SUMMARY
To take the best actions, we often need to maintain and update beliefs about variables that cannot be directly observed. To understand the principles underlying such belief updates, we need tools to uncover subjects’ belief dynamics from natural behaviour. We tested whether eye movements could be used to infer subjects’ beliefs about latent variables using a naturalistic navigation task. Humans and monkeys navigated to a remembered goal location in a virtual environment that provided optic flow but lacked explicit position cues. We observed eye movements that appeared to continuously track the goal location even when no visible target was present there. Accurate goal-tracking was associated with improved task performance, and inhibiting eye movements in humans impaired navigation precision. These results suggest that gaze dynamics play a key role in action-selection during challenging visuomotor behaviours, and may possibly serve as a window into the subject’s dynamically evolving internal beliefs.
eTOC blurb
Humans and animals constantly maintain and update beliefs about latent world-states, but those belief dynamics are notoriously difficult to measure experimentally. Using a naturalistic task in which subjects navigated to a hidden goal in virtual reality, Lakshminarasimhan et al. demonstrate that this could be achieved by tracking eye movements.
INTRODUCTION
Rational behaviour often requires predicting latent states from sensory observations. Since latent variables cannot be directly observed, and since the utility of actions depends on the status of latent variables in the future, we must use statistical regularities in space and in time to predict them. There is a large body of studies that not only demonstrate that humans exploit regularities in feature space (Knill and Pouget, 2004), but also show how to infer the associated subjective priors from data (Houlsby et al., 2013; Paninski, 2006; Smith et al., 2012; Stocker and Simoncelli, 2006; Turnham et al., 2011). In contrast, we know relatively little about how physical laws that govern the temporal dynamics of inputs are internalized and used to guide time-evolving beliefs in the absence of reliable observations (Lee et al., 2014). We refer to these subjective beliefs about time-varying latent states as belief dynamics.
The reasons for limited progress in understanding belief dynamics are twofold. First, psychophysics continues to be dominated by experimental paradigms in which actions are discrete (e.g., binary choice) and sporadic (e.g., at the end of the trial). In contrast, continuous tasks (Bonnen et al., 2015; Huk et al., 2018; Knoll et al., 2018; Pitkow and Angelaki, 2017) provide subjects the opportunity to reveal more information about their beliefs and predictions as they unfold in time. Second, although theoretical techniques to infer latent beliefs from actions are slowly becoming available (Kumar et al., 2019; Reddy et al., 2018; Wu et al., 2018, 2019), they have yet to be successfully applied to settings in which state and action spaces are both continuous. Consequently, principled ways to reliably uncover subjects’ belief dynamics from natural behaviour are still lacking. Meanwhile, a practical way to overcome this hurdle would be by covertly ‘measuring’ those beliefs. One candidate tool to accomplish this is eye-tracking (Spivey, 2007). Saccadic eye movements have previously been used to understand mental processes underlying a wide variety of abstract tasks such as language comprehension (Tanenhaus et al., 1995), reading (Rayner, 1998), mental imagery (Spivey and Geng, 2001), evidence accumulation (Gold and Shadlen, 2000), visual search (Zhang et al., 2018), and even random number generation (Loetscher et al., 2010). Furthermore, it has recently been argued that smooth-pursuit eye movements may be influenced by short-term memory (Deravet et al., 2018; Orban de Xivry et al., 2013). By formulating oculomotor pursuit to transiently occluded moving targets as an active inference process, these eye movements have been used to infer subjects’ internal beliefs (Adams et al., 2015). We wanted to know whether eye movements also reflect belief dynamics for extended periods of time under more naturalistic conditions.
To test this, we created a virtual environment with an unstructured ground plane on which subjects steered to a transiently cued target location. To successfully perform the task, subjects had to first infer their own movements based on the sparse optic flow cues generated while steering, and then integrate them over time to estimate the relative target location. Although the target appears briefly at the beginning of the trial, the location of the target relative to the subject becomes latent as soon as they start steering because thereafter, the relative location is not directly observed, only inferred by discounting one’s own displacements. This task differs from traditional paradigms used to study latent state inference in two important ways that make it both challenging and better suited to understanding belief dynamics in the real-world. First, in contrast to tasks in which latent states remain unchanged throughout the trial (e.g. right or left in a random-dot motion (Britten et al., 1992) or heading-discrimination task (Britten, 2008)), here the latent state dynamically varies over the course of each trial, under the subject’s control. Second, unlike tasks that use pulsatile evidence and discrete numbers of latent states (e.g. auditory-clicks (Brunton et al., 2013) or accumulating-towers task (Pinto et al., 2018)), both the sensory input (self-motion) and latent states (relative target position) are continuous-valued. To test whether eye movements were informative regarding subjective beliefs about those time-varying, continuous-valued latent states, we recorded the gaze behaviour of humans and rhesus macaques while they performed this task. Parallel experiments in the two species allowed us to test whether the eye movements were evolutionarily conserved. We found that both humans and monkeys tend to follow the location of the unseen target with their gaze until they reach it, and their success in tracking the target over time predicted their final behavioural accuracy. These findings suggest that gaze dynamics reflect internal beliefs, and could help shed light on the computations that transform visual perception to action in naturalistic settings.
RESULTS
Monkeys and humans performed a visual navigation task in which they used a joystick to steer to a transiently cued target location in a three-dimensional virtual reality (VR) environment without allocentric reference cues (i.e. stable landmarks) (Fig 1A, Methods). Individual visual elements comprising the ground plane were transient and could not be used as landmarks. At the beginning of each trial, a circular target blinked briefly at a random location within the field of view on the ground plane, and then disappeared. The joystick controlled forward and angular velocities, allowing subjects to steer freely in two dimensions (Fig 1B). The subjects’ goal was to steer towards the target, and stop when they believed their position fell within a circular reward zone centered on the target. They received feedback about their performance at the end of each trial.
Figure 1. Prim ates can navigate by integrating optic flow.
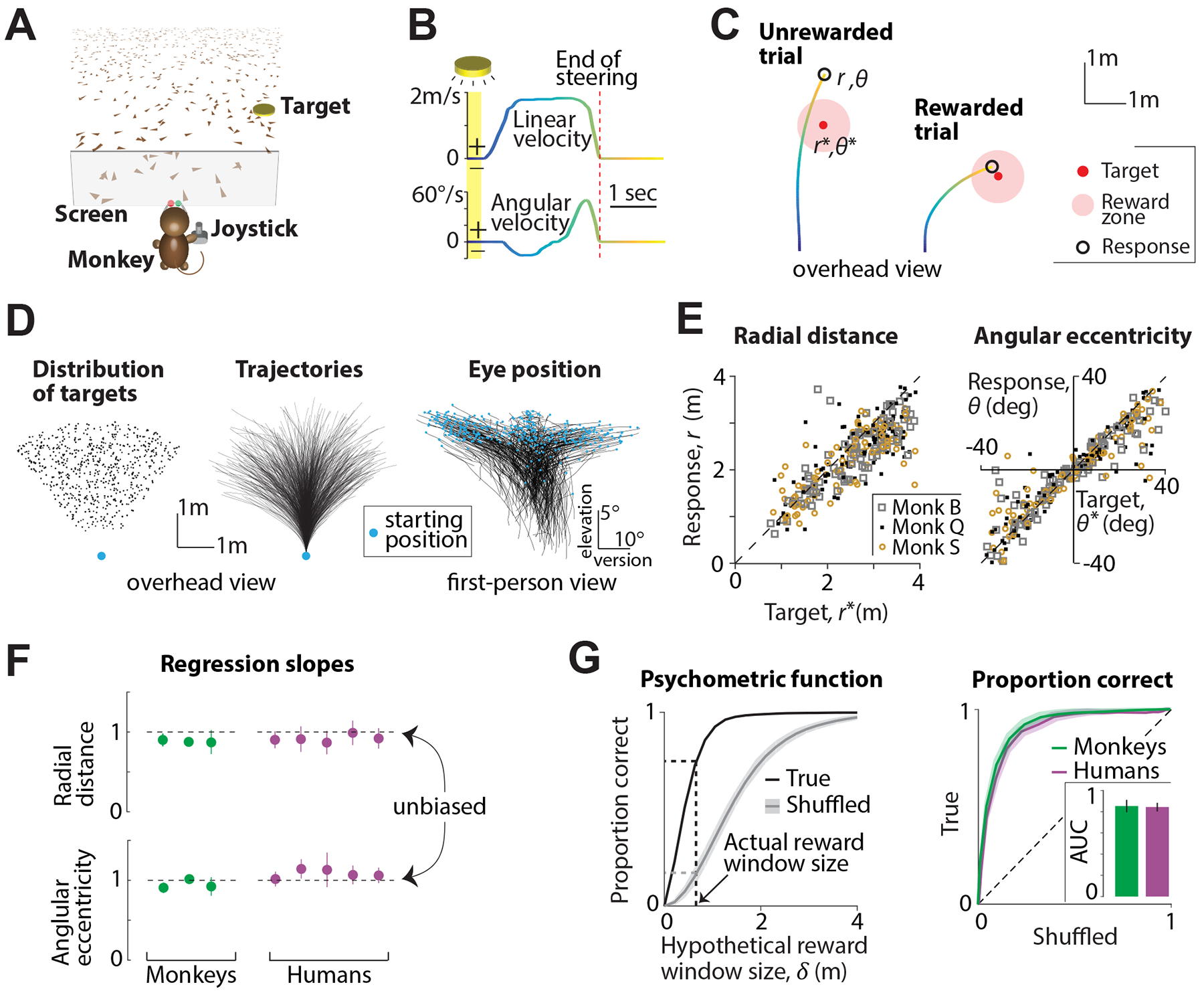
A. Monkeys and human subjects use a joystick to navigate to a cued target (yellow disc) using optic flow cues generated by ground plane elements (brown triangles). B. The time-course of linear (top) and angular (bottom) velocities during one example trial. Yellow shaded region corresponds to the time period when the target was visible on the screen. Time is also coded by color. C. Example trials showing incorrect (left) and correct (right) responses of a monkey. D. Left. Overhead view of the spatial distribution of target positions across trials. Middle: Movement trajectories of one monkey during a representative subset of trials. Blue dot denotes starting location. Right. First-person view of the trajectories of eye movements during the same trials. Abscissa and ordinate show horizontal version and elevation of the eyes. Blue dots represent the initial eye position (when the target was turned OFF) on each trial. E. Left. Comparison of the radial distance of the monkey’s response (stopping location) against radial distance of the target across trials. Right: Angular eccentricity of the response vs target angle. Black dashed lines have unity slope. The subject’s starting location was taken as the origin. F. Subjects’ accuracy in radial distance (top) and angular eccentricity (bottom) were quantified as the slopes of the corresponding linear regressions and plotted for individual monkeys and human subjects. Horizontal dashed lines denote the value of the slope that corresponds to unbiased behaviour. Error bars denote ±1 SEM across trials. G. Left. The proportion of correct trials of one monkey for various values of hypothetical reward window size (black). Shuffled estimates are shown in gray. Right. ROC curves for all subjects, obtained by plotting their true proportion of correct trials (from unshuffled data) against the corresponding chance-level proportions (from shuffled data) for a range of reward windows. Shaded area denotes standard deviation across subjects. Inset shows the average area under the curve (AUC) for monkeys and human subjects. See also Figure S1.
