Abstract
OBJECTIVE:
To use patient-level clinical variables to develop and validate a parsimonious model to predict hospital readmissions from home health care (HHC) in Medicare fee-for-servic beneficiaries.
DESIGN:
Retrospective analysis using multivariable logistic regression and gradient boosting machine (GBM) learning to develop and validate a predictive model.
SETTING/PARTICIPANTS/MEAUREMENTS:
A 5% national sample of patients ≥65 years with Medicare fee-for-service who received skilled HHC services within 5 days of hospital discharge in 2012 (n = 43,407). Multiple datasets were merged including Medicare OASIS, Home Health Claims, MedPAR, and Master Beneficiary Summary Files to extract patient-level variables from the first HHC visit after discharge and measure 30-day readmission outcomes.
RESULTS:
Among 43,407 patients with inpatient hospitalizations followed by HHC, 14.7% were readmitted within 30 days. Of the 53 candidate variables, seven remained in the final model: Elixhauser comorbidity index, index hospital length of stay ), urinary catheter presence, patient status (i.e., fragile health with high risk of complications or serious progressive condition), two or more hospitalizations in prior year, pressure ulcer risk or presence, and surgical wound presence. Of interest, surgical wounds, either from a total hip or total knee arthroplasty procedure or another surgical procedure, was associated with fewer readmissions. The optimism-corrected c-statistics for the full model and parsimonious model were 0.67 and 0.66, respectively, indicating fair discrimination. The Brier score for both models was 0.120, indicating good calibration. The GBM model identified similar predictive variables.
CONCLUSION:
Variables available to HHC clinicians at the first post-discharge HHC visit can predict readmission risk and inform care plans in HHC. Future analyses incorporating measures of social determinants of health such as housing instability or social support have the potential to enhance prediction of this outcome.
Keywords: Home health care, care transitions, hospital readmission
INTRODUCTION
Hospitals increasingly rely upon home health care (HHC) agencies to provide skilled services including nursing and therapies (e.g., physical, occupational, speech) for patients after hospitalization.1 In 2013, there were over 3.9 million HHC referrals following hospitalization, and HHC referrals exceeded skilled nursing facility referrals after hospitalization (11.2% versus 9.0%) across all payers.2 In addition, nearly 65% of patients discharged with HHC in 2013 were Medicare beneficiaries.2
Recently, Medicare has begun to measure and publicly report outcomes for HHC following hospitalization, including risk-adjusted rehospitalization during the first 30 days of HHC at the agency level.3 In addition, Medicare also recently initiated a value-based purchasing model for home health agencies in 9 states that includes a rehospitalization reduction incentive to more closely tie payment to performance.4 The rehospitalization measure for HHC represents a substantial overlap between 30-day rehospitalization metrics for which acute care hospitals and HHC agencies are accountable.
At present, Medicare public reporting is at the home health agency level to allow for comparison of performance between agencies. Yet, as home health agencies aim to improve care transitions for recently-discharged patients, rigorous evidence on how to identify HHC patients at high readmission risk is lacking.5 In addition, readmission models often include a large number of variables drawn from retrospective administrative data that may be cumbersome and unavailable for clinicians to use in real-time to guide transitional care.6–8 In this study, our goal was to develop and validate a parsimonious predictive model using variables that would be available to clinicians at the first HHC visit to identify patients at high risk of readmission.
METHODS
Study Design, Setting, and Population
The dataset includes a 5% sample of national Medicare Outcome and Assessment Information Set (OASIS-C), Home Health Claims, Medicare Provider Analysis and Review (MedPAR), and Master Beneficiary Summary File data from 2012, and the first month of 2013 (to capture 30-day readmissions after December 2012 discharges). Because a majority of Medicare beneficiaries who use HHC services are ages 65 and above,9 beneficiaries younger than 65 years old were excluded from the cohort. The Medicare Home Health Claims-Based Rehospitalization Measure was used to further restrict the cohort to include Medicare fee-for-service beneficiaries with an acute inpatient hospitalization followed by the HHC start of care within five days of hospital discharge occurring in 2012.8 To ensure the OASIS record had a corresponding HHC claim, the OASIS start of care and the HHC claim from the Home Health Claim file were required to be within five days of each other. Using the MedPAR and the Master Beneficiary Summary File, the same criteria as the Medicare All-Cause Rehospitalization Measure was applied to exclude cases in which patients left against clinical advice from the index hospitalization, died within 30 days of index hospitalization discharge, had an index hospitalization for inpatient rehabilitation, or had an index hospitalization for medical treatment of cancer or a primary psychiatric diagnosis.10 For beneficiaries with multiple hospitalization-HHC episodes, only the initial episode was included for analysis. This study was approved by the Colorado Multiple Institutional Regulatory Board (15-2174).
