E, Namujju PB, Obiri S, Dzodzomenyo M, Association of arsenic with adverse pregnancy outcomes/infant mortality: a systematic review and meta-analysis, Environ Health Perspect 123(5) (2015) 412–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Claus Henn B, Ettinger AS, Hopkins MR, Jim R, Amarasiriwardena C, Christiani DC, Coull BA, Bellinger DC, Wright RO, Prenatal Arsenic Exposure and Birth Outcomes among a Population Residing near a Mining-Related Superfund Site, Environ Health Perspect 124(8) (2016) 1308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Laine JE, Bailey KA, Rubio-Andrade M, Olshan AF, Smeester L, Drobna Z, Herring AH, Styblo M, Garcia-Vargas GG, Fry RC, Maternal arsenic exposure, arsenic methylation efficiency, and birth outcomes in the Biomarkers of Exposure to ARsenic (BEAR) pregnancy cohort in Mexico, Environ Health Perspect 123(2) (2015) 186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rahman A, Vahter M, Smith AH, Nermell B, Yunus M, El Arifeen S, Persson LA, Ekstrom EC, Arsenic exposure during pregnancy and size at birth: a prospective cohort study in Bangladesh, Am J Epidemiol 169(3) (2009) 304–12. [DOI] [PubMed] [Google Scholar]
- [14].Tokar EJ, Diwan BA, Ward JM, Delker DA, Waalkes MP, Carcinogenic effects of “whole-life” exposure to inorganic arsenic in CD1 mice, Toxicol Sci 119(1) (2011) 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Garry MR, Santamaria AB, Williams AL, DeSesso JM, In utero arsenic exposure in mice and early life susceptibility to cancer, Regul Toxicol Pharmacol 73(1) (2015) 378–90. [DOI] [PubMed] [Google Scholar]
- [16].Waalkes MP, Keefer LK, Diwan BA, Induction of proliferative lesions of the uterus, testes, and liver in swiss mice given repeated injections of sodium arsenate: possible estrogenic mode of action, Toxicol Appl Pharmacol 166(1) (2000) 24–35. [DOI] [PubMed] [Google Scholar]
- [17].Rodriguez KF, Ungewitter EK, Crespo-Mejias Y, Liu C, Nicol B, Kissling GE, Yao HH, Effects of in Utero Exposure to Arsenic during the Second Half of Gestation on Reproductive End Points and Metabolic Parameters in Female CD-1 Mice, Environ Health Perspect 124(3) (2016) 336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Waalkes MP, Qu W, Tokar EJ, Kissling GE, Dixon D, Lung tumors in mice induced by “whole-life” inorganic arsenic exposure at human-relevant doses, Arch Toxicol 88(8) (2014) 1619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Waalkes MP, Ward JM, Liu J, Diwan BA, Transplacental carcinogenicity of inorganic arsenic in the drinking water: induction of hepatic, ovarian, pulmonary, and adrenal tumors in mice, Toxicol Appl Pharmacol 186(1) (2003) 7–17. [DOI] [PubMed] [Google Scholar]
- [20].Ditzel EJ, Nguyen T, Parker P, Camenisch TD, Effects of Arsenite Exposure during Fetal Development on Energy Metabolism and Susceptibility to Diet-Induced Fatty Liver Disease in Male Mice, Environ Health Perspect 124(2) (2016) 201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Huang MC, Douillet C, Dover EN, Styblo M, Prenatal arsenic exposure and dietary folate and methylcobalamin supplementation alter the metabolic phenotype of C57BL/6J mice in a sex-specific manner, Arch Toxicol 92(6) (2018) 1925–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Paul DS, Hernandez-Zavala A, Walton FS, Adair BM, Dedina J, Matousek T, Styblo M, Examination of the effects of arsenic on glucose homeostasis in cell culture and animal studies: development of a mouse model for arsenic-induced diabetes, Toxicol Appl Pharmacol 222(3) (2007)305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F, Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes, Genome Biol 3(7) (2002) RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Creasy D, Bube A, de Rijk E, Kandori H, Kuwahara M, Masson R, Nolte T, Reams R, Regan K, Rehm S, Rogerson P, Whitney K, Proliferative and nonproliferative lesions of the rat and mouse male reproductive system, Toxicol Pathol 40(6 Suppl) (2012) 40S–121S. [DOI] [PubMed] [Google Scholar]
