Abstract
The flame retardant and plasticizer, tetrabromobisphenol-A (TBBPA) has rapidly become a common component in the manufacture of circuit boards and plastics worldwide. It is also an analog of bisphenol A (BPA), an endocrine disrupting chemical identified by the Endocrine Society. As such, TBBPA needs to be investigated for similar potential human health risks. Using rats as a model, we exposed pregnant dams and their progeny to 0, 0.1,25, or 250 mg TBBPA/kg of body weight until the offspring reached adulthood and assessed the first generation of males for any reproductive tract abnormalities. We found no differences in the morphology of testes, sperm, prostates, or secondary sex organs from post-natal day 21 through one-year of age. A delay in the time to preputial separation was found with the 250 mg/kg treatment. Also, minor differences of sperm count at one-year old with the 25 mg/kg treatment and expression levels of two steroidogenic pathway enzymes at either post-natal day 90 or one-year old in the 250 mg/kg treatment group were detected, but spermatogenesis was not disrupted. While these results may lead to the supposition that TBBPA is less harmful than its parent compound BPA, more studies need to be conducted to assess long-term exposure effects.
Keywords: Tetrabromobisphenol A, Endocrine disruption, Bisphenol A, Male reproduction
1. Introduction
The Endocrine Society published a statement in 2009 defining endocrine-disrupting chemicals (EDCs) as “exogenous chemical(s), or mixtures of chemicals, that interfere with any aspect of hormone action” [1]. A follow-up report in 2015 focused on endocrine systems shown to be targets of EDCs, including male reproductive organs [2]. Bisphenol A (BPA), a widely used compound in the manufacture of plastics, has been rigorously studied because it has endocrine disruption properties, including interference with or activation of steroid hormone receptors. For example, exposure to BPA is implicated in altered gene expression of germ cells during oogenesis in mouse embryos [3], polycystic ovarian syndrome of post-natally exposed rats (PND one – ten) [4], and changes in adult uterine functions after treatment from gestation day (GD) nine through weaning [5]. In male rodents, BPA exposure either in utero through weaning or in adulthood led to decreased numbers and quality of spermatozoa [6–8].
Although the consequences of BPA exposure may depend upon the route, timing, and amount of administration, this body of research has led to a ban of BPA from use in baby bottles and cups, as well as the coating of infant formula cans in many industrialized countries of the world, including the US, Canada, and EU member nations. Therefore, industries have turned to analogs of BPA as substitutes. Due to the reproductive hazards of BPA, it is critical to investigate whether congeners with structural similarities to BPA could negatively impact human health.
Tetrabromobisphenol A (TBBPA) is a brominated form of BPA, which commanded 58.7% of the global market for all brominated flame retardants in 2001 [9]. This chemical is mainly used to coat circuit boards in electronic devices, but is also used in the production of plastic resins. There have been studies of TBBPA in the context of human exposure with varying but detectable levels found in maternal and cord serum [10], breast milk [11, 12], fish and shellfish [13], dust of daycare centers and classrooms [14], outdoor air [15] and in occupational settings such as electronic waste recycling facilities [16]. Schauer et al. [17] reported a comparison of TBBPA and its metabolites found in serum after oral doses to both humans (0.1 mg/kg) and rats (300 mg/kg). In humans the level of plasma TBBPA was below the level of detection and area under the curve (AUC) value for the glucuronide form was 0.79 nmol/ml per hour, while the plasma AUC for TBBPA and TBBPA glucuronide in rats were calculated at 1028 and 161 nmol/ml per hour, respectively. Thus indicating a low bioavailability and rapid metabolism of TBBPA in mammals.
Due to the increasing use of TBBPA in consumer products, the number of published experiments using animal models to examine effects of exposure to TBBPA have also increased. However, results were varying, mostly due to the differences in exposure timing, route, level of dosing, animal models, and sex of the animals. There are few reports which indicated adverse outcomes to the male reproductive system. Dunnick JK. et al. [18] observed testicular interstitial cell adenomas in rats exposed to 1000 mg/kg of TBBPA, while Zatecka E. et al. [19] discovered DNA damage to spermatozoa with an estimated dose of 35 μg/kg of body weight in mice. The US Environmental Protection Agency set the lowest observed adverse effect level of BPA at 50 mg/kg/day in 1988 based on body weight loss found in chronic oral dose studies. There is now a large compendium reporting poor outcomes from lower doses [20]. In this study, we aimed to examine not only if TBBPA exposure could induce male reproductive phenotypes similar to BPA exposure, but also used low level exposures which are closer to an estimated human oral exposure reported by the European Food Safety Authority [21].
2. Materials & Methods
2.1. Animals and study design
All animals were treated humanely and in accordance with the approved protocols of the NIEHS Animal Care and Use Committee specific to this study. A fully detailed report of the study design has been published [22]. To summarize, time pregnant adult Wistar Han dams (Charles Rivers, Raleigh, NC) with observed copulatory plugs, designated on gestation day (GD) zero, were delivered to NIEHS on GD four and allowed two days to acclimate prior to dosing. Animals were maintained on a low phytoestrogen diet without soybean protein and less than 20 ppm isoflavones (Teklad 2919, Envigo Laboratories, Madison, Wl). Pregnant dams were singly housed in polysulfone cages with Sani-Chip bedding (PJ Murphy Forest Products, Montville, NJ) and Enviro-Dri enrichment (Shepherd Specialty Papers, Watertown, TN), on ventilated racks. Animals were housed in humidity-and temperature-controlled rooms, 25 °C and 45–60% average humidity, with standard 12 h light cycles (not reversed). Food and RO/DI water were provided ad libitum.