Primary Outcome and Variables
The Medicare Hospital-Wide All-Cause Unplanned Readmission measure methodology was applied to MedPAR data to identify 30-day readmissions, in which the index inpatient discharge claim is followed by a subsequent inpatient admission within ≤30 days.10 We defined readmissions as occurring at least one day after discharge to allow time for an initial HHC visit to occur. In alignment with measure methodology, readmissions that are always planned, such as organ transplantation, maintenance chemotherapy, and others were excluded from the outcome.10
OASIS variables used in this analysis were collected at the start of HHC and include unique measures of rehospitalization factors (e.g., multiple prior hospitalizations, falls, medications), social situation (i.e., lives alone), cognition, depression, anxiety, activities of daily living, and others.11 OASIS variables that would only be available for a small subset of patients in the cohort (e.g., pressure wound measurements) were not included in the final set of variables. In instances in which multiple OASIS variables collect similar information (e.g., pain, presence of a urinary catheter, behavior symptoms), only one of the variables collecting this information was included in the analysis. For example, OASIS items M1240 and M1242 both assess pain. OASIS item Ml240 asks “Has this patient had a formal pain assessment using a standardized pain assessment tool?” and three potential responses: (a) no standardized assessment conducted, (b) yes, and it does not indicate severe pain, or (c) yes, and it indicates severe pain. The OASIS item M1242 asks about the “Frequency of pain interfering with patient’s activity or movement” and has five potential responses ranging from “no pain” to “all of the time” pain. In this case, the M1240 item was not used and the Ml242 item was retained because it provided a more global assessment of pain. In cases of OASIS activities of daily living (ADLs) assessments that were similar (e.g., Ml870 Feeding or Eating, and Ml880 Ability to Plan and Prepare light meals), we included only the item that would be retained in future versions of the OASIS-D (i.e., Ml 870).12
The depression measure was determined by either screening positive for the PHQ2 scale included in the OASIS,13 or screening yes with a different instrument as noted by the OASIS assessor. The prior activities of daily living variables were dichotomized to indicate whether assistance is needed or not. The measure for “Overall patient status” is based on question M1034 on the OASIS instrument, which asks “Which description best fits the patient’s overall status?” Categories include: 0 - stable with no heightened risks for serious complications or death, 1 - temporarily facing high health risks but is likely to return to being stable without heightened risks for serious complications and death, 2 - likely to remain in fragile health and have ongoing high risks of serious complications and death, and 3 - serious progressive conditions that could lead to death within a year. This variable was dichotomized into 0–1 and 2–3 for analysis. The surgical wound variable and the total hip arthroplasty/total knee arthroplasty (THA/TKA) diagnosis variable were noted to have high collinearity, therefore a 3-level variable was created from the surgical wound OASIS variable and THA/TKA primary diagnosis from MedPAR data to include the following 3 values: no surgical wound, a surgical wound from THA/TKA, or surgical wound from other surgery.
In total, 53 variables were included for model development. MedPAR variables were used to determine race/ethnicity, dual eligibility for Medicare and Medicaid, index hospital length of stay (LOS), intensive care during the index hospitalization, primary diagnoses, surgical procedures, and comorbidities to determine Elixhauser comorbidity index (ECI) scores. For LOS, the inpatient admission date was subtracted from the discharge date in MedPAR, and only values of one or above were included as inpatient index hospitalizations. For the ECI, the presence of 31 comorbidities are assessed (e.g., heart failure, diabetes, liver disease), with higher scores associated with increased hospital length of stay, charges, mortality, and readmissions. Index hospitalization variables including LOS and the ECI scores were modeled categorically as they took on a limited number of discrete values. For LOS, the referent group was one day, and the referent for ECI was a score of zero.
