Abstract
Cannabinoid subtype 1 receptor (CB1R) antagonists were originally developed as antiobesity agents. Unfortunately, SR1417116A (rimonabant), the first marketed inverse agonist of CB1R, produced CNS-related adverse effects including depression and suicidal ideation, and thus it was withdrawn from the market. These effects of rimonabant became evident in patients following chronic dosing. Standard preclinical toxicity studies failed to detect these adverse effects. The goal of these studies was to perform an integrated battery of behavioral assays to better understand the behavioral effects of rimonabant following both acute and chronic administration. In the present study, acute dosing with rimonabant in rats significantly decreased food consumption; decreased measures of locomotor activity; increased scratching, grooming and wet-dog shakes; and increased defecation. Subsequently, animals were tested using a chronic dosing regimen but prior to drug administration for that day. The highest dose of rimonabant tested significantly decreased marble burying behavior, presumably anxiolysis. There were also significant effects in social interaction after chronic dosing. Our results did not reveal significant rimonabant-induced anxiogenic behaviors. Future studies to characterize behavioral screens for anxiogenic effects of CB1 antagonists in rodents should further explore social interaction paradigms and potential comorbid factors of rimonabant dosing such as sex, age, and obesity.
Keywords: Cannabinoids, CB1, rimonabant, behavior, depression, chronic
4. Introduction
Cannabinoid receptors belong to the endocannabinoid (EC) system, which consists of receptors, transporters, endocannabinoids, and the enzymes involved in synthesis and degradation of endocannabinoids.1,2 To date, two different receptors have been identified – CB1R and CB2R. Both CB1R and CB2R are G protein–coupled receptors (GPCRs) primarily activating inhibitory G proteins (Gi/o). In recent years, CB1R antagonists have received attention in the treatment of obesity and metabolic syndrome.3,4 However, basic science and clinical studies suggest that CB1R antagonists have significant metabolic effects that extend beyond merely decreasing caloric intake.5–7 This is due to the expression of CB1R in peripheral tissues outside of the central nervous system (CNS) including liver, skeletal muscle, pancreas and kidneys. Rimonabant was the first CB1R antagonist/inverse agonist approved as a drug to treat obesity in Europe.8 However, the United States Food and Drug Administration (FDA) voted against the approval of this drug citing concerns with adverse psychiatric effects. This was due to rimonabant treatment being linked to an increase in anxiety, depression and suicidal ideation in some patients. The drug was eventually withdrawn from Europe for these same reasons.9 Rimonabant significantly increased anxiety scores as measured on the hospital anxiety and depression (HAD) scale, and participants receiving rimonabant were more likely to drop out of studies due to depressive mood disorders.10 The effects appear to occur at higher rates in individuals with a history of mental illness, and many studies included a history of psychiatric disorders in their exclusion criteria.11
Consequently, clinical development of several other CB1R antagonists was halted. The endocannabinoid system is integral to the CNS and regulates several important functions including tolerance to pain, regulation of relaxation and pleasure, and protection against stroke.2,10 Thus, there is the potential for harm from long-term disruption of central endocannabinoid signaling. To combat the potential liability of brain penetrant CB1R antagonists, various groups are developing peripherally selective antagonists that are expected to have little to no adverse neuropsychiatric effects. However, to have a clear path forward, the behavioral effects of long-term treatment with CB1R antagonists must be established. Specifically, behaviors that are analogous to the side effects previously seen in post-market monitoring (e.g. depression, see above) should be evaluated for effects due to long-term rimonabant treatment.
In sharp contrast to humans, rimonabant has been paradoxically reported to mediate anxiolytic-like behaviors in rodents, at least when administered acutely in certain paradigms.11 The most immediate explanations could be that the dysphoric effects in humans are due to chronic treatment or are a result of an interaction between rimonabant-induced weight loss and CB1R blockade. Another possibility is that the drug induced precipitated withdrawal in users with overactive endocannabinoid system. Previous studies of chronic rimonabant dosing in rodents have been performed to assess both depressive phenotypes and tolerance to acute effects of rimonabant over time.12,13 These studies have shown significant effects on phenotypes such as the forced swim test and sucrose preference, but there is no consensus on a highly reproducible set of phenotypes to screen for negative psychiatric phenotypes of CB1 antagonists in preclinical studies. To evaluate both the acute effects of rimonabant and the effects of chronic dosing on behavior, we devised a behavioral battery that incorporated a number of paradigms that may correlate with neuropsychiatric side-effects, including attempts to reproduce phenotypes observed previously in the literature. This study was performed across two experimental groups of animals. In experiment 1, two doses of rimonabant were compared across a number of behavioral tests. The second experiment utilizes the higher of the two doses of rimonabant from experiment 1 to test the reproducibility of those findings, to incorporate tests of social interaction, and to test for interactions between chronic rimonabant dosing and acute pharmacological challenges. The goal was two-fold: (1) identify behaviors disrupted by long-term treatment with rimonabant, and (2) to devise a testing strategy that could be then adapted for the evaluation of compounds that are devoid of such effects such as peripherally selective compounds with limited brain exposure and neutral antagonists of CB1R.
5. Materials and methods
5.1. Subjects and Housing
Male Sprague-Dawley rats (Charles River Laboratories) were housed in plastic cages (45 cm × 23 cm × 20 cm) with water ad libitum and free access to standard lab chow under a 12 hr light/dark cycle. The weights of the animals were recorded daily. All animals were habituated to the testing room one hour before experiments began. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the State University of New York at Buffalo and conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory animals.
5.2. Intraperitoneal (I.P.) Injections
Animals were habituated to the handling which would be required for an I.P. injection beginning the day after arrival in the vivarium, with daily handling continuing throughout the study. Rimonabant (NIDA Drug Supply Program) was prepared in a vehicle consisting of 2% Tween-80, 0.5% methylcellulose, and de-ionized sterile water.
5.3. Experiment One: Comparison of Two Doses of Rimonabant
The first experiment investigated the effects of acute dosing, followed by chronic dosing of rimonabant. The goal was to identify robust behavioral measures of CB1R antagonism that may be correlated with unwanted psychiatric side effects. There were three experimental groups (all groups, n=12), and doses were selected based on literature (0, 3, and 10 mg/kg;12,14,15). Behavioral testing was conducted as outlined in Figure 1A.
Figure 1: Experimental Timelines.
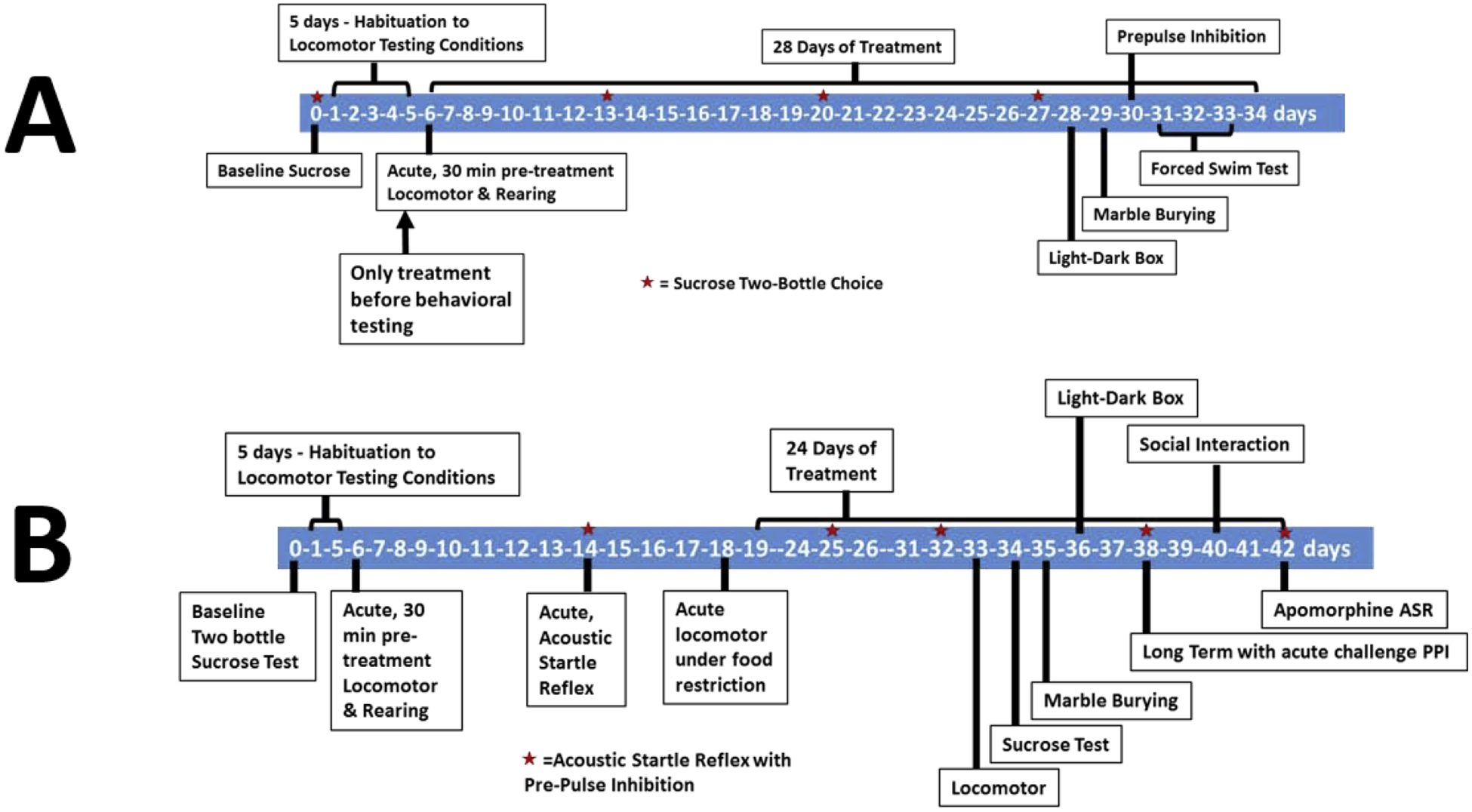
The timelines of Experiment 1 (A) and Experiment 2 (B) are outlined here.