Dams were assigned to a dose group by blocked randomization based on arrival body weight and were exposed via oral gavage once daily within one hour of lights on with TBBPA solubilized in sesame oil at 0, 0.1,25, or 250 mg per kilogram of daily measured body weight from GD six through weaning (PND 21). The rat strain and doses administered were based on previous studies conducted by the National Toxicology Program at National Institute of Environmental Health Sciences [23] and a pilot study examining behavior effects of TBBPA [22]. After birth occurred on the evening of GD 21, the following day (PND one) litter sizes were equalized to eight pups (four males and four females per dam if possible) with 20-24 litters in each dosing group. After weaning, pups were group housed with no more than three siblings of the same sex per cage and maintained on Teklad 2916 diet for growth and maintenance. Weanlings were dosed by gavage with the same concentration as their respective dams beginning on PND 22 and continued to PND 90. Dosing was performed blinded to treatment identification to minimize bias. Anogenital distance (AGD), the distance from the midpoint of the anus to the base of the penis, was measured with a ruler in millimeters on PND four. Male pups were monitored daily for preputial separation (PPS) as indicators of androgen activity beginning on PND 36, at the same time each day (+/− one hour). Several males from each group were allowed to age to one year without further dosing of TBBPA.
Dosing stock solutions were prepared by dissolving TBBPA Sigma-Aldrich (CAS# 79-94-7, 97% purity, lot #MKBG4059 V) in 100% ethanol (Warner-Graham Company, Cockeysville, MD) and adding appropriate volumes to known volumes of refined sesame seed oil (Jedwards International, Inc, Braintree, MA). Ethanol was removed after mixing under a stream of nitrogen with gentle stirring in a fume hood. Dose solutions were made fresh weekly and stored at room temperature in color-coded glass vials for dosing.
2.2. Tissue Collection
Samples from one male per litter (N=12 litters) in each treatment group were collected on PND 21 (prepubertal), 42 (pubertal), and 90 (adult). Animals were randomly selected from each litter based on their assigned number using a random number generator. We also collected at least nine males from each treatment group at one year old (senescence). At necropsy, animals were euthanized 20 minutes after dosing by rapid decapitation using a guillotine to limit the metabolism of TBBPA [17]. Animals were fasted for two hours prior to necropsy, which occurred within the first five hours of lights on. The left testis was snap frozen in liquid nitrogen, the right testis was weighed and then fixed in Bouin’s solution (Sigma-Aldrich, Burlington, MA) overnight at 4 °C. For PND 90 and one-year groups, the right cauda epididymis with vas deferens attached was isolated in 1x PBS for sperm collection. The epididymis was trimmed on a watch glass and placed in 2 ml of 1x PBS-CMF for swim-out analysis. Sperm was expelled from the vas deferens, the cauda epididymis was cut with scissors six times, then placed on a 37 °C warming tray for 20 minutes. Sperm were transferred to 5 ml tubes and diluted for counting on a hemocytometer. Prostate and other secondary sex organs were collected at necropsy. In half of each treatment group, the tissues were placed flat on the dorsal surface on an index card and submerged in 10% neutral buffered formalin for 48 hours at 4 °C followed by a transfer to 1X PBS. The fixed samples were then embedded in paraffin for histology. The remaining samples were frozen in liquid nitrogen.
2.3. Histology
Fixed testes were washed five times with 1x PBS and gradually dehydrated to 70% ethanol prior to processing and embedding in paraffin. Testis sections were cut at six microns and stained with hematoxylin and eosin (H&E) for gross morphology evaluation. Prostates with adjoining reproductive tract were trimmed and sections were prepared by the NIEHS Histopathology Core and independently evaluated at Experimental Pathology Laboratories, Inc. (Durham, NC).
2.4. Quantification of mRNA Expression
Testes were ground with a stone mortar and pestle under liquid nitrogen to a fine powder. Approximately 100 mg powdered tissue was weighed out for total RNA isolation using a QIAGEN Midi kit (Germanton, MD, USA) according to manufacturer’s recommendation. RNA concentrations were measured on a NanoDrop 2000c spectrophotometer (ThermoFisher Scientific, Waltham, MA, USA) and 1.0 μg was converted to cDNA using Superscript II reverse transcriptase (Thermo Fisher Scientific). Samples were then diluted to 2.5 ng/μl for use in TaqMan probe (Thermo Fisher Scientific) reactions for quantitative PCR analysis and normalized to β-actin levels. All TaqMan probes were designed for the Rattus norvegicus genome (Supplemental Table 1).