Monkeys were first trained extensively using a staircase procedure (see Methods) until their performance stopped improving. Here, we will focus only on their post-training behaviour. At this point, the radius of the reward zone was fixed across trials (see Methods) and they received juice reward at the end of the trial for correctly stopping within this zone (Fig 1C). In contrast, human subjects received no prior training on this task. Instead, we used an adaptive feedback scheme in which the radius of the reward zone was dynamically scaled using a staircase procedure to match individual subjects’ abilities (Fig S1A, see Methods). In practice, it took less than fifty trials for the performance of humans to stabilize (Fig S1B). Therefore, we ignored the first fifty trials collected from human subjects and focused our analyses on the remaining data.
Target locations were uniformly distributed at random over the ground plane area within the subject’s field of view (Fig 1D - left). The stimulus was nearly identical for both species except for minor details such as the range of target distances and the duration for which the target was visible (see Methods). All subjects were head-fixed, and we recorded each subject’s movement trajectory (Fig 1D - middle) as well as eye position (Fig 1D - right) throughout each trial.
Behavioural performance
Figure 1E shows the performance of the monkeys in this task. Both radial distance (Fig 1E - left) and angular eccentricity (Fig 1E - right) of the monkeys’ responses (stopping location) were highly correlated with the target location across trials (n = 3 monkeys, Pearson’s r ± standard deviation, radial distance: 0.72 ± 0.1, angle: 0.84 ± 0.1) suggesting that their behaviour was appropriate for the task. To test whether their performance was accurate, we regressed their responses against target locations. The slope of the regression was close to unity both for radial distance (mean ± standard deviation = 0.92 ± 0.06) and angle (0.98 ± 0.1) suggesting that the monkeys were nearly unbiased (Fig 1F - green). We did notice modest undershooting for distant targets, an effect that is likely due to growing position uncertainty described in previous work (Lakshminarasimhan et al., 2018a).
We showed previously that humans are systematically biased when performing this task without feedback (Lakshminarasimhan et al., 2018a). Consistent with those findings, human subjects overshot the target in an initial block of trials in which no feedback was provided (Fig S1C; n = 5, mean slope ±standard deviation, radial distance: 1.21 ± 0.2, angle: 1.78 ± 0.3), to a degree that was proportional to target distance. With feedback, however, the same subjects quickly adapted their responses to produce nearly unbiased performance (Fig 1F - purple, see Fig S1D for individual trials; mean slope ± standard deviation, radial distance: 0.95 ± 0.1, angle: 1.15 ± 0.2). Notably, this improvement in performance was maintained in a final block of trials in which feedback was withheld (Fig S1E–F; radial distance: 1.03 ± 0.15, angle: 1.2 ± 0.2) suggesting that learning of this task was stable. To be consistent with monkey data, we only consider human subjects’ data collected during the block of trials with feedback in the remainder of this work.
We wanted to know whether humans and monkeys had comparable accuracies. Because we used a slightly larger range of target distances for humans (see Methods), travel durations were longer (median travel time ± IQR: monkeys: 1.9 ± 0.8s, humans: 2.7 ± 0.6s). Consequently, we could not directly compare the mean error magnitude of the subjects as it ignores differences in task difficulty. Instead, we used an approach that is conceptually similar to receiver operating characteristic (ROC) analysis to objectively compare the performance of monkeys and human subjects on a common scale. For each subject, we constructed a ‘psychometric function’ of performance as a function of hypothetical reward window size (Fig 1G; see Methods). By plotting the true psychometric function against one obtained by shuffling target locations across trials, we obtain the subject’s ROC curve. Chance-level performance would correspond to an area under the ROC curve (AUC) of 0.5, while perfectly accurate responses (zero error) will yield an AUC of one. The AUCs for both monkey and human subjects were quite large and statistically indistinguishable (mean ± standard deviation, monkeys: 0.85 ± 0.03, humans: 0.84 ± 0.05; t-test: p = 0.41) suggesting that they performed comparably. Although it is possible, in principle, to avoid integrating optic flow by learning the precise sensorimotor transformation implemented by the joystick controller, we previously showed that the variability of human subjects is greatly affected by removing optic flow cues (Lakshminarasimhan et al., 2018a) (Fig S1G). Likewise, monkeys rapidly adapt their actions in response to gain changes of the joystick controller (Fig S1H). This suggests that both monkeys and humans use optic flow to perform this task.
Pattern of eye movements
To understand the role of eye movements, we recorded the position of the subjects’ eyes while they performed the task. Figure 2A shows the vertical and horizontal eye positions of one monkey during an example trial. On this trial, we noticed saccades (eye movements exceeding 200°/s) before the target was turned off (henceforth called start of the trial) and around the time when the monkey stopped moving (end of steering), but not in-between. This pattern was evident across trials, as seen in the trial-averaged density of saccades (Fig 2B). Across all datasets from monkeys, the average frequency of saccades during the trial was significantly smaller than that during the inter-trial interval (mean saccade rate ± standard deviation, during trials: 0.5 ± 0.3 Hz, between trials: 0.9 ± 0.5 Hz; paired t-test: p = 0.02). We noticed a similar tendency among human subjects although the comparison was not statistically significant (Fig S2A; during trials: 0.8 ± 0.5 Hz, between trials: 1.4 ± 1 Hz; p = 0.11). Moreover, the velocity of eye movements during steering was generally low, with magnitudes well below 20°/s both in monkeys (Fig 2C; mean ± std.: 16.2 ± 2.1 °/s) and in humans (Fig S2B; 11.4 ± 3.2 °/s).
Figure 2. Eye movement dynamics during the task.

A. Time-course of vertical and horizontal (bottom) positions of the left and right eyes of a monkey during one example trial. Yellow region shows the period when a target was visible on the screen. Red dashed line corresponds to the end of steering in this trial. B. The time-course of the rate of saccades during the trial, averaged across all trials separately for each monkey. Trial-averaging was done by aligning trials relative to target onset (yellow region, before break on the x-axis) and end of steering (red dashed line, following the break). Grey line denotes mean saccade rate across monkeys during the period between trials. C. Joint probability density of the distribution over horizontal and vertical eye velocities, averaged across monkeys, while they steered towards the target. Marginals are shown in black. D. Comparison of the predicted and true eye positions in a subset of trials for all monkeys at the moment when the target was just turned OFF. E. Time-course of the eye position during a random subset of trials taken from one monkey. Blue and red dots denote the times at which the target was turned OFF and the end of steering, respectively. F. Target-tracking index when the target turned OFF for individual monkeys and humans. Error bars denote ±1 SEM obtained either by averaging across recording sessions (for monkeys) or bootstrapping (for humans). G. Time-course of the target-tracking index, averaged across monkeys and humans. Grey arrow denotes the chance level tracking-index verified by shuffling procedure. Shaded region denotes ±1 SEM across datasets. See also Figures S2–S4A.
Because saccades were mostly confined to periods when the animal was not actively steering and subjects appeared to make slowly-varying eye movements while steering, we asked whether they may be continuously ‘tracking’ the (invisible) target with their eyes while they navigated to it. Note that as one steers towards the target location, the target becomes progressively less eccentric and moves downward in the visual field. Therefore, if subjects’ eyes were to track the target, the magnitude of lateral version would tend towards zero and the eye elevation would become more negative with time (Fig S2C). To quantitatively test whether subjects tracked the target, we first generated ground truth theoretical predictions for the binocular position of their eyes during each trial, assuming that they maintained fixation at the center of the target throughout the trial (Fig S2D; Methods – Equation 1). Note that at each moment in time, the predicted eye position depends only the relative target position at that moment regardless of whether the subject accurately stopped on target, but we will examine the relationship to the latter in the next section. We then compared this prediction against the observed eye position of the subject by expressing both quantities in terms of three standard components - lateral version, elevation and vergence (Fig S2E; see Methods).
We expect subjects’ eyes to be drawn to the target when it appears on the screen. Indeed, the model predictions were highly correlated with the measured values of lateral version (Fig 2D - left and Fig S3A - left; Pearson’s r ± standard deviation, monkeys: 0.91 ± 0.1, humans: 0.85 ± 0.1) as well as elevation (Fig 2D - right and Fig S3A - right; monkeys: 0.60 ± 0.2, humans: 0.42 ± 0.2) at the beginning of the trial. The somewhat lower correlations for the latter are understandable because it is difficult to precisely fixate at the elevations for distant targets since they subtend a smaller visual angle. Next, we examined the time-course of eye movements during the trial and found a striking qualitative correspondence to the predicted dynamics (Fig 2E, S3B): as the trial progressed, lateral version became increasingly more concentrated around zero (Fig S3C - left) while eye elevation was significantly lower (Fig S3C - right). The correlation between predicted and observed values remained significantly greater than zero throughout the trial for both components (Fig S3D). This is quite remarkable because the target appeared only transiently at the beginning of the trial.
On the other hand, the correspondence between predicted and observed vergence was less clear. Performing this comparison for our task was challenging because about 90% of the full range of vergence angles is known to occur within gaze distances below one meter (Howard, 2012) and the predicted change in vergence is negligible for gaze distances beyond 2m (Fig S2E - bottom right). Only two of the monkeys exhibited vergence values that weakly correlated with the predictions at trial onset (Fig S3A) and a tendency to make convergent eye movements as they approached the target (Fig S3B), an effect that was also absent in human subjects (Fig S3B–D). It is possible that this inconsistency is due to the previously documented difficulty in executing voluntary vergence movements to imagined moving targets (Erkelens et al., 1989). This difficulty is likely exacerbated in VR where vergence eye movements must be executed without changing accommodation to maintain a clear retinal image of onscreen objects (Hoffman et al., 2008; Shibata et al., 2011). Therefore, we did not consider the vergence component for further analyses.
To quantify how well subject’s eyes tracked the target, we expressed the eye position as a two-dimensional vector comprised of lateral version and elevation, and computed a target-tracking index. Specifically, this quantity was given by the square root of the fraction of variance in the observed eye position that was explained by the prediction (Methods – Equation 2). An index of one implies that the subject consistently looked at the center of the (invisible) target while steering towards it, while zero denotes lack of correspondence between target and gaze locations. The target-tracking index was quite high at trial onset (during the first 500ms) when the target had just disappeared (Fig 2F; mean ± standard deviation, monkeys: 0.73 ± 0.05, humans: 0.71 ±0.05). Although this slowly dropped during the trial, the index at the end of the trial (during the last 500ms) remained well above zero (Fig 2G; mean ± standard deviation, monkeys: 0.35 ± 0.1, humans: 0.18±0.05), implying that subjects tend to maintain gaze at the target location while they steer towards it. To estimate this timescale of the correlation between gaze and target location, we analyzed the cross-correlogram between them and found that subjects’ eye positions did not systematically lead or lag the predictions based on the relative target location (Fig S4A). This suggests that eye movements reflect the current relative target position rather than predict its future value (although the computations used to estimate it could still be predictive).
