Summary
GATA1 is an essential regulator of erythroid cell gene expression and maturation. In its absence, erythroid progenitors are arrested in differentiation and undergo apoptosis. Much has been learned about GATA1 function through animal models, which include genetic knockouts as well as ones with decreased levels of expression. However, even greater insights have come from the finding that a number of rare red cell disorders, including Diamond Blackfan Anemia, are associated with GATA1 mutations. These mutations affect the amino-terminal zinc finger (N-ZF) and the amino-terminus of the protein, and in both cases can alter the DNA binding activity, which is primarily conferred by the third functional domain, the carboxyl-terminal zinc finger (C-ZF). Here we discuss the role of GATA1 in erythropoiesis with an emphasis on the mutations found in human patients with red cell disorders.
GATA1 Is Required for Erythropoiesis
The GATA family is composed of six zinc-finger transcription factors that bind the consensus DNA sequence (T/A)GATA(A/G). GATA½/3 predominantly function in hematopoietic system, while GATA4/5/6 are critical for non-hematopoietic tissues. GATA1 was originally identified as a trans-acting factor of globin and other erythroid-specific genes, and subsequently found to be expressed in megakaryocytes, mast cells, basophils and eosinophils but not at substantial levels in other hematopoietic lineages1–9. The murine Gata1 gene has two promoters, the testis-specific promoter/first exon (IT) located ≈ 8 kb 5′ of the erythroid promoter and first exon (IE), erythroid promoter, which is predominantly used in hematopoietic cells10. Although, Gata1 mRNA is detectable in mouse Sertoli cells of the testis, its function in this tissue remains uncertain10–13. Transcripts from the testis promoter have been reported to increase during the differentiation of erythroid precursors purified from the spleen of mice treated with phenylhydrazine (PHZ) or infected with the anemia-inducing strain of the Friend virus (FVA cells)14. Disruption of erythroid promoter, which doesn’t diminish expression from the testis promoter, leads to embryonic lethality at E12.5 as a result of a major defect in primitive erythropoiesis15.
During human hematopoiesis, GATA1 is expressed in hematopoietic stem and progenitor cells (HSPCs), and progressively increases in expression in CD34+/CD38− primitive cells and CD34+/CD38+ committed progenitors16,17. With respect to the erythroid lineage, its expression is initially increased during specification, but is downregulated at the late stages; it is similarly downregulated late in megakaryocytic maturation18. An important animal study of loss of function of Gata1 was reported by Costantini and colleagues19. Using chimeric mice, the investigators found that GATA1 is required for maturation of hemoglobinized red blood cells during the fetal stages and in adults. Notably, no other transcription factors could compensate effectively for the erythropoietic deficit of GATA1-null ES cells19. The Gata1 germ line knockout mouse model was generated and characterized by the Orkin laboratory in 1996. In this study, they found that Gata1 hemizygous deficient male embryos died between embryonic days 10.5 and 11.5 (E10.5-E11.5) from profound anemia20. They further demonstrated that erythropoiesis is arrested at the proerythroblast stage in the absence of GATA1 and that yolk sacs yielded pale CFU-E colonies with dying cells as well as large pale BFU-E colonies that included an abundance of megakaryocyte-like cells20. Subsequent studies demonstrated that Gata1 deficient cells failed to contribute to maturation of red blood cells by arresting erythropoiesis at the proerythroblast stage through apoptosis of the cells21.
Other studies have investigated the effect of decreasing GATA1 expression. For example, replacement of upstream sequences including a DNase I hypersensitive (HS) region with a neomycin-resistance cassette impaired erythropoiesis through a modest (4 to 5-fold) decrease of GATA1 expression, suggesting that erythroid differentiation is dose-dependent with respect to GATA122. A second study of cells that express 5% of the normal level of GATA1, referred to GATA1.05 mice, also showed that, although this low level of GATA1 maintained the viability of definitive erythroid cells, there was aberrant expression of erythroid-specific transcription factors and heme biosynthetic enzymes. Moreover, the GATA1.05 ES cells were found to differentiated into blast-like cells23.
Collectively, these studies with various animal models of a GATA1 deficiency showed that GATA1 is essential for both primitive and definitive erythropoiesis (Table 1). Although mice that express GATA2 or GATA3 from the GATA1 locus are rescued from embryonic lethality due to the absence of Gata1, the mice develop anemia after birth, indicating that there are functional differences among the family members24.
Table 1:
Animal models of Gata1 deficiencies
| Genotype | Hematopoietic phenotype | Reference |
|---|---|---|
|
Gata1ΔIE (erythroid first exon (IE) deleted) |
Gata1ΔIE mRNA level is significantly down-regulated in the megakaryocyte lineage but is expressed in the erythroid lineage. These Gata1ΔIE mRNA fail to produce full-length GATA1 protein, but instead generate GATA1 lacking the N-terminal domain inefficiently. Hemizygous male embryos die by E12.5 showing severe anemia. Conditional knockout male mice (3–4 weeks) suffer from severe thrombocytopenia and anemia. Nucleated erythrocytes can be found on the peripheral blood smear. Splenomegaly with apparent destruction of the splenic architecture, due to expansion of the red pulp, can be observed. Markedly increased CD41+CD61+ megakaryocytes and accumulation of CD71hiTer119low in the bone marrow can be detected by flow cytometric analysis. | 111 |
|
Gata1ΔN (Δ 3–63 AA) and Gata1Δe2 (Δ 1–83 AA) |
Adult hemizygotes have normal RBC, PLT, normal spleen structure and normal numbers of megakaryocytes in spleen. 10–15% of the mutant mice were lost in utero. Fetal liver pallor and reduced cellularity (by ∼50%) can be observed in E12.5 embryos from hemizygous and homozygous compared to the wild type. At E12.5 more CD41+ cells can be detected by flow cytometric analysis, and megakaryocytes are hyperproliferative in liquid medium containing thrombopoietin. Erythroid colony-forming units (CFU-Es) in Gata1ΔN fetal liver are reduced by ∼50%. At E14.5 and thereafter, Gata1ΔN fetal liver had normal cellularity and comparable Ter119+ cells as wild type. | 110 |
|
Gata1− (Start codon Disrupted) |
Male embryos die between E10.5-E11.5 of gestation because of severe anemia. Female heterozygotes are born pale and recover after birth. Embryonic erythroid cells are arrested at an early proerythroblast-like stage of their development and die thereafter by apoptosis. Abundant acetylcholinesterase positive cells can be found in methylcellulose culture supplemented with Epo and kit ligand. | 20 |
|
Gata1low or Gata1neoΔHS (Disrupt DNase I-hypersensitive region by replacing ∼8 kb of upstream sequences, including the IT and HS regions, with a PGK-NeoR cassette flanked by loxP sites) |
