Abstract
Liver cancer incidence has increased for several decades in the US. Recently, reports suggested that rates of hepatocellular carcinoma (HCC), the dominant form of liver cancer, had declined in certain groups. However, the most recent histology-specific liver cancer rates have not been reported. Thus, we examined HCC and intrahepatic cholangiocarcinoma (ICC) incidence from 1992 to 2016 using data from the Surveillance, Epidemiology, and End Results (SEER) Registries. Age-standardized incidence rates (ASR) were calculated by histology, sex, race/ethnicity, and age. Trends were analyzed using Joinpoint regression to estimate annual percent change (APC). Between 2011 and 2016, HCC rates significantly declined (APC=−1.9%), with more prominent declines among males, Asian/Pacific Islanders, and individuals <50 years of age. Conversely, ICC rates increased from 2002–2016. Declining HCC rates may persist due to improved hepatitis C virus (HCV) treatment and/or competing causes of mortality among persons with fatty liver disease.
Keywords: incidence, hepatocellular carcinoma, intrahepatic cholangiocarcinoma
Precis
Between 2011 and 2016, HCC rates significantly declined (APC=−1.9%), driven by declines among males, Asian/Pacific Islanders, and individuals <50 years of age. Conversely, ICC rates increased from 2002–2016.
Hepatocellular carcinoma (HCC) rates have been rising in the US since the mid-1970s.1 Additionally, rates of intrahepatic cholangiocarcinoma (ICC), the second most common type of liver cancer,2 have also been increasing.3 We previously forecast that HCC rates would continue to increase through 2030, based on data from 2000 to 2012 drawn from the Surveillance, Epidemiology, and End Results (SEER) Registries.4 However, we noted that rates were decreasing in Asians/Pacific Islanders, individuals younger than age 65, and cohorts born after 1960, portending possible declines in incidence of HCC in future years. A recent publication reported that the incidence of HCC in the U.S. started to plateau between 2010 and 2015.5 However, the most recent HCC and ICC incidence rates have not been reported, which are needed as risk factors for liver cancer are in rapid transition.6 Thus, we examined recent trends of HCC and ICC incidence, using high-quality cancer registry data from SEER.
We utilized the SEER-13 registries, which include cases diagnosed from 1992–2016 and cover approximately 14% of the US population.7 Primary liver cancer was identified by the International Classification of Diseases for Oncology (ICD-O), 3rd edition topography code C22.8 Cases were classified as HCC (morphology codes 8170–8175) or ICC (morphology codes 8032–8033, 8041, 8050, 8070–8071, 8140–8141, 8160, 8260, 8480, 8481, 8490, 8560).2 Age-standardized incidence rates (ASR) per 100,000 persons and 95% confidence intervals (CIs) were calculated by year of diagnosis. In addition to overall rates, incidence was examined by sex, race/ethnicity (non-Hispanic white [NHW], non-Hispanic black [NHB], Hispanic, Asian/Pacific Islander [API], American Indian/Alaskan Native [AIAN]), and age at diagnosis (<50, 50–69, 70–84 years).
Using Joinpoint regression, annual percent change (APC), average annual percent change (AAPC), and 95% CIs were estimated for all subgroups. A maximum of four segments were allowed in the model to fit age-adjusted trends, utilizing the permutation test.9 A segment was considered significant if the slope of the regression line was statistically different from zero (p<0.05). All case and population data were obtained from SEER*Stat (v8.3.6, IMS, Silver Spring, MD). Joinpoint regression analyses were conducted in the Joinpoint Program (v4.6.0.0, IMS, Silver Spring, MD).
As a sensitivity analysis, we also examined HCC incidence in SEER-9 and 18 registries and the National Program of Cancer Registries (NPCR) database (data not shown). We observed similar trends across these databases and only report SEER-13 registry data herein, as these allow the most robust trend analysis by histology and race/ethnicity. SEER-9 registries allow for a longer trend analysis but do not contain information on ethnicity, while SEER-18 and NPCR cover a larger proportion of the U.S. .population but only having data starting around 2000.