The tracking index quantifies how subjects’ dynamical state (relative target position) is encoded in their continuous-valued eye position, while they navigate towards the target. However, recent work has highlighted the importance of discrete saccadic eye movements in mediating flow-tracking behaviour of humans and primates (Knoll et al., 2018). Therefore, we wanted to know whether saccades aided the target-tracking behaviour of our subjects. To test this, we first compared the distribution of saccade amplitudes during three non-overlapping epochs of the experiment - inter-trial periods when saccades tend to be exploratory, target-presentation phase when saccades are guided by the external stimulus, and the ensuing task phase when subjects steered using optic flow (Fig 3A). We found that across monkeys, the amplitude of saccades was much lower during the task phase than during other epochs (Fig 3B; mean ± SE: inter-trial −10.2 ± 1.6°, target-presentation - 14.4 ± 2.2°, task phase - 7.1 ± 1.2°) suggesting that saccades made while steering were qualitatively different from saccades made at other times. To directly test whether those saccades served to correct errors in target-tracking, we computed a saccade-triggered average of the target-tracking error and found that this error dropped significantly (peak decrease of 2.4 ± 0.4°; p < 10−10, t-test) shortly after saccade onset (Fig 3C). Following Knoll et al. (2018), we used lagged regression analysis to determine the precise relationship between saccade amplitude and the dynamics of target-tracking error (Methods). The amplitude of both vertical and horizontal components of the saccade were influenced by tracking error during the previous 200ms suggesting that these saccades were indeed made towards the target (Fig 3D). Moreover, the regression kernels were biphasic implying that the saccades overcompensated for the tracking errors. Finally, if these saccades were corrective, they should depend on the subjects’ internal estimate of the target location making them increasingly unreliable over time due to the buildup of uncertainty. Indeed, the strength of the regression kernel was weaker for later saccades (Fig 3E; peak-to-peak difference in weights for vertical component: first saccade - 0.44 ± 0.1, third - 0.20 ± 0.15; horizontal component: first saccade - 1.5 ± 0.4, third - 0.15 ± 0.2), thereby signaling a drop in saccadic precision over time. This suggests that these saccades were not stereotyped reflexive responses but were dynamically modulated by ongoing cognitive computations analogous to “catch-up” saccades observed during smooth pursuit of visible targets (Daye et al., 2014; Orban de Xivry et al., 2008). Although the amplitude of saccades made by human subjects were not significantly smaller while steering (Fig S4B), regression analysis revealed a strong association between tracking error and saccade amplitude but with slightly shorter integration windows. As observed for monkeys, the strength of this association was lower for saccades that happened later (Fig S4C) reflecting a potential influence of noisy integration.
Figure 3. Saccadic eye movements aid target-tracking.

A. Time-course of observed (black) and predicted (gray) vertical position of the eyes of a monkey. Black arrows indicate saccades made during three different task epochs (inter-trial, target presentation, and steering periods). Yellow region shows the period when a target was visible on the screen. B. Empirical cumulative distribution function of saccade amplitudes conditioned on the task epoch, averaged across monkeys. Inset shows amplitudes of individual saccades as a function of their timing. C. Average saccade-triggered target tracking error during a time-window around saccades made during steering. D. The time-course of coefficients obtained by linearly regressing the amplitudes of the two components of saccades (blue – vertical, red – horizontal) against the corresponding components of the target tracking error (Methods). E. Similar to D, but showing coefficients for regression done separately for the first, second, and third saccades made during steering. See also Figure S4B–C.
Eyes convey internal beliefs about target
Subjects could not have possibly been tracking the observed target location because the target disappeared at the beginning of the trial. A plausible explanation for their pattern of eye movements is that they tracked the location at which they believed the target was present. As they integrate their movements, subjects need to continuously update their internal estimate of the relative goal location, and perhaps their eye movements reveal those estimates. If this is the case, then we should be able to better predict their eye position when their beliefs are more accurate. We tested this both across subjects and across trials within each subject.
To test this across subjects, we used the variability in subjects’ stopping positions to first quantify the level of uncertainty in their position estimates (Methods). Due to the low trial count of individual human subjects, we pooled trials from all humans into a single dataset. Because uncertainty in knowing one’s location should limit one’s ability to visually track the target, we used the estimated uncertainties to calculate an approximate upper bound on the target-tracking index for each dataset (Fig 4A, Methods – equations 3). This upper bound serves to capture the heterogeneity in the spatial profile of uncertainty both across subjects (Fig 4B - left) and across sessions within each monkey (Fig S4D). Across all datasets (subjects × sessions), the target-tracking index observed towards the end of the trial (during the last 500ms) was weakly but significantly correlated with the theoretical upper bounds (Fig 4B - right, Pearson’s r = 0.26, p = 0.029). This suggests that differences in the ability to track the target with the eyes is due, at least in part, to differences in the magnitudes of positional uncertainty between subjects.
Figure 4. Accurate target-tracking is associated with increased task performance.
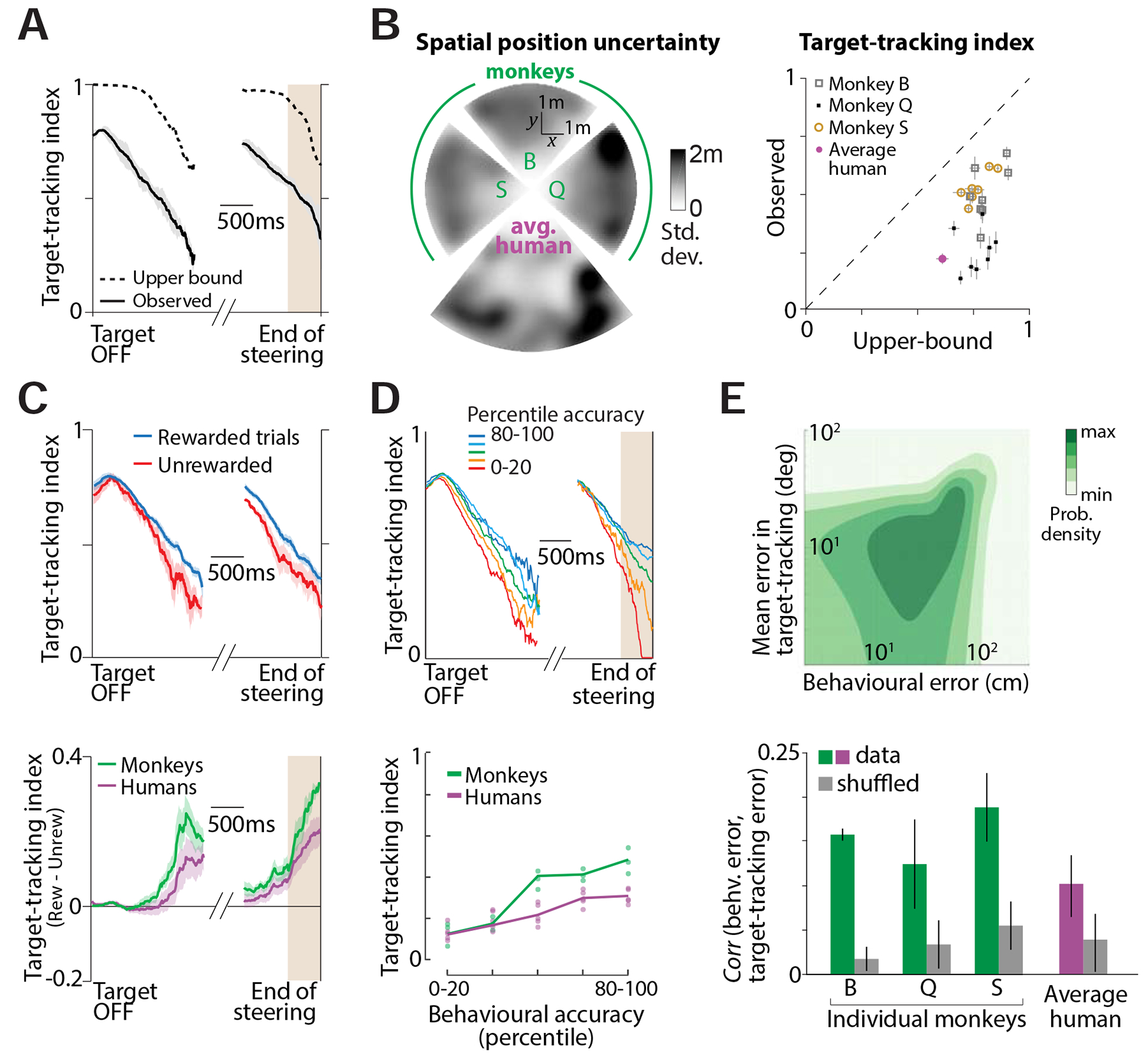
A. Time-course of the target-tracking index for one session computed using a monkey’s actual eye movements (black solid) and its theoretical upper-bound (black dashed) determined using variability in stopping positions (Methods, equations 3–4). B. Left. Overhead view of the spatial maps showing the standard deviation of stopping positions as a function of target location for individual monkeys and the average human subject. Each wedge corresponds to the map of one subject, calculated by binning target locations (see Fig. 1D) and smoothed using a Gaussian filter. The maps of monkey S & Q, and of the humans, have been rotated for compactness. Right: Comparison of the observed target-tracking index against the theoretical upper bound (averaged over the last 500ms of the trials) across all individual datasets. Dashed line has unity slope and error bars denote ±1 SEM obtained by bootstrapping. C. Top: Time-course of the target-tracking index for one example monkey shown separately for trials in which he stopped within the reward zone (blue), or stopped outside it (red). Shaded regions denote ± 1 standard error estimated by bootstrapping. Bottom: The difference between tracking coefficients the two sets of trials for all subjects. For human subjects, trials in which the subject’s final position was within 0.6m of the center of the target were considered ‘rewarded’. D. Top: We divided trials into five groups based on the magnitude of behavioural error. Time-courses of the target-tracking index for the five trial groups from one monkey (dark blue: most accurate; dark red: least accurate). Bottom: Average value of the target-tracking index just before the end of steering (brown region in the top panel) as a function of percentile accuracy for individual subjects. Solid lines show average across subjects. Across subjects (humans and monkeys), there was a significant correlation between accuracy and tracking coefficient (Pearson’s r = 0.68, p = 3.1 × 10−5). E. Top: Joint distribution of the behavioural error and the target-tracking error across trials of one session from one monkey. Bottom: The mean correlation between behavioural and target-tracking errors of individual subjects. Error bar denotes ±1 SEM obtained by bootstrapping. See also Figure S4D–E.