2.5. Statistics
Statistical analyses were performed using GraphPad Prism, version 8 (La Jolla, CA, USA). A ROUT outliers test (Q=1%) was used to identify and remove statistical outliers from for preputial separation (PPS), testis weight ratios, and sperm counts. For all analyses, statistical significance was defined as p ≤0.05. Data sets were evaluated by one-way ANOVA to test for a main effect of exposure and followed up with a Dunnett’s post hoc test for multiple comparisons when significant. AGD was normalized to body weight and analyzed by mixed effects analysis of variance with dam as an effect. Time to PPS was compared using mixed effects randomized blocks analysis of covariance with adjusted Dunnett’s test. A linear trend test was used to analyze dose responsivity and effect size was calculated by Cohen’s d which are defined as small at 0.2, medium at 0.5, and large at 0.8.
3. Results
H&E-stained testis sections were examined for spermatogenesis, composition of cell types, and the presence of any abnormalities (Fig. 1B). Testes from males of all treatment groups were normal in every category compared to their age matched vehicle controls. Seminiferous tubules of PND 21 males contained spermatogonia and primary spermatocytes. Spermatogenesis at PND 42 had progressed from the first wave to round and elongating spermatids. Testes of PND 90 and one-year-old male rats contained germ cells at all stages of development and no abnormal tissue was observed. The AGD of male offspring was measured on PND four and related to body weight. No significant differences were found between treated and control animals (Fig. 1C). Figure 1D shows males in the 250 mg/kg group monitored for preputial separation (PPS) were significantly delayed compared to controls (p = 0.0335, d = 0.1855).
Figure 1.
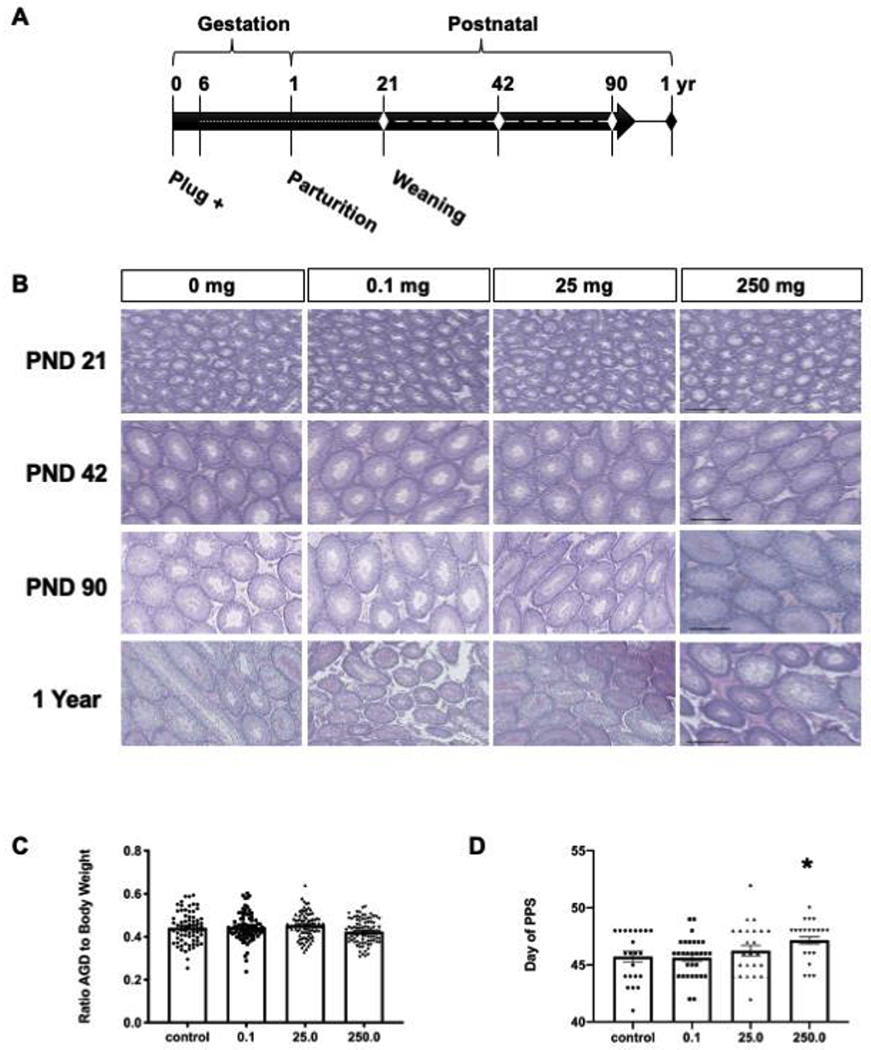
(A) The schematic shows the study timeline. The dotted line represents exposure via dam and dashed line direct exposure to progeny. The diamonds indicate ages collected. (B) Representative testis sections stained with H&E for morphological assessment are shown. At least four males were examined in each age and dose group. Scale bars = 200 μm. (C) Anogenital distance was measured on PND four. Mean anogenital distance normalized to body weight, (n ≥ 73 per dose group). (D) The average age at preputial separation (PPS, n ≤ 24 per dose group). A significant delay (*) was observed in the 250 mg/kg group compared to control (Dunnett’s p = 0.0335, d = 0.1855). Error bars indicate standard error of the mean.