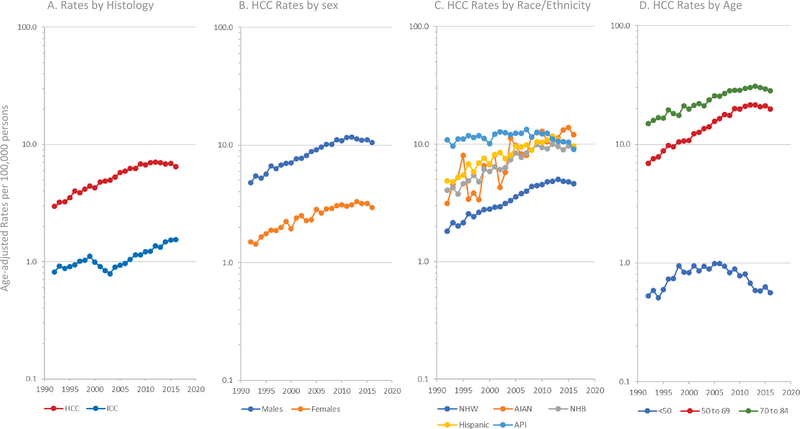
HCC incidence rates are shown in Figure 1A. For HCC, we further graphed rates by sex (Figure 1B), race/ethnicity (Figure 1C), and age (Figure 1D). Overall, HCC rates significantly declined (APC=−1.9%) between 2011 and 2016. Declines in rates were evident among almost all groups but were especially notable among men (APC=−2.0%), APIs (APC=−4.2%), and individuals <50 years of age (APC=−5.5, Table 1). The only group, however, whose rates did not follow this pattern were AIAN, who experienced significantly increasing HCC rates (APC=4.7%).
Figure 1.
Age-adjusted incidence rates per 100,000 persons, 1A) by histology, 1B) by sex, 1C) by race/ethnicity, and 1D) by age.
Table 1.
Age-standardized incidence rates (ASR) and 95% confidence intervals (CI) of hepatocellular carcinoma in the United States; Surveillance, Epidemiology, and End Results Program 13 Registries, 1992–2016.
| Population Group | 1992 |
2016 |
APC (%)1 |
AAPC 1992–20161 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | ASR | 95% CI | Cases | ASR | 95% CI | Trend 1 | Trend 2 | Trend 3 | Joinpoint 1 | Joinpoint 2 | (%) | 95% CI | |
| Hepatocellular Carcinoma Overall | 924 | 2.95 | (2.76, 3.15) | 3,398 | 6.49 | (6.27, 6.72) | 4.5* | −1.9* | - | 2011 | - | 3.2* | (2.8, 3.5) |
| Sex | |||||||||||||
| Males | 661 | 4.78 | (4.42, 5.17) | 2,587 | 10.52 | (10.11, 10.95) | 4.5* | −2.0* | - | 2011 | - | 3.1* | (2.7, 3.5) |
| Females | 263 | 1.51 | (1.33, 1.70) | 811 | 2.95 | (2.75, 3.16) | 4.2* | 0.1 | - | 2009 | - | 3.0* | (2.3, 3.7) |
| Race/Ethnicity2 | |||||||||||||
| Non-Hispanic White | 428 | 1.82 | (1.65, 2.00) | 1,498 | 4.60 | (4.36, 4.85) | 4.9* | −1.8 | - | 2012 | - | 3.7* | (3.3, 4.2) |
| Non-Hispanic Black | 103 | 4.07 | (3.30, 4.94) | 492 | 9.66 | (8.93, 10.44) | 5.4* | −0.2 | - | 2009 | - | 3.7* | (3.1, 4.4) |
| Hispanic | 125 | 4.86 | (3.97, 5.78) | 705 | 9.66 | (8.93, 10.44) | 4.1* | −4.2 | - | 2012 | - | 2.6* | (1.8, 3.5) |
| Asian/Pacific Islander | 261 | 10.84 | (9.51, 12.28) | 627 | 9.07 | (8.36, 9.83) | 1.1* | −4.2* | - | 2009 | - | −0.5 | (−1.2, 0.2) |
| American Indian/Alaskan Native | 6 | 3.14 | (0.99, 6.99) | 60 | 11.41 | (8.62, 14.84) | 4.7* | - | - | - | - | 4.7* | (3.2, 6.1) |
| Age at Diagnosis | |||||||||||||
| <50 years | 128 | 0.53 | (0.44, 0.63) | 158 | 0.56 | (0.48, 0.66) | 4.6* | −5.5* | - | 2005 | - | −0.2 | (−1.3, 1.0) |
| 50–69 years | 411 | 6.94 | (6.28, 7.65) | 2,206 | 19.88 | (19.05, 20.73) | 5.9* | −1.2 | - | 2011 | - | 4.4* | (4.0, 4.8) |
| 70–84 years | 344 | 14.92 | (13.38, 16.58) | 894 | 28.28 | (26.45, 30.20) | 3.6* | −2.3 | - | 2012 | - | 2.6* | (2.0, 3.1) |