Yolk sac erythropoiesis and fetal erythropoiesis are disturbed. Embryos are pale or dead by E13.5–14.5 and definitive erythroid cells are largely arrested at the proerythroblast stage of development. CFU-E and BFU-E are morphologically abnormal in colony forming assay with fetal liver cells. About 5% of hemizygous males survive fetal anemia into adult life. Abnormal nucleated erythroid precursors and erythrocytes can be found in peripheral blood smears of newborns, but anemia phenotypes at birth disappear by 4–5 weeks after birth. Mutant mice also loss of megakaryocyte GATA1 expression, resulting in markedly reduced PLT associated with deregulated megakaryocyte proliferation and severely impaired maturation. Female mice heterozygous have normal platelet numbers, but with increased megakaryocytes in hematopoietic tissues. | 22,112 |
|
Gata1.05 (Introduce a neo cassette into the intergenic region between an important regulatory region of the erythroid promoter and the mRNA cap site) |
The expression of GATA1 mRNA in the mutant male embryos is less than 5% of the level present in wild-type embryos (E9.5). The yolk sacs of mutant males are pale and almost no blood vessels are present. Embryos die in utero due to impaired primitive erythropoiesis. There are decreased number of erythroid cells and CFU-E in mutant fetal livers. | 15,113 |
GATA1 Functional Domains and Partners
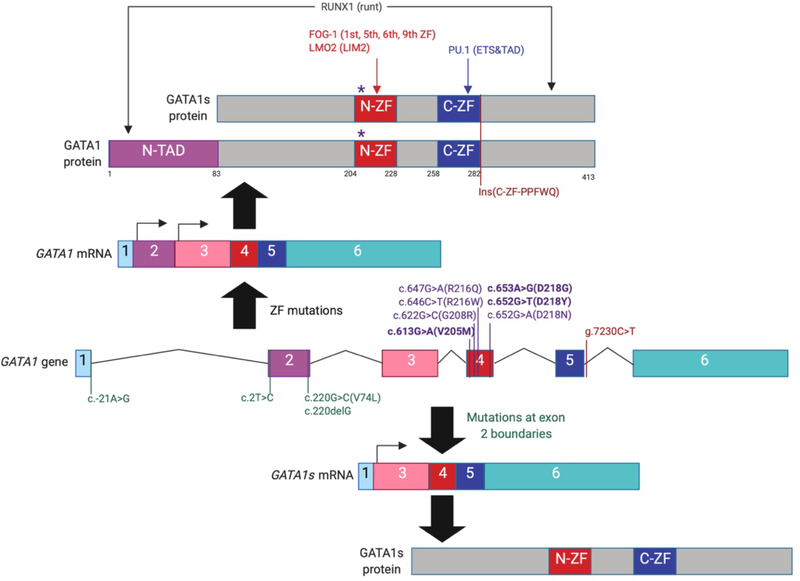
GATA1 contains three functional domains: the N-terminal transcription activation domain (N-TAD), an amino-terminal zinc finger (N-ZF) domain and a carboxyl-terminal zinc finger domain (C-ZF) (Figure 1). The two zinc fingers have been found to have different DNA binding specificities25; the C-ZF provides the primary DNA binding activity, as mutant isoforms containing only the N-ZF is deficient for binding 26,27. However, the N-ZF domain contributes to the strength of binding to some sites, such as the GATApal element, which contains one complete [(A/T)GATA(A/G)] and one partial (GAT) canonical motif28. Furthermore, the two zinc fingers can form a composite binding domain improving the DNA-binding specificity from single C-ZF, and the covalent linkage of them is required for influencing one another29. Zinc fingers of GATA1 have been shown to partially rescue erythroid and megakaryocytic differentiation30,31, which indicates they may have other functions in addition to mediating DNA binding.
Figure 1. Schematic of the GATA1 gene and known mutations in patients with congenital erythroid disorders.
GATA1 is composed of six exons which encode two transcripts that produce the full-length isoform (upper) or the N-terminally truncated GATA1s isoform (lower). The locations of GATA1 mutations found in patients with congenital erythroid disorders are indicated along GATA1 gene. Mutations at the boundaries of exon 2 (green), mutations within the sequences that encode the N-terminal zinc finger (purple), and those within intron 5 (red) are depicted. The mutations at the boundaries of exon 2 cause splicing defects that result in the exclusive generation of GATA1s, while mutations within exon 4 create variants that attenuate the GATA1-FOG1 interaction or impair DNA binding. The g.7230C>T alteration causes a five-amino acid (PPFWQ) insertion at the C-terminus of the C-terminal zinc finger. GATA1 cofactors and their interacting domains are shown on top. N-TAD, amino-terminal trans-activation domain; N-ZF, amino-terminal zinc finger; C-ZF, carboxyl-terminal zinc finger.
Of note, both N- and C-terminal domains of GATA1 have been shown to be important for regulation of expression of its target genes. For example, the Orkin laboratory showed that N-terminus is required for trans-activation by structure-function analysis32. Work by other groups demonstrated that both the N-terminus and C-terminus are important for GATA1 trans-activation of downstream target genes, and deletion of either of those transactivation regions led to deceased expression of downstream targets without affecting DNA binding or self-association activities33. Previous studies revealed that the N-terminus is required for proper chromatin occupancy of GATA1s in the G1ME model of erythron-megakaryocytic differentiation34,35. Genes that were not bound by GATA1s included prominent erythroid regulatory genes such as KLF134. More recently, our group has found that loss of the N-terminus alters chromatin accessibility and methylation of H3K27, resulting in impaired expression of erythroid genes36. Although a prior study reported that the N-terminus associated with Rb and regulates erythropoiesis37, the protein partners that bind the N-terminus to mediate its function remain largely undefined.
Previous studies have shown that GATA1 is a pioneer transcription factor that is sufficient to reprogram other hematopoietic cells to an erythroid fate38,39. Moreover, there is growing evidence that GATA1 temporally and spatially regulates downstream genes by interacting with other transcriptional partners. For example, GATA1 self-association (i.e. dimerization) was shown to be essential for its transcription regulation activity, and the N-ZF-C-ZF contact has been reported to be important for GATA1 intermolecular dimerization40,41. Furthermore, the Orkin laboratory demonstrated that there is a physical interaction between GATA1 and SP1, and EKLF in the absence of DNA42. SP1 was found to recognize GC and/or GT/CACC motifs and recruit GATA1 to promoters by interacting with the C-ZF of GATA142. The function of the GATA1/SP1 or EKLF interactions has been confirmed in Drosophila S2 cells with erythroid promoter driven reporters43.
Another key cofactor of GATA1 is Friend of GATA1 (FOG1), which was first identified through the yeast two-hybrid system as a binding partner of GATA144. The sixth zinc finger of FOG1, a protein with nine zinc fingers, interacts with the N-ZF of GATA145. Mutations within the GATA1 N-ZF that abolish the interaction between GATA1 and FOG1, but not the interaction between GATA1 and DNA, such as E203V, V205G and H222R, fail to induce differentiation of erythroid cells46. Restoring the interaction by creating second site mutations in FOG1 which can bind the GATA1 V205G mutant, rescued erythropoiesis, confirming that the interaction is critical for red cells46. As discussed below, further evidence of the requirement for the interaction between GATA1 and FOG1 in erythropoiesis was provided by the identification of FOG1 non-interacting GATA1 mutations in patients with dyserythropoietic anemia47.
A surprising discovery was the observation that a GATA1 mutant (V205), which failed to bind FOG1, displayed a striking redistribution on chromatin. Instead of being primary bound to erythroid and megakaryocytic genes, the V205G variant bound to a unique set of genes that are associated with mast cells48. Expression of FOG1 in mast cells led to displacement of GATA1 from mast cell genes and reduced their expression. Overall these studies demonstrate that FOG1 can alter GATA1 chromatin binding and thus impact lineage decisions.