Testis weights relative to body weights were not different across treatment groups in three of the four ages examined (Fig. 2A,C,D). However, PND 42 males showed a significant dose response (p = 0.0128) and a weight difference (p = 0.0409, d = 1.17) in the 250 mg/kg category (Fig. 2B) compared to controls. The numbers of sperm collected from the cauda epididymis and vas deferens of the PND 90 group was comparable to the control (Fig. 2E). A significant difference was observed in the one-year old 25 mg/kg treated males (Fig. 2F) which had fewer sperm than control animals (p = 0.0284, d = 1.12)
Figure 2.
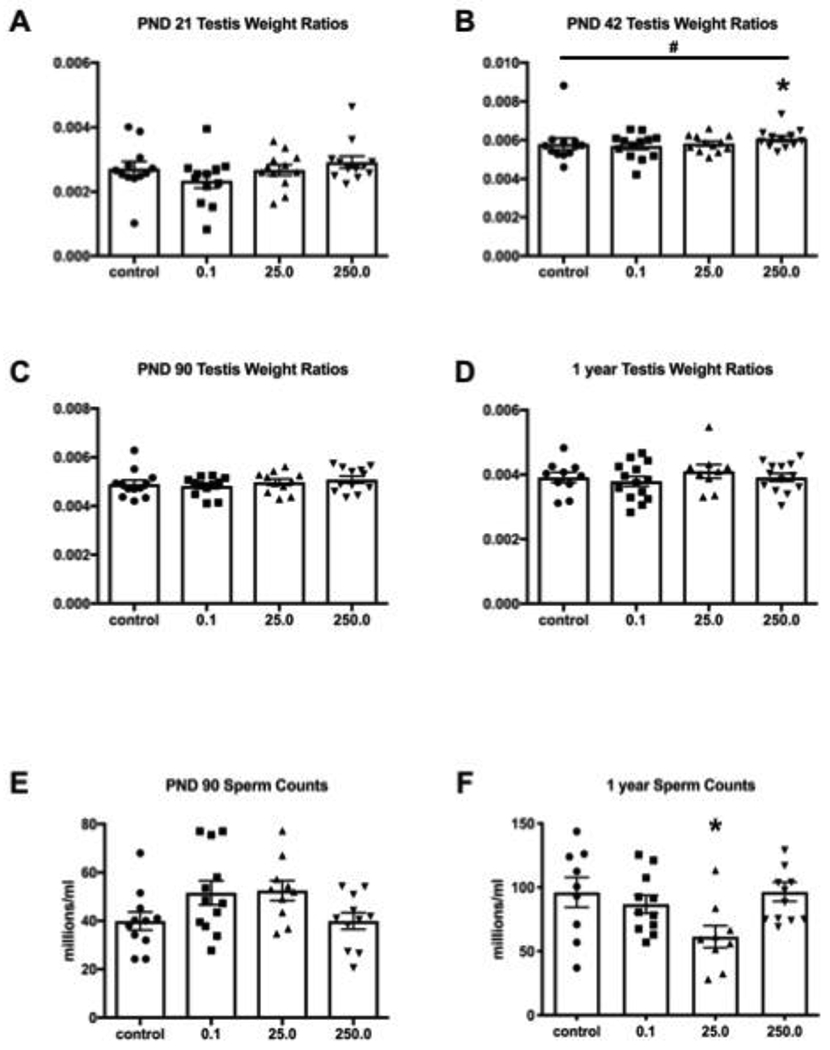
(A-D) Testes were weighed at collection and normalized to body weight in grams (n ≥ 9). A significant difference (*) was found at PND 42 at the highest dose (Dunnett’s p = 0.0409, d = 1.17) as well as a dose response indicated by # (p = 0.0128) (E-F) Cauda epididymal sperm from adult rats (n ≥ 9) were collected via swim-out and counted. The 25 mg/kg treated animals had significantly fewer sperm than controls (* p = 0.0284, d = 1.12). Error bars show standard error of the mean.
The mRNA expression levels of some steroidogenic enzymes and receptors in the testes were different from that in controls in PND 90 and one-year-old time points. At PND 90 cholesterol side-chain cleavage (Cyp11a1) showed a significant dose response effect (p = 0.0405) and a decrease of expression level in the 250 mg/kg group (Dunnett’s p = 0.0304, d = 2.3594) compared to controls (Fig. 3A), but no difference at one year (Fig. 3B). Similarly, three-beta-hydroxysteroid dehydrogenase (Hsd3b1) had a significant downward trend (p = 0.0188) and difference of expression in the 250 mg/kg treatment at one year (Dunnett’s p = 0.0443, d = 1.7381, Fig. 3D), but not at PND 90 (Fig. 3C). There was no change of expression at either age in hydroxysteroid (17beta) dehydrogenase 3 (Hsd17b3, Fig. 3E–F), 5α-reductase (Srd5a1, Fig. 3G–H), or estrogen receptors alpha (Esr1, Fig. 3I–J) or beta (Esr2, Fig. 3K–L).
Figure. 3.