5.3.1. Two-bottle Choice- Sucrose Consumption
Sucrose was prepared as a 1% solution in tap water. On the day before establishing baseline sucrose consumption, rats were given brief access (30 minutes) to the sucrose to reduce neophobic effects to the novel solution. In Experiment 1, animals were water restricted overnight before testing to drive drinking behavior as per Beyer et al. 2010.12 For consumption experiments, rats were given a two-bottle choice between a 1% sucrose solution and water for one hour. Data are presented as a preference for sucrose (preference = ((sucrose consumed)/ (sucrose+ water)) × 100).
5.3.2. Habituation to Locomotor Testing Conditions
Habituation to testing conditions consisted of an I.P. injection of vehicle followed by a 30-minute period in their home cage and a subsequent 30-minute exposure to the open field apparatus with access to wet mash (standard chow with water). After testing, all animals were returned to the colony room.
5.3.3. Feeding and Locomotor Behavior after Acute Dosing
All animals were dosed 30 minutes before the locomotor test. Animals were placed into computer interfaced infrared beam open field boxes (OmniTech, Columbus, OH) (40cm × 40 cm) with access to wet mash during the 1-hour locomotor assessment. Both locomotion (distance travelled) and rearing were scored by infrared beam breaks. The wet mash was weighed before and after the hour test to measure how much food was consumed. Locomotor behavior was also digitally recorded and hand scored for grooming, scratching, and “wet dog shakes”.
5.3.4. Chronic Treatment
Following the acute locomotor testing all animals then proceeded to long-term treatment of rimonabant. This consisted of daily I.P. injections starting the day after acute locomotor testing and lasted for 28 days. All behavioral testing was performed prior to that day’s rimonabant administration such that the animals were at their lowest levels of on-board drug for testing. The two-bottle sucrose test was again given after overnight water deprivation at 7, 14, and 21 days. Below are descriptions of other behaviors that were tested during the 28 days, on the days specified in Figure 1A.
5.3.5. Light-Dark Box
The Light-Dark Box test was conducted on day 22 of treatment and consisted of a box (40 cm × 40 cm) with one half clear and the other black Plexiglas. The two sides were separated by a partition with a doorway to allow access to both sides. Once the animals were placed in the apparatus, they were tracked by infrared beam tracking for 10 minutes. The time spent on the light side is thought to correlate with anxiolytic-like behavior.
5.3.6. Marble Burying
On day 23 of treatment, the Marble Burying paradigm was conducted. This test included 15 marbles (25 mm diameter) evenly spaced atop of corncob bedding (5 cm deep) in novel homecages (45 cm × 23 cm × 20 cm). Once placed in the cage, rats were given 30 minutes to bury marbles. After the 30 minutes, rats were returned to their homecages and digital images of the marbles were taken for later scoring.
5.3.7. Acoustic Startle Reflex (ASR)
On day 24 of chronic treatment, rats were tested for acoustic startle reflex (ASR) and prepulse inhibition (PPI) using Kinder Scientific Startle Monitor boxes (Poway, CA). The program consisted of 88 quasi-random trials to assess the ASR at 120 dB, and prepulses of varying levels (70, 75, 80, 85 dB). PPI data is presented as a percentage, calculated as [(mean 120 dB ASR amplitude on pulse alone trials – mean ASR amplitude on prepulse pulse trials)/mean 120 dB ASR amplitude on pulse alone trials)] × 100 as per MacLaren et al. 2014 and Clark et al. 2005.16,17.
5.3.8. Forced Swim Test (FST)
The forced swim test spanned across three days (days 25, 26, 27 of chronic treatment). The rats were placed in a cylinder tank with water at a depth of 30 cm and video recorded. The FST test sessions were 15 minutes the first day, and 10 minutes for the second and third days. FST is presented as time spent immobile, which is the amount of time where the rat is doing the least amount of movement to keep its nose above the water as per Reynolds et al. 2004.18 There are a variable number of animals per group per experiment because a subset of animals in each run were able to propel themselves through the water and leap to catch the edge of the container and escape (sample numbers are in the figure legend).
5.4. Experiment Two: Social interaction, Long-term Dosing with Acute Pharmacological Challenges
For experiment two, a new cohort of 30 rats were purchased from Charles River Laboratories. The order of the behavioral paradigms is outlined in Figure 1B.
5.4.1. Acute Testing
After five days of habituation to testing conditions, rats were injected 30 minutes before undergoing a 1-hour acute locomotor test with wet mash present, as described above. Animals did not receive another injection for 8 days, when they underwent ASR testing. The same 88-trial startle program from Experiment 1 was utilized to assess the acute effects of treatments (30-minute pretreatment) on ASR and PPI. Once again, these rats did not receive another injection for 4 days, at which time they underwent the final acute test. The last acute locomotor test was conducted while under food restriction (overnight, 18 hours).
5.4.2. Long-Term Rimonabant Treatment
Beginning the day following the acute locomotor testing under food restriction (day 19), rats underwent 24 days of chronic treatment. The same assays were completed as outlined in Experiment 1 with the addition of social interaction behavior, the acute challenge of rimonabant in ASR, and acute apomorphine challenge in ASR, as described below.
5.4.3. Long-term treatment with acute rimonabant challenge ASR
Animals were typically dosed after the completion of that day’s behavioral testing. In this one instance, the animals were given their respective dose before the ASR testing (30-minute pretreatment). The same testing paradigm was used as outlined above.
5.4.4. Social Interaction
Two 10-minute phases were used to determine if social behavior is impacted by chronic rimonabant treatment. This paradigm consisted of rectangular locomotor boxes (39.4 cm × 50 cm) with a hanging pen (11.5 cm × 39 cm × 13 cm, 15 cm above the floor of the box) inserted at one end. The first phase included the subject in the presence of an empty pen and in the second phase there was an ‘intruder’ rat present in the pen. A surrogate score of social behavior was generated by tracking (via infrared beams) the time spent near the pen and the number of times the rat entered the area around the pen near the intruder. In addition, videos of the encounters were scored for the time spent directly interacting with the intruder, as defined as any behavior (sniffing/touching/climbing) directed toward the intruder animal. Intruders were of the same sex, strain, and age as the test subjects.
5.4.5. Apomorphine ASR
Animals were given a sub-effective dose of apomorphine (0.2 mg/kg) five minutes before the ASR testing via subcutaneous (s.c.) injection. Apomorphine at 1 mg/kg, s.c, has been previously shown to robustly disrupt PPI.19 The use of the lower dose of apomorphine was to determine if chronic CB1R antagonist treatment exacerbated or made the rats more susceptible to the effects of apomorphine. Again, the same ASR/PPI program as described above was used. The rats did not receive injections of rimonabant, as it was the last day of testing.
5.5. Data Analysis
Statistical analyses were performed by t-test, one-way ANOVA, and two-way ANOVA with Sidak’s or Dunnet’s multiple comparisons test analysis, where appropriate, within the GraphPad Prism 8 software (GraphPad Software, San Diego California, USA). All results are expressed as mean values ± SEM. Group means were considered significantly different when p < 0.05. On graphs, a single asterisk (*) indicates significant difference at the p = 0.01 – 0.05 confidence level; two asterisks (**) indicates significance at p ≤ 0.01; three asterisks (***) indicates significance at p ≤ 0.001; four asterisks (****) indicates significance at p ≤ 0.0001.
6. Results
6.1.1. Experiment 1 Results
All experimental groups had similar weights at the beginning of the study (Fig 2). The day after the acute locomotor and feeding experiment (below), animals were dosed once per day, after behavioral assessment. With chronic treatment at 10 mg/kg rimonabant, there was a significant reduction in weight gain (Fig 2; a repeated measures ANOVA with treatment as the between-subjects factor and testing day as the within-subjects factor confirmed that there was a significant effect of treatment F(2, 33) = 4.393p = 0.0204, time F(28, 924) = 282.9p < 0.0001, interaction F(56, 924) = 2.785p < 0.0001; post hoc analysis found significant effects between vehicle and the 10 mg/kg dose group at day 23 p < 0.05, day 24 p < 0.05, day 25 p < 0.01, day 26 p < 0.01, day 27 p < 0.01, day 28 p < 0.01). The 3 mg/kg dose appeared to reduce weight gain, but statistical significance was not reached.
Figure 2: Rimonabant Attenuates Weight Gain.
