Abstract
Complications related to treatment of long bone fractures still stand as a major challenge for orthopaedic surgeons. Elucidation of the mechanisms of bone healing and development, and the subsequent alteration of these mechanisms to improve outcomes, typically requires animal models as an intermediary between in vitro and human clinical studies. Murine models are some of the most commonly used in translational research, and mouse fracture models are particularly diverse, offering a wide variety of customization with distinct benefits and limitations depending on the study. This review critically examines three common femur fracture models in the mouse, namely cortical hole, 3-point fracture (Einhorn), and segmental bone defect. We lay out the general procedure for execution of each model, evaluate the practical implications and important advantages/disadvantages of each and describe recent innovations. Furthermore, we explore the applications that each model is best adapted for in the context of the current state of murine orthopaedic research.
Keywords: Bone healing, Fracture, Animal models, Mouse, Defect, Segmental defect
INTRODUCTION
Fractures are the most common injury type in older patients, with women aged 60 years having a residual lifetime fracture risk of 56%1. The proportion of US citizens greater than 65 years old is also set to increase exponentially, with projections showing them as 16.9% of the population by 2030 and 25.8% of the population by 20602. Fracture malunion, a broken bone that does not heal properly, and nonunion, a broken bone that does not heal, are of great concern in orthopaedics. Nonunions account for roughly 4.9% of all fractures, and femur fracture nonunion rates range from 0–12% across multiple studies depending on fixation type3. The effect of nonunion on patients can be devastating4, 5. Lifelong disability is common, and estimations of the average hospital cost of nonunion exceed $11,000 in the U.S.6. As the demographic shifts to an older, more fracture prone population, the burden of malunion and nonunion on the healthcare system will increase substantially. Due to the increasing demand for effective bone healing therapies, future innovation is required. Translational research using animal models is foundational in bridging the gap from benchtop to human subjects.
Murine models have been extensively used to analyze the biology of fracture healing and bone development. Several factors contribute to the utility of mouse models, particularly their low cost, availability of genetically modified strains, rapid fracture healing time course, and the development of intricate fixation systems capable of repairing such small bones. Several different bones in the mouse have been used to investigate fracture healing, namely the femur, tibia, radius, ulna, mandible, and calvarium7, 8. Of these, only the femur and tibia are available for accurate biomechanical testing7. However, the tibia has inherent limitations; for example, the intramedullary canal of the mouse tibia is curved and declines in caliber distally7. This significantly complicates the design of fixation devices, leads to high variability in callus formation with differing fracture placement, and diminishes the reproducibility of rotational mechanical testing7. Soft tissue coverage is also sparse in the lower leg, reducing the ability to analyze the role of soft tissue in the bone healing process8. The presence of the fibula complicates these models and the reproducibility of single bone breaks. In contrast, the femur is straight, larger in diameter, and has ample soft tissue coverage, considerably reducing the complexity and increasing the reproducibility of the model7.
An ideal fracture model would be inexpensive, reproducible, not encumbered with technical difficulties, and have high throughput. Although the fracture models presented in this paper do not fit all of these criteria, they best represent each category to a varying degree. It is known that bone healing may occur through different pathways, most prominently through endochondral and intramembranous ossification. Different factors, including distance between bony ends, viability of periosteum, and rigidity of fixation help determine which mechanism predominates9, 10. The ability to modulate these factors to study each pathway is also a desirable characteristic of a fracture model. Measures of fracture healing and the effects of different treatments are most commonly assessed by mechanical testing, X-ray, micro computed tomography (μCT), histology, and, more recently, magnetic resonance imaging. The ability to accomplish these assessments, and the difficulty in performing these assessments, differs between models and fixation types. Recent innovations in materials and techniques have contributed greatly to the diversity by which fracture models are able to accommodate these measures. Although this diversity is good for basic science research, all fracture models using osteosynthesis modulate bone healing to some degree. Consequently, it is important for the external validity of a study that a fracture model use a fixation type similar to those used clinically. Therefore, in this review, we outline the general procedure for the cortical-hole, 3-point fracture (Einhorn), and segmental defect models, discuss the practical implications of each model, and compare them based upon the factors set out above (Figure 1).
Fig. 1.
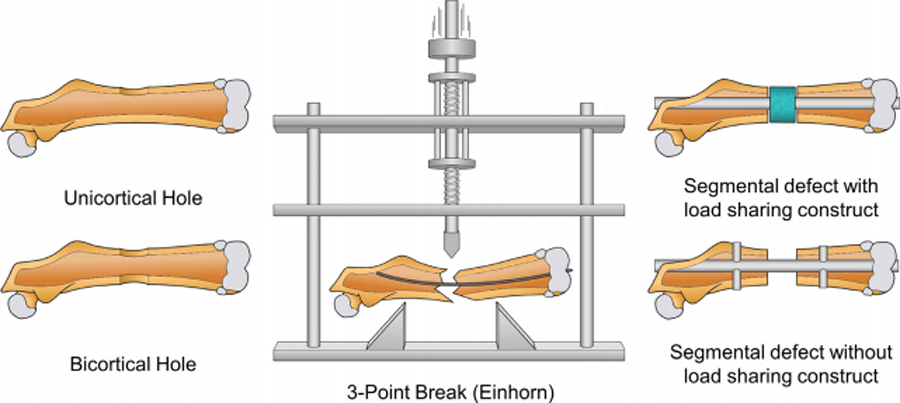
Illustration of bone healing models in the murine femoral diaphysis: unicortical hole, bicortical hole. 3-point break, segmental defect with load sharing construct, and segmental defect without load sharing construct.
CORTICAL HOLE MODEL
The cortical hole (i.e., corticotomy) model provides an adequate way to systematically deliver testing agents directly to the site of the defect as well as investigate bone healing mechanisms. In general, the procedure creates small boreholes in the mid-diaphysis of the femur. The location and size limit the defect to the cortical area.
Procedure
A longitudinal incision is made on the lateral aspect of the thigh with further dissection of the fascial plane to expose the femur where the defect will be implemented. A borehole typically between 0.8–1mm in diameter is made through the mid-shaft, penetrating down to the level of bone marrow using a Dremel tool, dental drill, or another comparable device. The defect is made under constant saline irrigation in order to cleanse the exposed area from debris and prevent heat transfer to the bone. The bi-cortical variant follows the same general procedure as the unilateral with an additional borehole continued through the opposite side of the femur (Figure 1)11, 12.