Primary medical and surgical diagnoses included in Medicare Readmissions Reduction Program and/or public reporting on Hospital Compare were chosen as covariates when they were not already included as part of the ECI. These additional covariates included: acute myocardial infarction, pneumonia, stroke, coronary artery bypass grafting (CABG) and TKA/THA procedures.19,20 To avoid duplicating variables, comorbidities that were already included in the ECI (i.e., heart failure, chronic obstructive pulmonary disease, depression, obesity, drug and alcohol use) were not included as additional covariates in the model. A clustering term to adjust for index hospitals was not included in the model because little variance between sites was observed. A single pass imputation was performed to fill in missing values for five variables, all of which had <1% missing values.
Statistical Analysis
A predictive model was developed using logistic regression. An initial model comprised of 53 predictors of interest was fit, then a series of progressively more parsimonious models were created using backward variable elimination at increasingly restrictive p-value cutoffs. The optimism corrected c-statistic using bootstrapping and Brier score were calculated to assess relative model discrimination and calibration.21,22
A gradient boosting machine (GBM) was used as an adjunct to the logistic regression model to identify the relative influence of variables to separate the classes of interest (i.e. readmitted versus not readmitted) compared to other variables in the model, and to identify notable interactions between variables that could improve predictive performance of the logistic regression model.23 Second-order interactions were assessed using the gbm.interactions function in the dismo package in R, but no strong interactions were noted. A final model was created with the intent of gaining maximum parsimony while avoiding meaningful degradation in model performance based on the results from the aforementioned analyses.
Data preparation was conducted using SAS 9.4 (Cary, NC) and R version 3.5.1.24 Logistic regression models were fit using the logistic regression model (lrm) and generalized linear model (glm) functions from the regression modeling strategies (rms) and base stats package in R, respectively. The GBM was run at an interaction depth of 5, a learning rate of 0.005 and 1600 trees (the number of trees was determined using the gbm.step function from the dismo package in R).
RESULTS
In the year 2012, among Medicare fee for service beneficiaries, there were 522,346 OASIS observations. After restricting to beneficiaries who were ≥65 years old, had Medicare fee-for-service with OASIS and HHC claims within five days, and removal of duplicate records, 167,665 OASIS observations remained (Supplementary Figure 1). In 2012, 335,486 inpatient hospitalizations were identified using MedPAR files. After exclusions outlined in Supplementary Figure 1, 218,134 MedPAR inpatient hospitalizations remained. OASIS and MedPAR datasets were then merged and the cohort was restricted to: episodes in which an OASIS start of care occurred within 5 days following a MedPAR inpatient discharge, and the first hospitalization to HHC episode in 2012 for a given subject, 43,407 hospital to HHC episodes remained, which were followed by 6,380 inpatient readmissions within 30 days of discharge (14.7% readmission rate).
The characteristics of subjects in the cohort are shown in Supplementary Table 1, stratified by readmission status. Readmitted patients were slightly older, more likely to be dually eligible for Medicare and Medicaid, and more likely to have received intensive care during hospitalization. Urinary incontinence, UTIs, and urinary catheters were all more frequent in readmitted patients. Medicare readmissions penalty conditions including MI, COPD, Pneumonia, and HF were more frequent in readmitted patients, but the surgical conditions including CABG and THA/TKA were less frequent. Readmitted patients were more likely to have had two or more hospitalizations during the prior year, multiple falls, frailty indicators, and cognitive impairment. Readmitted patients were more likely to need assistance for ADLs both before and after hospitalization.
The c-statistics for the full 53-variable model and the parsimonious model were 0.67 and 0.66, respectively, indicating fair discrimination. Results including odds ratios (OR) and 95% confidence intervals (CI) for each variable in the full model are shown in Supplementary Table 2. The final variables in the parsimonious model were: categorical LOS, categorical ECI, presence of urinary catheter, overall patient status, multiple (i.e., two or more) hospitalizations in prior year, and pressure ulcer (either risk for or presence of ulcer), and a three-level variable for surgical wound (lower with TKA/THA surgical wound or other surgical wound compared to no surgical wound). Forest plots showing OR and 95% CI for variables in the parsimonious model are shown in Figures 1 and 2. An index hospitalization LOS of one day (the reference) was associated with higher odds of readmission compared to LOS categories of two days. For ECI scores, in which higher scores are known to be associated with a higher risk for mortality and readmissions, the highest odds of readmission were noted with scores of 7 and above. Brier scores were 0.120 for both the full and parsimonious models, indicating good calibration. The results of the GBM analysis identified similar variables (Supplementary Table 3). No strong interactions were noted in the GBM analysis and performance of the GBM model and model calibration were nearly identical to the logistic regression model.