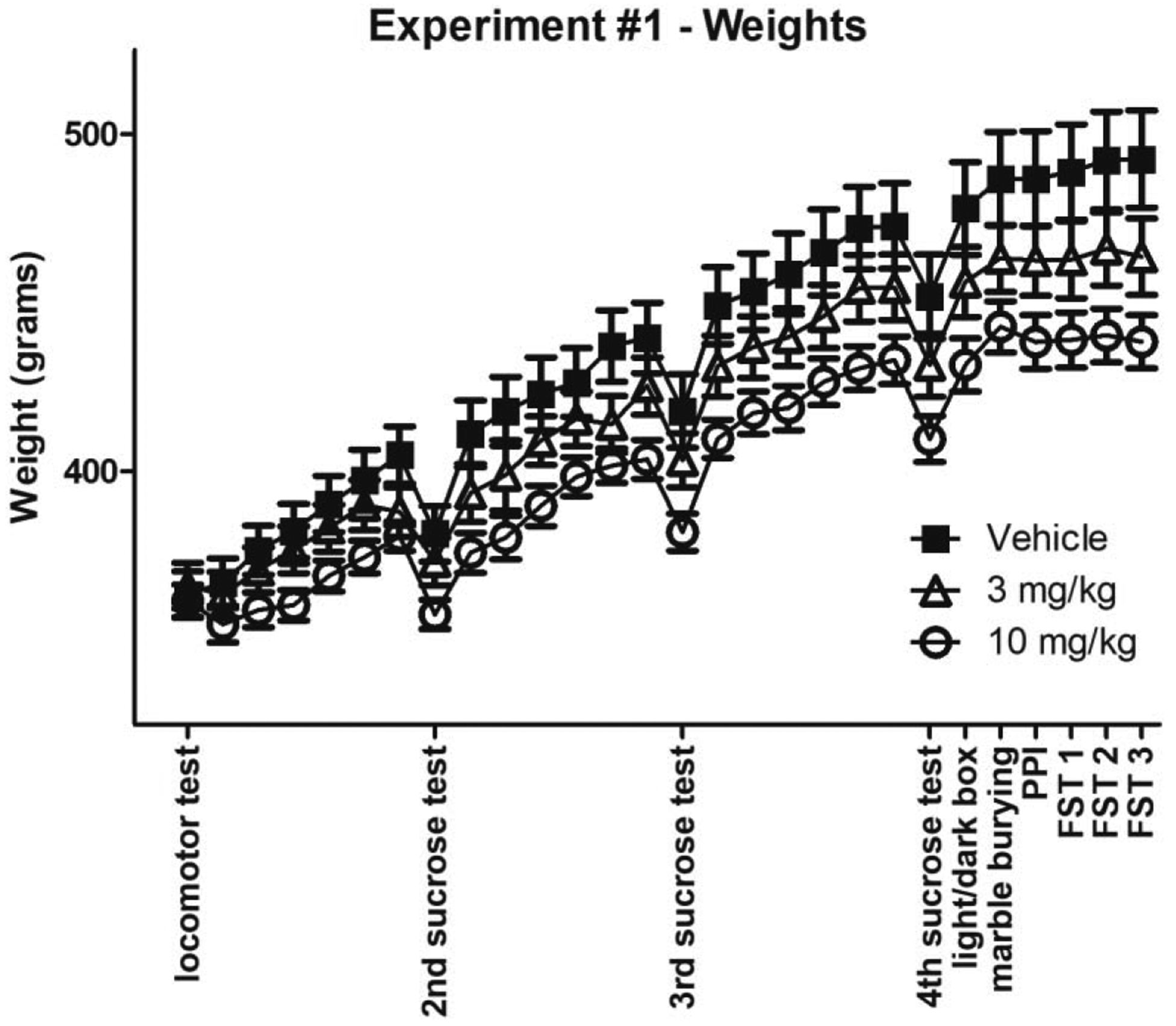
Rat weight over time, including experimentation days (all groups n=12). Note the dips in weight on days of two-bottle sucrose tests are due to water restriction the night before.
6.1.2. Feeding and Locomotor Activity – Acute Dosing
Animals were pretreated for 30 minutes prior to a 60-minute behavioral assessment. Acute dosing with rimonabant in Expt 1 produced decreases in palatable food ingestion (Fig 3A; one-way ANOVA with overall effect F(2, 33) = 0.3882, p = 0.0016, and Dunnett’s Multiple Comparison Test vehicle vs. 3 mg/kg p < 0.05, vehicle vs. 10 mg/kg p < 0.01). Correspondingly, the time spent feeding (Fig 3B; one-way ANOVA with overall effect F(2, 31) = 3.953, p = 0.0002, and Dunnett’s Multiple Comparison Test vehicle vs. 3 mg/kg p < 0.05, vehicle vs. 10 mg/kg p < 0.001) and the number of feeding episodes (Fig 3C; one-way ANOVA with overall effect F(2, 31) = 7.003, p = 0.0001, and Dunnett’s Multiple Comparison Test vehicle vs. 3 mg/kg p < 0.05, vehicle vs. 10 mg/kg p < 0.001) were significantly decreased. There was a non-significant increase in defecation (Fig 3D; oneway ANOVA F(2, 33) = 0.1921, p = 0.1429). Our results are similar to a number of groups which described decreased food intake and an increase in defecation after rimonabant administration.20–22
Figure 3: Rimonabant Decreases Food Intake.
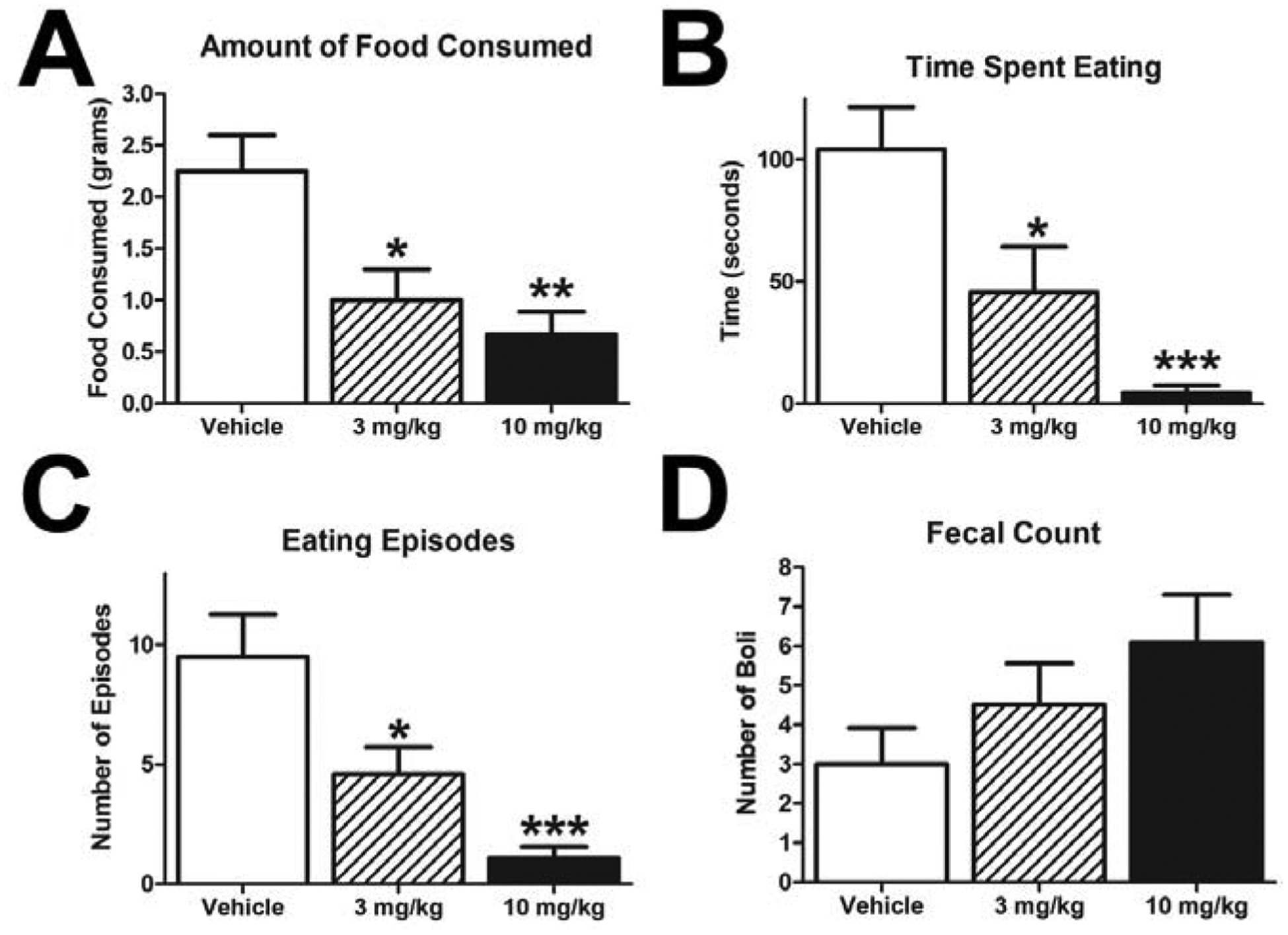
Within a 1 hour session after a single dose of rimonabant, all measures of food intake were decreased: mass of food consumed (A), the total time eating (B), and number of eating episodes (C). Rimonabant non-significantly increased the number of fecal boli shed during the testing session (D). (*p < 0.05, **p < 0.01, ***p<0.001; all groups n=12).
Locomotor activity was measured during the feeding test described above. Measures of locomotor activity (Fig 4A; one-way ANOVA with overall effect F(2, 32) =0.5412, p = 0.0014, and Dunnett’s Multiple Comparison Test vehicle vs. 3 mg/kg p < 0.001, vehicle vs. 10 mg/kg p < 0.05) and rearing behavior were decreased. Specifically, rimonabant treatment significantly reduced time spent rearing (Fig 4C; one-way ANOVA with overall effect F(2, 32) = 1.934, p = 0.0211, and Dunnett’s Multiple Comparison Test vehicle vs. 3 mg/kg p < 0.05) and the number of rearing episodes (Fig 4D; one-way ANOVA with overall effect F(2, 32) = 2.216, p = 0.0143, and Dunnett’s Multiple Comparison Test vehicle vs. 3 mg/kg p < 0.05, vehicle vs. 10 mg/kg p < 0.05). The decrease in locomotor activity was seen over the entirety of the 60 minute session (Fig 4B; a repeated measures ANOVA with treatment as the between-subjects factor and time as the within-subjects factor confirmed that there was a significant effect of treatment F(2, 33) = 4.759, p=0.0153 and time F(5, 165) = 64.69 p<0.0001, but no interaction F(10, 165) = 0.8229 p=0.6070). These results are in agreement with previous studies that acutely dosed rats with rimonabant.14,23,24
Figure 4: Rimonabant Decreases Locomotor Activity and Increases Scratching.