| Intrahepatic Cholangiocarcinoma Overall | 251 | 0.82 | (0.72, 0.93) | 775 | 1.55 | (1.44, 1.67) | 3.8* | −8.9 | 4.8* | 1999 | 2002 | 2.7* | (1.4, 4.1) |
| Sex | |||||||||||||
| Males | 138 | 1.08 | (0.90, 1.28) | 417 | 1.81 | (1.63, 1.99) | 2.6* | −6.8 | 4.8* | 1999 | 2003 | 2.1* | (0.6, 3.7) |
| Females | 113 | 0.64 | (0.53, 0.77) | 358 | 1.34 | (1.20, 1.49) | 4.5* | −7.6 | 5.1* | 1999 | 2002 | 3.3* | (0.6, 6.0) |
| Race/Ethnicity2 | |||||||||||||
| Non-Hispanic White | 161 | 0.69 | (0.58, 0.80) | 446 | 1.44 | (1.31, 1.59) | 5.3* | −12.2 | 5.6* | 1999 | 2002 | 3.1* | (1.2, 5.1) |
| Non-Hispanic Black | 18 | 0.81 | (0.47, 1.27) | 67 | 1.49 | (1.15, 1.91) | 2.7* | - | - | - | - | 2.7* | (1.7, 3.7) |
| Hispanic | 34 | 1.33 | (0.89, 1.89) | 126 | 1.89 | (1.56, 2.26) | 2.2* | - | - | - | - | 2.2* | (1.3, 3.1) |
| Asian/Pacific Islander | 33 | 1.44 | (0.97, 2.04) | 123 | 1.78 | (1.48, 2.13) | 1.0* | - | - | - | - | 1.0* | (0.3, 1.8) |
| American Indian/Alaskan Native | 5 | 3.14 | (0.95, 7.09) | 10 | 2.10 | (0.96, 3.97) | −1.2 | - | - | - | - | −1.2 | (−3.7, 1.4) |
| Age at Diagnosis | |||||||||||||
| <50 years | 30 | 0.12 | (0.08, 0.18) | 48 | 0.17 | (0.13, 0.23) | 1.8* | - | - | - | - | 1.8* | (0.7, 2.8) |
| 50–69 years | 88 | 1.49 | (1.19, 1.83) | 379 | 3.42 | (3.08, 3.78) | 1.5 | 4.8* | - | 2004 | - | 3.2* | (2.2, 4.2) |
| 70–84 years | 106 | 4.63 | (3.79, 5.60) | 273 | 8.62 | (7.62, 9.71) | 4.4* | −11.7 | 5.2* | 1999 | 2002 | 2.7 | (−0.4, 5.8) |
Abbreviations: APC, annual percent change; AAPC, average annual percent change.
Asterisk denotes trend is statistically different from zero at the alpha=0.05 level.
Cases may not add up to overall due to unknown race/ethnicity categories (data not shown).
In contract to HCC rates, ICC rates significantly increased between 2002 and 2016 (Figure 1A, APC=4.8%) overall, and among almost all subgroups (Table 1). Among AIAN, however, ICC rates declined (APC=−1.2%).
In the current study, we report that HCC rates have declined or plateaued in all groups in the U.S. with the exception of AIAN. While there was a more prominent decline in rates among men than women starting around 2011, the overall trends by sex were very similar, with rates rising until 2009–2011, then declining thereafter. Similarly, the decline in rates among all race/ethnic groups except AIAN started around 2009–2012. Why the HCC trend among AIAN differs from other racial/ethnic groups is not certain, although rates among smaller populations are often challenging to characterize.