Benefits and Limitations
The cortical hole fracture model has a few notable benefits. The procedure is relatively simple, quick, and easily reproducible. The cortical hole model does not require a fixation device, and does not carry the same risk for post-operative fractures as the other models11, 12. In contrast to the 3-point fracture model, the cortical hole requires open access to the femur, which makes it optimal for administration of pharmaceutical and tissue engineering therapies. It has also seen use in the study of metabolic activity in diseased states on fracture healing13. Due to the natural rigidity of the bone and the small size of the defect itself, intermembranous ossification is the primary fracture healing mechanism. Thus, the model neglects endochondral ossification12, 14. Some drawbacks do exist. Outside of orthopaedic oncology and bone infections, the borehole is not a common clinical presentation. In the process of creating the borehole, there is risk of soft tissue damage as it must be retracted to visualize the bone injury site at the midline as well as prevent entanglement with the drill shaft.
Applications
The cortical hole model has numerous applications including, genetic, pharmaceutical, and bone healing studies. A model studying intramembranous ossification was created using the cortical hole12. This model allows for robust ability to study the genomics and cellular/molecular mechanisms of cortical bone healing12. Knockout studies have successfully utilized the cortical hole model to look at various aspects of metabolic states and healing mechanisms15, 16. Importantly, many Clinical Research Organizations (CROs) choose to utilize to cortical hole model to complete pre-clinical safety studies for testing bone healing agents, likely owing to the simplicity of this model (personal communication MAK).
3-POINT FRACTURE (EINHORN)
The fracture model first developed by Bonnarens and Einhorn is a commonly used model17. Modifications to the original procedure for murine models have proved to be effective at creating closed, diaphyseal, transverse femoral fractures.17 The closed model is clinically relevant as it mimics simple fractures commonly seen from trauma. Below, procedural information, benefits and limitations to the 3-point model, improvements and modifications to the procedure, and different fixation techniques will be outlined and discussed.
Procedure
The 3-point fracture/Einhorn fracture model is a procedure that has been adapted for mice from a rat protocol18. This closed fracture model typically requires intramedullary nail (IM nail) fixation to maintain consistency in the break. To achieve this, a small incision is made over the knee of the hind limb and a hypodermic needle is driven through the intercondylar fossa and passed into the medullary cavity of the femur19. Since lateral force will be applied to the femur to create the defect, the needle is then removed and replaced with a small gauge guide wire. This allows for easy replacement of the needle after the break and prevents deflection of the needle. A custom Einhorn 3-point bending device is then used to apply brief lateral force the mid diaphysis of the femur in a guillotine like fashion to create the break (Figure 1). The hypodermic needle is then loaded onto the guide wire and inserted into the medullary cavity to align and fixate the fracture. The guide wire is removed, the needle is clipped close to the knee joint, and the knee wound is closed with suture or wound clips.
Procedural Modifications
The model developed by Einhorn and Bonnarens was originally a rat model. This was repurposed for murine use with some modifications. The thin IM nails used in mice are typically angulated to an unacceptable level for most investigators when the guillotine is applied. Guidewires remedy this problem by retaining patency of the medullary canal for insertion of the nail after use of the guillotine18. The guidewires are commonly medical-grade stainless steel, tungsten, or aluminum20. Tungsten and stainless steel are stiffer than aluminum and thus better suited for this use. The procedure is generally minimally invasive, requiring an anterior, longitudinal incision along the knee joint, and subluxation of the patella to expose the femoral condyles. The surgeon may make a second incision on the lateral surface of the thigh and bluntly dissect down to the femur. The femur can then be grasped with forceps to make pin placement less difficult21. While this does inherently cause more soft tissue damage, it reduces the risk of pin misplacement which could result in termination of the mouse. Another option is to perform the same procedure and use a surgical blade to make a notch on the medial surface of the femur in order to predispose a fracture in the diaphysis22. This modification poses an increased risk to soft tissue damage but potentially may lower chance of displacement and comminution at the moment of fracture.
Depending on the fixation device, insertion into the canal can be accomplished by either a dedicated drill or twisting the implant by hand through the bone of the intercondylar fossa. The implant can then be advanced until the proximal end of the medullary canal is felt. Drilling is not absolutely necessary with pin insertion as the size of the mouse femur allows twisting to be a reliable method for penetration21, 23.
The guillotine device itself is an important tool in this protocol. The two variables that manipulate the force applied to the extremity is the initial height of the load to be dropped and the weight of the load. Factors such as sex, weight, and strain should be accounted for when operating, as force needed to generate the fracture will be different24. The load can be modified to cause less severe trauma, such as bone contusions, allowing for different objectives to be studied25. Furthermore, improvement of the guillotine itself mitigates problems associated with reproducibility. The development of a springless electromagnetic assembly and mouse positioning system produced an approximately 85% favorable transverse fracture rate26. Another potential option is the use of a modified 3-point break adapted from a tibial procedure for better control of the loading weight27.
Model subtypes
Proper fixation of a closed femoral fracture is paramount for the success of union and creating a reproducible method to study fracture healing. Stiffness and rigidity of the fracture fixation directly correlates to the amount of motion within the fracture site and developing callus. This amount of motion correlates to the type and degree of healing20. Fracture motion outside of a range impairs callus formation and fracture healing28. For this reason, optimization of fixation is critical to consider, and IM nails/pins, locking nails, micro/compression screws are all viable options. Pins are the most diverse fixation device for the 3-point fracture as they vary in size. While affordable, easily implemented, and reproducible, simple pins lack rotational and axial stiffness compromising bone healing pathways. Variations in pin size are possible with commonly used ranging from 27- to 22-gauge18, 21, 29. Gauges may be used in series from smallest to largest to gradually introduce a larger hole through the medullary canal22. The locking nail is essentially a pin/nail placed within the medullary canal with the distal and proximal ends of the pin “locking in” to the bone. As an example, a modified 24-gauge needle is placed with a 0.1 mm tungsten guidewire running through the center. The distal and proximal ends are flattened. Torsional testing indicated that femoral rotational stability was greater when using the flattened locking nail rather than simple pin implementation30. The flattened locking nail is a reliable option that is cost effective and relatively easy to modify from simple pin implementation with increased rotational stability, but it still lacks axial stiffness31. Compression screws made of medical grade stainless steel are commercially available from RISystem and are 17.2 mm in length with a diameter of 0.5mm. A drill is used to create a hole in the intercondylar fossa of the distal femur, and a tungsten guidewire is inserted through the hole. The micro-screw is then threaded along the guide wire and screwed into the proximal portion of the femur using a drill32. The micro-screw provides longitudinal compression along the femur. Biomechanical testing reveled the screw improved rotational and axial stiffness thereby optimizing fixational performance7. Additionally, the screw has been shown to decrease the amount of fibrous tissue in the healing process and increase cartilage generation33. All three mechanisms provide some level of fixation, but none are as rigid as an unaffected femur33. Compression/micro-screws provide the most global rigidity, with axial and rotational stiffness being greater than the locking nail or simple pin. However, the micro-screw procedure can present with particular challenges, including patellar ligament rupture, condylar rupture, and availability in a single size only33. The flattened locking nail has better rotational stability than a simple pin but lacks the axial stiffness of the compression/micro-screw30. The simple pin with a guidewire is the most routine option and can be used with knowledge that it has little stiffness axially or rotationally30. In any case, fixation must be monitored closely, and radiography of the fractured femur must be used after implementation of any device in order to ensure proper placement. Improper technique has the potential to damage the interior of the medullary canal with any fixation instrument.