The N-ZF can also interact with LMO2 (LIM2 domain) in a complex that includes FOG1 and TAL1/E2A/LMO2/LDB1. This complex can co-occupy E-box/(T/A)GATA(A/G) sites to facilitate GATA1-mediated activation of transcription49,50. Additional studies by Blobel and colleagues revealed that this complex is a positive regulator of gene expression, and that specifically the inclusion of TAL1 biases the activity towards transcriptional activation51. FOG1 can activate expression of the p45 NF-E2 promoter52, and also activate expression of some genes. Of note, FOG1 also acts a transcriptional repressor through its interaction with the co-repressor CtBP252,53. More recent studies reveal that GATA1 can associate with epigenetic factors such as PRC2 and NuRD to control gene expression50,54–56. PRC2 has been implicated in suppression of a number of GATA1 targets, including c-Kit, Gata2, and Myb 50,55,56
Another partner of GATA1 is the Ets family member PU.1 (Spi-1), a master transcription regulator for myeloid lineage development. The C-ZF of GATA1 interacts with the Ets domain of PU.1, and the GATA1/PU.1 interaction has been shown critical for erythroid differentiation by impeding the activity of PU.1 through blocking its interaction with c-Jun57. The N-terminus of PU.1 has been also shown to repress the function of GATA1 by reducing the binding of GATA1 to DNA58. Moreover, GATA1 can inhibit the ability of PU.1 to transactivation of myeloid target promoters, and overexpression of PU.1 represses GATA1-mediated transactivation and block terminal differentiation of erythroid cells59–61.
Studies of post-translational modifications (PTM) provide a different angle to better understand GATA1 function. CBP/p300 physically interacts with GATA1 to stimulate GATA1’s transcriptional activity, and preventing the interaction between these proteins blocked erythroid differentiation62. Mechanistically, it has been reported that p300 acetylates GATA1 and enhances the GATA1-DNA interaction, which can stimulate GATA1-dependent transcription63. Phosphorylation of GATA1 also has been shown to increase the DNA-binding affinity of GATA1, and this is correlated with erythroid induction of K562 cells64. Finally, a recent study revealed that the deubiquitinase USP7 binds to GATA1 and catalyzes the removal of polyubiquitylation chains conjugated on K48, which results in stabilizing GATA1 during human terminal erythroid differentiation65.
GATA1 Dysregulation in Human Red Blood Cell Disorders
GATA1 is mutated in a number of human red blood cell disorders (Figure 1 and Table 2). These mutations include missense mutations in the N-ZF and the N-terminus. Alterations in regulatory elements that affect GATA1 occupancy are also seen in rare cases of red cell disorders.
Table 2.
GATA1 mutations identified in patients with congenital red blood cell disorders
| Pathogenic mutations | Annotation of mutation | Red blood cell disorders | Reference | |
|---|---|---|---|---|
| Genomic change | Predicted protein change | |||
| c.613G>A | p.Val205Met (V205M) | Mutation in N-ZF of GATA1, strongly reduced FOG1 binding | Dyserythropoietic anemia | 47 |
| c.622G>C | p.Gly208Arg (G208R) | Mutation in N-ZF of GATA1 | Dyserythropoietic anemia | 114,115 |
| c.647G>A | p.Arg216Gln (R216Q) | Mutation in N-ZF of GATA1 | β-Thalassemia, dyserythropoietic anemia | 79,116–118 |
| c.646C>T | p.Arg216Trp (R216W) | Mutation in N-ZF of GATA1 | β-Thalassemia, microcytic anemia, congenital erythropoietic porphyria | 119 |
| c.653A>G | p.Asp218Gly (D218G) | Mutation in N-ZF of GATA1, reduced FOG1 binding | Dysmorphic red blood cells | 120 |
| c.652G>T | p.Asp218Tyr (D218Y) | Mutation in N-ZF of GATA1, strongly reduced FOG1 binding | Dyserythropoietic anemia | 121 |
| c.652G>A | p.Asp218Asn (D218N) | Mutation in N-ZF of GATA1 | Dyserythropoiesis | 122 |
| g.7230C>T | Five–amino acid insertion at the C-terminus of the C-ZF | Dyserythropoietic anemia | 84 | |
| c.220G>C | Splicing defect; Loss of GATA1, increased GATA1s | Macrocytic anemia | 83 | |
| c.220G>C | Splicing defect; Loss of GATA1, favoring GATA1s production | Diamond–Blackfan Anemia | 98,101 | |
| c.220delG | Splicing defect; Loss of GATA1, favoring GATA1s production | Diamond–Blackfan Anemia | 98 | |
| c.-21A>G | Splicing defect; Loss of GATA1, GATA1s only | Dyserythropoietic Anemia | 123 | |
| c.2T>C | Translation defect; favoring GATA1s production | Diamond–Blackfan Anemia | 99,100 | |
Cis element substitutions that attenuate the interaction of GATA1 with DNA
Genetic mutations in cis regulatory elements, which affect the ability of GATA1 to bind DNA and properly regulate downstream gene expression can result in human blood disorders. For example, a single change at −175 (T to C) in the human γ-globin promoter, which disrupts the interaction between GATA1 and the GATA motif, is associated with increased fetal hemoglobin in nondeletion hereditary persistence of fetal hemoglobin (HPFH)66. Congenital erythropoietic porphyria (CEP), also known as Günther disease, is another disorder associated with cis-element mutations; a −70T->C substitution in the uroporphyrinogen III synthase (URO-synthase) locus was found in a CEP patient67. This change alters the binding of GATA1 and impairs erythroid-specific transcription/heme biosynthesis.
Another example includes the cis-element of 5-aminolevulinate synthase (ALAS2), which encodes an enzyme necessary for heme biosynthesis. Mutations in a GATA1 binding enhancer (int-1-GATA) of ALAS2 was identified in patients with sideroblastic anemia68,69. Further investigation showed that deletion of the int-1-GATA site disrupts the recruitment of the GATA1/TAL1/LMO2/LDB1 complex and fails to fully activate ALAS2 transcription, leading to a failure of developing mature red blood cells and embryonic lethality of mutant males at E12.570,71. A fourth example can be found in the PKLR gene, which encodes pyruvate kinase, an enzyme that catalyzes the final step of glycolysis. Pyruvate kinase deficiency (PKD) is one of the most common causes of hereditary non-spherocytic hemolytic anemia. A mutation in a GATA-motif in the PKLR promoter results in substantial decrease in gene expression and results in the anemia72,73.
Genome-wide association studies (GWAS) have identified 75 genetic loci associated with erythroid phenotypes, with many of the SNPs postulated to influence gene regulation74. The Sankaran laboratory leveraged a massively parallel reporter assay (MPRA) to explore the association of GWAS variants with human erythroid phenotypes, leading to the identification of 32 massively parallel reporter assay functional variants (MFVs) representing 23 of the original 75 GWAS hits75. Further functional investigation of three MFVs confirmed endogenous enhancer regulatory activity that affected expression of nearby target genes, which included SMIM1, RBM38, and CD16475. In a subsequent study, this group integrated data from chromatin accessibility studies and variants associated with erythroid traits. The study led to the discovery that genetic variants affect differentiation at various stages. Moreover, the work revealed that the GATA1 complex temporally and stage-specifically regulates accessibility regulatory elements and associated gene expression76.