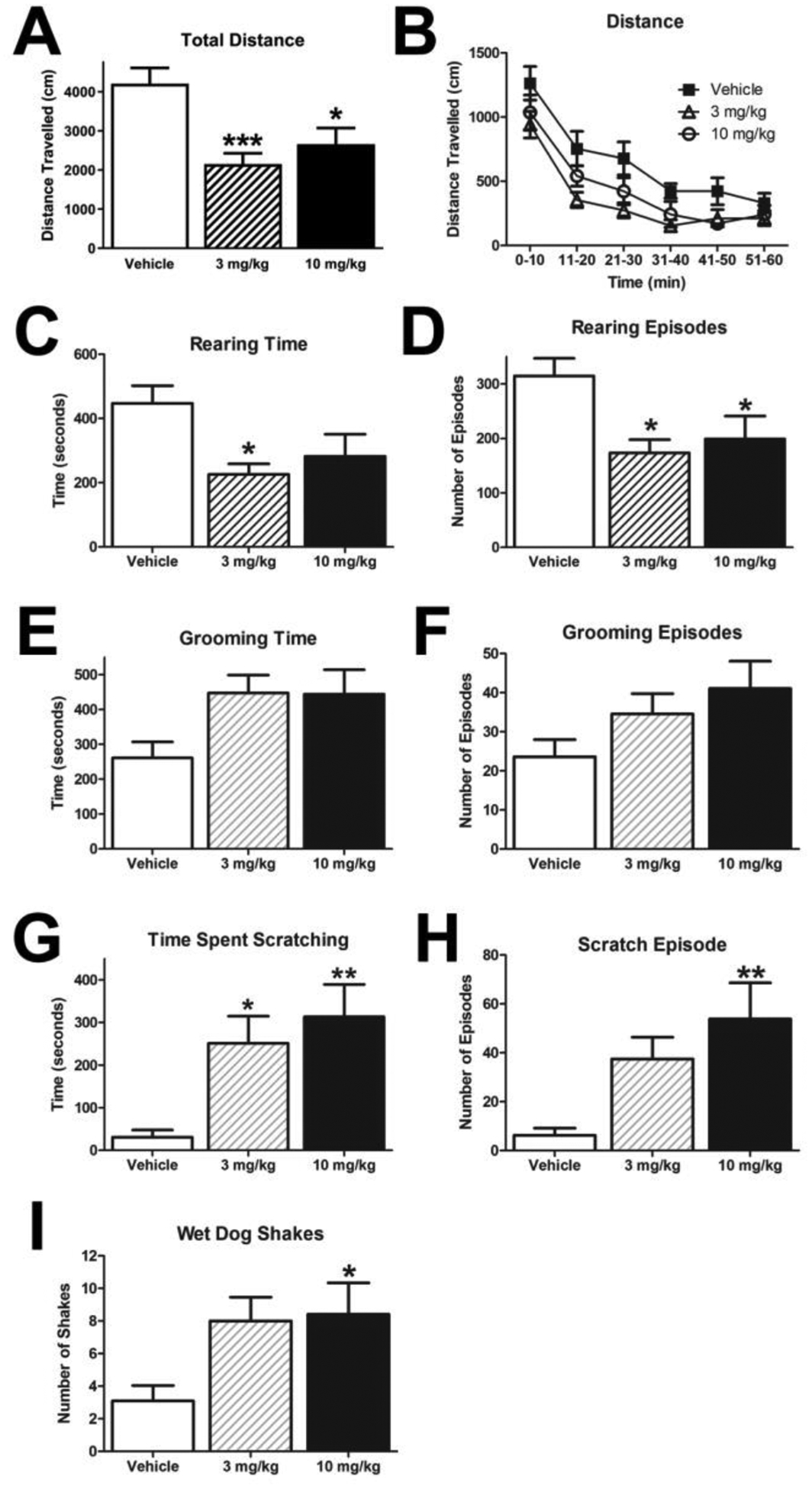
Acute administration of rimonabant decreased both total distance traveled (A-B) and rearing behavior during the one hour session (C-D). (*p < 0.05, **p < 0.01 ; all groups n=12, except vehicle group n = 11 due to device failure in rearing (A,B,C,D)) While acute administration of rimonabant only had a non-significant effect on grooming behavior (E-F), it did produce a significant increase in scratching (G-H) and in the number of wetdog shakes (I) (*p < 0.05, **p < 0.01 ; all groups n=12, except vehicle group n = 10 (G-I))
Other behaviors assessed during the feeding test after acute administration of rimonabant included scratching and grooming (scored via video recordings). Rimonabant trended to increase grooming that reached near significance (Fig 4E - time; one-way ANOVA F(2, 31) = 0.4761, p = 0.0587; Fig 4F - episodes; one-way ANOVA F(2, 31) = 0.9413, p = 0.1246), and it significantly increased the time spent scratching (Fig 4G; oneway ANOVA with overall effect F(2, 31) = 5.868, p = 0.0092, and Dunnett’s Multiple Comparison Test vehicle vs. 3 mg/kg p < 0.05, vehicle vs. 10 mg/kg p < 0.01) and the number of scratching episodes (Fig 4H; one-way ANOVA with overall effect F(2, 31) = 3.772, p = 0.0151, and Dunnett’s Multiple Comparison Test vehicle vs. 10 mg/kg p < 0.01). In addition, rimonabant increased the number of wet-dog shakes during the 1 hour session (Fig 4I; one-way ANOVA with overall effect F(2, 31) = 2.964, p = 0.0477, and Dunnett’s Multiple Comparison Test vehicle vs. 10 mg/kg p < 0.05). These data are consistent with previous observations wherein acute administration of rimonabant increased scratching and grooming behavior14,23,24.
6.1.3. Sucrose Preference Test
A past study indicates that chronic administration of rimonabant decreases preference for sucrose12, but it is unclear from the authors’ description whether this was given prior to the access to sucrose (pretreatment) or given at some other time during the day. However, our data suggest that irrespective of the length of the chronic treatment, rimonabant does not change the preference for sucrose in chronic dosing regimen prior to daily drug administration (Supplementary Fig 1 ; two-way ANOVA; treatment F(2,33) = 0.1443, p=0.8662; time F(2.805, 92.57) = 1.215, p=0.3080; interaction F(6, 99) = 3.015, p = 0.0095). We observe high variability, however, with no consistent trends across the study.
6.1.4. Anxiety-like Behaviors
Previous reports describe anxiolytic-like effects with acute doses of rimonabant, while chronic dosing produced anxiogenic effects25,26. It is unclear in the case of the previous chronic dosing study whether rimonabant administration took place before or after the behavioral assessment. In the present study, the rats were dosed after behavioral testing and there was no significant impact on behavior in the Light-Dark Box paradigm (Fig 5A - total; one-way ANOVA F(2, 32) = 0.2850, p = 0.5744; Fig 5B – five minute bins; a repeated measures ANOVA with treatment as the between-subjects factor and time as the within-subjects factor revealed no significant effect of treatment F(2, 32) = 0.5642, p=0.5744, time F(1, 32) = 1.109, p=0.3002, interaction F(2, 32) = 0.4830, p=0.6214). In Expt 1, chronic administration of rimonabant decreased the number of marbles buried after 30 minutes (Fig 5C; one-way ANOVA with overall effect F(2, 33) = 0.4653, p = 0.0402, and Dunnett’s Multiple Comparison Test vehicle vs. 10 mg/kg p < 0.05).
Figure 5: Chronic Rimonabant Treatment Decreases Marble Burying.