Benefits and Limitations
The 3-point fracture model is relatively cheap, requiring a guillotine and IM fixation (micro screw, locking nail, or pin) all of which are comparatively more cost effective than plates or external fixators29. Furthermore, the procedure is reproducible and requires less surgical technical ability than creating an open defect17. The relative simplicity of the procedure shortens the time taken to perform it (around 15 minutes per mouse) 21, 29. The model causes minimal soft tissue damage compared to open fracture models and is optimal for studying endochondral ossification pathways in the healing process33.
Compared to the corticotomy and segmental bone defect, this model is limited in its capacity to study the effects of locally applied therapeutics and pharmaceuticals34. Since the model does not expose the bone injury site, treatments are limited to a percutaneous or systemic delivery. Distribution of intravenous drugs to bone is limited in most cases and could result in unknown systemic toxicity. Direct injection into the developing callus has been proposed, but this is difficult and may require subjective manipulation and potential damage to the callus34.
The 3-point bending fracture mechanism with the guillotine also presents some issues with fracture creation. The procedure is less reproducible than open models as the femur is not exposed and the surgeon must use bony landmarks to place the guillotine over the diaphysis. The fracture can become displaced, poorly positioned, and has a higher probability of sustaining comminution26. In one study 36/92 of mice were removed from the study after undergoing the procedure due to the aforementioned issues. In contrast, in O’Connor’s developmental study only 8/246 had the same fate, possibly due to the addition of a guidewire into the fixation procedure, which makes post fracture needle placement easier18, 23.
Applications
Several studies mimicking trauma have incorporated the 3-point fracture into novel models. In a novel study concurrent bilateral lung contusions and femoral fracture via the 3-point technique sought to mimic a high impact accident35. Another study examined the effect of trauma-induced hemorrhagic shock on bone healing. The study identified inhibited fracture healing after the subject was placed under hemorrhagic shock conditions36. Additionally, the 3-point fracture model has been an effective model in some pain studies, such as those looking at use of anti-nerve growth factor to reduce pain after orthopaedic procedures23. Recent studies by Drissi et al. have used the 3-point fracture model to investigate the effect of RunX1 on endochondral ossification. In this study, Runx1 was ablated from mesenchymal stem cells of mice, and these ablated mice showed delayed cartilaginous callus formation37.
SEGMENTAL BONE DEFECT
The segmental bone defect (SBD) is one of the oldest models for studying bone healing and development. The simplicity and customizability of the model has attracted investigators to its use for decades, and thus a host of different defect sizes and fixation types have been developed. Recent calls for standardization to allow for the comparison of different studies have led to the commercialization of fixation equipment. Although there is still much work to be done in the way of standardization, the following outlines the general procedure, defect sizes, fixation types, and current applications, along the with advantages and disadvantages to different methods to help guide the investigator in choosing the best practice for their experiment.
Procedure
There are a multitude of methods to create SBDs in the mouse femur. Most commonly, the skin on the lateral hind limb is incised and the femur is exposed via dissection through the fascial plane between the anterior and posterior muscle compartments. The femur is then bluntly stripped of its muscular attachments, and fixation is applied. This may be a small gauge hypodermic needle passed through the intercondylar fossa and into the medullary canal identical to the procedure used in the 3-point fracture (IM nail)19 or a plate fixation to the outside of the femur38. The needle or plate is then removed, and the defect is created with a dental drill, Dremel tool, or gigli saw. In the case of intramedullary implant without axial control, a structural support is typically required to maintain the space within the defect. Most commonly, a scaffold of osteoconductive material is placed into the defect site, but other investigators have also used clip implants that traverse the defect site and stabilize the space39. Both IM and plate fixation methods can accommodate a collagen sponge, which can be wrapped around or inserted into the defect site and imbued with experimental treatment, such as BMP-219. Muscle fascia and skin incision are then closed with sutures and wound clips. We refer the interested reader to more thorough descriptions of each surgical technique: IM nail with scaffold19, plate fixation38, pin-clip39, and external fixator40.
Model Subtypes
There are various subtypes within the murine segmental femoral defect. These are primarily stratified by the fixation type and defect size but can also be stratified by the presence of scaffold and concurrent use of disease models. Defect sizes used in the mouse femur typically range from 0 mm to 5 mm41. The bone healing mechanisms that are to be studied and the degree of stress placed on the fixation and biology can be fine-tuned using different defect sizes. Small rigidly fixed defects, like the single cut osteotomy with abutting bone ends (0 mm defect), heal by direct bone healing, while larger, less rigid defects heal by indirect bone healing pathways42. Smaller defects also heal faster and more often than larger defects. A tipping point is achieved when the defect becomes large enough that it is unable to heal over the lifetime of the mouse. This defect is termed a critical sized defect. The search for the length of the defect in mice femora required to create a critical size defect (CSD) is ongoing. Many research groups have come to the conclusion that 4 mm constitutes a CSD in a mouse femur fixated with an IM nail and bridged by allograft43–48. Notable exceptions to this size include work by Menger and colleagues where 1.8 mm diaphyseal femoral defects with pin-clip fixation and 2 mm of periosteal stripping produced a 100% non-union rate39. Similar experiments by our group demonstrated non-union at 9 weeks post operation in single cut osteotomies. These included 2 mm of periosteal stripping proximally and distally to the defect and were stabilized with IM nail fixation49. Petite et al. have also more recently established a 3.5 mm CSD fixated with a 4-hole locking plate50. Livne and colleagues describe a 2 mm CSD braced with an external fixator in an immunodeficient mouse model51. The variability in length of the critical sized femoral defect in mice between experiments demonstrates the salient characteristics of fracture healing, including periosteal and vascular integrity, type and rigidity of fixation, and disease state. These must all be considered when choosing to do an experiment with a CSD model as they can significantly affect the results. The CSD is a model most easily and consistently achieved in the mouse femur with the segmental defect. Although high energy trauma that leads to nonunion clinically rarely occurs with clean, symmetrical bone removal, these nonunions are often plagued by large defects, soft tissue and periosteal stripping, and devascularization. The CSD models created by SBD are particularly well suited for imitating these common clinical scenarios as they allow for control of these clinically relevant variables. These clinical realities also frequently require intervention in the form of bone autograft to promote union, and the SBD uniquely allows for the implantation of bone grafts or synthetic scaffolds into the defect site to test interventions for SBD healing.