Mutations in the N-ZF of GATA1
Residues that are critical for the interaction between GATA1 and FOG1 were initially identified through a yeast-two hybrid assay46. These include E203, V205, and H222, all contained within the N-ZF. Additional mutations in the four cysteine residues were identified; these likely affect the folding of the entire finger leading to impaired FOG1 binding.
Remarkably, Nichols and colleagues discovered that GATA1 was mutated in a family with a rare form of dyserythropoietic anemia and thrombocytopenia77. The affected individuals harbored the V205M mutation, which like V205G, fails to interact with FOG1. Subsequent work by other groups identified additional N-ZF mutations that alter FOG1 binding and lead to a spectrum of related erythroid and platelet disorder78. Distinct mutations in GATA1 that alter DNA binding, were discovered in affected members of other families with congenital erythroid disorders78. For example, the R216Q missense mutation in the GATA1 N-ZF was identified in a patient with X-linked thrombocytopenia with thalassemia; this change was associated with comparable affinity to single GATA motifs but decreased affinity to palindromic sites79.
GATA1 mutations that lead to expression of the GATA1s isoform
In 1995, Calligaris and colleagues were the first to report that a ~40 kDa protein could be recognized by GATA1 specific antibodies80. This protein, determined to be a shorter isoform of GATA1 that begins at methionine at position 84 at the end of the N-TAD domain, is referred to as GATA1s. Genetic studies in 2002 identified mutations within exon 2 of GATA1 that lead to loss of the full-length protein but allow for expression of GATA1s: these mutations were initially found in leukemic blasts from patients with acute megakaryoblastic leukemia who had Down syndrome (DS-AMKL)81. The same mutations are also seen in the transient leukemia of Down syndrome, transient abnormal myelopoiesis82.
Germline mutations that lead to the favored/exclusive production of the GATA1s isoform have also been identified in a small subset of patients with Diamond Blackfan Anemia (DBA) who lack ribosomal gene mutations (Table 2) (discussed below under GATA1 mutations in DBA). The mutation has also been reported in a family with impaired erythropoiesis83.
An Intronic GATA1 mutation
An intronic mutation in GATA1 (chrX:48,652,176 C>T in hg19) was recently found in two patients with dyserythropoietic anemia by the Sankaran laboratory84. This mutation is located 24 nucleotides upstream of the canonical splice acceptor site in the fifth intron of GATA1, and its alteration results in reduced splicing and a five-amino acid insertion at the C-terminus of the C-ZF. Of note, the mutant protein displayed no observable transactivation activity, suggesting a mechanism by which this allele impairs erythropoiesis.
GATA1 Mutations in DBA
DBA is a rare congenital and inherited bone marrow disorder characterized by erythroid hypoplasia and minimal defects in other hematopoietic cells. Congenital abnormalities and a predisposition to cancer accompany the erythroid defect. DBA was first characterized by Hugh Josephs in 193685, and then described by pediatricians Louis Diamond and Kenneth Blackfan in 193886. Most DBA patients are diagnosed in their first year after birth and have a lifelong impairment in erythropoiesis. Cells within colony-forming unit-erythroid (CFU-E) and burst-forming unit-erythroid (BFU-E) from DBA patients rapidly undergo apoptosis upon erythropoietin deprivation87.
After the first genetic mutation, RPS19, was found in DBA patients in 199988, much attention has been drawn to studies of ribosome protein (RP) encoding genes. About 70% DBA patients have loss-of-function mutations of ribosomal protein encoding genes, which suggests that a malfunctioning translational machinery is a major contributor to the pathogenesis of DBA89–91 (Figure 2). Alterations in ribosome biogenesis are known to trigger activation of the P53 pathway and cell-cycle arrest92–95, and the increased P53 activity likely contributes to the impaired erythropoiesis.
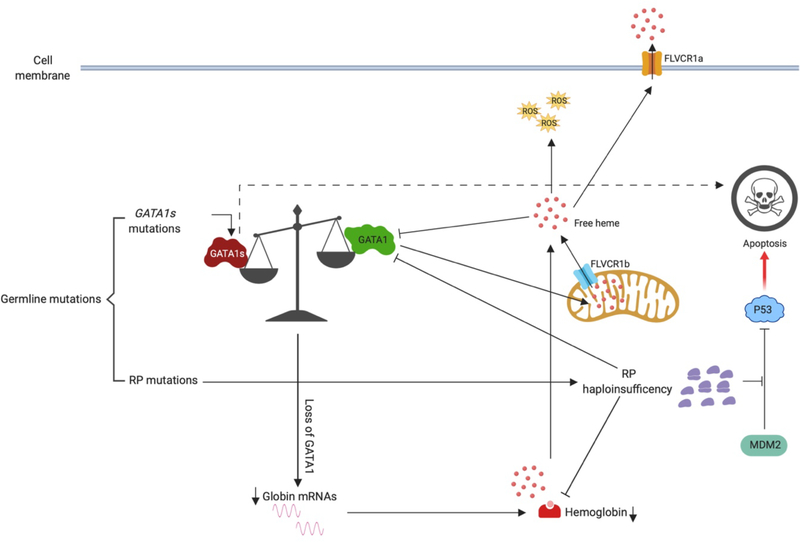
Figure 2. Mechanisms that lead to Diamond-Blackfan anemia.
The majority of germline mutations found in DBA patients reside in ribosome protein (RP) coding genes. The resulting RP haploinsufficiency leads to inefficient translation of a number of erythroid genes, such as globin and GATA1, resulting in impaired erythropoiesis. Moreover, free ribosomal subunits, such as RPL5 and RPL23, block MDM2-mediated P53 ubiquitination and degradation, leading an increase of P53-dependent apoptosis of the erythroid progenitors. In other patients, GATA1 gene mutations result in loss of the full-length protein but allow for expression of the shorter isoform (GATA1s mutations), which also impairs erythropoiesis. Finally, an altered globin-heme balance has been shown to lead to the accumulation of free heme in cytoplasm, which downregulates GATA1 at both the mRNA and protein level. FLVCR1a and FLVCR1b are heme transporters on cell membrane and mitochondrial membrane respectively.
Mutations in RPS19 alter ribosome biogenesis through impairing 18S rRNA synthesis and 40S subunits assembly, which subsequently promotes apoptosis96. Indeed, by comparing transcriptomes of three populations, CD34+CD71−CD45RA−, CD34+CD71hiCD45RA− and CD34+CD71lowCD45RA+, from DBA patients versus control donors, Gazda and colleagues found that apoptotic genes were strongly upregulated in diseased erythroid cells harboring the RPS19 mutation97.