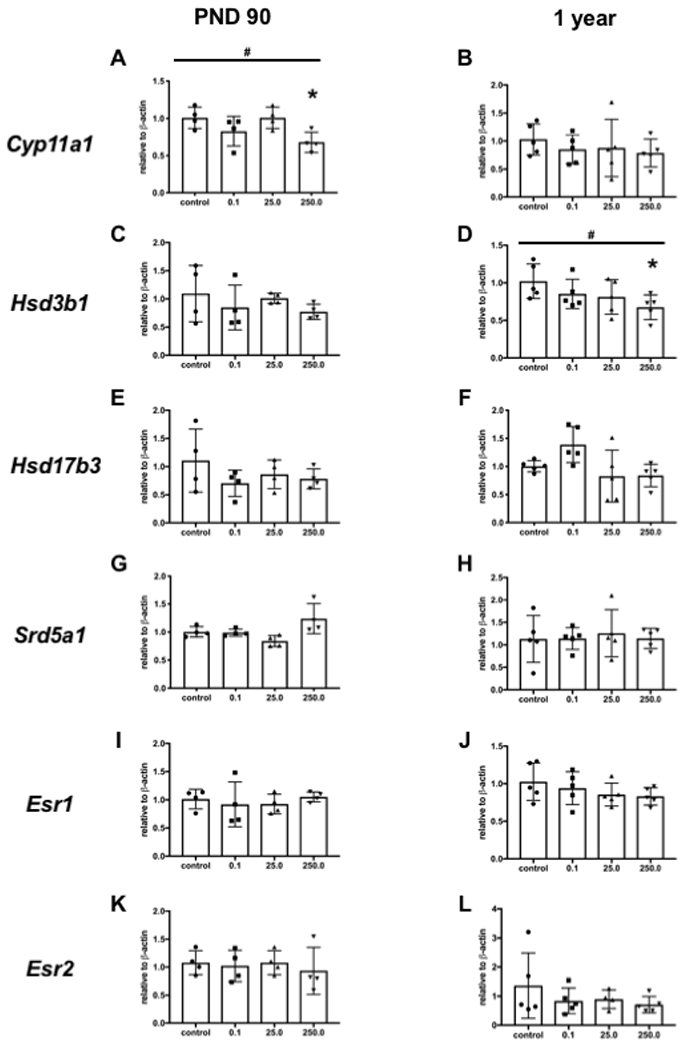
Selected steroidogenic enzymes and receptors were quantitated for mRNA expression using real time PCR. Levels of expression of cholesterol side-chain cleavage (Cyp11a1), 3-beta-hydroxysteroid dehydrogenase (Hsd3b1), 17-beta-hydroxysteroid dehydrogenase (17B-Hsd), 5α-reductase (Srd5a1), and estrogen receptors alpha (Esr1) and beta (Esr2) were evaluated. A dose response was detected in A (p = 0.0405) and D (p = 0.0188) indicated by #. A significant difference was found in the 250 mg/kg dose compared to the control in A (* p = 0.0304, d = 2.36) and D (* p = 0.0443, d = 1.74). Error bars indicate standard error of the mean.
Tissue morphology of the three prostate lobes, the ampullary gland, the seminal vesicles, and the coagulating gland was normal with no evidence of treatment-related differences in lesions or maturity (Fig. 4), therefore no further evaluations were conducted.
Figure 4.
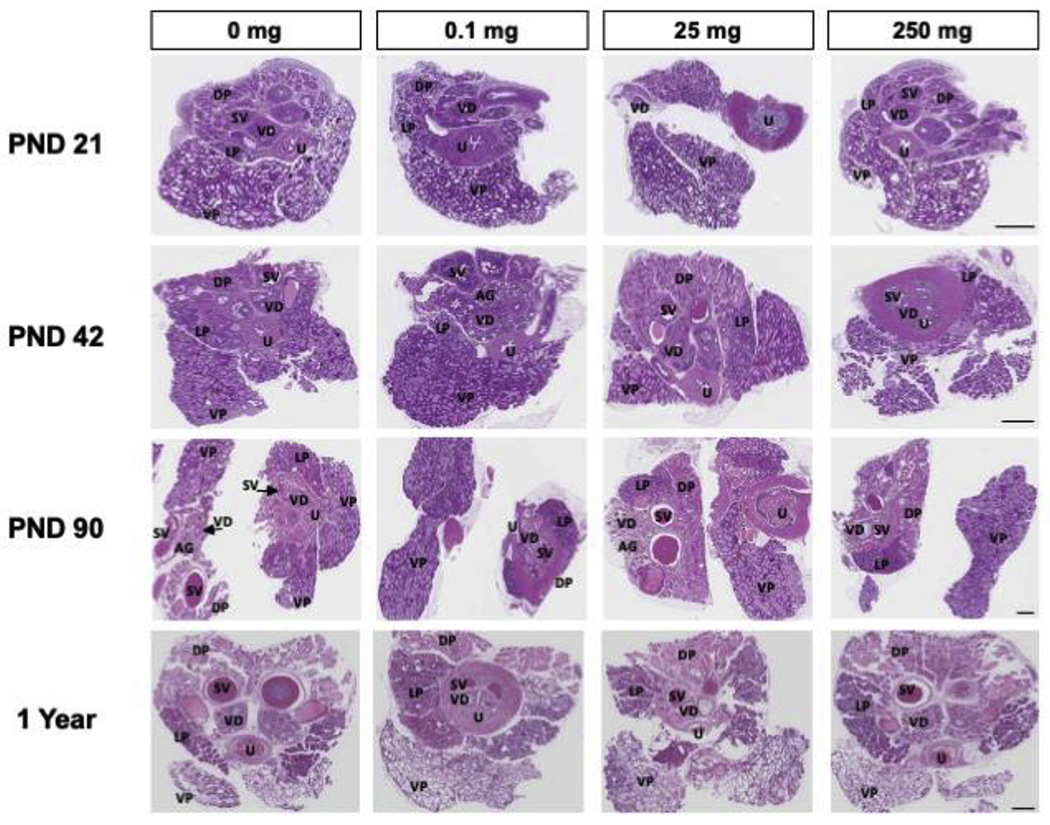
Representative prostate and surrounding reproductive tract sections stained with H&E for morphological assessment were evaluated (n = 3 of each age and dose). Scale bars = 1.1 mm. Abbreviations: AG ampullary gland; DP dorsal prostate lobe; LP lateral prostate lobe; SV seminal vesicle; VD vas deferens; VP ventral prostate lobe; U urethra.