Four main methods of fixation exist for segmental femoral defects in mice. These are the IM nail, plate, pin-clip, and external fixation. These fixation types vary in difficulty and cost, and they have their own specific advantages and disadvantages. A brief summary of these factors, model subtypes that have been developed, and example references for each fixation type can be found in Table 1.
Table 1.
Summary of fixation types and factors affecting the choice of each for the segmental bone defect model.
| Fixation Type | IM Nail | Plate | Pin-Clip | Ex-Fix |
|---|---|---|---|---|
| Subtypes | Flattened Locking Nail [31] Pseudo-locking Nail [52] Mouse Nail [53],[54] |
Locking Plate [50] PEEK Plate [56] Flexible Plate [59] |
N/A | MRI Compatible [40],[68] Flexible [67] |
| Difficulty Cost | Easy – Moderate $–$$ |
Hard $$–$$$ |
Moderate $ |
Hard $$–$$$ |
| Advantages | Simple and easy Scaffold securely placed Mimics clinical application |
Rigid fixation Accurate osteotomy with jig Different stiffnesses available Available in radiolucent PEEK Mimics clinical application |
Greater rigidity than nonlocking nails Does not need a scaffold to maintain defect size |
Most rigid fixation Can perform distraction osteogenesis Different stiffnesses available MRI compatible versions |
| Disadvantages | Nonlocking nails have low rigidity and require scaffold to maintain defect size Inherent damage to medullary canal |
Long procedure Removal of plate for histology or μCT is riskier Scaffold not as securely placed |
More complicated procedure than IM nail | Infections in pin tracts Weight of the fixator affects locomotion |
| Example References | [31],[ 52–54] | [38],[50],[55–62] | [39],[63],[64] | [40],[51],[65–71] |
IM Nail
IM nail fixation of SBDs in the femur of mice is the simplest and most cost-effective form for stabilizing these defects (Figure 2). The implant used can vary, but typically a hypodermic needle between 27- and 22-gauge is used. Increasing needle diameter increases the rigidity of the osteosynthesis but increases medullary canal and articular surface damage, so the investigator must weigh these two factors when considering implant size. Although some investigators use the more complicated anterograde technique (beginning at the hip joint), the nail is usually implanted in a retrograde fashion (beginning at the knee joint). In the retrograde technique, the hypodermic needle can be driven by hand through the intercondylar fossa of the femur and passed into the medullary canal. To prevent anterograde movement of the implant, the needle can be drilled through the proximal end of the femur and bent over the greater trochanter. The lack of specialized equipment, simplicity, speed, and low cost of the procedure, as well as the rigidity with which scaffolds and other intercalary implants can be fixed are the major advantages of IM nail fixation. Disadvantages to the IM nail include a requirement for a load sharing device such as a scaffold or allograft to maintain defect size, inherent damage to the medullary canal, and low axial and rotational stability30. Recent advances in this technique have overcome some of these challenges using different types of locking nails. Pohlemann and colleagues have developed what is the most cost-effective locking nail by simply flattening the proximal and distal ends of a 24-gauge hypodermic needle after implantation (Flattened Locking Nail)31. This showed vastly increased rotational stability when compared to simple IM nail controls, and the technique also prevents migration of the nail axially within the canal31. Gregory et al. have recently developed a pseudo-locking IM nail to increase axial stability but not rotational stability52. The IM nail in this case is a self-made construct consisting of a 3 mm length of 19-gauge hypodermic tubing crushed onto the center of a longer, 9 mm length of 22-gauge hypodermic tubing. The custom nail is then inserted into the medullary cavity through the defect site rather than a hole at the intercondylar fossa and abuts the ends of the medullary cavity to increase axial stability and remove the requirement for a scaffold. Removal of the custom nail is difficult, though, and may complicate μCT, histologic, and mechanical analyses52. Pohlemann and colleagues have more recently developed a locking IM nail for mice that is very similar to the type used in humans53. The model uses a nail with holes for cortical pins and simple jig, both of which are now available from RISystem as MouseNail. The MouseNail is depicted in Figure 1 as the segmental defect without load sharing construct. The MouseNail jig allows for retrograde placement of the nail, accurate osteotomy with gigli saw, and insertion of transcortical pins into the nail. This is the standard technique for IM nail fixation in the human femur and provides much more axial and rotational stability than the simple hypodermic needle technique or screw type locking nail30, 53. Removal of the pin is less difficult than in the method developed by Gregory et al., and therefore analyses requiring hardware removal may be done more easily53, 54.
Fig. 2.
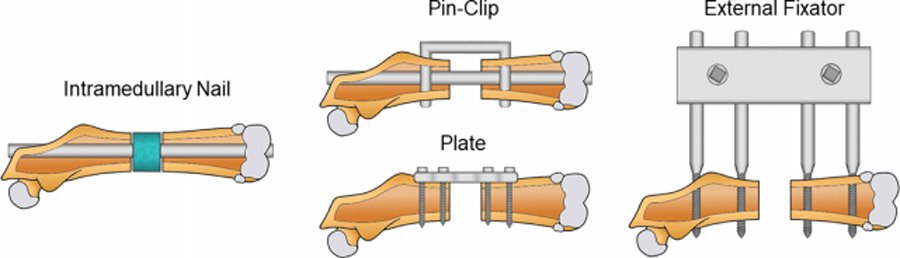
Illustration of fixation types for the segmental femoral defect: intramedullary (IM) nail, plate, pin-clip, and external fixator.
Plate
More recently, plate fixation has become a popular choice for fixation of mouse segmental femoral defects38, 50, 55–62. Plate fixation requires the attachment of a 3 to 6-hole plate to the mouse femur via bicortical locking or nonlocking screws (Figure 2). This requires a custom-made jig, if deemed necessary, and a plate, screws, and a drill. Although some investigators use rotary tools to remove the bone segment, jigs created for the plate systems interface with the plate to allow for accurate creation of the SBD with a gigli saw. Plate fixation offers comparable stiffness to the highly rigid external fixator for repair of mouse femur SBDs, but it is also the most costly and difficult procedure30. Use of the jig typically requires two personnel, and initial placement of the screws can be challenging. This also increases the length of the procedure which is a critical factor in high volume studies. Most plates are made of stainless steel and therefore complicate histologic and μCT analysis. Due to osseointegration of the plate, the removal for analysis can be more time intensive and risky than the other methods. Recent, exciting advances in mouse plate materials have overcome this problem with radiolucent PEEK plates that do not need to be removed for analysis56. The rigidity of mouse plates can also be altered to shift fracture healing mechanisms from a more intramembranous repair with a stiff plate to a more endochondral recovery with a flexible plate59. In larger defects, a scaffold or other treatment carrying drug or material can be placed within the defect site. But, unlike with the IM nail, rigid fixation of these inserts is not as straight forward. Most investigators simply close the muscle around the insert, allowing for some motion50, 60, while others suture the insert to the plate38, 56.