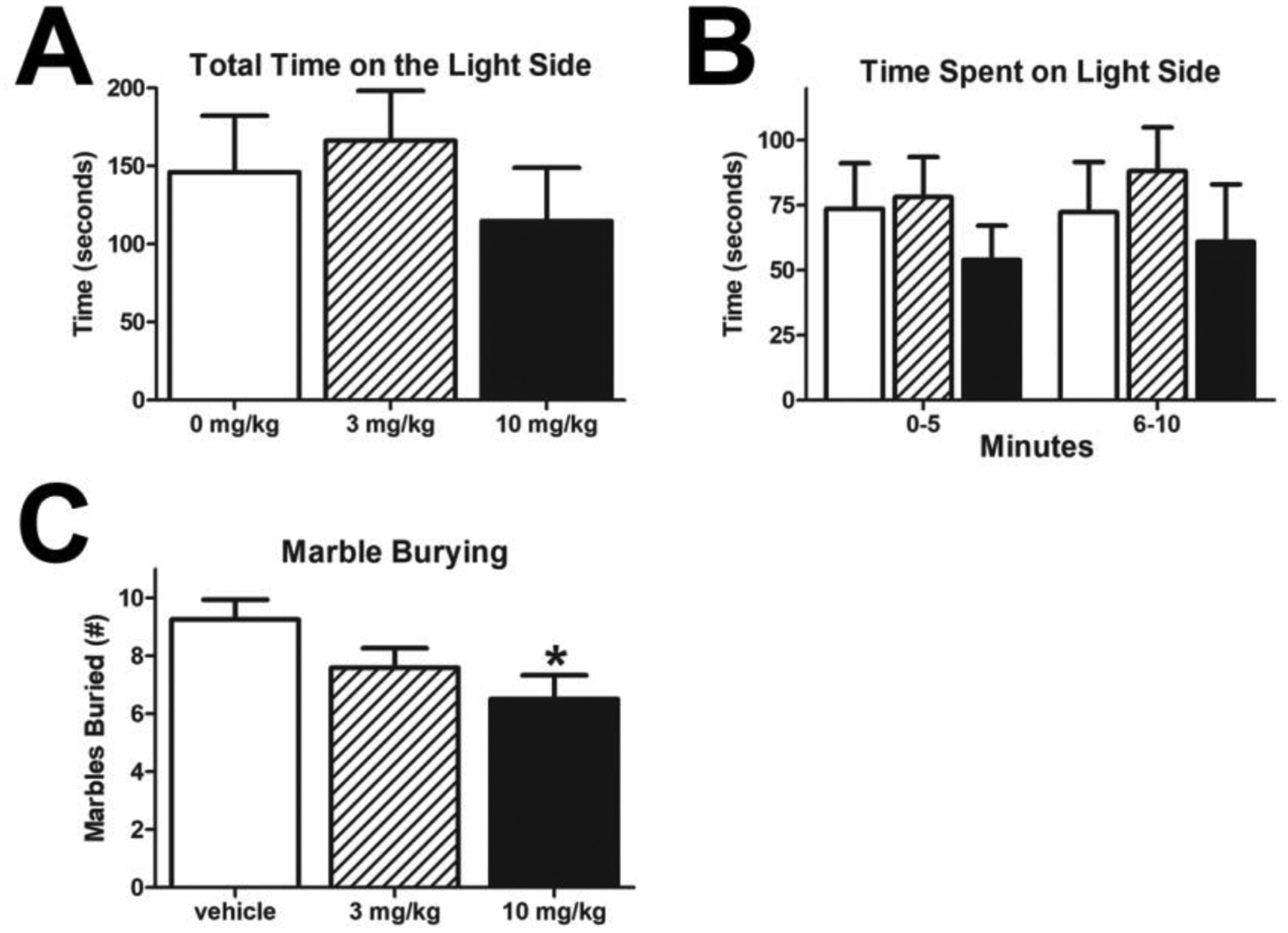
Neither dose of rimonabant produced significant effects in Light-Dark Box paradigm (A-B). (vehicle n=12; 3 mg/kg rimonabant n=12; 10 mg/kg rimonabant n=11). However, the highest dose of rimonabant tested decreased the number of marbles buried in a 30-minute session as compared to vehicle controls (*p<0.05; all groups n=12).
6.1.5. Acoustic Startle
Prepulse Inhibition (PPI) is a widely used surrogate of sensorimotor gating, which is disrupted in some neuropsychiatric conditions and was used here to assess whether rimonabant impacted the processing of sensory information. Animals that had been chronically treated with rimonabant tended to have decreased PPI (Supplementary Figure 2B). However, this was a non-significant effect in either the ASR or PPI (Supplementary Fig 2A - ASR; one-way ANOVA F(2, 31) = 0.5516, p = 0.6212; Supplementary Fig 2B – PPI); a repeated measures ANOVA with treatment as the between-subjects factor and prepulse intensity as the within-subjects factor confirmed that there was no significant effect of treatment F(2, 31) = 0.9248, p=0.4073, with the expected significant effect of prepulse intensity F(3, 93) = 40.58, p<0.0001, and no interaction F(6, 93) = 1.529, p=0.1774). For analysis, any animal the exhibited a negative PPI value was removed.
6.1.6. Forced Swim Test
The majority of previous research suggests that rimonabant, both acutely and chronically, decreases immobility time in the forced swim test (FST; anti-depressive-like effect11,15,27). However, two reports have also concluded there is no effect and one reported an increase in immobility12,25,28. Our results are in agreement that there is no robust effect of chronic rimonabant treatment on behavior in the FST: Day 1 – Supplementary Figure 3A – total, one-way ANOVA F(2, 27) = 2.024, p = 0.7572, Supplementary Figure 3B – five minute bins, a repeated measures ANOVA with treatment as the between-subjects factor and time as the within-subjects factor confirmed that there was no significant effect of treatment F(2, 27) = 0.3062, p=0.7388, or time F(2, 54) = 45.61, p<0.0001, and no interaction F(4, 54) = 1.145, p=0.3453; Day 2 – Supplementary Figure 3C - total; one-way ANOVA F(2, 27) = 0.3508, p = 0.7643, Supplementary Figure 3D – five minute bins, a repeated measures ANOVA with treatment as the between-subjects factor and time as the within-subjects factor confirmed that there was no significant effect of treatment F(2, 27) = 0.1224, p=0.8853, or time F(1, 27) = 109.0, p<0.0001, and no interaction F(2, 27) = 1.694, p=0.2027; Day 3 – Supplementary Figure 3E – total, one-way ANOVA F(2, 24) = 0.9463, p = 0.7920, Supplementary Figure 3F – five minute bins, a repeated measures ANOVA with treatment as the between-subjects factor and time as the within-subjects factor confirmed that there was no significant effect of treatment F(2, 24) = 0.2355, p=0.7920, or time F(1, 24) = 32.98, p<0.0001, and no interaction F(2, 24) = 0.5297, p=0.5955).
6.2.1. Experiment Two Results
Both the vehicle and rimonabant (10 mg/kg) groups had similar weights at the beginning of the study (Fig 6A, t-test p = 0.9242). As seen in Expt 1, rimonabant produced a significant decrease in weight gain over the course of the study (Fig 6B; a repeated measures ANOVA with treatment as the between-subjects factor and testing day as the within-subjects factor confirmed that there was a significant effect of treatment F(1, 28) = 8.697, p=0.0064, time F(2.808, 78.62) = 454.3, p<0.0001, and a significant interaction F(24, 672) = 11.49, p<0.0001, post hoc testing showed significant differences for nearly all points after the second PPI testing p < 0.05).
Figure 6: Experiment 2 - Chronic Treatment with Rimonabant Decreases Weight Gain.
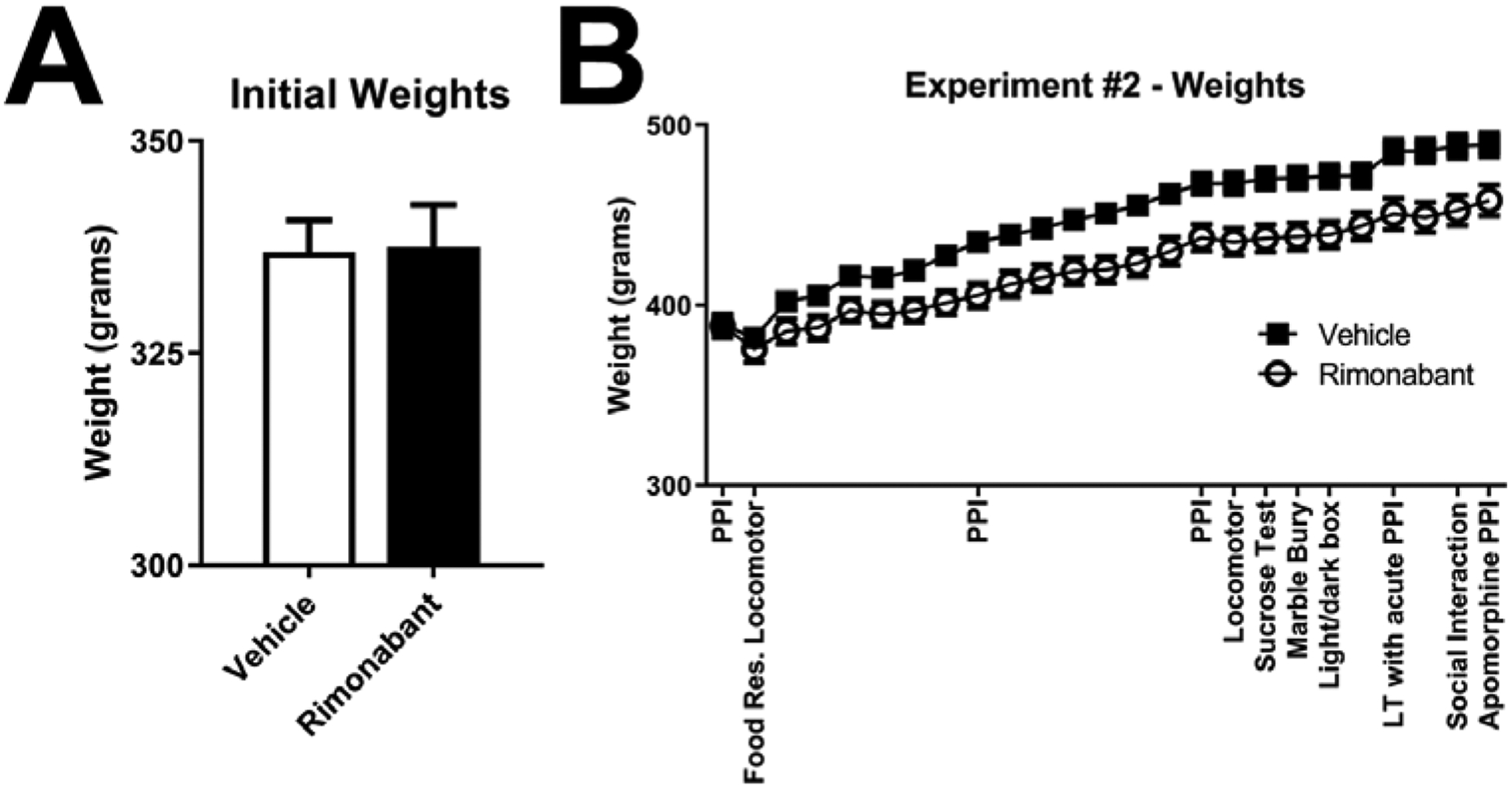
At the beginning of the experiment (food ad libitum feeding test) weights did not significantly differ between test groups (A). Similarly, weights were unchanged for the testing of acute effects of rimonabant in PPI and under food restriction conditions (B). With chronic treatment, rimonabant decreased weight gain (as see previously Fig 3; (both groups n=15). Note: the dip in weights before the second locomotor experiment is due to the food restriction conditions for the one night prior to testing (B).