4. Discussion
In this study, we focused on the impact of oral exposure to TBBPA, an analog of BPA, on the reproductive system of male Wistar Han rats. The exposure to dams began on gestation day six and was continued through lactation. The same treatment was then resumed in their progeny at weaning and continued through PND 90. Several males were aged to one year without further treatment. Masculinization of the male reproductive organs requires proper timing and production of testosterone in juveniles as well as adults. Unfortunately, the ELISA assay data which measured sex steroids was unreliable and we had to turn to other biological end points to estimate any effect on testosterone. The results indicated no overt abnormalities in male reproductive organs surveyed at any dose. There were no significant histological changes of testes at any age. A minimally significant difference was found in PND 42 testis weights, but not at other time points. Minor decreases in sperm counts were observed compared to controls in one-year-olds in the 25 mg/kg treatment group. However, there is an accepted wide range of adult rat sperm counts, which may account for this variance [24]. There were significant changes of expression at the mRNA level of two of the steroidogenic pathway components tested in the testes at different ages. But, since spermatogenesis progressed normally in these animals, we surmise any slight alteration of these factors was not sufficient to compromise fertility. There was a delay to PPS timing with the highest dose, but we found exposure to TBBPA had no impact on anogenital distance or morphology of the prostate and other secondary sex organs at any developmental stage examined. Since these parameters require androgens for growth, development, and semen production, these data imply that steroidogenesis was not detrimentally disrupted following exposure to TBBPA.
Many in vivo studies have reported limited effects of TBBPA exposure on the male reproductive system during organ development. Van der Ven et al. [25] exposed male and female Wistar rats prior to mating then continued it through pregnancy and laction with a large range of doses from 3 – 3000 mg/kg of body weight. The offspring were dosed the same concentrations their parents received for 14 weeks. While there was a dose response increase of testis weights at PND 21 and 14 weeks, there were no effects on preputial gland separation, testis morphology, or sperm counts in the F1 generation. Cope and colleagues [26] found no adverse effects on male fertility of Sprague-Dawley offspring from parents dosed up to ten weeks prior to mating, which continued through pregnancy and laction in a pair of studies where the doses were either ten or 100 mg/kg body weight per day up to 1000 mg/kg. Similarly, Saegusa et al. [27] reported no differences in anogenital distance, onset of puberty, or male reproductive organ weights between control and treatment of up to 10,0000 ppm of TBBPA in Sprague-Dawley male offspring from pregnant dams exposed beginning on GD ten through weaning. The windows of exposure (gestation to young adult), the oral administration of TBBPA (via gavage or chow), and concentration of TBBPA used in these studies are similar to what we report here.
Due to the findings that BPA, the parent compound of TBBPA, is an endocrine disrupting chemical affecting both male and female reproductive systems, along with the public pressure to remove BPA from products for children, any substitute compound must be examined for similar affects. Studies have reported an increase of germ cell apoptosis and decreased sperm counts from in utero exposure to BPA using rat as a model. Quan et al. [28] found an increase of oxidative stress and apoptosis with 10 and 100 mg/kg per day. Hass and colleagues [29] showed prenatal and postnatal exposure to BPA of 25 μg/kg/day significantly decreased the number of spermatozoa from Wistar rats. There appears to be a lack of severe adverse effects upon the male reproductive system in rats from our findings examining TBBPA exposure compared to BPA insults.
The global production of TBBPA is increasing [30]. It is routinely used as a brominated flame retardant in electronic circuit boards and in the production of plastics [31]. Therefore, human exposure to TBBPA may have also increased. Our results showed minimal impact to the male reproductive system in rats after a thorough exposure to TBBPA, from in utero to adulthood. However, additional studies are needed to investigate any longer term effects or any that may be passed on to future generations.
Supplementary Material
Highlights.