We also tested whether eye movements reflect fluctuations in the subject’s belief about their location across trials. Because subjects were more precise during rewarded (Fig S4E - left) than during unrewarded trials (Fig S4E - middle), we expect them to track the target more accurately during rewarded trials (Fig S4E - right). We computed the target-tracking index separately for the two groups of trials and found that it was indeed higher during rewarded trials (Fig 4C - top). The difference between the target-tracking indices during the two sets of trials grew as the trial progressed, and was significantly greater than zero at the end of the trial (Fig 4C - bottom; mean difference ± standard deviation during the period shaded in grey - monkeys: 0.19 ± 0.05, p = 4.8 × 10−3; humans: 0.13 ± 0.05, p = 3.1 × 10−2; bootstrap test, 10,000 bootstrap samples). In fact, when trials were stratified based on behavioural accuracy, we found that the tracking index increased with behavioural accuracy (Fig 4D). To more directly test for a fine-grained relationship between eye movements and task performance, we estimated the correlation between the behavioural error (distance between the stopping location and the target) and the target-tracking error (mean absolute difference between the actual eye position and the theoretical prediction, see Methods) across trials (Fig 4E - top). To control for possible spurious effects of trial difficulty, we computed a shuffled estimate by subdividing the trials into groups based on initial target distance and then shuffling the trials within each group (see Methods). We found that the behavioural and target-tracking errors were significantly correlated across trials (Fig 4E - bottom, Pearson’s r ± standard deviation across all datasets – true: 0.14 ± 0.04, controlled shuffle: 0.04 ± 0.02; p = 9.1 × 10–3, paired t-test) further reinforcing the view that subjects track their internally estimated goal location with their eyes.
Purely reflexive eye movements do not explain target-tracking behaviour
In principle, the above results could also be produced by purely reflexive eye movements, driven solely by optic flow (ocular following response or OFR). For instance, if subjects’ eye velocity is perfectly correlated with their perceived movement velocity, then oculomotor errors would be proportional to perceptual errors, explaining the relatively poor target-tracking in erroneous trials. However, past studies have shown that errors in reflexive eye movements are uncorrelated with perceptual errors (Blum and Price, 2014; Bostrom and Warzecha, 2010; Glasser and Tadin, 2014; Price and Blum, 2014) suggesting that the observed eye movements are not reflexive. Two further pieces of evidence in our own monkey data support this.
First, in a subset of sessions we recorded the stimulus movie of the complete block of trials and replayed them back to the animal at the end of the session, but with the joystick withheld (see Methods). All aspects of the task structure during this replay block were identical to the initial block of trials (e.g. the monkey still received juice reward at the end of the corresponding trials), except the animal only viewed a movie of the stimulus rather than actively performing the task. Importantly, monkeys were still free to move their eyes. Eye movements were weaker during passive viewing than during active task (Fig S5A, B) and the magnitude of eye velocity was much smaller during passive block even though both blocks had identical visual stimuli (Fig S5C). We analysed the target tracking behaviour by computing the target-tracking index separately for the two blocks of trials. Figure 5A (top panel) shows the time-course of the target-tracking index of one monkey during the both blocks of trials. In this monkey, the tracking index was much lower during passive viewing (red vs blue). Because OFR is, by definition, involuntary and difficult to suppress, this suggests that eye movements contributing to the high target-tracking index during active steering must be voluntary. Note however that the tracking index during passive viewing is poor right from trial onset, perhaps because the monkey did not consistently look at the target initially when it appeared on the screen. We wanted to know whether OFR dynamics, coupled with the appropriate boundary condition (looking at the target when it initially appears) might be sufficient to give the impression that the animal is tracking the target. We simulated this model by shifting the initial eye position on each trial of the passive block to match the corresponding trial in the active block, a procedure that left the eye movement dynamics unaltered (Fig 5A - black). The tracking index of this simulated model was substantially lower than that observed during the active block of trials, suggesting that the target-tracking behaviour is voluntary. In all monkeys, the target tracking during the active task was significantly stronger than during either the passive viewing condition or the OFR model (Fig 5A - bottom; mean difference ± standard deviation during the period shaded in brown, active: 0.27 ± 0.1, passive: 0.08 ± 0.1, OFR model: 0.07 ± 0.1; p < 0.01, bootstrap test). The difference between conditions was small in one monkey (labelled ‘Q’ in Fig 5A - bottom; Fig S5 - rightmost), possibly because this animal was mentally performing the task even during passive viewing.
Figure 5. Steering-induced eye movements are not reflexive.

A. Top: Time-course of target-tracking index for one monkey during trials in which he performed the task (blue) or passively viewed the stimulus identical to the one generated when performing the task (red). Black trace shows the tracking index of the OFR model. Tracking indices at time points with negative variance explained were clipped to zero. Bottom: The time-course of the cumulative difference between the target-tracking index on active trials and the OFR model for individual monkeys. B. Top: Time-course of the tracking index of one monkey during trials in which the ground plane density was either high (blue) or low (red). Bottom: The difference between target-tracking index under high and low density conditions for individual monkeys. Brown shaded regions in the bottom panels correspond to the time-window considered for statistical testing. See also Figure S5.
Second, OFR is known to be sensitive to signal strength (Barthelemy et al., 2009; Quaia et al., 2012). To test whether target tracking depends on signal strength, we manipulated stimulus reliability by randomly interleaving trials with two different densities of ground plane elements by more than an order of magnitude (see Methods). We analysed the two sets of trials separately, but found no significant difference between the target-tracking index (Fig 5B; mean ± standard deviation across subjects, low density: 0.28 ± 0.1, high density: 0.31 ± 0.1). Therefore, the pattern of eye movements observed during this task likely represent volitional movements, rather than reflexive ones.
Inhibiting eye movements worsens task performance
Since eye movements were predictive of subjects’ navigational performance, we wanted to know if they were essential for performing the task. To test this, we asked five human subjects to perform a variation of the task in which we overlaid a cross on top of the target location and instructed them to fixate on this cross for as long as it appeared on the screen. In half the trials (‘Eyes-moving’ condition), the fixation cross disappeared along with the target so that subjects were free to produce eye movements as before. In the remaining trials (‘Eyes-fixed’ condition), the cross remained at the same location on the screen throughout the trial and subjects had to perform the task without moving their eyes (see Methods). Although we did not penalize subjects for breaking fixation, we verified offline that they maintained fixation as instructed (Fig 6A and Fig S6). We assessed their behavioural performance by comparing the area under the ROC curve (AUC), and found that performance was significantly impaired in the ‘Eyes-fixed’ condition (Fig 6B; n = 5 humans, mean AUC ± standard deviation; Eyes-moving: 0.85 ± 0.07, Eyes-fixed: 0.77 ± 0.07, p = 2.5 × 10−3, paired t-test). Figure 6C shows the responses of individual subjects. Although subjects were nearly unbiased under both conditions, the correlation between target and response locations was significantly lower in the absence of eye movements (Fig 6D; mean ± standard deviation; C orr(r, r*), Eyes-moving: 0.71 ± 0.1, Eyes-fixed: 0.49 ± 0.2, p = 0.011, paired t-test; Corr(|θ |, |θ*|), Eyes-moving: 0.92±0.03, Eyes-fixed: 0.82±0.1, p = 0.035). These results suggest that subjects benefit when their eyes can track the internally estimated goal location in this task.
Figure 6. Fixation affects task performance.
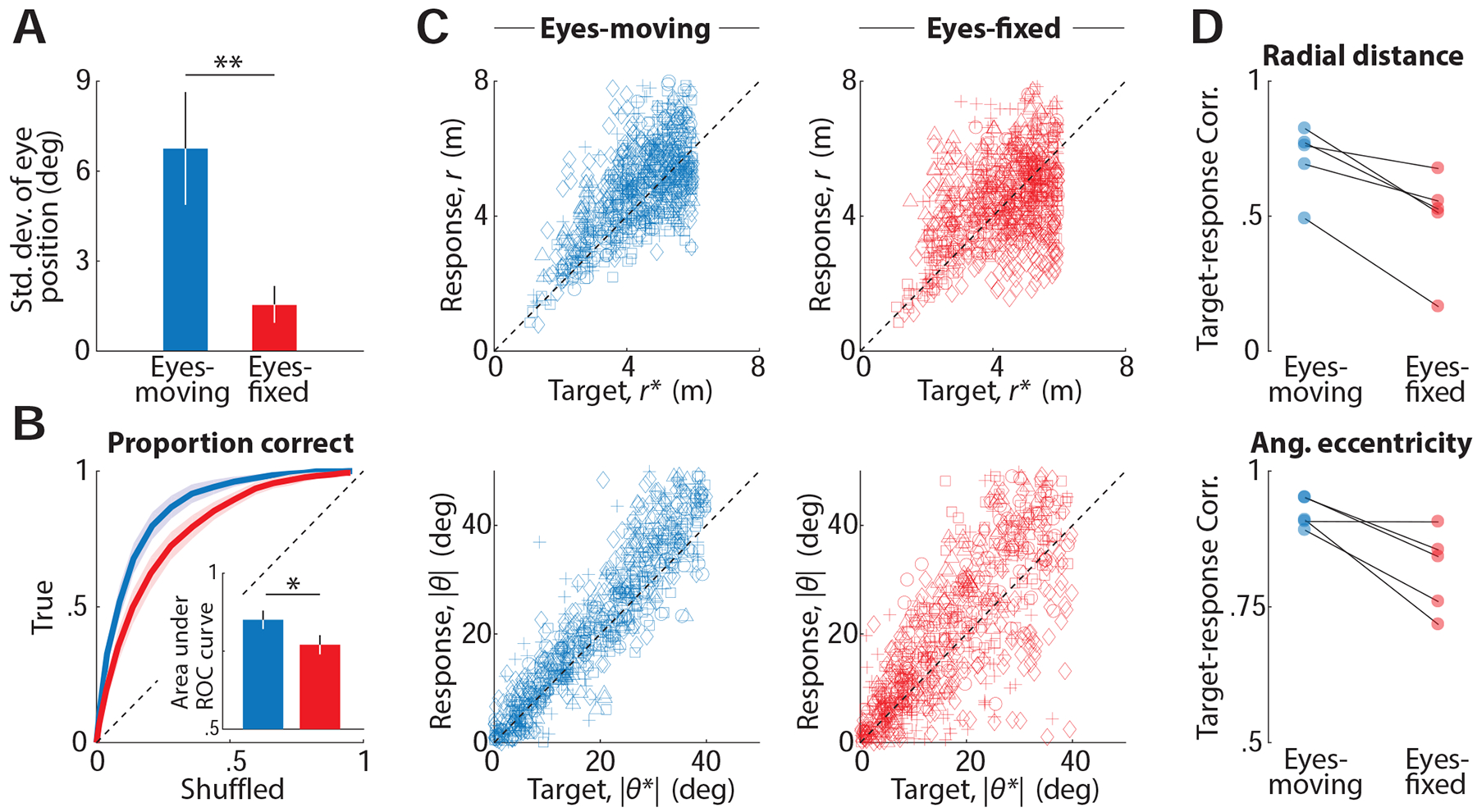
A. Trial- averaged temporal variability of subjects’ eye position, quantified by standard deviation (see Methods) during ‘Eyes-moving’ (blue) and ‘Eyes-fixed’ (red) trials. Error bars denote standard deviation across subjects (** p = 1.2 × 10−3, paired t-test). B. ROC curves averaged across subjects, for trials in the ‘Eyes-moving’ (blue) and the ‘Eyes-fixed’ condition (red). Inset shows the area under the two curves. Error bars denote standard deviation across subjects (* p = 2.5 × 10−3, paired t-test). C. Top: Comparison of the radial distances of the response and the target on trials under the two conditions. Different symbols denote different human subjects. Bottom: Comparison of the (absolute) angular eccentricity of the response and target. D. Top: Pearson’s correlation coefficient between the radial distance of subjects’ response and the target for all individual subjects. Bottom: Similar comparison for the absolute angular eccentricity of target and response under the two conditions. See also Figure S6.