6.2.2. Feeding and Locomotor Activity – Acute Dosing
Replicating the results from Expt 1, rimonabant decreased the amount of food consumed (Supplementary Figure 4A; t-test p = 0.0006, n = 15 per group), decreased locomotor activity (Supplementary Figure 4B; t-test p < 0.0001, n = 15 per group), and decreased rearing time (Supplementary Figure 4C; t-test p < 0.0001, n = 15 per group) during the feeding experiment. After 8 days of “washout” with no rimonabant dosing since the feeding/locomotion test, rimonabant or vehicle was given acutely before testing ASR and PPI (30 minute pretreatment). There was no evidence that rimonabant given acutely impacts acoustic startle reflex or sensorimotor integration (Supplementary Figure 5A, ASR – t-test p = 0.3844; Supplementary Figure 5B, PPI - a repeated measures ANOVA with treatment as the between-subjects factor and prepulse intensity as the within-subjects factor confirmed that there was no significant effect of treatment F(1, 52) = 0.6963, p=0.4078, but had the expected effect of prepulse intensity F(3, 52) = 11.48, p<0.0001, and no interaction F(3, 52) = 0.1692, p=0.9167). For analysis, any animal that exhibited a negative PPI value was removed (vehicle n=8; rimonabant n=7).
To determine whether the effects seen during the feeding tests were due to under-motivation of the animals, rats were tested 4 days after the ASR testing and were food restricted overnight. Under food restricted conditions, the rimonabant-mediated decrease in food consumption, locomotor activity, and rearing behavior remained (Supplementary Figure 6A, food consumption – t-test p < 0.0001, n = 15 per group; Supplementary Figure 6B, locomotion – t-test p = 0.0052, n = 15 per group; Supplementary Figure 6C, rearing time – t-test p = 0.0006, n = 15 per group). This suggests that the effects of this dose of rimonabant, under conditions of high motivation, are not diminished.
Animals that received chronic rimonabant treatment consistently had higher startle values in ASR. However, this trend never reached the level of significance (Supplementary Figure 7A, 7 Days – t-test p = 0.2622; Supplemntal Figure 7B, 14 Days – t-test p = 0.1504). To control for a weight-to-force differential in the rimonabant group, which did not gain as much weight, force exerted was normalized to the mass of the animal. In addition, chronic treatment of rimonabant did not alter PPI at the two time points tested (Supplementary Figure 7C, 7 Days - a repeated measures ANOVA with treatment as the between-subjects factor and prepulse intensity as the within-subjects factor confirmed that there was no significant effect of treatment F(1, 19) = 0.8702, p=0.3626, with the expected effect of prepulse intensity F(2.611, 49.61) = 41.74, p<0.0001, and no interaction F(3, 57) = 0.2320, p=0.8737; Supplementary Figure 7D, 14 Days - a repeated measures ANOVA with treatment as the between-subjects factor and prepulse intensity as the within-subjects factor confirmed that there was no significant effect of treatment F(1, 23) = 0.7411, p=0.3982, with the expected effect of prepulse intensity F(2.446, 56.25) = 43.58, p<0.0001, and no interaction F(3, 69) = 0.3733, p=0.7725). For analysis, any animal that exhibited a negative PPI was removed (7 Days, vehicle n=10; rimonabant n=11; 14 Days, vehicle n=13; rimonabant n=12). At 15 days of chronic administration, locomotor activity was tested to determine whether chronic treatment had effects on exploratory activity, which could impact measures in other tests. There were no differences in total distance (Supplementary Figure 8A; distance – t-test p = 0.5282, n = 15 per group) or rearing behavior (Supplementary Figure 8B; rearing time – t-test p = 0.6419, n = 15 per group). Therefore, effects seen with acute dosing persist.
6.2.3. Emotionality Tests
In contrast to Expt 1, rimonabant produced a near significant decrease in marble burying (Supplementary Figure 9; marbles buried – t-test p = 0.0685, n = 15 per group). However, the difference between Expt 1 and Expt 2 appears to be driven by a difference in behavior among control rats. As seen in Expt 1, rimonabant had no influence on the preference of a sucrose solution over that of water in Expt 2 (Supplementary Figure 10; t-test p = 0.9627, n = 15 per group). Also mirroring Expt 1, rimonabant had a numerical decrease in the time spent on the light side of the Light-Dark Box, but again this did not reach significance (Supplementary Figure 11; total time – t-test p = 0.2198, n = 15 per group; 5 min bins, a repeated measures ANOVA with treatment as the between-subjects factor and time as the within-subjects factor confirmed that there was no significant effect of treatment F(1, 28) = 1.575, p=0.2198, or time F(1, 28) = 0.8550, =0.3630, and no interaction F(1, 28) = 0.5527, p=0.4634).
6.2.4. Acute Challenge of Chronically Treated Animals – ASR
Unlike previous testing of ASR and PPI, at 20 days of chronic treatment rats were administered rimonabant or vehicle 30 minutes before being placed in the startle chambers. This pretreatment served as the daily administration for day 20. This experiment was performed to gauge whether there is an interaction of chronic treatment with acute dosing. The acute challenge did not produce any significant differences in behavior, but there was a trend for decreased PPI (Supplementary Figure 12; ASR – t-test p = 0.4986; PPI - a repeated measures ANOVA with treatment as the between- subjects factor and prepulse intensity as the within-subjects factor confirmed that there was no significant effect of treatment F(1, 27) = 3.804, p=0.0616, with the expected effect of prepulse intensity F(2.269, 61.26) = 48.45, p<0.0001, and no interaction F(3, 81) = 0.4086, p=0.7472). For analysis, any animal that exhibited a negative PPI was removed (vehicle n=14; rimonabant n=15).
Due to a trend in Expt1 and Expt2 that rimonabant disrupts PPI, it was reasoned that challenging the rats with a sub-effectual dose of apomorphine (0.2 mg/kg) may reveal a more robust effect. Apomorphine is a potent disruptor of PPI, and has been used historically as a “pharmacological model” of schizophrenia29. However, apomorphine treatment did not produce a significant difference in the groups that had received chronic treatment of rimonabant (Supplementary Figure 13A, ASR – t-test p = 0.1812; Supplementary Figure 13B, PPI - a repeated measures ANOVA with treatment as the between-subjects factor and prepulse intensity as the within-subjects factor confirmed that there was no significant effect of treatment F(1, 25) = 2.317, p=0.1405, with the expected effect of prepulse intensity F(2.209, 55.23) = 53.16, p<0.0001, and no interaction F(3, 75) = 0.5135, p=0.6742). For analysis, any animal that exhibited a negative PPI was removed (vehicle n=14; rimonabant n=13). Moreover, control animals receiving apomorphine had similar PPI values as seen in vehicle treated in Supplementary Figure 2 (~25% at a prepulse intensity of 70dB).
6.2.5. Social Interaction
The social interaction paradigm was set-up such that the intruder was placed in a Plexiglas box with many 1 cm holes on the sides and bottom and was suspended 15 cm above the floor of the chamber. This allowed: 1) the subject rat to be able to sniff and see the intruder but not physically interact, 2) for the use of infrared beam detection of the subject rat with no interference of the intruder, and 3) video recording of the session. The chamber was virtually separated into three sections using the “zone analysis” of the Fusion Activity Monitor software (Omnitech, Columbus, OH; Fig 7A). One third was under the suspended intruder pen (under intruder zone), another third in the middle of the chamber (approach zone), and the last third was furthest from the pen. Using this analysis, both groups spent similar time under or near (approach zone) the pen before the intruder was introduced (Fig 7B, approach zone – t-test p = 0.1261; Fig 7C, under pen – t-test p = 0.1375). After the intruder was placed in the pen, the subject rats that received chronic treatment of rimonabant (10 mg/kg) spent significantly more time in the approach zone (Fig 7D, t-test p = 0.0010) and significantly less time under the intruder (Fig 7E, t-test p = 0.0016) compared to vehicle controls. The difference between time spent under the pen before and after the intruder was introduced was calculated for individual animals, and it was found that the rimonabant treated animals had a significantly lower difference score (Fig 7F, t-test p = 0.0093). Interestingly, the score revealed that the rimonabant treated animals did not alter their behavior in response to the intruder, and the difference between the groups was due to the vehicle treated animals changing their behavior (vehicle 115+/− 24 seconds; rimonabant 24+/−21 seconds). There was no difference in the total distance traveled between the two groups (Fig 7G, t-test p = 0.8611).
Figure 7: Proximity to the Intruder Rat Differ After Chronic Rimonabant Treatment.