Pin-Clip
Developed in 2008 by Menger and colleagues, the pin-clip fixation for femoral shaft defects in mice is a combination of a conventional IM nail with a custom transcortical clip, resembling a staple, to add stability (Figure 2)39. The clip is typically made of a blunted hypodermic needle or a solid steel pin that is bent into a U shape. A small drill is required to create the cortical holes through which the clip will pass. The primary advantage to the pin-clip is the enhanced axial and rotational rigidity when compared to most other IM nail types. Biomechanical testing by both Menger and Pohlemann showed the torsional strength of the pin-clip construct to be much greater than that of the conventional IM nail and crimped locking nail. The pin-clip showed similar torsional strength and rigidity to the MouseNail30, 63. Like the IM nail, the pin-clip can be used to make defects of different sizes, and an established CSD of 1.8 mm with periosteal stripping exists for the pin-clip39. The clip also has advantage over the IM nail because it allows for defect length maintenance with or without a load sharing insert such as a scaffold or bone graft. In fact, pliable substances such as collagen sponges soaked in test drug as well as other soft experimental materials may be placed within large gaps without loss of gap length. These inserts can be held more firmly in place due to the presence of an IM nail than can those placed with an external fixator. Although the procedure for the pin-clip is more complicated than the simple IM nail, the cost of the procedure is relatively the same as the clip can be made by simply bending a hypodermic needle. Some investigators propose that the pin-clip causes more intramedullary trauma, and therefore, may affect endosteal repair more than the other fixation methods8. This has not been substantiated however, and experiments with the pin-clip method that show high endosteal vascularization, as well as the use of medullary reamers in clinical orthopaedics, suggest that this not the case64.
External Fixation
External fixation of diaphyseal femoral defects in mice requires custom made devices that span the length of the defect from the outside of the animal and use bicortical pins penetrating the skin and soft tissue to align the bone (Figure 2). This method was first introduced in mice by Cheah et al. in 2003 and was sparsely used until more recent commercial availability and standardization of the external fixators40, 51, 65–72. The effort in development of this model and its application has been led by Ignatius and colleagues68. The advantages of the external fixator are many. The most profound advantages are the unique ability to perform experiments with distraction osteogenesis as well as the recent introduction of ceramic pins to allow for longitudinal MRI analysis of fracture healing. MRI analysis can be accomplished with a plastic block attached to the external fixator to reduce motion of the femur during imaging. This model was found to be highly accurate in predicting fibrous tissue and cartilage values, both critical in the early and intermediate phases of fracture healing 68. This is important because it allows for the examination of bone healing parameters without euthanasia of mice, therefore reducing the number of mice required per experiment and empowering more accurate longitudinal analysis of individual mice. Although the long moment arms of the pins do not immediately suggest so, external fixation is also the most axially and rotationally rigid system for fixation of mouse femoral fractures30. More successful than the newer plate models, external fixation allows for accurate adjustment of fixation rigidity as well67. The main concern with modern external fixators is their weight. These can affect gait and utilization of the affected limb, which is known to be an important factor in fracture healing. To combat this problem, Livne and colleagues have devised a simple, very light external fixator which can be made by the investigator51. The construct uses a set of 4 transverse, bicortical steel pins glued together outside of the skin by acrylic dental paste. The paste weighs much less than the normal external block, and unlike the conventional external fixator, mechanical testing can be done without removal of the pins51. Mice equipped with an external fixator also show highly variable amounts of locomotion, making it difficult to reduce inter-mouse variability66. Pin tract infections and clinical application have also been concerns. External fixation is not commonly used for definitive fixation of long bone fractures in humans, and therefore, the generalizability of these experiments to the clinic may be limited.
Applications
The SBD lends itself to the use of vectors for local drug delivery, development of biocompatible materials for replacement of clinical autografting procedures, and tissue engineering. Drug vectors commonly include drug impregnated load bearing scaffolds and non-load bearing collagen sponges. Scaffolds are typically made of poly(propylene) fumarate/tricalcium phosphate, polycaprolactone, or synthetic collagen in combination with hydroxyapatite or beta tricalcium phosphate. More thorough discussions of scaffold design and materials, as well as the state of scaffold research can be found in the following references42, 73. Much of the recent research in the segmental defect has revolved around adjunctive and replacement therapy to bone morphogenic protein 2 (BMP-2), a growth factor clinically approved for use in delayed union and nonunion of tibial shaft fractures. In a mouse segmental defect with an external fixator, Stiehler and colleagues have made breakthrough discoveries in the adjunctive use of SDF-1α with BMP-2 to reduce the required dose of BMP-2 for bone healing74. In the realm of tissue engineering, Wanner et al. have used a mouse segmental defect with a plate to demonstrate the utility of native pericytes in healing a critical sized bone defect when implanted on a cancellous bone graft75. Other emerging tissue engineering research has looked at replacing the lost osteogenic and osteointegrative potential of devitalized bone allografts with tissue engineered periosteum76–78. These experiments are of high clinical relevancy, as autografts are not always available or adequate in the clinic due to the patient’s condition, bone quality, or volume of autograft required, and devitalized allografts are not nearly as effective at inducing bone healing77.
CONCLUSION
The present and future relevance of fracture healing complications in the clinical setting have driven the need for translational research to optimize reliable models. The aforementioned models offer a variety of modifications that cater to a wider array of studies. The cortical hole model (corticotomy) is a technically simple and efficient way to create a mid-diaphyseal bony defect. The simplicity of the model and open access to the defect site lend it to use in high throughput pharmaceutical and transgenic studies. The Einhorn, or 3-point fracture, model is currently the most viable option for creation of closed diaphyseal femur fractures in mice. Of the models discussed above the 3-point fracture produces the least soft tissue damage if performed correctly. Additionally, the procedure is relatively simple, quick, and cost effective, especially when compared to the segmental defect. It is most compatible with studies investigating multisystem trauma, shock, and bone contusion. However, the 3-point fracture is limited in its ability to study locally applied pharmaceuticals as the blunt fracture does not always create a clean defect. In some cases, many animals may need to be excluded based on poor fracture creation. The segmental defect is the oldest studied model and the most customizable. It is best suited for materials/tissue engineering paradigms, understanding healing of critical sized defects, locally applied pharmaceuticals, and the exciting new realm of periosteal reconstruction studies. Multiple fixation options exist for the segmental defect, and new innovations in design have brought MRI compatible, radiolucent, variable stiffness, and more clinically relevant fixations to the forefront. Together, these models represent a diverse, highly applicable mode of translational research for the development of fracture healing therapies and elucidation of bone healing mechanisms. The insights into clinical relevance, experimental procedures, relevant parameters, modifications, and current applications presented in this review hopefully provide the reader with the base knowledge required to perform and strategically modify their research design to serve their needs while retaining a standard by which other studies can be compared.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Clement ND, Beauchamp NJ, Duckworth AD, McQueen MM, Court-Brown CM. The outcome of tibial diaphyseal fractures in the elderly. The bone & joint journal. 2013;95-b:1255–62. [DOI] [PubMed] [Google Scholar]
- 2.Colby SL, Ortman, Jennifer M. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. In: Commerve USDo, editor. www.census.gov2015.