DISCUSSION
Although the tracking index remained significantly above chance throughout the trial in these experiments, it nonetheless decreased over time. This is expected because the target disappears, so subjects cannot directly measure its true position but must instead rely on an internal estimate computed by integrating optic flow with knowledge of the controller dynamics. We have previously shown that human subjects perform near-perfect integration in this task (Lakshminarasimhan et al., 2018a). Nevertheless, due to noise in the integration process, the error in the internal estimate of target location on any given trial should grow over time. Consequently, even if those estimates are unbiased, their precision worsens, leading to a decrease in the target-tracking index (Fig 4A - dashed line). Consistent with this, the precision of error-correcting saccades gradually deteriorated as the trial progressed (Fig 3E). Therefore, the observed decrease in target tracking is an inevitable consequence of noisy observations and noisy integration, and in fact serves to expose the growing uncertainty in subjective beliefs.
Inferring belief dynamics
The task design used in this study was motivated by the need to ultimately understand neural computations governing belief dynamics that transform sensory inputs to motor output. In the real world, these belief dynamics correspond to subjective estimates of latent-variable dynamics, thus making them difficult to measure and relate to neural activity. Classic experimental paradigms used with primates attempt to measure this by training animals to provide discrete responses to simple stimuli, which reduces the dimensionality of state and action spaces, limiting their potential to shed light on natural computations. On the other hand, paradigms that let rodents loose in open arenas mimic natural behaviour at the expense of sacrificing control over the nature of computations they perform. One exception is a recent paper (Knöll et al., 2018) in which the authors used stimuli with rich spatiotemporal dynamics to elicit continuous oculomotor behaviour from primates with minimal reinforcement. While the Knoll et al. (2018) task is similar to ours in that it leverages unconstrained eye movements to remedy several shortcomings of historical approaches, the computation performed by the animals in their task was somewhat straightforward: inferring the current focus of expansion from noisy optic flow. In contrast, we asked animals to use optic flow to navigate to a goal location without providing explicit position cues. This requires the animal to first infer their self-motion from optic flow and then temporally integrate the resulting time-varying estimates to track the dynamics of a latent variable (position of the target relative to them) — a more challenging set of computations. Moreover, we trained our animals only by rewarding them for reaching the goal location. Eye movements were not explicitly reinforced, yet our post-hoc analysis revealed that the continuous-valued, time-varying eye position encoded subjective beliefs about the time-varying latent variable. We hope that this approach of covertly measuring belief dynamics will serve as a useful template for future studies.
The nature of eye movements
To understand the nature of slow eye movements made while steering towards the target, we analyzed individual components of eye position and found that both lateral version and elevation were largely smooth and consistent with the predicted dynamics for pursuing the invisible target. By analyzing eye movements during stimulus playback, we ruled out the possibility that the smooth dynamics correspond to pure ocular following reflex (OFR) induced by optic flow. Because these eye movements were always preceded by fixating a visible target and occurred in parallel with computations for mentally tracking that same target, they are functionally more similar to smooth-pursuit eye movements. Despite ample evidence for smooth-pursuit eye movements in the absence of foveal stimulation in humans (Becker and Fuchs, 1985; Missal and Heinen, 2017; Wyatt et al., 1994) and rhesus macaques (Ilg and Thier, 1999), smoothly tracking a purely imaginary object is thought to be difficult (Spering and Montagnini, 2011). This is because, in the absence of dynamic information about target motion, the pursuit velocity gradually decays to zero (Barnes, 2008; Missal and Heinen, 2017). However, when the underlying model for target motion is known, subjects can use their dynamic internal representation of the target to make predictive smooth pursuit during target blanking (Adams et al., 2012; Orban de Xivry et al., 2013, 2008). In our task, the dynamics of optic flow completely determine the (relative) motion of the target and can subsequently drive eye movements. Furthermore, the flow fields were self-generated rather than simulated, a condition that has previously been shown to improve pursuit of occluded targets (Danion et al., 2017; Gauthier et al., 1988; Vercher and Gauthier, 1992). Nevertheless, a moderate contribution of OFR induced by optic flow cannot be completely excluded, so it is possible that the eye movements reported here is composed of a mixture of reflexive signals that encode velocity of self-motion and predictive signals that encode the latent state.
Finally, saccadic eye movements, although infrequent, contributed to tracking the target. The amplitude of these saccade was largely influenced by target-tracking error during the previous ~200ms. These results suggest that the mechanism responsible for generating saccades in this paradigm are similar to the ones at play in flow-tracking (Knöll et al., 2018) and smooth pursuit of visible objects (Daye et al., 2014; Orban de Xivry et al., 2008). One reason for the relatively low frequency of saccades in this study could be that motion in our task was self-generated and predominantly smooth, whereas saccades in smooth-pursuit experiments are primarily due to unexpected jumps in target velocity (de Brouwer et al., 2002).
Computational role of tracking eye movements
The experimental task was specifically designed to ensure that subjects would attempt to mentally track the goal location by integrating momentary sensory evidence about movement provided by optic flow. In principle, this can be accomplished without physically tracking the believed goal location with one’s eyes. Yet we noticed a significant decline in task performance when eye movements were suppressed. This is consistent with previous results that demonstrated that real-world driving performance is impaired when eye movements are constrained (Wilson et al., 2008). Although this does not demonstrate a need to make tracking eye movements, it suggests that eye movements play an important role in neural computations for navigation. Indirect evidence of a role for slow eye movements in visually-guided navigation comes from a recent study of path integration, in which subjects used a joystick to reproduce previously-experienced self-motion (Churan et al., 2018). Eye movements during the reproduction phase were similar to those during initial exposure even when optic flow was removed. This suggested that eye movements constitute a form of mental imagery that, if suppressed, hampered memory retrieval (Johansson and Johansson, 2014; Johansson et al., 2012). Our findings extend this to naturalistic settings and argue that eye movements have a more dynamic role in path integration. The precise computational advantage of the specific eye movement dynamics observed in our task is unclear. Below, we propose two potential theories.
One possibility is that eye movements directed towards the intended goal location stabilizes the mental image of the goal, and could reduce the computational complexity of estimating self-motion from optic flow similar to the effect of foveal image stabilization (Lappe et al., 1999; Longuet-Higgins and Prazdny, 1980; Perrone and Stone, 1994; Sandini and Tistarelli, 1990). Normative mathematical theories posit that maintaining gaze at a point on the intended path can greatly simplify the problem of exploiting optic flow (Glennerster et al., 2001; Kim and Turvey, 1999; Wann and Swapp, 2000). Therefore, the eye movements reported here may constitute a closed-loop visuomotor process in which subjects integrate sense data (optic flow) to dynamically update their beliefs about the relative goal location, and in turn, use them to guide future eye movements in order to acquire new sense data in a computationally useful format. In this view, eye movements primarily aid optic flow processing.
Alternatively, the observed eye movements might simply be an embodiment of subjects’ dynamically evolving internal beliefs about the goal. Humans have a well-documented tendency for externalizing their internal representations (Barsalou, 2008; Spivey, 2007), with eye movements sometimes employed as a pointing device to visible as well as invisible objects, much like one’s index finger (Ballard et al., 1995, 1997; Spivey and Geng, 2001). By allowing dynamic beliefs about the relative target location to continuously modulate eye movements in this task, the brain could piggyback on the oculomotor circuit and reduce the computational burden on working memory. Consistent with this interpretation, there is overwhelming evidence for decision-related responses in primate oculomotor brain areas (de Lafuente et al., 2015; Shadlen and Newsome, 1996), and such responses are thought to drive eye movements (Joo et al., 2016). Therefore, in this view, primates use gaze as an affordance to efficiently update and store the output of integrating optic flow.
Although the above accounts are not mutually exclusive, simultaneously recording the neural activity from the primate sensory, oculomotor, and decision areas during this task might shed light on the dominant role of eye movements and how they link perception and action. A candidate brain area is the primate posterior parietal cortex where there is ample evidence for convergence of self-motion (Avila et al., 2019; Britten, 2008; Gu et al., 2012), gaze (Andersen, 1989; Andersen et al., 1987), and decision-related (Gold and Shadlen, 2007; Ibos and Freedman, 2017; Lakshminarasimhan et al., 2018b) signals. In any case, regardless of the mechanistic and computational explanations for these eye movements, the paradigm used here offers a useful approach to directly readout dynamical internal beliefs in real-time, simply by tracking subjects’ eyes.
STAR METHODS
Lead Contact and Materials Availability
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact, Kaushik Lakshminarasimhan (jkl9@nyu.edu).
Experimental Model and Subject Details
Three rhesus macaques (all male, 7–8 yrs. old) and ten human subjects (six males, all adults in the age group 18–32 yrs.) participated in the experiments. All but one subject were unaware of the purpose of the study. All surgeries and experimental procedures were approved by the Institutional Review Board at Baylor College of Medicine, and were in accordance with National Institutes of Health guidelines. All human subjects signed an approved consent form. In the following sections, the term subject is used to denote both monkey and human subjects, unless specified otherwise or implied by the context.