While we previously reported the decline in HCC rates among APIs,4, 10 which has continued through 2016, we also forecast that HCC rates would not begin to plateau in other groups until 2025. Our prior study was based on incidence data from 2000–2012 and provided the best model of future HCC incidence at the time. Other attempts to model the US HCC epidemic curve also suggested that rates would likely increase until 2020 or 2025.11, 12 A recent publication examined HCC incidence through 2015, however, observed plateauing rates.5 Herein, we show that HCC rates have not only plateaued, but significantly declined between 2011 and 2016. While the reasons for the decline in HCC rates are not clear, factors that may have had an effect include halting the spread of HCV via testing of the blood supply, improvement in HCV treatment13, 14 and competing mortality risks.15 Of these potential factors however, it should be noted that direct-acting antiviral (DAA) therapy for HCV was not approved by the FDA until 2011 and the all-oral DAA combinations were not available until 2014. 16 As the downturn in rates began around 2011 before widespread availability of these drugs, other factors must have had an effect on the decline.
A potential factor that could have affected HCC rates is the competing mortality risk related to non-alcoholic fatty liver disease (NAFLD). The prevalence of NAFLD is now estimated to be 30% in the general US population 17–19 and NAFLD, particularly in its most severe form, non-alcoholic steatohepatitis (NASH), has become an important risk factor for HCCs in many countries, including the U.S. 20 However, persons with NAFLD are more likely to die of cirrhosis, cardiovascular disease, diabetes, and non-HCC cancer than they are to die of HCC. 21, 22 This suggests that the rising prevalence of NAFLD could potentially decrease the incidence of HCC.
In the US, known risk factors for ICC largely overlap with HCC (e.g., metabolic conditions, alcohol consumption, and HBV/HCV infection).23–29 Thus, it is unclear why HCC rates have significantly declined while ICC rates increased. However, these trends may be related to how transitioning risk factors affect the tumor microenvironment.6, 30, 31 For example, a recent study demonstrated that ICC can arise from hepatocytes in a necroptotic microenvironment,30 and studies have also suggested that necroptosis could be a fundamental element in the pathogenesis of NAFLD.31
In summary, HCC rates have declined while ICC rates have increased. Due to HCV removal from the blood supply and advances in treatment, and/or competing mortality risks among persons with NAFLD, HCC rates may continue to decrease. Further research is needed to elucidate the etiology and molecular mechanisms of ICC.
Acknowledgments
Funding: NCI Intramural Program.
Abbreviations
- HCC
hepatocellular carcinoma
- ICC
intrahepatic cholangiocarcinoma
- SEER
Surveillance, Epidemiology, and End Results
- ASR
Age-standardized incidence rates
- APC
annual percent change
- ICD-O
International Classification of Diseases for Oncology
- CIs
confidence intervals
- NHW
non-Hispanic white
- NHB
non-Hispanic black
- API
Hispanic, Asian/Pacific Islander
- AIAN
American Indian/Alaskan Native
- AAPC
average annual percent change
- HCV
hepatitis C virus
- DAA
direct-acting antiviral
- NAFLD
non-alcoholic fatty liver disease
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular Carcinoma Incidence, Mortality, and Survival Trends in the United States From 1975 to 2005. Journal of Clinical Oncology. 2009;27: 1485–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altekruse SF, Devesa SS, Dickie LA, McGlynn KA, Kleiner DE. Histological classification of liver and intrahepatic bile duct cancers in SEER registries. J Registry Manag. 2011;38: 201–205. [PMC free article] [PubMed] [Google Scholar]
- 3.Van Dyke AL, Shiels MS, Jones GS, et al. Biliary tract cancer incidence and trends in the United States by demographic group, 1999–2013. Cancer. 2019;125: 1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. Journal of Clinical Oncology. 2016;34: 1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rich NE, Yopp AC, Singal AG, Murphy CC. Hepatocellular Carcinoma Incidence Is Decreasing Among Younger Adults in the United States. Clin Gastroenterol Hepatol. 2020;18: 242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrick JL, McGlynn KA. The changing epidemiology of primary liver cancer. Curr Epidemiol Rep. 2019;6: 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Surveillance E, and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER 13 Regs Research Data, November 2018. Sub (1992–2016) <Katrina/Rita Population Adjustment> - Linked to County Attributes - Total U.S., 1969–2017 Counties. In: National Cancer Institute D, Surveillance Research Program., editor, released April 2019, based on the November 2018 submission. [Google Scholar]