- 3.Tzioupis C, Giannoudis PV. Prevalence of long-bone non-unions. Injury. 2007;38:S3–S9. [DOI] [PubMed] [Google Scholar]
- 4.Schottel PC, O’Connor DP, Brinker MR. Time Trade-Off as a Measure of Health-Related Quality of Life: Long Bone Nonunions Have a Devastating Impact. The Journal of bone and joint surgery American volume. 2015;97:1406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinker MR, Trivedi A, O’Connor DP. Debilitating Effects of Femoral Nonunion on Health-Related Quality of Life. Journal of orthopaedic trauma. 2017;31:e37–e42. [DOI] [PubMed] [Google Scholar]
- 6.Hak DJ, Fitzpatrick D, Bishop JA, Marsh JL, Tilp S, Schnettler R, et al. Delayed union and nonunions: Epidemiology, clinical issues, and financial aspects. Injury. 2014;45:S3–S7. [DOI] [PubMed] [Google Scholar]
- 7.Holstein JH, Garcia P, Histing T, Kristen A, Scheuer C, Menger MD, et al. Advances in the establishment of defined mouse models for the study of fracture healing and bone regeneration. J Orthop Trauma. 2009;23:S31–8. [DOI] [PubMed] [Google Scholar]
- 8.Histing T, Garcia P, Holstein JH, Klein M, Matthys R, Nuetzi R, et al. Small animal bone healing models: Standards, tips, and pitfalls results of a consensus meeting. Bone. 2011;49:591–9. [DOI] [PubMed] [Google Scholar]
- 9.Einhorn TA. The science of fracture healing. J Orthop Trauma. 2005;19:S4–6. [DOI] [PubMed] [Google Scholar]
- 10.McKibbin B The biology of fracture healing in long bones. J Bone Joint Surg Br. 1978;60-b:150–62. [DOI] [PubMed] [Google Scholar]
- 11.He Y-X, Zhang G, Pan X-H, Liu Z, Zheng L-z, Chan C-W, et al. Impaired bone healing pattern in mice with ovariectomy-induced osteoporosis: A drill-hole defect model. Bone. 2011;48:1388–400. [DOI] [PubMed] [Google Scholar]
- 12.Monfoulet L, Rabier B, Chassande O, Fricain JC. Drilled hole defects in mouse femur as models of intramembranous cortical and cancellous bone regeneration. Calcif Tissue Int. 2010;86:72–81. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Shi X, Wang L, Li X, Zheng C, Gao B, et al. Intramembranous ossification and endochondral ossification are impaired differently between glucocorticoid-induced osteoporosis and estrogen deficiency-induced osteoporosis. Scientific reports. 2018;8:3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGee-Lawrence ME, Razidlo DF. Induction of fully stabilized cortical bone defects to study intramembranous bone regeneration. Methods Mol Biol. 2015;1226:183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGee-Lawrence ME, Ryan ZC, Carpio LR, Kakar S, Westendorf JJ, Kumar R. Sclerostin deficient mice rapidly heal bone defects by activating beta-catenin and increasing intramembranous ossification. Biochem Biophys Res Commun. 2013;441:886–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh KB, Dixit M, Dev K, Maurya R, Singh D. Formononetin, a methoxy isoflavone, enhances bone regeneration in a mouse model of cortical bone defect. Br J Nutr. 2017;117:1511–22. [DOI] [PubMed] [Google Scholar]
- 17.Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 1984;2:97–101. [DOI] [PubMed] [Google Scholar]
- 18.Manigrasso MB, O’Connor JP. Characterization of a closed femur fracture model in mice. J Orthop Trauma. 2004;18:687–95. [DOI] [PubMed] [Google Scholar]
- 19.Scofield DC, Rytlewski JD, Childress P, Shah K, Tucker A, Khan F, et al. Development of a step-down method for altering male C57BL/6 mouse housing density and hierarchical structure: Preparations for spaceflight studies. Life Sci Space Res (Amst). 2018;17:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gardner MJ, Putnam SM, Wong A, Streubel PN, Kotiya A, Silva MJ. Differential fracture healing resulting from fixation stiffness variability: a mouse model. J Orthop Sci. 2011;16:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Giacomo A, Morgan EF, Gerstenfeld LC. Generation of closed transverse fractures in small animals. Methods Mol Biol. 2014;1130:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, McKenzie JA, Maschhoff CW, Gardner MJ, Silva MJ. Exogenous hedgehog antagonist delays but does not prevent fracture healing in young mice. Bone. 2017;103:241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majuta LA, Longo G, Fealk MN, McCaffrey G, Mantyh PW. Orthopedic surgery and bone fracture pain are both significantly attenuated by sustained blockade of nerve growth factor. [DOI] [PMC free article] [PubMed]
- 24.Au - Williams JN, Au - Li Y, Au - Valiya Kambrath A, Au - Sankar U. The Generation of Closed Femoral Fractures in Mice: A Model to Study Bone Healing. JoVE. 2018:e58122. [DOI] [PMC free article] [PubMed]
- 25.Lamb LE, Siggins MK, Scudamore C, Macdonald W, Turner CE, Lynskey NN, et al. Impact of contusion injury on intramuscular emm1 group a streptococcus infection and lymphatic spread. Virulence. 2018;9:1074–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marturano JE, Cleveland BC, Byrne MA, O’Connell SL, Wixted JJ, Billiar KL. An improved murine femur fracture device for bone healing studies. Journal of biomechanics. 2008;41:1222–8. [DOI] [PubMed] [Google Scholar]
- 27.Hiltunen A, Vuorio E, Aro HT. A standardized experimental fracture in the mouse tibia. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 1993;11:305–12. [DOI] [PubMed] [Google Scholar]
- 28.Epari DR, Kassi JP, Schell H, Duda GN. Timely fracture-healing requires optimization of axial fixation stability. The Journal of bone and joint surgery American volume. 2007;89:1575–85. [DOI] [PubMed] [Google Scholar]
- 29.Williams JN, Li Y, Valiya Kambrath A, Sankar U. The Generation of Closed Femoral Fractures in Mice: A Model to Study Bone Healing. J Vis Exp. 2018. [DOI] [PMC free article] [PubMed]
- 30.Histing T, Holstein JH, Garcia P, Matthys R, Kristen A, Claes L, et al. Ex vivo analysis of rotational stiffness of different osteosynthesis techniques in mouse femur fracture. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2009;27:1152–6. [DOI] [PubMed] [Google Scholar]