Method Details
Experimental setup
Monkeys were chronically implanted with a lightweight polyacetal ring for head restraint, and scleral coils for monitoring eye movements (CNC Engineering, Seattle WA, USA). At the beginning of each experimental session, monkeys were head-fixed and secured in a primate chair placed on top of a platform (Kollmorgen, Radford, VA, USA). A 3-chip DLP projector (Christie Digital Mirage 2000, Cypress, CA, USA) was mounted on top of the platform and rear-projected images onto a 60 × 60 cm tangent screen that was attached to the front of the field coil frame, ~30cm in front of the monkey. The projector was capable of rendering stereoscopic images generated by an OpenGL accelerator board (Nvidia Quadro FX 3000G).
Human subjects wore a custom-fit thermoplastic mask (CIVCO Medical Solutions) that was screwed to the back of the chair to restrain their head. The mask was mounted with a binocular eye tracker (ISCAN Inc.) to record the position of the subjects’ pupils at 60Hz. All other aspects of the setup were similar to the one used for monkeys, but with subjects seated 67.5cm in front of a 149 × 127 cm2 (width × height) rectangular screen. Although humans and monkeys were head-fixed, they were both free to move their eyes when performing the task, except under one experimental manipulation in humans (noted towards the end of the section below).
Behavioural Task
Subjects used an analog joystick (M20U9T-N82, CTI electronics) with two degrees of freedom and a circular displacement boundary to control their linear and angular speeds in a virtual environment. This virtual world comprised a ground plane whose textural elements had limited lifetime (~250m s) to avoid serving as landmarks. The ground plane was circular with a radius of 70m (near and far clipping planes at 5cm and 4000cm respectively), with the subject positioned at its center at the beginning of each trial. Each texture element was an isosceles triangle (base × height: 8.5 × 18.5 cm2) that was randomly repositioned and reoriented anywhere in the arena at the end of its lifetime, making it impossible to use as a landmark. The maximum linear and angular speeds were fixed to νmax = 2 ms−1 and (ωmax = 90°/s respectively, and the density of the ground plane was either held fixed at ρ = 2.5 elements/m2 or varied randomly between two values (ρ = 2.5 elements/m2 and ρ = 0.1 elements/m2) in a subset of recording sessions (see below). The stimulus was rendered as a red-green anaglyph and projected onto the screen in front of the subject’s eyes. Subjects wore goggles fitted with Kodak Wratten filters (red #29 and green #61) to view the stimulus. The binocular crosstalk for the green and red channels was 1.7% and 2.3% respectively.
Human subjects pressed a button on the joystick to initiate each trial, and the task was to steer to a random target location that was cued briefly at the beginning of the trial (Fig 1A). Monkeys performed the same task, but each trial was programmed to start after a variable random delay (0.5 – 1.1s) following the end of the previous trial. The target was a circular disc of radius 20cm whose luminance was matched to the texture elements. It appeared at a random location between θ = ±40° of visual angle at a distance of r = 0.7 — 4m (up to 6m for human subjects) relative the subject at the beginning of the trial. For human subjects, the target disappeared after one second, which was a cue for the subject to start steering, and the joystick controller was activated. In the case of monkeys, the target only appeared on the screen for 300ms, and the joystick was always active.
Monkeys typically performed two blocks of ~750 trials in each experimental session, and received feedback at the end of each trial. Monkeys performed a total of ~6,000 trials (4 sessions) each. Eye tracking was performed either using scleral coils (monkey Q & B) or a head-mounted eye tracker (monkey S). In one of the above recording sessions in each monkey, we saved the stimulus movie and replayed them to the animal at the end of the block. Both the visual stimulus and the schedule of rewards during this replay block were identical to the active navigation block, with the only difference being that the joystick was withheld and monkeys passively viewed the stimulus. Furthermore, a subset of the recording sessions (two sessions in each monkey) contained two randomly interleaved sets of trials that differed in terms of the density of optic flow (ρ = 0.1 elements/m2 and ρ = 2.5 elements/m2).
Of the ten human subjects, five subjects performed a total of 600 trials spread equally across three blocks. The blocks were identical in all respects, except no feedback was provided at the end of the trials in the first and third blocks. The purpose of using this block structure was to study how feedback affected learning in humans. Although data collected in the absence of feedback (first and last blocks) are briefly described in Fig. S1, the key results of the paper are based only on data collected during the intermittent block with feedback. Furthermore, during the block with feedback, the performance of human subjects typically stabilized within fifty trials (Fig. S1B). Because we wanted to ensure that the performance was stable during the course of testing, we ignored the first fifty trials of this block for all our analysis (Figs 1,2,4). The remaining five human subjects participated in a version of the experiment that was designed to study the effect of inhibiting eye movements on task performance (Fig 6). These subjects first performed a block of fifty trials with feedback to allow their performance to stabilize. Following this pre-training block, they performed a test block comprising 400 trials of a version of this task in which a fixation cross was overlaid on top of the target in each trial, again with feedback. In a random subset of trials (50%), this fixation cross remained on the screen even after the target disappeared and subjects were instructed to maintain fixation on the cross while steering to the target. The location of the cross remained fixed in screen coordinates and thus carried no dynamic information about stimulus location.
Feedback
Monkeys received binary feedback following a variable waiting period after stopping (range: 0.1–0.6s, mean waiting period: 0.25s). They received a drop of juice if their stopping position was within 0.6m away from the center of the target. No juice was provided otherwise. The fixed reward boundary of 0.6m was determined using a staircase procedure prior to the experiment to ensure that monkeys received reward in approximately two-thirds of the trials.
Human subjects received a somewhat richer, adaptive feedback in the form of a bullseye pattern that appeared on the ground at the end of steering upon pushing a button. The bullseye was centered on the target, with the innermost region having the highest luminance. The pattern comprised of five zones (Fig S1A), and the radii of the rings were continuously scaled (up or down by 5%) during the experiment using a 1-up 2-down staircase procedure. Additionally, an arrowhead pointing to the target also appeared on the ground in front of the subjects, colored green or red depending on whether the subject’s stopping position was inside or outside the reward boundary. The adaptive feedback procedure ensured that human subjects, like monkeys, stopped within the reward boundary in roughly two-thirds of the trials. Unlike monkeys, human subjects did not receive juice at the end of each successful trial, but instead received monetary compensation that was commensurate with their performance.
Stimulus and Data acquisition
All stimuli were generated and rendered using C++ Open Graphics Library (OpenGL) by continuously repositioning the camera based on joystick inputs to update the visual scene at 60 Hz. The camera was positioned at a height of 1m above the ground plane (10cm for monkeys). Spike2 software (Power 1401 MkII data acquisition system from Cambridge Electronic Design Ltd.) was used to record and store the target location (r*, θ*), subject’s position (r, θ), horizontal positions of left and right eyes (αl and αr), vertical eye positions (βl and βr) and all event markers for offline analysis at a sampling rate of .
Model predicted eye position
To test whether subjects’ eyes tracked the location of the (invisible) target, we generated predictions for subjects’ instantaneous eye positions by assuming that they maintained fixation at the center of the target. (xt, yt, zt) denotes the location of the target relative to the mid-point of the subject’s eyes at time t. The mean predicted lateral displacement (relative to fixating at the point (0, ∞, 0)) of the left and right eyes ( and ) are geometrically related to the target location and the inter-ocular distance (2Δ) as (Figs S2D):
| (1.1) |
Likewise, the vertical displacement of the two eyes ( and ) should be:
| (1.2) |
Note that zt is determined entirely by the camera height and hence time-invariant. In contrast, xt and yt change continuously as the subject steers to the target, and are both equal to zero in the special case when the subject’s location coincides with the center of the target. The predicted eye positions also have variances associated with them, which we derive in a later section (equation 4).
Quantification and Statistical Analysis
Customised MATLAB code was written to analyse data and to fit models. Depending on the quantity estimated, we report statistical dispersions either using 95% confidence interval, standard deviation, or standard error in the mean. The specific dispersion measure is identified in the portion of the text accompanying the estimates. For error bars in figures, we provide this information in the caption of the corresponding figure. We report exact p-values for all statistical tests, and describe the outcome as significant if p < 0.05.
Bias estimation
We regressed (with an intercept term) each subject’s response positions (r, θ) against target positions (r*, θ*) separately for the radial (r vs r*) and angular (θ vs θ*) co-ordinates, and the radial and angular multiplicative biases were quantified as the slope of the respective regressions (Fig 1F). The intercept terms of the regression models denote additive bias. For each subject, we estimated the 95% confidence intervals for the biases by bootstrapping.
Psychometric analysis
As described in the section on feedback, reward boundaries were chosen to ensure that all subjects correctly stopped within the reward zone in about two-thirds of the trials. However, the precise radius of these boundaries varied across human subjects, as well as between humans and monkeys. To objectively compare the performance of different subjects on a common scale, we performed ROC analysis as follows. For each subject, we first constructed a psychometric function by calculating the proportion of correct trials as a function of (hypothetical) reward boundary (Fig 1G). In keeping with the range of target distances used for the two species, we varied the reward boundary between 0–4m for monkeys and 0–6m for human subjects. Whereas an infinitesimally small boundary will result in all trials being classified as incorrect, a large enough reward boundary will yield near-perfect accuracy. To define a chance-level psychometric function, we repeated the above procedure but now by shuffling the target locations across trials, thereby destroying the relationship between target and response locations. Finally, we obtained the ROC curve by plotting the proportion of correct trials in the original dataset (true positives) against the shuffled dataset (false positives) for each value of hypothetical reward boundary. We used the area under this ROC curve to obtain an accuracy measure that was independent of the reward boundary used for various subject.
Characterizing eye position
For convenience, we express the subject’s actual eye position using the following three standard degrees of freedom: (i) Conjunctive horizontal movement of the two eyes or ‘lateral version’ quantified here as the mean lateral position of the two eyes, α = (αl + αr)/2, (ii) Conjunctive vertical movement of the two eyes or ‘elevation’ quantified here as β = (βl + βr)/2, (iii) Disjunctive horizontal eye movements or ‘vergence’ quantified here as γ = (αl – αr)/2. Disjunctive eye movements along the vertical direction (vertical vergence) were an order of magnitude smaller than the precision of our measurements, and therefore we ignored them in all our analyses. We also transformed the predicted eye positions given by Equation 1 into the above three degrees of freedom using analogous definitions to obtain , , and .
Saccade detection and pre-processing
We estimated the instantaneous speed of eye movements as where α and β denote lateral version and elevation respectively (as defined above), and a dot denotes a time derivative. Saccades were detected by identifying the time points at which the speed of eye movements crossed a threshold of 200°/s from below (a threshold of 50°/s yielded similar results). Although saccades were mostly confined to periods immediately following target onset and end of steering (Fig 2B), we removed a period of 100ms immediately following the onset of saccades for visualizing the time-course of eye movements during the trial (Fig 2E) and for all subsequent temporal analyses described below. We verified that this procedure had minimal effect on the results. In approximately 10% of the trials in monkeys and ~30% in human subjects, the subject travelled beyond the target. The predicted eye positions towards the end of these trials were outside the range that was physically possible. Therefore, we removed time points at which any of the four predicted components of eye movements in Equation 1 exceeded 60° before further analysis. Such time points constituted less than 3% of the dataset, and including them did not qualitatively alter the results.