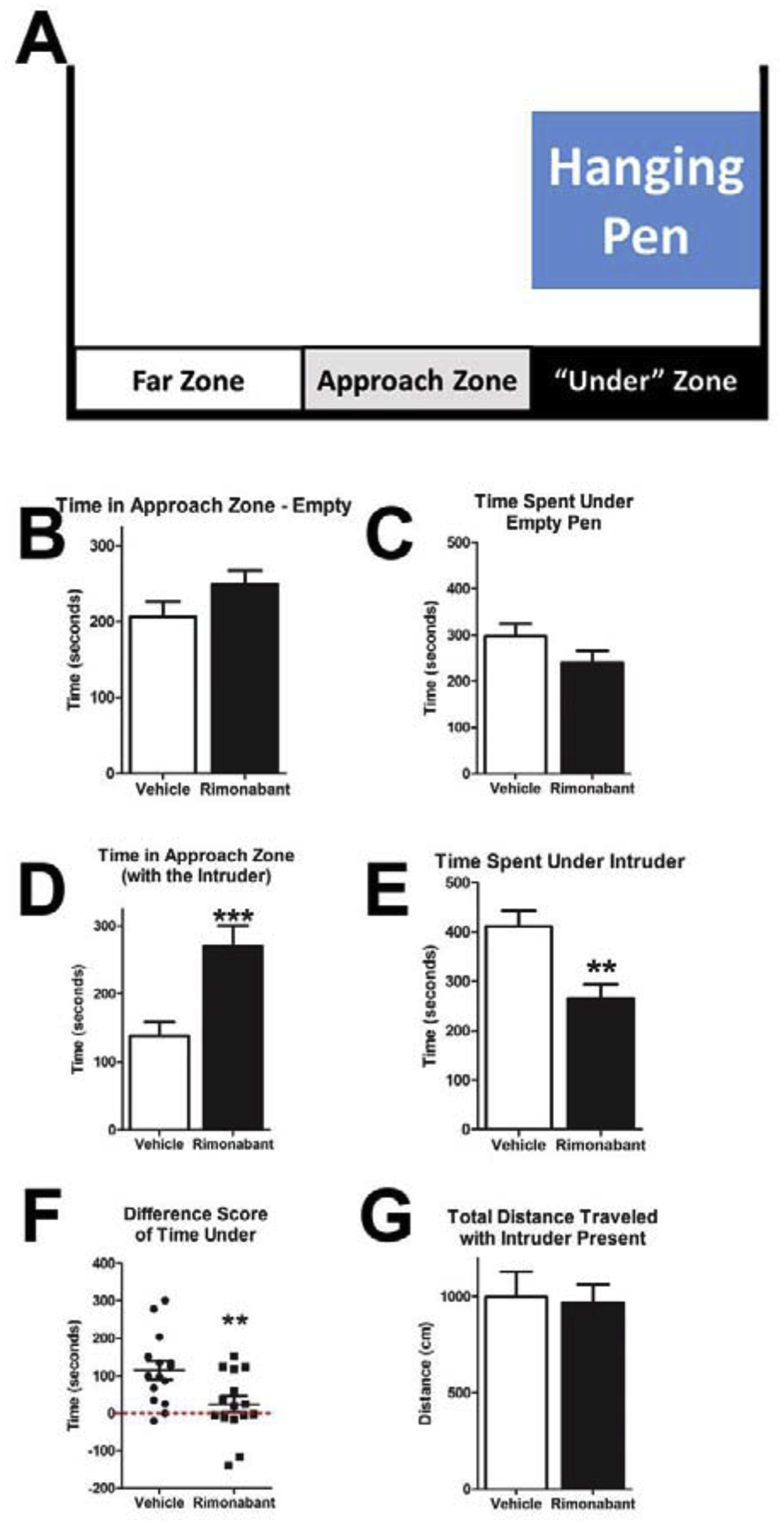
The location of the rat during social interaction testing was determined by infrared beam breaks. The intruder was placed in a pen suspended above the infrared beams. For analysis, the arena was broken into three zones in relation to the proximity to the pen (A). During the first 10 minutes of the testing period, all groups spent similar time near (B) or under the empty pen (C). In the subsequent 10 minutes with an intruder present in the pen, the rimonabant treated group (10 mg/kg) spent significantly more in the approach zone (D), and also spent significant less time under the intruder (E). To better understand the observed behavior, difference scores for individual animals were calculated between their time under the pen before and after the introduction of the intruder. Rimonabant treated animals did not change their behavior (mean score near zero), while the vehicle treated animals shifted their behavior to be under the pen (F; dotted line is at zero difference). There was no difference between groups in the total distance travelled during the 10 minute session while the intruder was present (G). (**p < 0.01, ***p < 0.001, all groups n=15)
There were no differences in the interactions with the intruder as assessed by video scoring: Supplementary Figure 14C, time spent interacting – t-test p = 0.7499; Supplementary Figure 14D, number of episodes interacting – t-test p = 0.5857; Supplementary Figure 14E, time spent per episode interacting – t-test p = 0.9234). These scores were also not different before the intruder entered the pen (empty pen): Supplementary Figure 14A, time spent interacting – t-test p = 0.8074; Supplementary Figure 14B, number of episodes interacting – t-test p = 0.3436.
7. Discussion
Acute rimonabant treatment produced many of the effects previously shown by others (e.g. increased grooming), while animals tested after chronic dosing showed differences in measures of emotionality (e.g. social interaction) and produced attenuated weight gain on ad libitium food access.
In Expt 1 acute dosing without food restriction decreased locomotion, rearing time, and rearing episodes at both doses. The higher dose of rimonabant significantly increased scratching and wet-dog shakes, and it trended to increase grooming (Fig 4). Our interpretation is that the reduction in locomotion and rearing was due to an increase in time spent grooming/scratching. In an attempt to address any potential behavioral competition between food consumption and grooming, these tests were performed after overnight food restriction in Expt 2 to increase motivation to eat, but the effects noted in Expt 1 persist. This suggests that behavioral competition was likely not driving decreased food consumption, and previous results in the literature additionally demonstrate that the anorectic effects of rimonabant remain when increased grooming and scratching behaviors are blocked.32 The metabolic effects of rimonabant could manifest through other mechanisms such as regulation of leptin or ghrelin circuitry.6,30,31 Additionally, the anorectic effects of rimonabant treatment were also noted following chronic dosing.
In Expt 1 and Expt 2, chronic rimonabant treatment (24 days Expt 1 and 20 days Expt 2) produces trends in PPI disruption, and any possible effects are not likely to be due to changes within the dopaminergic pathways, as we failed to see an exacerbation with the administration of apomorphine. It may be worthwhile to revisit this testing with larger subject numbers, and any further studies should probe other circuity well known to be involved in PPI (e.g., glutamate → phencyclidine).
A previous report indicated that chronic rimonabant administration decreased sucrose intake and increased immobility in the forced swim test12, signs of anhedonia. Other groups have found that rimonabant produced a decrease in immobility11,15,27. Our experiments dosed animals after testing and found no apparent anhedonia with chronic treatment (Supplementary Figure 1; sucrose consumption). The current study also did not find compelling evidence of increased anxiety (Fig 5, Supplementary Figure 11), although both experiments had the rimonabant groups having a numerically lower amount of time spent on the light-side of a light-dark box. In addition, there was no effect of chronic rimonabant treatment in FST (Supplementary Figure 3) when the test was performed prior to drug administration for the day. Conversely, rimonabant treated (10 mg/kg) animals had decreased marble burying (Fig 5C), which is often interpreted as anxiolytic. There are other plausible explanations as to why a rodent would not bury foreign objects in a novel homecage environment, but exploration of these alternative reasons is beyond the scope of the present study. To our knowledge, the effect of chronic rimonabant administration on marble burying behavior has not been previously reported.
Together the data are suggestive that at least in the measures that were performed in this study, there is no evidence of depressive or anxiety-like phenotypes at the doses tested. To probe the effects of rimonabant on “emotional” type behaviors further, rats were tested for their social interaction with a novel same sex “intruder”. Here it was seen that chronic rimonabant treatment significantly reduced the time spent under the intruder (Fig 7). It was revealed by calculating a difference score (time spent in a particular zone before versus after intruder placement) that the rimonabant animals did not significantly change their behavior. The vehicle treated animals shifted the time they spent from the “approach” zone to under the intruder. However, the rimonabant animals clearly interact with the intruder (Supplementary Figure 14). Therefore, it appears that chronic rimonabant treatment blocks a shift in behavior (Fig 7). Due to the unique construction of our testing apparatus with an added dimension (hanging pen), it should be considered that there may be a difference in motivation between when an animal is sniffing from below the intruder versus when they are interacting merely from the sides. More detailed and more elaborate paradigms will be required to determine the underlying cause of this behavioral difference, and the reasons could be complex.