- 31.Holstein JH, Menger MD, Culemann U, Meier C, Pohlemann T. Development of a locking femur nail for mice. Journal of biomechanics. 2007;40:215–9. [DOI] [PubMed] [Google Scholar]
- 32.Holstein JH, Matthys R, Histing T, Becker SC, Fiedler M, Garcia P, et al. Development of a stable closed femoral fracture model in mice. J Surg Res. 2009;153:71–5. [DOI] [PubMed] [Google Scholar]
- 33.Histing T, Bremer P, Rollmann MF, Herath S, Klein M, Pohlemann T, et al. A Minimally Invasive Model to Analyze Endochondral Fracture Healing in Mice Under Standardized Biomechanical Conditions. J Vis Exp. 2018. [DOI] [PMC free article] [PubMed]
- 34.Einhorn TA, Majeska RJ, Mohaideen A, Kagel EM, Bouxsein ML, Turek TJ, et al. A single percutaneous injection of recombinant human bone morphogenetic protein-2 accelerates fracture repair. The Journal of bone and joint surgery American volume. 2003;85:1425–35. [DOI] [PubMed] [Google Scholar]
- 35.Fitschen-Oestern S, Lippross S, Klueter T, Weuster M, Varoga D, Tohidnezhad M, et al. A new multiple trauma model of the mouse. BMC Musculoskelet Disord. 2017;18:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lichte P, Kobbe P, Pfeifer R, Campbell GC, Beckmann R, Tohidnezhad M, et al. Impaired Fracture Healing after Hemorrhagic Shock. Mediators Inflamm. 2015;2015:132451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soung do Y, Talebian L, Matheny CJ, Guzzo R, Speck ME, Lieberman JR, et al. Runx1 dose-dependently regulates endochondral ossification during skeletal development and fracture healing. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012;27:1585–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xing J, Jin H, Hou T, Chang Z, Luo F, Wang P, et al. Establishment of a bilateral femoral large segmental bone defect mouse model potentially applicable to basic research in bone tissue engineering. Journal of Surgical Research. 2014;192:454–63. [DOI] [PubMed] [Google Scholar]
- 39.Garcia P, Holstein JH, Maier S, Schaumlöffel H, Al-Marrawi F, Hannig M, et al. Development of a Reliable Non-Union Model in Mice. Journal of Surgical Research. 2008;147:84–91. [DOI] [PubMed] [Google Scholar]
- 40.Haffner-Luntzer M, Muller-Graf F, Matthys R, Abaei A, Jonas R, Gebhard F, et al. In Vivo Evaluation of Fracture Callus Development During Bone Healing in Mice Using an MRI-compatible Osteosynthesis Device for the Mouse Femur. J Vis Exp. 2017. [DOI] [PMC free article] [PubMed]
- 41.Kanczler JM, Ginty PJ, White L, Clarke NMP, Howdle SM, Shakesheff KM, et al. The effect of the delivery of vascular endothelial growth factor and bone morphogenic protein-2 to osteoprogenitor cell populations on bone formation. Biomaterials. 2010;31:1242–50. [DOI] [PubMed] [Google Scholar]
- 42.Harris JS, Bemenderfer TB, Wessel AR, Kacena MA. A review of mouse critical size defect models in weight bearing bones. Bone. 2013;55:241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reynolds DG, Hock C, Shaikh S, Jacobson J, Zhang X, Rubery PT, et al. Micro-computed tomography prediction of biomechanical strength in murine structural bone grafts. Journal of biomechanics. 2007;40:3178–86. [DOI] [PubMed] [Google Scholar]
- 44.Reynolds DG, Takahata M, Lerner AL, O’Keefe RJ, Schwarz EM, Awad HA. Teriparatide therapy enhances devitalized femoral allograft osseointegration and biomechanics in a murine model. Bone. 2011;48:562–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie C, Reynolds D, Awad H, Rubery PT, Pelled G, Gazit D, et al. Structural bone allograft combined with genetically engineered mesenchymal stem cells as a novel platform for bone tissue engineering. Tissue Eng. 2007;13:435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koefoed M, Ito H, Gromov K, Reynolds DG, Awad HA, Rubery PT, et al. Biological effects of rAAV-caAlk2 coating on structural allograft healing. Mol Ther. 2005;12:212–8. [DOI] [PubMed] [Google Scholar]
- 47.Jacobson JA, Yanoso-Scholl L, Reynolds DG, Dadali T, Bradica G, Bukata S, et al. Teriparatide therapy and beta-tricalcium phosphate enhance scaffold reconstruction of mouse femoral defects. Tissue engineering Part A. 2011;17:389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu H, Vandevord PJ, Gong W, Wu B, Song Z, Matthew HW, et al. Promotion of osteogenesis in tissue-engineered bone by pre-seeding endothelial progenitor cells-derived endothelial cells. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2008;26:1147–52. [DOI] [PubMed] [Google Scholar]
- 49.Oetgen ME, Merrell GA, Troiano NW, Horowitz MC, Kacena MA. Development of a femoral non-union model in the mouse. Injury. 2008;39:1119–26. [DOI] [PubMed] [Google Scholar]
- 50.Manassero M, Decambron A, Huu Thong BT, Viateau V, Bensidhoum M, Petite H. Establishment of a Segmental Femoral Critical-size Defect Model in Mice Stabilized by Plate Osteosynthesis. J Vis Exp. 2016. [DOI] [PMC free article] [PubMed]
- 51.Srouji S, Ben-David D, Kohler T, Muller R, Zussman E, Livne E. A model for tissue engineering applications: femoral critical size defect in immunodeficient mice. Tissue Eng Part C Methods. 2011;17:597–606. [DOI] [PubMed] [Google Scholar]
- 52.Clough BH, McCarley MR, Gregory CA. A simple critical-sized femoral defect model in mice. [DOI] [PMC free article] [PubMed]
- 53.Au - Histing T, Au - Menger MD, Au - Pohlemann T, Au - Matthys R, Au - Fritz T, Au - Garcia P, et al. An Intramedullary Locking Nail for Standardized Fixation of Femur Osteotomies to Analyze Normal and Defective Bone Healing in Mice. JoVE. 2016:e54472. [DOI] [PMC free article] [PubMed]
- 54.Garcia P, Herwerth S, Matthys R, Holstein JH, Histing T, Menger MD, et al. The LockingMouseNail—A New Implant for Standardized Stable Osteosynthesis in Mice. Journal of Surgical Research. 2011;169:220–6. [DOI] [PubMed] [Google Scholar]