Comparing predicted and observed eye positions
Let φt = (αt, βt) and denote the observed and mean predicted eye positions respectively at time 9. For each subject, we computed the square root of the fraction of variance in their eye movements explained by the predictions:
| (2) |
where ||·||2 denotes the L2 norm, 〈·〉 denotes expectation across trials, and denotes the mean observed eye position across trials at time t. Because the predictions are based on a model that assumes subjects’ eyes track the center of the target, we call ρ the ‘ target- tracking index’, or simply ‘ tracking-index’. A value of 1 corresponds to perfect prediction while zero implies that the predictions were no better than the mean observation. In principle, the deviation from the predictions can be larger than the intrinsic variability of the data. We clipped the target-tracking index to zero whenever this happened. Since trial durations were variable, we aligned all trials relative to the time at which the target was turned off (t = 0) to estimate the time course of tracking coefficient . corresponds to the similarity between observed and predicted eye position at the moment when the target was turned off (Fig 2F). We also computed the tracking coefficient by aligning trials with respect to the end of steering (t = T) to estimate . To visualize the time-course of the tracking coefficient, we plot both and with a break in the A-axis (Fig 2G, 4 & 5). To assess standard errors and statistical significance of differences between tracking coefficients from pairs of conditions (e.g. rewarded vs unrewarded trials), we used a bootstrap test with 10,000 bootstrap samples.
Correlation between saccade amplitude and target-tracking error
Because saccades were not very frequent while steering, we pooled data from all subjects (separately for monkeys and humans) for analyzing saccades. The amplitude of saccades was taken to be the average displacement of the position of the two eyes from saccade onset to 100ms later (; Fig 3B). We quantified the effect of saccadic eye movements on target-tracking error by computing the saccade-triggered average (STA) of the tracking error within a 400ms window centered around time ts of saccade onset (i.e., ). To quantify the precise relationship between saccade amplitude and tracking error, we simultaneously regressed horizontal and vertical amplitudes of the saccade (Δα and Δβ) on horizontal and vertical tracking errors ( and ), respectively, at various lags between ±0.5s with l2 regularization.
Estimation of position uncertainty
We estimated subjects’ position uncertainty by binning the 2D space into 10×10 cm2 bins. For each bin, we computed the variance in the subject’s stopping position across trials in which targets fell in that bin. The resulting spatial map of variability was then convolved with a two-dimensional isotropic Gaussian kernel of width 40cm (equal to the diameter of the target) to yield a smooth estimate of variability as a function of space (Fig 4B - left). Because subjects aimed to stop on the target, variability in their stopping position can be interpreted as the uncertainty in subjects’ posterior estimate about their own position.
Deriving an upper bound on the target-tracking index
Once the target disappears, subjects no longer get to directly observe it. To reach the target location, they update their beliefs about the relative location of the target by integrating their self-motion, which in turn must be estimated from the observed optic flow. Even if those beliefs are accurate on average, the uncertainty in believed target location will grow over time on any given trial due to noise both in the observations and in the integration process. Consequently, the degree to which subjects’ eyes can track the target (quantified by the tracking index, ρ) should decrease over time. Using the variability in subjects’ stopping positions to model their uncertainty in their believed location (see section above), we derived an approximate upper-bound on the temporal dynamics of the tracking-index ρt at time t assuming inter-ocular distance Δ ≈ 0:
| (3) |
where denotes the mean predicted eye position at time t. Note that this represents an upper-bound insofar as the variability in subject’s stopping positions stems entirely from uncertainty in their believed location. To derive this approximate bound, we first used the first-order Taylor series approximation of equation (1) to express the variance of the predicted eye position in terms of the variance of the relative target position (xt, yt, zt) as: , and , where and from equation (1), and we have used the fact that Var(zt) = 0 because there is no motion component perpendicular to the ground plane· Substituting the derivatives, we get:
| (4.1) |
| (4.2) |
The above equations are based on first-order Taylor series approximation and hold as long as the higher-order terms are relatively small. Although we cannot not directly measure Var(xt) and Var(yt), we could estimate them from the data (see previous section) and use it to determine the variability in predicted eye positions given by equation (4). Variability in the predictions then implies a lower bound in the mean squared error achievable by any observation . Substituting this in (2), we obtain an upper bound on the tracking-index given by equation (3). Note that, in deriving this approximate upper-bound, we ignored the noise in generating an eye movement to an intended location (process noise). So in principle, it is possible to derive a tighter bound by incorporating it. Note that as subjects approach the target, xt and yt approach zero, whereas the uncertainty grows so both Var(xt) and Var(yt) increase. Together, this leads to an increase in the variance of the predicted eye positions (equation 4) and consequently, a gradual decrease in the fraction of explainable variance over time (equation 3).
Comparing behavioural and target-tracking errors
To test whether poor target-tracking was associated with poor behavioural accuracy, we estimated the correlation between behavioural and target-tracking errors across trials of individual recording sessions. Behavioural error was given by the Euclidean distance between the target location and the subject’s response (stopping location) on individual trials, while the target-tracking error was given by the Euclidean distance between actual and predicted eye position, averaged over the entire time period of the trial, except for the last 300ms (as the predictions typically broke down when the subject was too close to the target). Because trial difficulty could affect both errors thereby inducing spurious correlations, we estimated the null distribution of correlations using a shuffling procedure where we grouped the trials from each recording session into ten quantiles based on target distance and shuffling only trials within the same group. The results were quite robust to the number of quantiles.
Assessing performance in the fixation task
To assess the behavioural effect of inhibiting eye movements, we compared human subjects’ performance across ‘eyes-moving’ and ‘eyes-fixed’ trials. Because we did not control for fixation breaks that happened in the ‘eyes-fixed’ condition during the experiment, we identified and removed such trials offline. Specifically, we removed the trials in which the temporal standard deviation (σ) of subject’s eye position during the trial (i.e. from the time when the target disappeared until the end of steering) exceeded 3° (roughly half-width of the fixation cross), from our analysis (~10% of the fixation trials across all subjects). The standard deviation was quantified as where σ(α) and σ(β) denote the temporal standard deviation of lateral version and elevation respectively. To evaluate the role of eye movements, we compared subjects’ performance in the fixation trials (‘eyes-fixed’) with trials that did not require fixation (‘eyes-moving’). For both sets of trials, we computed ROC curves for distinguishing ‘rewarded’ and ‘unrewarded’ trials (see section ‘psychometric analysis’ above) and used a paired t-test to test whether the mean area under the curves were different. We also computed the correlation between target and response locations and then used a paired t-test to test whether there was a significant difference between the correlation coefficients in the two sets of trials across subjects (Fig 6D).
Data and Software availability.
MATLAB code implementing all quantitative analyses in this study is available online (see Key Resources Table). Datasets generated by this study are available from the corresponding author upon reasonable request.
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Equipment, Software and Algorithms | ||
| M20U9T-N82 USB joystick | CTI Electronics | http://www.ctielectronics.com/ |
| Power 1401 MkII data acquisition system and Spike2 software | Cambridge Electronic Design Ltd. | http://ced.co.uk/ |
| Open Graphics Library (OpenGL) | Khronos Group | https://www.opengl.org/ |
| Custom-built analysis code | MATLAB | https://github.com/kaushik-l/monkey-dj |
Supplementary Material
Highlights.
Humans and monkeys virtually navigated to a memorized goal by integrating optic flow
Eye movements tracked the goal location as subjects steered towards it
Better goal tracking was associated with better navigation performance
Forcing humans to fixate impaired navigation precision
ACKNOWLEDGEMENTS
We thank Samir Saidi for assisting with the human experiments, Jing Lin and Jian Chen for their help in programming the stimulus. This work was supported by grants EY025538 and R01-DC014678. G.C.D. was supported by EY 016178.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors declare no competing interests.