- 8.Fritz AJA, Shanmugaratnam K, Sobin LH, Parkin MD, editors. International Classification of Diseases for Oncology, 3rd ed. Geneva, Switzerland: World Health Organization, 2000. [Google Scholar]
- 9.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19: 335–351. [DOI] [PubMed] [Google Scholar]
- 10.Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol. 2014;109: 542–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138: 513–521. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka Y, Hanada K, Mizokami M, et al. A comparison of the molecular clock of hepatitis C virus in the United States and Japan predicts that hepatocellular carcinoma incidence in the United States will increase over the next two decades. Proc Natl Acad Sci U S A. 2002;99: 15584–15589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ditah I, Ditah F, Devaki P, et al. The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. J Hepatol. 2014;60: 691–698. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg ES, Rosenthal EM, Hall EW, et al. Prevalence of Hepatitis C Virus Infection in US States and the District of Columbia, 2013 to 2016. JAMA Netw Open. 2018;1: e186371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drake I, Gullberg B, Sonestedt E, et al. Type 2 diabetes, adiposity and cancer morbidity and mortality risk taking into account competing risk of noncancer deaths in a prospective cohort setting. Int J Cancer. 2017;141: 1170–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fung J Era of direct acting antivirals in chronic hepatitis C: Who will benefit? World J Hepatol. 2015;7: 2543–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313: 2263–2273. [DOI] [PubMed] [Google Scholar]
- 18.Lazo M, Hernaez R, Bonekamp S, et al. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011;343: d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan JJ, Fallon MB. Gender and racial differences in nonalcoholic fatty liver disease. World J Hepatol. 2014;6: 274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol. 2019;70: 531–544. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez CS, Graubard BI, Thistle JE, Petrick JL, McGlynn KA. Attributable Fractions of NAFLD for Mortality in the United States: Results From NHANES III With 27 Years of Follow-up. Hepatology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paik JM, Henry L, De Avila L, Younossi E, Racila A, Younossi ZM. Mortality Related to Nonalcoholic Fatty Liver Disease Is Increasing in the United States. Hepatol Commun. 2019;3: 1459–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donato F, Gelatti U, Tagger A, et al. Intrahepatic cholangiocarcinoma and hepatitis C and B virus infection, alcohol intake, and hepatolithiasis: a case–control study in Italy. Cancer Causes & Control. 2001;12: 959–964. [DOI] [PubMed] [Google Scholar]
- 24.Lee TY, Lee SS, Jung SW, et al. Hepatitis B virus infection and intrahepatic cholangiocarcinoma in Korea: a case-control study. Am J Gastroenterol. 2008;103: 1716–1720. [DOI] [PubMed] [Google Scholar]
- 25.Petrick JL, Yang B, Altekruse SF, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: A population-based study in SEER-Medicare. PLoS One. 2017;12: e0186643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005;128: 620–626. [DOI] [PubMed] [Google Scholar]
- 27.Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54: 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto S, Kubo S, Hai S, et al. Hepatitis C virus infection as a likely etiology of intrahepatic cholangiocarcinoma. Cancer Sci. 2004;95: 592–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou YM, Yin ZF, Yang JM, et al. Risk factors for intrahepatic cholangiocarcinoma: a case-control study in China. World J Gastroenterol. 2008;14: 632–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seehawer M, Heinzmann F, D’Artista L, et al. Necroptosis microenvironment directs lineage commitment in liver cancer. Nature. 2018;562: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwabe RF, Luedde T. Apoptosis and necroptosis in the liver: a matter of life and death. Nature Reviews Gastroenterology & Hepatology. 2018;15: 738–752. [DOI] [PMC free article] [PubMed] [Google Scholar]