- [25].Maull EA, Ahsan H, Edwards J, Longnecker MP, Navas-Acien A, Pi J, Silbergeld EK, Styblo M, Tseng CH, Thayer KA, Loomis D, Evaluation of the association between arsenic and diabetes: a National Toxicology Program workshop review, Environ Health Perspect 120(12) (2012) 1658–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang D, Zhu W, Yan S, Meng Z, Yan J, Teng M, Jia M, Li R, Zhou Z, Impaired lipid and glucose homeostasis in male mice offspring after combined exposure to low-dose bisphenol A and arsenic during the second half of gestation, Chemosphere 210 (2018) 998–1005. [DOI] [PubMed] [Google Scholar]
- [27].Kozul-Horvath CD, Zandbergen F, Jackson BP, Enelow RI, Hamilton JW, Effects of low-dose drinking water arsenic on mouse fetal and postnatal growth and development, PLoS One 7(5) (2012) e38249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chang SI, Jin B, Youn P, Park C, Park JD, Ryu DY, Arsenic-induced toxicity and the protective role of ascorbic acid in mouse testis, Toxicol Appl Pharmacol 218(2) (2007) 196–203. [DOI] [PubMed] [Google Scholar]
- [29].da Silva RF, Borges CDS, de Almeida Lamas C, Cagnon VHA, de Grava Kempinas W, Arsenic trioxide exposure impairs testicular morphology in adult male mice and consequent fetus viability, J Toxicol Environ Health A 80(19–21) (2017) 1166–1179. [DOI] [PubMed] [Google Scholar]
- [30].Thankamony A, Lek N, Carroll D, Williams M, Dunger DB, Acerini CL, Ong KK, Hughes IA, Anogenital distance and penile length in infants with hypospadias or cryptorchidism: comparison with normative data, Environ Health Perspect 122(2) (2014) 207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Welsh M, Saunders PT, Fisken M, Scott HM, Hutchison GR, Smith LB, Sharpe RM, Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism, J Clin Invest 118(4) (2008) 1479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Alamdar A, Xi G, Huang Q, Tian M, Eqani S, Shen H, Arsenic activates the expression of 3beta-HSD in mouse Leydig cells through repression of histone H3K9 methylation, Toxicol Appl Pharmacol 326 (2017) 7–14. [DOI] [PubMed] [Google Scholar]
- [33].Saito K, O’Donnell L, McLachlan RI, Robertson DM, Spermiation failure is a major contributor to early spermatogenic suppression caused by hormone withdrawal in adult rats, Endocrinology 141(8) (2000) 2779–85. [DOI] [PubMed] [Google Scholar]
- [34].Loffredo CA, Aposhian HV, Cebrian ME, Yamauchi H, Silbergeld EK, Variability in human metabolism of arsenic, Environ Res 92(2) (2003) 85–91. [DOI] [PubMed] [Google Scholar]
- [35].Vahter M, Mechanisms of arsenic biotransformation, Toxicology 181–182 (2002) 211–7. [DOI] [PubMed] [Google Scholar]
- [36].Drobna Z, Naranmandura H, Kubachka KM, Edwards BC, Herbin-Davis K, Styblo M, Le XC, Creed JT, Maeda N, Hughes MF, Thomas DJ, Disruption of the arsenic (+3 oxidation state) methyltransferase gene in the mouse alters the phenotype for methylation of arsenic and affects distribution and retention of orally administered arsenate, Chem Res Toxicol 22(10) (2009) 1713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Huang MC, Douillet C, Su M, Zhou K, Wu T, Chen W, Galanko JA, Drobna Z, Saunders RJ, Martin E, Fry RC, Jia W, Styblo M, Metabolomic profiles of arsenic (+3 oxidation state) methyltransferase knockout mice: effect of sex and arsenic exposure, Arch Toxicol 91(1) (2017) 189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lopez S, Miyashita Y, Simons SS Jr., Structurally based, selective interaction of arsenite with steroid receptors, J Biol Chem 265(27) (1990) 16039–42. [PubMed] [Google Scholar]
- [39].Chatterjee A, Chatterji U, Arsenic abrogates the estrogen-signaling pathway in the rat uterus, Reprod Biol Endocrinol 8 (2010) 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chattopadhyay S, Ghosh D, The involvement of hypophyseal-gonadal and hypophyseal-adrenal axes in arsenic-mediated ovarian and uterine toxicity: modulation by hCG, J Biochem Mol Toxicol 24(1) (2010) 29–41. [DOI] [PubMed] [Google Scholar]
- [41].Jana K, Jana S, Samanta PK, Effects of chronic exposure to sodium arsenite on hypothalamo-pituitary-testicular activities in adult rats: possible an estrogenic mode of action, Reprod Biol Endocrinol 4 (2006) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]