Moving forward with attempts to establish a reproducible preclinical screen for depressive phenotypes induced by CB1R antagonists should further explore potential co-morbidities among the population being tested. Many of the adverse events associated with rimonabant come from studies of obesity, which may be important for the development of adverse psychiatric phenotypes. First, obesity can drive increased activity of the endocannabinoid system in humans and rodent models of obesity32,33, and obesity is associated with depression.34,35 This combination of factors may increase the likelihood of adverse events from administration of CNS penetrant CB1 antagonists like rimonabant. It has further been shown that incidence rates of psychiatric side effects were higher when a history or psychiatric disorders was not an exclusion criteria for participants.36,37 Age also appeared to be a predictor of serious adverse events.38 However, a lower dose of rimonabant (5 mg) significantly reduced number of adverse events during phase III clinical trials suggesting that peripheralized compounds with lower brain penetration may be appropriate for further development.39,40 Future preclinical screens for adverse psychiatric phenotypes may be well-served by including obesity and/or increased age in rodent models. Sex may be an important factor to explore further in rodent models as well. Anxiety-like phenotypes arising in Cnr1 knockout models differ by sex.41,42
8. Conclusions
The goal of the present study was to identify behavior(s) in rats that could be used to screen for potential negative CNS-mediated neuropsychiatric side effects of CB1R antagonism. Our data suggest that social interaction paradigms may be worthy of further testing to probe for possible negative neuropsychiatric effects of CB1R antagonists. This may be because the social interaction paradigm is multidimensional and that other paradigms are too narrow in their scope and metric (light-dark box) to capture a complex phenotype. To answer the question of whether the rimonabant-mediated effect is pharmacological induced neophobia, multiple day habituation to the social interaction testing arena may normalize the behavior of the rimonabant treated animals. Alternatively, testing for neophagic responses may be a more efficient and sensitive test. A follow-up on the present study with a more targeted and streamlined behavioral battery, including a full dose response, is planned to assess adverse liabilities associated with centrally acting antagonists of CB1R and to compare them to peripherally selective compounds as well as neutral/silent antagonists. Additional studies to identify useful behavioral tests for preclinical screens of CB1R antagonists may we warranted with potential co-morbid factors such as obesity, age, and sex.
Supplementary Material
9. Acknowledgements
We thank the dedicated staff of the Laboratory Animal Facility for daily care of our rats. We wish to thank the NIDA drug supply program for supplying rimonabant. This work was made possible by AA023256 and DK100414 to RM.
3. Abbreviations:
- ASR
Acoustic Startle Reflex
- CB1R
cannabinoid receptor 1
- EC
endocannabinoid
- FST
forced swim test
- PPI
Prepulse inhibition
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simon V & Cota D MECHANISMS IN ENDOCRINOLOGY: Endocannabinoids and metabolism: past, present and future. Eur J Endocrinol 176, R309–R324 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Lu Y & Anderson HD Cannabinoid signaling in health and disease. Can J Physiol Pharmacol 95, 311–327 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Sharma MK, Murumkar PR, Barmade MA, Giridhar R & Yadav MR A comprehensive patents review on cannabinoid 1 receptor antagonists as antiobesity agents. Expert Opin Ther Pat 25, 1093–1116 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Chorvat RJ Peripherally restricted CB1R blockers. Bioorg Med Chem Lett 23, 4751–4760 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Cinar R, et al. Hepatic CB receptors mediate diet-induced insulin resistance by increasing de novo synthesis of long chain ceramides. Hepatology (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tam J, et al. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab 16, 167–179 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, et al. Hepatic cannabinoid receptor-1 mediates diet-induced insulin resistance via inhibition of insulin signaling and clearance in mice. Gastroenterology 142, 1218–1228 e1211 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curioni C & Andre C Rimonabant for overweight or obesity. Cochrane Database Syst Rev, CD006162 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akbas F, Gasteyger C, Sjodin A, Astrup A & Larsen TM A critical review of the cannabinoid receptor as a drug target for obesity management. Obes Rev 10, 58–67 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Pacher P & Kunos G Modulating the endocannabinoid system in human health and disease--successes and failures. FEBS J 280, 1918–1943 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griebel G, Stemmelin J & Scatton B Effects of the cannabinoid CB1R antagonist rimonabant in models of emotional reactivity in rodents. Biol Psychiatry 57, 261–267 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Beyer CE, et al. Depression-like phenotype following chronic CB1R antagonism. Neurobiol Dis 39, 148–155 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Rubino T, et al. Cannabinoid-Precipitated Withdrawal: A Time-Course Study of the Behavioral Aspect and its Correlation with Cannabinoid Receptors and G Protein Expression. Journal of Pharmacology and Experimental Therapeutics 285, 813–819 (1998). [PubMed] [Google Scholar]
- 14.Jarbe TU, Ross T, DiPatrizio NV, Pandarinathan L & Makriyannis A Effects of the CB1R agonist WIN-55,212–2 and the CB1R antagonists SR-141716 and AM-1387: open-field examination in rats. Pharmacology, biochemistry, and behavior 85, 243–252 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Tzavara ET, et al. The CB1R antagonist SR141716A selectively increases monoaminergic neurotransmission in the medial prefrontal cortex: implications for therapeutic actions. British journal of pharmacology 138, 544–553 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacLaren DA, Markovic T & Clark SD Assessment of sensorimotor gating following selective lesions of cholinergic pedunculopontine neurons. The European journal of neuroscience 40, 3526–3537 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Clark SD, et al. Urotensin II acts as a modulator of mesopontine cholinergic neurons. Brain research 1059, 139–148 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Reynolds JL, Ignatowski TA, Sud R & Spengler RN Brain-derived tumor necrosis factor-alpha and its involvement in noradrenergic neuron functioning involved in the mechanism of action of an antidepressant. The Journal of pharmacology and experimental therapeutics 310, 1216–1225 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Suemaru K, et al. Nicotine blocks apomorphine-induced disruption of prepulse inhibition of the acoustic startle in rats: possible involvement of central nicotinic alpha7 receptors. Br J Pharmacol 142, 843–850 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa B & Colleoni M SR141716A induces in rats a behavioral pattern opposite to that of CB1R agonists. Zhongguo Yao Li Xue Bao 20, 1103–1108 (1999). [PubMed] [Google Scholar]
- 21.Darmani NA Methods evaluating cannabinoid and endocannabinoid effects on gastrointestinal functions. Methods Mol Med 123, 169–189 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Wiley JL, et al. Structural analogs of pyrazole and sulfonamide cannabinoids: effects on acute food intake in mice. Eur J Pharmacol 695, 62–70 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arevalo C, de Miguel R & Hernandez-Tristan R Cannabinoid effects on anxiety-related behaviours and hypothalamic neurotransmitters. Pharmacology, biochemistry, and behavior 70, 123–131 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Tallett AJ, Blundell JE & Rodgers RJ Grooming, scratching and feeding: role of response competition in acute anorectic response to rimonabant in male rats. Psychopharmacology 195, 27–39 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Zador F, et al. Low dosage of rimonabant leads to anxiolytic-like behavior via inhibiting expression levels and G-protein activity of kappa opioid receptors in a cannabinoid receptor independent manner. Neuropharmacology 89, 298–307 (2015). [DOI] [PubMed] [Google Scholar]
- 26.O’Brien LD, et al. Effect of chronic exposure to rimonabant and phytocannabinoids on anxiety-like behavior and saccharin palatability. Pharmacology, biochemistry, and behavior 103, 597–602 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Lockie SH, et al. CNS opioid signaling separates cannabinoid receptor 1-mediated effects on body weight and mood-related behavior in mice. Endocrinology 152, 3661–3667 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Lee S, Kim DH, Yoon SH & Ryu JH Sub-chronic administration of rimonabant causes loss of antidepressive activity and decreases doublecortin immunoreactivity in the mouse hippocampus. Neurosci Lett 467, 111–116 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Geyer MA & Ellenbroek B Animal behavior models of the mechanisms underlying antipsychotic atypicality. Progress in neuro-psychopharmacology & biological psychiatry 27, 1071–1079 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Lazzari P, et al. Weight loss induced by rimonabant is associated with an altered leptin expression and hypothalamic leptin signaling in diet-induced obese mice. Behav Brain Res 217, 432–438 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Kola B, et al. The CB1R mediates the peripheral effects of ghrelin on AMPK activity but not on growth hormone release. FASEB J (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monteleone AM, et al. Responses of peripheral endocannabinoids and endocannabinoid-related compounds to hedonic eating in obesity. Eur J Nutr 55, 1799–1805 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Perkins JM & Davis SN Endocannabinoid system overactivity and the metabolic syndrome: prospects for treatment. Current diabetes reports 8, 12–19 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Dixon JB, Dixon ME & O’Brien PE Depression in association with severe obesity: changes with weight loss. Arch Intern Med 163, 2058–2065 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Wadden TA, et al. Psychosocial aspects of obesity and obesity surgery. Surg Clin North Am 81, 1001–1024 (2001). [DOI] [PubMed] [Google Scholar]
- 36.Moreira FA, Grieb M & Lutz B Central side-effects of therapies based on CB1 cannabinoid receptor agonists and antagonists: focus on anxiety and depression. Best Pract Res Clin Endocrinol Metab 23, 133–144 (2009). [DOI] [PubMed] [Google Scholar]
- 37.Moreira FA & Crippa JA The psychiatric side-effects of rimonabant. Braz J Psychiatry 31, 145–153 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Christensen R, Kristensen PK, Bartels EM, Bliddal H & Astrup A Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet 370, 1706–1713 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Van Gaal L, Pi-Sunyer X, Despres JP, McCarthy C & Scheen A Efficacy and safety of rimonabant for improvement of multiple cardiometabolic risk factors in overweight/obese patients: pooled 1-year data from the Rimonabant in Obesity (RIO) program. Diabetes Care 31 Suppl 2, S229–240 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Van Gaal LF, et al. Long-term effect of CB1 blockade with rimonabant on cardiometabolic risk factors: two year results from the RIO-Europe Study. Eur Heart J 29, 1761–1771 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Bowers ME & Ressler KJ Sex-dependence of anxiety-like behavior in cannabinoid receptor 1 (Cnr1) knockout mice. Behav Brain Res 300, 65–69 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Craft RM, Wakley AA, Tsutsui KT & Laggart JD Sex differences in cannabinoid 1 vs. cannabinoid 2 receptor-selective antagonism of antinociception produced by delta9-tetrahydrocannabinol and CP55,940 in the rat. J Pharmacol Exp Ther 340, 787–800 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