In 2012 Sankaran and colleagues reported that two siblings and a third individual with DBA who lack ribosomal gene mutations carried a GATA1 mutation98. Subsequent studies identified additional cases of DBA patients with GATA1 mutations99–101. In several cases, the patients were found to harbor a GATA1 mutation (c.220G>C) that affects splicing and leads to a strong reduction in expression of full length GATA1, favoring expression of GATA1s98,101. An ATG to ACG mutation in the first translation initiation codon of GATA1 has also been found in DBA patients; this change also favors GATA1s expression, as it results in a near complete loss of full length GATA1 but persistent expression of GATA1s99,100. Together these findings in patients suggest that the disruption of the levels of GATA1 versus GATA1s contribute to the pathogenesis of DBA. In follow up studies, Sankaran and colleagues investigated the relationship between DBA cases with GATA1 mutations and those with ribosomal gene mutations99. They proposed that that ribosomal gene mutations result in anemia by reducing the production of full-length GATA1. Reduced translation of GATA1 was indeed observed upon knockdown of ribosome proteins; this was a selective effect in that other erythroid regulators such as EPOR, TAL1, transferrin receptor (CD71) and STAT5A were not significantly downregulated. Mechanistically, they found that the secondary structure of the GATA1 5’-UTR, which can affect the efficiency of translation, led to reduced translation under conditions of limiting ribosomes. Expression of a GATA1 cDNA with modified 5’-UTR, in which the ribosome levels sensitive structures were removed, was able to rescue erythroid differentiation of DBA CD34+ cells by two- to fourfold99.
A previous study in yeast revealed that a ribosome deficiency can affect expression of a specific subset of genes102. However, how the changes in the ribosome machinery in DBA selectively impairs erythropoiesis has been unclear. Recent work by the Sankaran laboratory revealed that transcripts with shorter 5’-UTR, which are predicted to have less complex secondary structure and fewer upstream start codons (uAUGs), are more sensitive to ribosome protein haploinsufficiency17. Significantly longer 5′ UTR lengths and more complex 5′-UTR structures were found in most hematopoietic master regulators compared to the group of transcripts showing sensitivity to ribosomal protein haploinsufficiency17. Intriguingly, although GATA1 has a relative short and unstructured 5’-UTR, it exhibits unique features among hematopoietic transcriptional regulators, which may give GATA1 mRNA a higher translation efficiency but make it more sensitive to RP haploinsufficiency in DBA patients17.
Another recent paper provided another piece of evidence in support of transcript-specific defects in translation of GATA1. This study showed that ribonuclease inhibitor 1 (RNH1)-deficient murine embryos died between E8.5 and E10 due to impaired maturation of erythroid progenitors. The authors found that RNH1 interacts with the 40S subunit of ribosomes and facilitates polysome formation on Gata1 mRNA to confer transcript-specific translation103.
Heme toxicity is thought to be another contributor to pathogenesis of DBA. In 2004, Abkowitz and colleagues identified Feline Leukemia Virus subgroup C Receptor (FLVCR), which exports cytoplasmic free heme, and reported it is required for erythroid cell development by protecting them from heme toxicity104. This study also showed that FLVCR was highly expressed on cell surface of CFU-E and decreased as erythropoiesis proceeds. FLVCR has two isoforms, FLVCR1a and FLVCR1b, and they function as heme transporters on cell surface membrane and mitochondrial membrane separately. Both FLVCR1a and FLVCR1b are required for heme homeostasis and for expansion of committed erythroid progenitors105. Later studies provided new insights into GATA1globin-heme feedback loop in the pathogenesis of DBA. Two recent papers suggest that decrease of GATA1 full length protein resulting from RP haploinsufficiency and deficiency of HSP70 can disturb the balance of globin-heme and leads to the accumulation of free cytoplasmic heme in erythroid progenitors, which can cause increase of P53-dependent apoptosis of DBA erythroid cells106–108. Of note, unlike in the presence of RP mutations in DBA patients, accumulation of heme upon only FLVCR1 depletion causes P53-independent apoptosis107.
Finally, work by Bodine and colleagues suggest yet another model for pathogenesis of DBA109. By culturing CD34+ cells from patients, they found that both RP and GATA1 mutant cells showed reduced proliferation and delayed differentiation. Interestingly, by RNA-seq they discovered that RP mutant cases had prominent upregulation of genes involved in heme biosynthesis while GATA1 mutant cases were associated with enrichment of genes in the translational apparatus. Thus, in this alternative model, dysregulation of translation appears to be a theme shared by these two groups of patients.
Animal models of GATA1s display a transient anemia with some parallels to DBA
A mouse model of Gata1s mutations was created by the Orkin laboratory110. Gata1s mutant animals display anemia in mid-gestation which improves with age. This fetal anemia is accompanied by a prominent transient expansion of megakaryocytes that bears some similarity to the transient leukemia seen in children with DS. Although the erythroid phenotype was initially reported to revert to normal by birth, ongoing studies reveal that there is a persistent underlying defect in erythropoiesis (TL and JDC unpublished observations). Mechanistically, the short isoform was found to be differentially occupy chromatin of a subset of target genes in Gata1s fetal liver erythroid36. Moreover, the loss of the N-TAD was associated with altered chromatin accessibility and reduced H3K27me3 modifications along the Gata2 and Runx1 loci, explaining their aberrant increased expression in Gata1s proerythroblasts. These observations further suggest that the N-TAD may be involved in the recruitment of other epigenetic factors to regulate the accessibility of regulatory elements of GATA1 downstream target genes. Of note, reducing the levels of GATA2 substantially rescued erythropoiesis in the Gata1s embryos indicating that the impaired downregulation by GATA1s is a key pathogenic event. Future studies will clarify the extent to which aberrant erythropoiesis in Gata1s mice is relevant to human DBA.
Conclusions
GATA1 was originally identified as a key regulator of erythroid cell development. In the absence of GATA1, mouse embryos die in mid-gestation from severe anemia. This essential function of Gata1 in mice supported the belief that GATA1 mutations would not be found in humans. However, studies over the past 20 years have shown that GATA1 mutations, although rare, can indeed be found in patients with a spectrum of red cell disorders, including congenital dyserythropoietic anemia and DBA. Molecular studies revealed that all three functional domains of GATA1 affect chromatin occupancy and its ability to properly regulate gene expression.
Acknowledgements
This review was supported by grants from the National Institutes of Health (DK101329) and the Samuel Waxman Cancer Research Foundation.
Footnotes
Conflict of Interest
JDC has research funding from Scholar Rock and Forma Therapeutics, is a consultant for Sierra Oncology and is the Scientific Advisor of the MPN Research Foundation.