Figure 1.
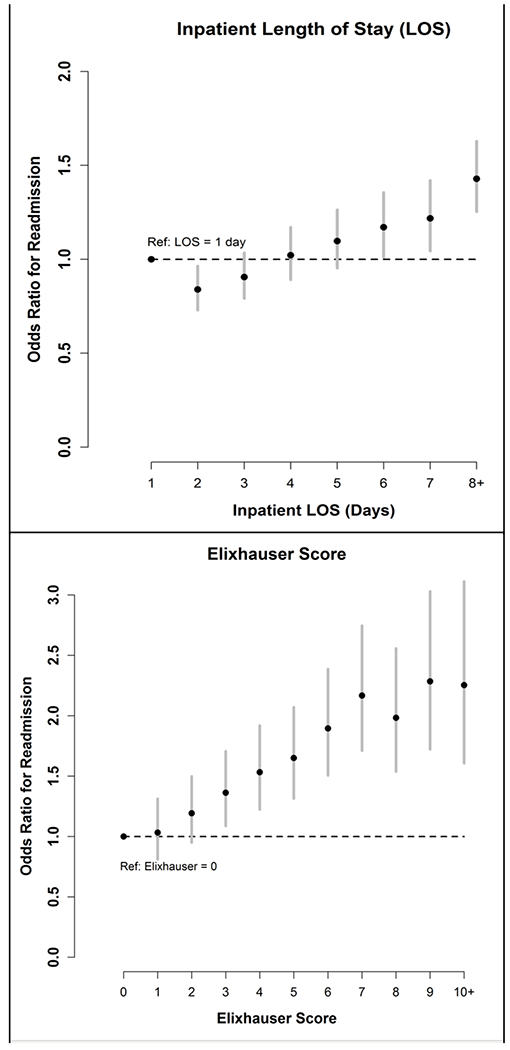
Odds of Readmission by Length of Stay and Elixhauser Score Categories
Figure 2.
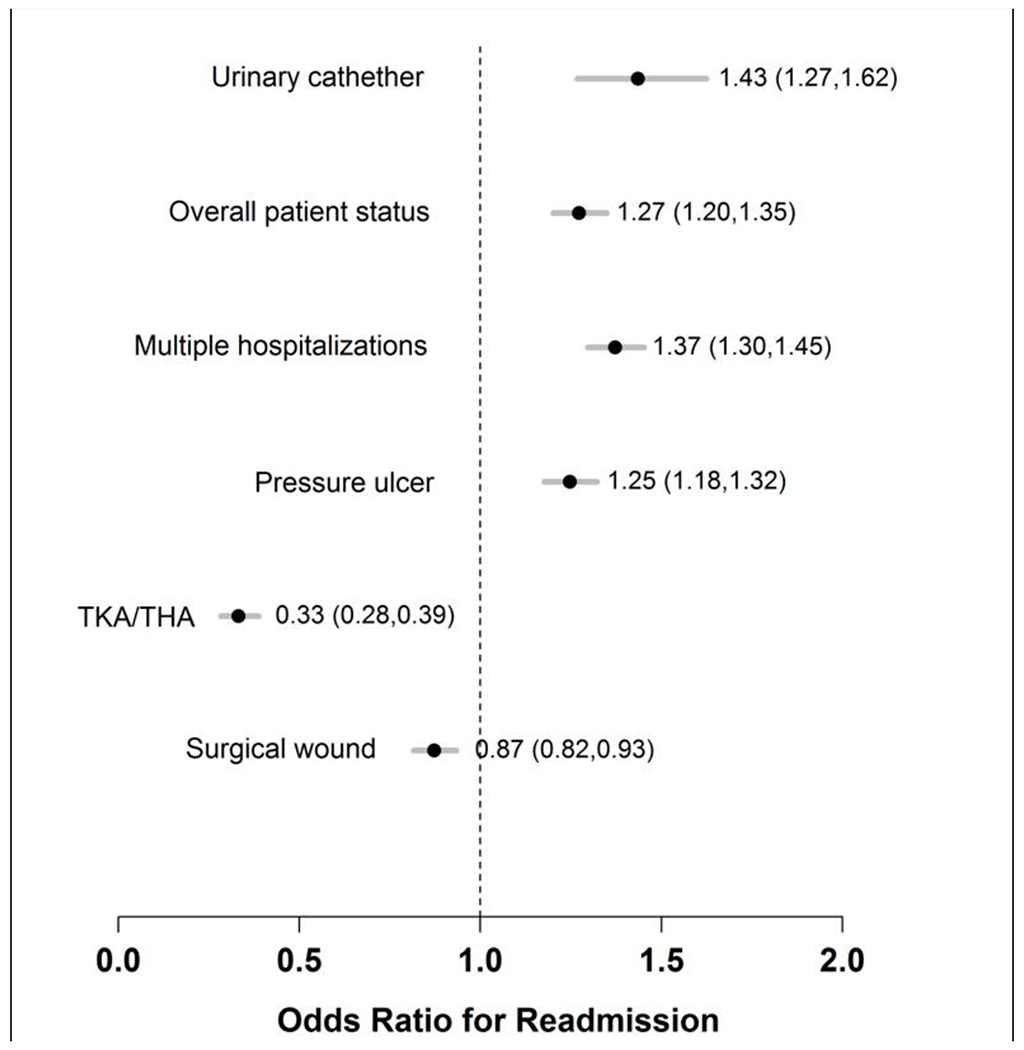
Forest Plot of Remaining Predictive Model Variables, Parsimonious Model
DISCUSSION
Findings from this analysis suggest that a combination of variables from the index hospitalization and HHC clinician assessment can predict risk for readmissions from HHC. Variables for this model would be readily available to HHC clinicians at the first HHC visit. The clinical implications of this work include the potential to evaluate for readmission risk at the first HHC visit to identify patients at high risk of readmission who may benefit from interventions to improve transitions of care. The full and parsimonious logistic regression models had fair discrimination, and good calibration. Logistic regression modeling and GBM yielded similar predictors.
In this predictive model, variables from the HHC clinician assessment included presence of a urinary catheter, overall patient status, and risk for or presence of a pressure ulcer (i.e., a pressure injury). These variables could be markers of high readmission risk without being mediators of the readmission risk. However, if the variables are mediators in this relationship, presence of a urinary catheter and pressure injury risk/presence could suggest the need for specific transitions of care plans that could be implemented for these patients. For example, HHC clinicians could follow a specific care plan for urinary catheters and pressure injuries including education and training for patients and caregivers, expedited outpatient follow-up, or potentially more intensive HHC visits immediately following hospital discharge. In addition, patients with high-risk features could benefit from evidence-based, nurse-led interventions including the Transitional Care Model or the Care Transitions Intervention.25–27
In our analysis, patients with index inpatient hospitalizations of one day had a higher odds of 30-day readmission compared to patients with LOS of two days, and a similar risk to patients with LOS of 4 days, which is notable and might contribute to speculation about the cause. This finding is of interest in the context of the Medicare Two-Midnight rule