- 55.Meesters DM, Neubert S, Wijnands KAP, Heyer FL, Zeiter S, Ito K, et al. Deficiency of inducible and endothelial nitric oxide synthase results in diminished bone formation and delayed union and nonunion development. Bone. 2016;83:111–8. [DOI] [PubMed] [Google Scholar]
- 56.Inzana JA, Schwarz EM, Kates SL, Awad HA. A novel murine model of established Staphylococcal bone infection in the presence of a fracture fixation plate to study therapies utilizing antibiotic-laden spacers after revision surgery. Bone. 2015;72:128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Histing T, Garcia P, Matthys R, Leidinger M, Holstein JH, Kristen A, et al. An internal locking plate to study intramembranous bone healing in a mouse femur fracture model. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2010;28:397–402. [DOI] [PubMed] [Google Scholar]
- 58.Liu K, Li D, Huang X, Lv K, Ongodia D, Zhu L, et al. A murine femoral segmental defect model for bone tissue engineering using a novel rigid internal fixation system. J Surg Res. 2013;183:493–502. [DOI] [PubMed] [Google Scholar]
- 59.Ueno M, Uchida K, Takaso M, Minehara H, Suto K, Takahira N, et al. Distribution of bone marrow-derived cells in the fracture callus during plate fixation in a green fluorescent protein-chimeric mouse model. Exp Anim. 2011;60:455–62. [DOI] [PubMed] [Google Scholar]
- 60.Oezel L, Büren C, Scholz AO, Windolf J, Windolf CD. Effect of antibiotic infused calcium sulfate/hydroxyapatite (CAS/HA) insets on implant-associated osteitis in a femur fracture model in mice. PLoS One. 2019;14:e0213590-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buren C, Hambuchen M, Windolf J, Logters T, Windolf CD. Histological score for degrees of severity in an implant-associated infection model in mice. Arch Orthop Trauma Surg. 2019. [DOI] [PubMed]
- 62.Buren C, Logters T, Oezel L, Rommelfanger G, Scholz AO, Windolf J, et al. Effect of hyperbaric oxygen therapy (HBO) on implant-associated osteitis in a femur fracture model in mice. PLoS One. 2018;13:e0191594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garcia P, Holstein JH, Histing T, Burkhardt M, Culemann U, Pizanis A, et al. A new technique for internal fixation of femoral fractures in mice: impact of stability on fracture healing. Journal of biomechanics. 2008;41:1689–96. [DOI] [PubMed] [Google Scholar]
- 64.Garcia P, Speidel V, Scheuer C, Laschke MW, Holstein JH, Histing T, et al. Low dose erythropoietin stimulates bone healing in mice. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2011;29:165–72. [DOI] [PubMed] [Google Scholar]
- 65.Cheung KMC, Kaluarachi K, Andrew G, Lu W, Chan D, Cheah KSE. An externally fixed femoral fracture model for mice. Journal of Orthopaedic Research. 2003;21:685–90. [DOI] [PubMed] [Google Scholar]
- 66.Connolly CK, Li G, Bunn JR, Mushipe M, Dickson GR, Marsh DR. A reliable externally fixated murine femoral fracture model that accounts for variation in movement between animals. [DOI] [PubMed]
- 67.Röntgen V, Blakytny R, Matthys R, Landauer M, Wehner T, Göckelmann M, et al. Fracture healing in mice under controlled rigid and flexible conditions using an adjustable external fixator. [DOI] [PubMed]
- 68.Haffner-Luntzer M, Muller-Graf F, Matthys R, Hagele Y, Fischer V, Jonas R, et al. Evaluation of high-resolution In Vivo MRI for longitudinal analysis of endochondral fracture healing in mice. PLoS One. 2017;12:e0174283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haffner-Luntzer M, Kovtun A, Lackner I, Modinger Y, Hacker S, Liedert A, et al. Estrogen receptor alpha- (ERalpha), but not ERbeta-signaling, is crucially involved in mechanostimulation of bone fracture healing by whole-body vibration. Bone. 2018;110:11–20. [DOI] [PubMed] [Google Scholar]
- 70.Recknagel S, Bindl R, Brochhausen C, Gockelmann M, Wehner T, Schoengraf P, et al. Systemic inflammation induced by a thoracic trauma alters the cellular composition of the early fracture callus. J Trauma Acute Care Surg. 2013;74:531–7. [DOI] [PubMed] [Google Scholar]
- 71.Recknagel S, Bindl R, Wehner T, Gockelmann M, Wehrle E, Gebhard F, et al. Conversion from external fixator to intramedullary nail causes a second hit and impairs fracture healing in a severe trauma model. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2013;31:465–71. [DOI] [PubMed] [Google Scholar]
- 72.Recknagel S, Bindl R, Kurz J, Wehner T, Schoengraf P, Ehrnthaller C, et al. C5aR-antagonist significantly reduces the deleterious effect of a blunt chest trauma on fracture healing. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2012;30:581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hollister SJ. Porous scaffold design for tissue engineering. Nature materials. 2005;4:518–24. [DOI] [PubMed] [Google Scholar]
- 74.Zwingenberger S, Yao Z, Jacobi A, Vater C, Valladares RD, Li C, et al. Enhancement of BMP-2 induced bone regeneration by SDF-1alpha mediated stem cell recruitment. Tissue engineering Part A. 2014;20:810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Konig MA, Canepa DD, Cadosch D, Casanova E, Heinzelmann M, Rittirsch D, et al. Direct transplantation of native pericytes from adipose tissue: A new perspective to stimulate healing in critical size bone defects. Cytotherapy. 2016;18:41–52. [DOI] [PubMed] [Google Scholar]
- 76.Wang T, Zhai Y, Nuzzo M, Yang X, Yang Y, Zhang X. Layer-by-layer nanofiber-enabled engineering of biomimetic periosteum for bone repair and reconstruction. Biomaterials. 2018;182:279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoffman MD, Benoit DSW. Emerging ideas: Engineering the periosteum: revitalizing allografts by mimicking autograft healing. Clin Orthop Relat Res. 2013;471:721–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hoffman MD, Benoit DSW. Emulating native periosteum cell population and subsequent paracrine factor production to promote tissue engineered periosteum-mediated allograft healing. Biomaterials. 2015;52:426–40. [DOI] [PMC free article] [PubMed] [Google Scholar]