References
- 1.Zon LI, Gurish MF, Stevens RL, et al. GATA-binding transcription factors in mast cells regulate the promoter of the mast cell carboxypeptidase A gene. J Biol Chem. 1991;266(34):22948–22953. [PubMed] [Google Scholar]
- 2.Martin DI, Zon LI, Mutter G, Orkin SH. Expression of an erythroid transcription factor in megakaryocytic and mast cell lineages. Nature. 1990;344(6265):444–447. [DOI] [PubMed] [Google Scholar]
- 3.Zon LI, Yamaguchi Y, Yee K, et al. Expression of mRNA for the GATA-binding proteins in human eosinophils and basophils: potential role in gene transcription. Blood. 1993;81(12):3234–3241. [PubMed] [Google Scholar]
- 4.Trainor CD, Evans T, Felsenfeld G, Boguski MS. Structure and evolution of a human erythroid transcription factor. Nature. 1990;343(6253):92–96. [DOI] [PubMed] [Google Scholar]
- 5.Zon LI, Tsai SF, Burgess S, Matsudaira P, Bruns GA, Orkin SH. The major human erythroid DNA-binding protein (GF-1): primary sequence and localization of the gene to the X chromosome. Proc Natl Acad Sci U S A. 1990;87(2):668–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai SF, Martin DI, Zon LI, D’Andrea AD, Wong GG, Orkin SH. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature. 1989;339(6224):446–451. [DOI] [PubMed] [Google Scholar]
- 7.Evans T, Felsenfeld G. The erythroid-specific transcription factor Eryf1: a new finger protein. Cell. 1989;58(5):877–885. [DOI] [PubMed] [Google Scholar]
- 8.Nei Y, Obata-Ninomiya K, Tsutsui H, et al. GATA-1 regulates the generation and function of basophils. Proc Natl Acad Sci U S A. 2013;110(46):18620–18625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drissen R, Buza-Vidas N, Woll P, et al. Distinct myeloid progenitor-differentiation pathways identified through single-cell RNA sequencing. Nat Immunol. 2016;17(6):666–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito E, Toki T, Ishihara H, et al. Erythroid transcription factor GATA-1 is abundantly transcribed in mouse testis. Nature. 1993;362(6419):466–468. [DOI] [PubMed] [Google Scholar]
- 11.Lindeboom F, Gillemans N, Karis A, et al. A tissue-specific knockout reveals that Gata1 is not essential for Sertoli cell function in the mouse. Nucleic Acids Res. 2003;31(18):5405–5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakabayashi J, Yomogida K, Nakajima O, et al. GATA-1 testis activation region is essential for Sertoli cell-specific expression of GATA-1 gene in transgenic mouse. Genes to Cells. 2003;8(7):619–630. [DOI] [PubMed] [Google Scholar]
- 13.Fujiwara Y, Chang AN, Williams AM, Orkin SH. Functional overlap of GATA-1 and GATA-2 in primitive hematopoietic development. Blood. 2004;103(2):583–585. [DOI] [PubMed] [Google Scholar]
- 14.Vannucchi AM, Linari S, Lin CS, Koury MJ, Bondurant MC, Migliaccio AR. Increased expression of the distal, but not of the proximal, Gata1 transcripts during differentiation of primary erythroid cells. J Cell Physiol. 1999;180(3):390–401. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi S, Onodera K, Motohashi H, et al. Arrest in primitive erythroid cell development caused by promoter-specific disruption of the GATA-1 gene. J Biol Chem. 1997;272(19):12611–12615. [DOI] [PubMed] [Google Scholar]
- 16.Notta F, Zandi S, Takayama N, et al. Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science. 2016;351(6269):aab2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khajuria RK, Munschauer M, Ulirsch JC, et al. Ribosome Levels Selectively Regulate Translation and Lineage Commitment in Human Hematopoiesis. Cell. 2018;173(1):90–103 e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mouthon MA, Bernard O, Mitjavila MT, Romeo PH, Vainchenker W, Mathieu-Mahul D. Expression of tal-1 and GATA-binding proteins during human hematopoiesis. Blood. 1993;81(3):647–655. [PubMed] [Google Scholar]
- 19.Pevny L, Simon MC, Robertson E, et al. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991;349(6306):257–260. [DOI] [PubMed] [Google Scholar]
- 20.Fujiwara Y, Browne CP, Cunniff K, Goff SC, Orkin SH. Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc Natl Acad Sci U S A. 1996;93(22):12355–12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss MJ, Orkin SH. Transcription factor GATA-1 permits survival and maturation of erythroid precursors by preventing apoptosis. Proc Natl Acad Sci U S A. 1995;92(21):9623–9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDevitt MA, Shivdasani RA, Fujiwara Y, Yang H, Orkin SH. A “knockdown” mutation created by cis-element gene targeting reveals the dependence of erythroid cell maturation on the level of transcription factor GATA-1. Proc Natl Acad Sci U S A. 1997;94(13):6781–6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suwabe N, Takahashi S, Nakano T, Yamamoto M. GATA-1 regulates growth and differentiation of definitive erythroid lineage cells during in vitro ES cell differentiation. Blood. 1998;92(11):4108–4118. [PubMed] [Google Scholar]
- 24.Takahashi S, Shimizu R, Suwabe N, et al. GATA factor transgenes under GATA-1 locus control rescue germline GATA-1 mutant deficiencies. Blood. 2000;96(3):910–916. [PubMed] [Google Scholar]
- 25.Whyatt DJ, deBoer E, Grosveld F. The two zinc finger-like domains of GATA-1 have different DNA binding specificities. EMBO J. 1993;12(13):4993–5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Omichinski JG, Clore GM, Schaad O, et al. NMR structure of a specific DNA complex of Zn-containing DNA binding domain of GATA-1. Science. 1993;261(5120):438–446. [DOI] [PubMed] [Google Scholar]
- 27.Omichinski JG, Trainor C, Evans T, Gronenborn AM, Clore GM, Felsenfeld G. A small single-”finger” peptide from the erythroid transcription factor GATA-1 binds specifically to DNA as a zinc or iron complex. Proc Natl Acad Sci U S A. 1993;90(5):1676–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trainor CD, Omichinski JG, Vandergon TL, Gronenborn AM, Clore GM, Felsenfeld G. A palindromic regulatory site within vertebrate GATA-1 promoters requires both zinc fingers of the GATA-1 DNA-binding domain for high-affinity interaction. Mol Cell Biol. 1996;16(5):2238–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trainor CD, Ghirlando R, Simpson MA. GATA zinc finger interactions modulate DNA binding and transactivation. J Biol Chem. 2000;275(36):28157–28166. [DOI] [PubMed] [Google Scholar]
- 30.Visvader JE, Crossley M, Hill J, Orkin SH, Adams JM. The C-Terminal Zinc-Finger of Gata-1 or Gata-2 Is Sufficient to Induce Megakaryocytic Differentiation of an Early Myeloid Cell-Line. Mol Cell Biol. 1995;15(2):634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blobel GA, Simon MC, Orkin SH. Rescue of GATA-1-deficient embryonic stem cells by heterologous GATA-binding proteins. Mol Cell Biol. 1995;15(2):626–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin DI, Orkin SH. Transcriptional activation and DNA binding by the erythroid factor GF-1/NF-E1/Eryf 1. Genes Dev. 1990;4(11):1886–1898. [DOI] [PubMed] [Google Scholar]