References
- Adams RA, Perrinet LU, and Friston K (2012). Smooth Pursuit and Visual Occlusion: Active Inference and Oculomotor Control in Schizophrenia. PLoS One. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RA, Aponte E, Marshall L, and Friston KJ (2015). Active inference and oculomotor pursuit: The dynamic causal modelling of eye movements. J. Neurosci. Methods 242, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen RA (1989). Visual and eye movement functions of the posterior parietal cortex. Annu. Rev. Neurosci 12, 377–403. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Essick GK, and Siegel RM (1987). Neurons of area 7 activated by both visual stimuli and oculomotor behavior. Exp. Brain Res 67, 316–322. [DOI] [PubMed] [Google Scholar]
- Avila E, Lakshminarasimhan KJ, DeAngelis GC, and Angelaki DE (2019). Visual and Vestibular Selectivity for Self-Motion in Macaque Posterior Parietal Area 7a. Cereb. Cortex [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard DH, Hayhoe MM, and Pelz JB (1995). Memory Representations in Natural Tasks. J. Cogn. Neurosci [DOI] [PubMed] [Google Scholar]
- Ballard DH, Hayhoe MM, Pook PK, and Rao RPN (1997). Deictic codes for the embodiment of cognition. Behav. Brain Sci [DOI] [PubMed] [Google Scholar]
- Barnes GR (2008). Cognitive processes involved in smooth pursuit eye movements. Brain Cogn. [DOI] [PubMed] [Google Scholar]
- Barsalou LW (2008). Grounded Cognition. Annu. Rev. Psychol 59, 617–645. [DOI] [PubMed] [Google Scholar]
- Barthelemy FV, Fleuriet J, and Masson GS (2009). Temporal Dynamics of {2D} Motion Integration for Ocular Following in Macaque Monkeys. J. Neurophysiol [DOI] [PubMed] [Google Scholar]
- Becker W, and Fuchs AF (1985). Prediction in the oculomotor system: smooth pursuit during transient disappearance of a visual target. Exp. Brain Res [DOI] [PubMed] [Google Scholar]
- Blum J, and Price NSC (2014). Reflexive tracking eye movements and motion perception: one or two neural populations? J. Vis [DOI] [PubMed] [Google Scholar]
- Bonnen K, Burge J, Yates J, Pillow J, and Cormack LK (2015). Continuous psychophysics: Target-tracking to measure visual sensitivity. J. Vis 15, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boström KJ, and Warzecha AK (2010). Open-loop speed discrimination performance of ocular following response and perception. Vision Res. [DOI] [PubMed] [Google Scholar]
- Britten KH (2008). Mechanisms of Self-Motion Perception. Annu. Rev. Neurosci [DOI] [PubMed] [Google Scholar]
- Britten KH, Shadlen MN, Newsome WT, and Movshon JA (1992). The analysis of visual motion: a comparison of neuronal and psychophysical performance. J. Neurosci 12, 4745–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brouwer S, Missal M, Barnes G, and Lefevre P (2002). Quantitative Analysis of Catch-Up Saccades During Sustained Pursuit. J. Neurophysiol 87, 1772–1780. [DOI] [PubMed] [Google Scholar]
- Brunton BW, Botvinick MM, and Brody CD (2013). Rats and humans can optimally accumulate evidence for decision-making. Science 340, 95–98. [DOI] [PubMed] [Google Scholar]
- Churan J, von Hopffgarten, A., and Bremmer F (2018). Eye movements during path integration. Physiol. Rep 6, e13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danion F, Mathew J, and Flanagan JR (2017). Eye Tracking of Occluded Self-Moved Targets: Role of Haptic Feedback and Hand-Target Dynamics. Eneuro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daye PM, Blohm G, and Lefevre P (2014). Catch-up saccades in head-unrestrained conditions reveal that saccade amplitude is corrected using an internal model of target movement. J. Vis 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deravet N, Blohm G, de Xivry J-JO, and Lefëvre P (2018). Weighted integration of short-term memory and sensory signals in the oculomotor system. J. Vis 18, 16. [DOI] [PubMed] [Google Scholar]
- Erkelens CJ, Van der Steen J, Steinman RM, and Collewijn H (1989). Ocular vergence under natural conditions. I. Continuous changes of target distance along the median plane. Proc. R. Soc. B Biol. Sci [DOI] [PubMed] [Google Scholar]
- Gauthier GM, Vercher JL, Mussa Ivaldi F, and Marchetti E (1988). Oculo-manual tracking of visual targets: control learning, coordination control and coordination model. Exp. Brain Res [DOI] [PubMed] [Google Scholar]
- Glasser DM, and Tadin D (2014). Modularity in the motion system: independent oculomotor and perceptual processing of brief moving stimuli. J. Vis [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennerster A, Hansard ME, and Fitzgibbon AW (2001). Fixation could simplify, not complicate, the interpretation of retinal flow. Vision Res. [DOI] [PubMed] [Google Scholar]
- Gold JI, and Shadlen MN (2000). Representation of a perceptual decision in developing oculomotor commands. Nature. [DOI] [PubMed] [Google Scholar]
- Gold JI, and Shadlen MN (2007). The neural basis of decision making. Annu. Rev. Neurosci 30, 535–574. [DOI] [PubMed] [Google Scholar]
- Gu Y, DeAngelis GC, and Angelaki DE (2012). Causal Links between Dorsal Medial Superior Temporal Area Neurons and Multisensory Heading Perception. J. Neurosci 32, 2299–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DM, Girshick AR, Akeley K, and Banks MS (2008). Vergence-accommodation conflicts hinder visual performance and cause visual fatigue. J. Vis 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlsby NMT, Huszár F, Ghassemi MM, Orbán G, Wolpert DM, and Lengyel M (2013). Cognitive Tomography Reveals Complex, Task-Independent Mental Representations. Curr. Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard IP (2012). Vergence Eye movements. In In Perceiving in Depth, pp. 475–548. [Google Scholar]
- Huk A, Bonnen K, and He BJ (2018). Beyond trial-based paradigms: Continuous behavior, ongoing neural activity, and natural stimuli. J. Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibos G, and Freedman DJ (2017). Sequential sensory and decision processing in posterior parietal cortex. Elife. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilg UJ, and Thier P (1999). Eye movements of rhesus monkeys directed towards imaginary targets. Vision Res. [DOI] [PubMed] [Google Scholar]
- Johansson R, and Johansson M (2014). Look Here, Eye Movements Play a Functional Role in Memory Retrieval. Psychol. Sci [DOI] [PubMed] [Google Scholar]
- Johansson R, Holsanova J, Dewhurst R, and Holmqvist K (2012). Eye movements during scene recollection have a functional role, but they are not reinstatements of those produced during encoding. J. Exp. Psychol. Hum. Percept. Perform [DOI] [PubMed] [Google Scholar]
- Joo SJ, Katz LN, and Huk AC (2016). Decision-related perturbations of decision-irrelevant eye movements. Proc. Natl. Acad. Sci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NG, and Turvey MT (1999). Eye Movements and a Rule for Perceiving Direction of Heading. Ecol. Psychol [Google Scholar]
- Knill DC, and Pouget A (2004). The Bayesian brain: The role of uncertainty in neural coding and computation. Trends Neurosci. [DOI] [PubMed] [Google Scholar]
- Knöll J, Pillow JW, and Huk AC (2018). Lawful tracking of visual motion in humans, macaques, and marmosets in a naturalistic, continuous, and untrained behavioral context. Proc. Natl. Acad. Sci. U. S. A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Wu Z, Pitkow X, and Schrater P (2019). Belief dynamics extraction. ArXiv. [PMC free article] [PubMed] [Google Scholar]
- de Lafuente V, Jazayeri M, and Shadlen MN (2015). Representation of Accumulating Evidence for a Decision in Two Parietal Areas. J. Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminarasimhan KJ, Petsalis M, Park H, DeAngelis GC, Pitkow X, and Angelaki DE (2018a). A Dynamic Bayesian Observer Model Reveals Origins of Bias in Visual Path Integration. Neuron 99, 194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminarasimhan KJ, Pouget A, DeAngelis GC, Angelaki DE, and Pitkow X (2018b). Inferring decoding strategies for multiple correlated neural populations. PLoS Comput. Biol 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappe M, Bremmer F, and Van Den Berg AV (1999). Perception of self-motion from visual flow. Trends Cogn. Sci [DOI] [PubMed] [Google Scholar]
- Lee DD, Ortega PA, and Stocker AA (2014). Dynamic belief state representations. Curr. Opin. Neurobiol [DOI] [PubMed] [Google Scholar]
- Loetscher T, Bockisch CJ, Nicholls MER, and Brugger P (2010). Eye position predicts what number you have in mind. Curr. Biol [DOI] [PubMed] [Google Scholar]
- Longuet-Higgins HC, and Prazdny K (1980). The interpretation of a moving retinal image. Proc. R. Soc. London -Biol. Sci [DOI] [PubMed] [Google Scholar]
- Missal M, and Heinen SJ (2017). Stopping smooth pursuit. Philos. Trans. R. Soc. B Biol. Sci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban de Xivry J-J, Coppe S, Blohm G, and Lefevre P (2013). Kalman Filtering Naturally Accounts for Visually Guided and Predictive Smooth Pursuit Dynamics. J. Neurosci 33, 17301–17313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban de Xivry JJ, Missal M, and Lefevre P (2008). A dynamic representation of target motion drives predictive smooth pursuit during target blanking. J. Vis [DOI] [PubMed] [Google Scholar]
- Paninski L (2006). Nonparametric inference of prior probabilities from Bayes-optimal behavior. Adv. Neural Inf. Process. Syst [Google Scholar]
- Perrone JA, and Stone LS (1994). A model of self-motion estimation within primate extrastriate visual cortex. Vision Res. [DOI] [PubMed] [Google Scholar]
- Pinto L, Koay SA, Engelhard B, Yoon AM, Deverett B, Thiberge SY, Witten IB, Tank DW, and Brody CD (2018). An accumulation-of-evidence task using visual pulses for mice navigating in virtual reality. Front. Behav. Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkow X, and Angelaki DE (2017). Inference in the Brain: Statistics Flowing in Redundant Population Codes. Neuron 94, 943–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price NSC, and Blum J (2014). Motion perception correlates with volitional but not reflexive eye movements. Neuroscience. [DOI] [PubMed] [Google Scholar]
- Quaia C, Sheliga BM, FitzGibbon EJ, and Optican LM (2012). Ocular following in humans: Spatial properties. J. Vis [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner K (1998). Eye Movements in Reading and Information Processing: 20 Years of Research. Psychol. Bull [DOI] [PubMed] [Google Scholar]
- Reddy S, Dragan AD, and Levine S (2018). Where Do You Think You’re Going?: Inferring Beliefs about Dynamics from Behavior. In NeurIPS, p. [Google Scholar]
- Sandini G, and Tistarelli M (1990). Active Tracking Strategy for Monocular Depth Inference Over Multiple Frames. IEEE Trans. Pattern Anal. Mach. Intell [Google Scholar]
- Shadlen MN, and Newsome WT (1996). Motion perception: seeing and deciding. Proc. Natl. Acad. Sci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T, Kim J, Hoffman DM, and Banks MS (2011). The zone of comfort: Predicting visual discomfort with stereo displays. J. Vis 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Gosselin F, and Schyns PG (2012). Measuring internal representations from behavioral and brain data. Curr. Biol [DOI] [PubMed] [Google Scholar]
- Spering M, and Montagnini A (2011). Do we track what we see? Common versus independent processing for motion perception and smooth pursuit eye movements: A review. Vision Res. [DOI] [PubMed] [Google Scholar]
- Spivey M (2007). The Continuity of Mind (Oxford University Press; ). [Google Scholar]
- Spivey MJ, and Geng JJ (2001). Oculomotor mechanisms activated by imagery and memory: Eye movements to absent objects. Psychol. Res [DOI] [PubMed] [Google Scholar]
- Stocker AA, and Simoncelli EP (2006). Noise characteristics and prior expectations in human visual speed perception. Nat. Neurosci [DOI] [PubMed] [Google Scholar]
- Tanenhaus MK, Spivey-Knowlton MJ, Eberhard KM, and Sedivy JC (1995). Integration of visual and linguistic information in spoken language comprehension. Science (80-.) [DOI] [PubMed] [Google Scholar]
- Turnham EJ, Braun DA, and Wolpert DM (2011). Inferring visuomotor priors for sensorimotor learning. PLoS Comput. Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercher JL, and Gauthier GM (1992). Oculo-manual coordination control: Ocular and manual tracking of visual targets with delayed visual feedback of the hand motion. Exp. Brain Res [DOI] [PubMed] [Google Scholar]
- Wann JP, and Swapp DK (2000). Why you should look where you are going. Nat. Neurosci [DOI] [PubMed] [Google Scholar]
- Wilson M, Chattington M, and Marple-Horvat DE (2008). Eye movements drive steering: Reduced eye movement distribution impairs steering and driving performance. J. Mot. Behav [DOI] [PubMed] [Google Scholar]
- Wu Z, Schrater P, and Pitkow X (2018). Inverse POMDP : Inferring Internal Model and Latent Beliefs. In Conference on Cognitive Computational Neuroscience, p. [Google Scholar]
- Wu Z, Kwon M, Daptardar S, Schrater P, and Pitkow X (2019). Rational Thoughts in Neural Codes. BioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt HJ, Pola J, Fortune B, and Posner M (1994). Smooth pursuit eye movements with imaginary targets defined by extrafoveal cues. Vision Res. [DOI] [PubMed] [Google Scholar]
- Zhang M, Feng J, Lim JH, Zhao Q, and Kreiman G (2018). What am I searching for? ArXiv 1807.11926. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