Tetrabromobisphenol A (TBBPA) is an analog of the plasticizer Bisphenol A
Rats were exposed to TBBPA early in gestation, throughout weaning, and to adulthood
Male reproductive organs were examined for abnormalities up to one year of age
No overt poor outcomes were found in tissues or spermatozoa
Acknowledgements
We are grateful for the expertise of Julie F. Foley and T. Beth Mahler, NIEHS, in conduct of the necropsies. We thank Dr. Gabe Knudensen and Adam Filgo for assistance in dose preparation and in obtaining tissues, Dr. Grace Kissling for guidance in statistical analyses, Hannah Harris for technical assistance including dosing the animals and Rodriquez Sutton, Gordon Caviness, Spencer Bridges, and Joseph Hensley for help with animal care and husbandry.
Funding
This study was funded in part by National Institute of Environmental Health Sciences Intramural Research Fund ES102965 to H.H-C.Y. and the Division of the National Toxicology Program Z01ES102785 to S.E.F. The authors declare no actual or potential competing financial interests.
Abbreviations:
- TBBPA
tetrabromobisphenol A
- BPA
bisphenol A
- GD
gestation day
- PND
post-natal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC, Endocrine-disrupting chemicals: an Endocrine Society scientific statement, Endocr Rev 30(4) (2009) 293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT, Executive Summary to EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals, Endocrine Reviews 36(6) (2015) 593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lawson C, Gieske M, Murdoch B, Ye P, Li Y, Hassold T, Hunt PA, Gene expression in the fetal mouse ovary is altered by exposure to low doses of bisphenol A, Biol Reprod 84(1) (2011) 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fernandez M, Bourguignon N, Lux-Lantos V, Libertun C, Neonatal Exposure to Bisphenol A and Reproductive and Endocrine Alterations Resembling the Polycystic Ovarian Syndrome in Adult Rats, Environ Health Persp 118(9) (2010) 1217–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vigezzi L, Bosquiazzo VL, Kass L, Ramos JG, Munoz-de-Toro M, Luque EH, Developmental exposure to bisphenol A alters the differentiation and functional response of the adult rat uterus to estrogen treatment, Reprod Toxicol 52 (2015) 83–92. [DOI] [PubMed] [Google Scholar]
- [6].Liu C, Duan W, Li R, Xu S, Zhang L, Chen C, He M, Lu Y, Wu H, Pi H, Luo X, Zhang Y, Zhong M, Yu Z, Zhou Z, Exposure to bisphenol A disrupts meiotic progression during spermatogenesis in adult rats through estrogen-like activity, Cell Death Dis 4 (2013) e676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Okada A, Kai O, Effects of estradiol-17beta and bisphenol A administered chronically to mice throughout pregnancy and lactation on the male pups’ reproductive system, Asian J Androl 10(2) (2008) 271–6. [DOI] [PubMed] [Google Scholar]
- [8].Qiu LL, Wang X, Zhang XH, Zhang Z, Gu J, Liu L, Wang Y, Wang X, Wang SL, Decreased androgen receptor expression may contribute to spermatogenesis failure in rats exposed to low concentration of bisphenol A, Toxicol Lett 219(2) (2013) 116–24. [DOI] [PubMed] [Google Scholar]
- [9].Morose G, An overview of alternatives to tetrabromobisphenol A (TBBPA) and hexabromocyclododecane (HBCD). The Jennifer Altman Foundation, Massachusetts, USA: Lowell Center for Sustainable Production University of Massachusetts Lowell, 2006. [Google Scholar]
- [10].Cariou R, Antignac JP, Zalko D, Berrebi A, Cravedi JP, Maume D, Marchand P, Monteau F, Riu A, Andre F, Le Bizec B, Exposure assessment of French women and their newborns to tetrabromobisphenol-A: occurrence measurements in maternal adipose tissue, serum, breast milk and cord serum, Chemosphere 73(7) (2008) 1036–41. [DOI] [PubMed] [Google Scholar]
- [11].Lankova D, Lacina O, Pulkrabova J, Hajslova J, The determination of perfluoroalkyl substances, brominated flame retardants and their metabolites in human breast milk and infant formula, Talanta 117 (2013) 318–25. [DOI] [PubMed] [Google Scholar]
- [12].Shi ZX, Wu YN, Li JG, Zhao YF, Feng JF, Dietary exposure assessment of Chinese adults and nursing infants to tetrabromobisphenol-A and hexabromocyclododecanes: occurrence measurements in foods and human milk, Environ Sci Technol 43(12) (2009) 4314–9. [DOI] [PubMed] [Google Scholar]
- [13].Morris S, Allchin CR, Zegers BN, Haftka JJ, Boon JP, Belpaire C, Leonards PE, Van Leeuwen SP, De Boer J, Distribution and fate of HBCD and TBBPA brominated flame retardants in North Sea estuaries and aquatic food webs, Environ Sci Technol 38(21) (2004) 5497–504. [DOI] [PubMed] [Google Scholar]