for inpatient status, which was fully implemented in 2015.28 For this rule, hospitalizations of fewer than two midnights require documentation that an inpatient stay, rather than an observation stay, was necessary,29 which is important because Medicare typically provides a higher payment for inpatient versus observation care.29 As a result of the documentation and enforcement from this rule, it is anticipated that hospitalizations of fewer than two midnights will be more frequently categorized as observation status. Repeating this analysis in the time period following the Two-Midnight rule could determine if a one-day LOS is still predictive of hospital readmissions compared to a LOS of two days or if these one-day hospitalizations are now more frequently categorized as observation status and therefore excluded from Medicare readmissions measures.
Our model performance is comparable to other readmission risk predictive models developed for use in a variety of patient populations. In a systematic review of readmission risk prediction models, c-statistics in 21 studies ranged from 0.55-0.83, with only six studies achieving a c-statistic above 0.70.6 Two models in this review identified improved predictive model performance with the addition of functional ability and social variables.31,32 In one study, inclusion of self-reported data including questions about self-rated general health, visual impairment, functional score, assistance with ADLs, and others improved the model performance from a c-statistic of 0.77 to 0.83 in a sample of Medicare patients.32 In another study, including social variables such as housing instability improved model performance.31 Moreover, in a recent study to evaluate clinical outcomes in Medicare beneficiaries using HHC, those dually enrolled in both Medicare and Medicaid were more likely to have 30-day readmissions and 30-day emergency department visits following hospital discharge (OR 1.08 p<0.001; OR 1.20 p<0.001, respectively).33 This latter study also noted a similar association for beneficiaries living in a low-income zip code and for black race.33 Overall, these findings suggest a need to consider and incorporate functional outcomes and social determinants of health into models that assess and address readmission risk.