- 33.Kaneko H, Kobayashi E, Yamamoto M, Shimizu R. N- and C-terminal transactivation domains of GATA1 protein coordinate hematopoietic program. J Biol Chem. 2012;287(25):21439–21449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chlon TM, McNulty M, Goldenson B, Rosinski A, Crispino JD. Global transcriptome and chromatin occupancy analysis reveal the short isoform of GATA1 is deficient for erythroid specification and gene expression. Haematologica. 2015;100(5):575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byrska-Bishop M, VanDorn D, Campbell AE, et al. Pluripotent stem cells reveal erythroid-specific activities of the GATA1 N-terminus. J Clin Invest. 2015;125(3):993–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ling T, Birger Y, Stankiewicz MJ, et al. Chromatin occupancy and epigenetic analysis reveal new insights into the function of GATA1 N-terminus in erythropoiesis. Blood. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kadri Z, Shimizu R, Ohneda O, et al. Direct binding of pRb/E2F-2 to GATA-1 regulates maturation and terminal cell division during erythropoiesis. PLoS Biol. 2009;7(6):e1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwasaki H, Mizuno S, Wells RA, Cantor AB, Watanabe S, Akashi K. GATA-1 converts lymphoid and myelomonocytic progenitors into the megakaryocyte/erythrocyte lineages. Immunity. 2003;19(3):451–462. [DOI] [PubMed] [Google Scholar]
- 39.Kulessa H, Frampton J, Graf T. Gata-1 Reprograms Avian Myelomonocytic Cell-Lines into Eosinophils, Thromboblasts, and Erythroblasts. Genes Dev. 1995;9(10):1250–1262. [DOI] [PubMed] [Google Scholar]
- 40.Mackay JP, Kowalski K, Fox AH, Czolij R, King GF, Crossley M. Involvement of the N-finger in the self-association of GATA-1. J Biol Chem. 1998;273(46):30560–30567. [DOI] [PubMed] [Google Scholar]
- 41.Crossley M, Merika M, Orkin SH. Self-association of the erythroid transcription factor GATA-1 mediated by its zinc finger domains. Mol Cell Biol. 1995;15(5):2448–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merika M, Orkin SH. Functional synergy and physical interactions of the erythroid transcription factor GATA-1 with the Kruppel family proteins Sp1 and EKLF. Mol Cell Biol. 1995;15(5):2437–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gregory RC, Taxman DJ, Seshasayee D, Kensinger MH, Bieker JJ, Wojchowski DM. Functional interaction of GATA1 with erythroid Kruppel-like factor and Sp1 at defined erythroid promoters. Blood. 1996;87(5):1793–1801. [PubMed] [Google Scholar]
- 44.Tsang AP, Visvader JE, Turner CA, et al. FOG, a multitype zinc finger protein, acts as a cofactor for transcription factor GATA-1 in erythroid and megakaryocytic differentiation. Cell. 1997;90(1):109–119. [DOI] [PubMed] [Google Scholar]
- 45.Fox AH, Kowalski K, King GF, Mackay JP, Crossley M. Key residues characteristic of GATA N-fingers are recognized by FOG. J Biol Chem. 1998;273(50):33595–33603. [DOI] [PubMed] [Google Scholar]
- 46.Crispino JD, Lodish MB, MacKay JP, Orkin SH. Use of altered specificity mutants to probe a specific protein-protein interaction in differentiation: The GATA-1 : FOG complex. Mol Cell. 1999;3(2):219–228. [DOI] [PubMed] [Google Scholar]
- 47.Nichols KE, Crispino JD, Poncz M, et al. Familial dyserythropoietic anaemia and thrombocytopenia due to an inherited mutation in GATA1. Nat Genet. 2000;24(3):266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chlon TM, Dore LC, Crispino JD. Cofactor-mediated restriction of GATA-1 chromatin occupancy coordinates lineage-specific gene expression. Mol Cell. 2012;47(4):608–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilkinson-White L, Gamsjaeger R, Dastmalchi S, et al. Structural basis of simultaneous recruitment of the transcriptional regulators LMO2 and FOG1/ZFPM1 by the transcription factor GATA1. Proc Natl Acad Sci U S A. 2011;108(35):14443–14448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng Y, Wu W, Kumar SA, et al. Erythroid GATA1 function revealed by genome-wide analysis of transcription factor occupancy, histone modifications, and mRNA expression. Genome Res. 2009;19(12):2172–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tripic T, Deng W, Cheng Y, et al. SCL and associated proteins distinguish active from repressive GATA transcription factor complexes. Blood. 2009;113(10):2191–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holmes M, Turner J, Fox A, Chisholm O, Crossley M, Chong B. hFOG-2, a novel zinc finger protein, binds the co-repressor mCtBP2 and modulates GATA-mediated activation. J Biol Chem. 1999;274(33):23491–23498. [DOI] [PubMed] [Google Scholar]
- 53.Deconinck AE, Mead PE, Tevosian SG, et al. FOG acts as a repressor of red blood cell development in Xenopus. Development. 2000;127(10):2031–2040. [DOI] [PubMed] [Google Scholar]
- 54.Xu J, Shao Z, Li D, et al. Developmental control of polycomb subunit composition by GATA factors mediates a switch to non-canonical functions. Mol Cell. 2015;57(2):304–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ross J, Mavoungou L, Bresnick EH, Milot E. GATA-1 utilizes Ikaros and polycomb repressive complex 2 to suppress Hes1 and to promote erythropoiesis. Mol Cell Biol. 2012;32(18):3624–3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu M, Riva L, Xie H, et al. Insights into GATA-1-mediated gene activation versus repression via genome-wide chromatin occupancy analysis. Mol Cell. 2009;36(4):682–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang P, Behre G, Pan J, et al. Negative cross-talk between hematopoietic regulators: GATA proteins repress PU.1. Proc Natl Acad Sci U S A. 1999;96(15):8705–8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang P, Zhang X, Iwama A, et al. PU.1 inhibits GATA-1 function and erythroid differentiation by blocking GATA-1 DNA binding. Blood. 2000;96(8):2641–2648. [PubMed] [Google Scholar]
- 59.Rekhtman N, Radparvar F, Evans T, Skoultchi AI. Direct interaction of hematopoietic transcription factors PU.1 and GATA-1: functional antagonism in erythroid cells. Genes Dev. 1999;13(11):1398–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nerlov C, Querfurth E, Kulessa H, Graf T. GATA-1 interacts with the myeloid PU.1 transcription factor and represses PU.1-dependent transcription. Blood. 2000;95(8):2543–2551. [PubMed] [Google Scholar]
- 61.Rekhtman N, Choe KS, Matushansky I, Murray S, Stopka T, Skoultchi AI. PU.1 and pRB interact and cooperate to repress GATA-1 and block erythroid differentiation. Mol Cell Biol. 2003;23(21):7460–7474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blobel GA, Nakajima T, Eckner R, Montminy M, Orkin SH. CREB-binding protein cooperates with transcription factor GATA-1 and is required for erythroid differentiation. Proc Natl Acad Sci U S A. 1998;95(5):2061–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boyes J, Byfield P, Nakatani Y, Ogryzko V. Regulation of activity of the transcription factor GATA-1 by acetylation. Nature. 1998;396(6711):594–598. [DOI] [PubMed] [Google Scholar]
- 64.Partington GA, Patient RK. Phosphorylation of GATA-1 increases its DNA-binding affinity and is correlated with induction of human K562 erythroleukaemia cells. Nucleic Acids Res. 1999;27(4):1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liang L, Peng Y, Zhang J, et al. Deubiquitylase USP7 regulates human terminal erythroid differentiation by stabilizing GATA1. Haematologica. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martin DI, Tsai SF, Orkin SH. Increased gamma-globin expression in a nondeletion HPFH mediated by an erythroid-specific DNA-binding factor. Nature. 1989;338(6214):435–438. [DOI] [PubMed] [Google Scholar]