- [14].Harrad S, Goosey E, Desborough J, Abdallah MAE, Roosens L, Covaci A, Dust from UK Primary School Classrooms and Daycare Centers: The Significance of Dust As a Pathway of Exposure of Young UK Children to Brominated Flame Retardants and Polychlorinated Biphenyls, Environ Sci Technol 44(11) (2010) 4198–4202. [DOI] [PubMed] [Google Scholar]
- [15].Xie Z, Ebinghaus R, Lohmann R, Heemken O, Caba A, Puttmann W, Trace determination of the flame retardant tetrabromobisphenol A in the atmosphere by gas chromatography-mass spectrometry, Anal Chim Acta 584(2) (2007) 333–42. [DOI] [PubMed] [Google Scholar]
- [16].Deng J, Guo J, Zhou X, Zhou P, Fu X, Zhang W, Lin K, Hazardous substances in indoor dust emitted from waste TV recycling facility, Environ Sci Pollut Res Int 21(12) (2014) 7656–67. [DOI] [PubMed] [Google Scholar]
- [17].Schauer UM, Volkel W, Dekant W, Toxicokinetics of tetrabromobisphenol a in humans and rats after oral administration, Toxicol Sci 91(1) (2006) 49–58. [DOI] [PubMed] [Google Scholar]
- [18].Dunnick JK, Sanders JM, Kissling GE, Johnson CL, Boyle MH, Elmore SA, Environmental chemical exposure may contribute to uterine cancer development: studies with tetrabromobisphenol A, Toxicol Pathol 43(4) (2015) 464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zatecka E, Castillo J, Elzeinova F, Kubatova A, Ded L, Peknicova J, Oliva R, The effect of tetrabromobisphenol A on protamine content and DNA integrity in mouse spermatozoa, Andrology-Us 2(6) (2014) 910–7. [DOI] [PubMed] [Google Scholar]
- [20].Siracusa JS, Yin L, Measel E, Liang S, Yu X, Effects of bisphenol A and its analogs on reproductive health: A mini review, Reprod Toxicol 79 (2018) 96–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].E.P.o.C.i.t.F.C. (CONTAM), Scientific Opinion on Tetrabromobisphenol A (TBBPA) and its derivatives in food, EFSA 9(12) (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rock KD, Gillera SEA, Devarasetty P, Horman B, Knudsen G, Birnbaum LS, Fenton SE, Patisaul HB, Sex-specific behavioral effects following developmental exposure to tetrabromobisphenol A (TBBPA) in Wistar rats, Neurotoxicology 75 (2019) 136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Program NT, TOXICOLOGY STUDIES OF TETRABROMOBISPHENOL A (CAS NO. 79-94-7) IN F344/NTac RATS AND B6C3F1/N MICE AND TOXICOLOGY AND CARCINOGENESIS STUDIES OF TETRABROMOBISPHENOL A IN WISTAR HAN [Crl:WI(Han)] RATS AND B6C3F1/N MICE, NTP Technical Report, National Institutes of Health, PHS, HHS; http://ntp.niehs.nih.gov, 2014. [Google Scholar]
- [24].Varisli O, Agca C, Agca Y, Short-term storage of rat sperm in the presence of various extenders, J Am Assoc Lab Anim Sci 52(6) (2013) 732–7. [PMC free article] [PubMed] [Google Scholar]
- [25].Van der Ven LT, Van de Kuil T, Verhoef A, Verwer CM, Lilienthal H, Leonards PE, Schauer UM, Canton RF, Litens S, De Jong FH, Visser TJ, Dekant W, Stern N, Hakansson H, Slob W, Van den Berg M, Vos JG, Piersma AH, Endocrine effects of tetrabromobisphenol-A (TBBPA) in Wistar rats as tested in a one-generation reproduction study and a subacute toxicity study, Toxicology 245(1–2) (2008) 76–89. [DOI] [PubMed] [Google Scholar]
- [26].Cope RB, Kacew S, Dourson M, A reproductive, developmental and neurobehavioral study following oral exposure of tetrabromobisphenol A on Sprague-Dawley rats, Toxicology 329 (2015) 49–59. [DOI] [PubMed] [Google Scholar]
- [27].Saegusa Y, Fujimoto H, Woo GH, Inoue K, Takahashi M, Mitsumori K, Hirose M, Nishikawa A, Shibutani M, Developmental toxicity of brominated flame retardants, tetrabromobisphenol A and 1,2,5,6,9,10-hexabromocyclododecane, in rat offspring after maternal exposure from mid-gestation through lactation, Reprod Toxicol 28(4) (2009) 456–67. [DOI] [PubMed] [Google Scholar]
- [28].Quan C, Wang C, Duan P, Huang W, Yang K, Prenatal bisphenol a exposure leads to reproductive hazards on male offspring via the Akt/mTOR and mitochondrial apoptosis pathways, Environ Toxicol 32(3) (2017) 1007–1023. [DOI] [PubMed] [Google Scholar]
- [29].Hass U, Christiansen S, Boberg J, Rasmussen MG, Mandrup K, Axelstad M, Low-dose effect of developmental bisphenol A exposure on sperm count and behaviour in rats, Andrology-Us 4(4) (2016) 594–607. [DOI] [PubMed] [Google Scholar]
- [30].de Wit CA, Herzke D, Vorkamp K, Brominated flame retardants in the Arctic environment--trends and new candidates, Sci Total Environ 408(15) (2010) 2885–918. [DOI] [PubMed] [Google Scholar]
- [31].Wikoff D, Thompson C, Perry C, White M, Borghoff S, Fitzgerald L, Haws LC, Development of toxicity values and exposure estimates for tetrabromobisphenol A: application in a margin of exposure assessment, J Appl Toxicol 35(11) (2015) 1292–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