Certain factors should be considered in the interpretation of our study. This was a retrospective analysis using data available in Medicare datasets. Certain variables that may be important predictors of readmission such as measures of social determinants of health are not available in these data sources. In addition, because only the first hospital-HHC episode in the calendar year was included in this analysis, the model is not intended to identify risk for multiple readmissions from HHC. In conclusion, variables collected by and available to HHC clinicians at the first post-discharge HHC visit can be used to assess readmission risk for patients. Future analyses could explore whether addition of social determinants of health measures improve the performance of this model.
Supplementary Material
Supplementary Figure 1. Cohort Derivation with Exclusions from OASIS and MedPAR 402 databases
Supplementary Table 1. Characteristics of Sample by Readmission from Home Health Care
Supplementary Table 2. Odds Ratios for Variables in Full Predictive Model
Supplementary Table 3. Top Ten Variables in Gradient Boosting Machine Predictive Model
Impact Statement:
We certify this work is novel and describes a new predictive model for home health care readmissions using variables available at the first home health visit, which could be used by home health clinicians to identify patients at high risk of readmission.
ACKNOWLEDGEMENTS
Funding sources: This work was supported by Grant 2015212 from the Doris Duke Charitable Foundation and University of Colorado School of Medicine. Dr. Christine Jones is supported by grant number K08HS024569 from the Agency for Healthcare Research and Quality. Dr. Jason Falvey was supported during this work by grant F31AG056069 from the National Institute on Aging, National Institutes of Health and is currently supported by T32AG019134. Dr. Jennifer Stevens-Lapsley is supported by the Veteran’s Affairs Eastern Colorado Health System Geriatrics, Research, Education, and Clinical Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Doris Duke Charitable Foundation, the University of Colorado School of Medicine, the Agency for Healthcare Research and Quality, or the National Institutes of Health.
Sponsor’s Role: The sponsors had no role in the design, conduct, analysis, interpretation, or presentation of the study.
Footnotes
Conflict of Interest: The authors have no conflicts.
References:
- 1.Jones CD, Ginde AA, Burke RE, Wald HL, Masoudi FA, Boxer RS. Increasing Home Healthcare Referrals upon Discharge from U.S. Hospitals: 2001-2012. J Am Geriatr Soc. 2015;63(6):1265–1266. [DOI] [PubMed] [Google Scholar]
- 2.Tian W An All-Payer View of Hospital Discharge to Postacute Care, 2013 HCUP Statistical Brief #205. Rockville, MD: 2016. [PubMed] [Google Scholar]
- 3.Medicare.gov Home Health Compare. 2019; https://www.medicare.gov/homehealthcompare/. Accessed January 4, 2019.
- 4.MedPAC. Medicare Payment Advisory Commission Report to the Congress: Medicare Payment Policy. Washington, DC: 2018. [Google Scholar]
- 5.Jones CD, Bowles KH, Richard A, Boxer RS, Masoudi FA. High-Value Home Health Care for Patients With Heart Failure: An Opportunity to Optimize Transitions From Hospital to Home. Circ Cardiovasc Qual Outcomes. 2017;10(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madigan EA, Gordon NH, Fortinsky RH, Koroukian SM, Pina I, Riggs JS. Rehospitalization in a national population of home health care patients with heart failure. Health Serv Res. 2012;47(6):2316–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.www.cms.gov Home Health Quality Measures. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HomeHealthQualityInits/Home-Health-Quality-Measures.html. Accessed December 17, 2018.
- 9.Avalere. Home Health Chartbook: Prepared for the Alliance for Home Health Quality and Innovation. 2018. [Google Scholar]
- 10.Dorsey KG JN; Horwitz LI; Li Z; Herrin J; DeBuhr J; Bernheim S; Krumholz HM 2016 All-Cause Hospital-Wide Measure Updates and Specifications Report, Hospital-level 30-Day Risk-Standardized Readmission Measure - Version 5.0. New Haven, CT: Yale New Haven Health Services;2016. [Google Scholar]
- 11.Home Health Patient Tracking Sheet. In: Services CfMM, ed. Baltimore, MD: Center for Medicaid & Medicare Services; 2009:24. [Google Scholar]
- 12.Services CfMM. Outcome and Assessment Information Set: OASIS-D Guidance Manual. In: DHHS, ed. July 2, 2018 ed. Baltimore, MD: 2019. [Google Scholar]
- 13.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284–1292. [DOI] [PubMed] [Google Scholar]
- 14.Gill TM. Disentangling the disabling process: insights from the precipitating events project. Gerontologist. 2014;54(4):533–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill TM, Murphy TE, Gahbauer EA, Allore HG. Association of injurious falls with disability outcomes and nursing home admissions in community-living older persons. Am J Epidemiol. 2013;178(3):418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 17.Garland AF R; Olafson K; Ramsey C; Yogendran M; Chateau D; McGowan K The Epidemiology and Outcomes of Critical Illness in Manitoba. Winnepeg, MB2012. [Google Scholar]
- 18.Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying Increased Risk of Readmission and In-hospital Mortality Using Hospital Administrative Data: The AHRQ Elixhauser Comorbidity Index. Med Care. 2017;55(7):698–705. [DOI] [PubMed] [Google Scholar]
- 19.CMS.gov Readmissions Reduction Program. 2018; http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Accessed December 21, 2018, 2018.
- 20.Hospital Compare. https://www.medicare.gov/hospitalcompare/search.html. Accessed May 1, 2017.
- 21.Harrell FE. rms: Regression Modeling Strategies. 2018; https://CRAN.R-proiect.org/package=rms. Accessed December 1, 2018.
- 22.Brier GW. Verification of forecasts expressed in terms of probabilities. Monthly Weather Review. 1950;78:1–3. [Google Scholar]
- 23.Friedman JH. Greedy function approximation: A gradient boosting machine. The Annals of Statistics. 2001;29(5):1189–1232. [Google Scholar]
- 24.R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2008. http://www.R-proiect.org. [Google Scholar]
- 25.Naylor MD, Brooten DA, Campbell RL, Maislin G, McCauley KM, Schwartz JS. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. JAm Geriatr Soc. 2004;52(5):675–684. [DOI] [PubMed] [Google Scholar]
- 26.Hirschman KB, Shaid E, McCauley K, Pauly MV, Naylor MD. Continuity of Care: The Transitional Care Model. Online J Issues Nurs. 2015;20(3):1. [PubMed] [Google Scholar]
- 27.Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828. [DOI] [PubMed] [Google Scholar]
- 28.www.cms.gov. Fact Sheet: Two-Midnight Rule. 2015; https://www.cms.gov/newsroom/fact-sheets/fact-sheet-two-midnight-rule-0. Accessed December 18, 2018.
- 29.Cassidy A Health Policy Brief: The Two-Midnight Rule. Health Affairs. 2015. [Google Scholar]
- 30.Wright S Memorandum Report: Hospitals’ Use of Observation Stays and Short Inpatient Stays for Medicare Beneficiaries, OEI-02-12-00040. In: General OoI, ed. Washington, DC: Department of Health and Human Services; 2013. [Google Scholar]
- 31.Amarasingham R, Moore BJ, Tabak YP, et al. An automated model to identify heart failure patients at risk for 30-day readmission or death using electronic medical record data. Med Care. 2010;48(11):981–988. [DOI] [PubMed] [Google Scholar]
- 32.Coleman EA, Min SJ, Chomiak A, Kramer AM. Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39(5):1449–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joynt Maddox KE, Chen LM, Zuckerman R, Epstein AM. Association Between Race, Neighborhood, and Medicaid Enrollment and Outcomes in Medicare Home Health Care. J Am Geriatr Soc. 2018;66(2):239–246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Cohort Derivation with Exclusions from OASIS and MedPAR 402 databases
Supplementary Table 1. Characteristics of Sample by Readmission from Home Health Care
Supplementary Table 2. Odds Ratios for Variables in Full Predictive Model
Supplementary Table 3. Top Ten Variables in Gradient Boosting Machine Predictive Model