- 67.Solis C, Aizencang GI, Astrin KH, Bishop DF, Desnick RJ. Uroporphyrinogen III synthase erythroid promoter mutations in adjacent GATA1 and CP2 elements cause congenital erythropoietic porphyria. J Clin Invest. 2001;107(6):753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Campagna DR, de Bie CI, Schmitz-Abe K, et al. X-linked sideroblastic anemia due to ALAS2 intron 1 enhancer element GATA-binding site mutations (vol 89, pg 315, 2014). Am J Hematol. 2014;89(6):670–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaneko K, Furuyama K, Fujiwara T, et al. Identification of a novel erythroid-specific enhancer for the ALAS2 gene and its loss-of-function mutation which is associated with congenital sideroblastic anemia. Haematologica. 2014;99(2):252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Y, Zhang J, An W, et al. Intron 1 GATA site enhances ALAS2 expression indispensably during erythroid differentiation. Nucleic Acids Res. 2017;45(2):657–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tanimura N, Miller E, Igarashi K, et al. Mechanism governing heme synthesis reveals a GATA factor/heme circuit that controls differentiation. Embo Reports. 2016;17(2):249–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wakabayashi A, Ulirsch JC, Ludwig LS, et al. Insight into GATA1 transcriptional activity through interrogation of cis elements disrupted in human erythroid disorders. Proc Natl Acad Sci U S A. 2016;113(16):4434–4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manco L, Ribeiro ML, Maximo V, et al. A new PKLR gene mutation in the R-type promoter region affects the gene transcription causing pyruvate kinase deficiency. Br J Haematol. 2000;110(4):993–997. [DOI] [PubMed] [Google Scholar]
- 74.van der Harst P, Zhang W, Mateo Leach I, et al. Seventy-five genetic loci influencing the human red blood cell. Nature. 2012;492(7429):369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ulirsch JC, Nandakumar SK, Wang L, et al. Systematic Functional Dissection of Common Genetic Variation Affecting Red Blood Cell Traits. Cell. 2016;165(6):1530–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ludwig LS, Lareau CA, Bao EL, et al. Transcriptional States and Chromatin Accessibility Underlying Human Erythropoiesis. Cell Rep. 2019;27(11):3228–3240 e3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nichols KE, Crispino JD, Poncz M, et al. Familial dyserythropoietic anaemia and thrombocytopenia due to an inherited mutation in GATA1. Nat Genet. 2000;24(3):266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Crispino JD, Horwitz MS. GATA factor mutations in hematologic disease. Blood. 2017;129(15):2103–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu C, Niakan KK, Matsushita M, Stamatoyannopoulos G, Orkin SH, Raskind WH. X-linked thrombocytopenia with thalassemia from a mutation in the amino finger of GATA-1 affecting DNA binding rather than FOG-1 interaction. Blood. 2002;100(6):2040–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Calligaris R, Bottardi S, Cogoi S, Apezteguia I, Santoro C. Alternative translation initiation site usage results in two functionally distinct forms of the GATA-1 transcription factor. Proc Natl Acad Sci U S A. 1995;92(25):11598–11602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wechsler J, Greene M, McDevitt MA, et al. Acquired mutations in GATA1 in the megakaryoblastic leukemia of Down syndrome. Nat Genet. 2002;32(1):148–152. [DOI] [PubMed] [Google Scholar]
- 82.Mundschau G, Gurbuxani S, Gamis AS, Greene ME, Arceci RJ, Crispino JD. Mutagenesis of GATA1 is an initiating event in Down syndrome leukemogenesis. Blood. 2003;101(11):4298–4300. [DOI] [PubMed] [Google Scholar]
- 83.Hollanda LM, Lima CS, Cunha AF, et al. An inherited mutation leading to production of only the short isoform of GATA-1 is associated with impaired erythropoiesis. Nat Genet. 2006;38(7):807–812. [DOI] [PubMed] [Google Scholar]
- 84.Abdulhay NJ, Fiorini C, Verboon JM, et al. Impaired human hematopoiesis due to a cryptic intronic GATA1 splicing mutation. J Exp Med. 2019;216(5):1050–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Josephs HW. Anaemia of infancy and early childhood. Medicine. 1936;15(3):307–451. [Google Scholar]
- 86.Diamond LK. Hypoplastic anemia. Am J Dis Child. 1938;56:464. [Google Scholar]
- 87.Perdahl EB, Naprstek BL, Wallace WC, Lipton JM. Erythroid Failure in Diamond-Blackfan Anemia Is Characterized by Apoptosis. Blood. 1994;83(3):645–650. [PubMed] [Google Scholar]
- 88.Draptchinskaia N, Gustavsson P, Andersson B, et al. The gene encoding ribosomal protein S19 is mutated in Diamond-Blackfan anaemia. Nat Genet. 1999;21(2):169–175. [DOI] [PubMed] [Google Scholar]
- 89.Boria I, Garelli E, Gazda HT, et al. The ribosomal basis of Diamond-Blackfan Anemia: mutation and database update. Hum Mutat. 2010;31(12):1269–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Clinton C, Gazda HT. Diamond-Blackfan Anemia In: Adam MP, Ardinger HH, Pagon RA, et al. , eds. GeneReviews((R)). Seattle (WA): University of Washington, Seattle; 1993. [PubMed] [Google Scholar]
- 91.Ulirsch JC, Verboon JM, Kazerounian S, et al. The Genetic Landscape of Diamond-Blackfan Anemia. Am J Hum Genet. 2018;103(6):930–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dai MS, Zeng SX, Jin Y, Sun XX, David L, Lu H. Ribosomal protein L23 activates p53 by inhibiting MDM2 function in response to ribosomal perturbation but not to translation inhibition. Mol Cell Biol. 2004;24(17):7654–7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dai MS, Lu H. Inhibition of MDM2-mediated p53 ubiquitination and degradation by ribosomal protein L5. J Biol Chem. 2004;279(43):44475–44482. [DOI] [PubMed] [Google Scholar]
- 94.Lohrum MA, Ludwig RL, Kubbutat MH, Hanlon M, Vousden KH. Regulation of HDM2 activity by the ribosomal protein L11. Cancer Cell. 2003;3(6):577–587. [DOI] [PubMed] [Google Scholar]
- 95.Dutt S, Narla A, Lin K, et al. Haploinsufficiency for ribosomal protein genes causes selective activation of p53 in human erythroid progenitor cells. Blood. 2011;117(9):2567–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Choesmel V, Bacqueville D, Rouquette J, et al. Impaired ribosome biogenesis in Diamond-Blackfan anemia. Blood. 2007;109(3):1275–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gazda HT, Kho AT, Sanoudou D, et al. Defective ribosomal protein gene expression alters transcription, translation, apoptosis, and oncogenic pathways in Diamond-Blackfan anemia. Stem Cells. 2006;24(9):2034–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sankaran VG, Ghazvinian R, Do R, et al. Exome sequencing identifies GATA1 mutations resulting in Diamond-Blackfan anemia. J Clin Invest. 2012;122(7):2439–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ludwig LS, Gazda HT, Eng JC, et al. Altered translation of GATA1 in Diamond-Blackfan anemia. Nat Med. 2014;20(7):748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Parrella S, Aspesi A, Quarello P, et al. Loss of GATA-1 full length as a cause of Diamond-Blackfan anemia phenotype. Pediatr Blood Cancer. 2014;61(7):1319–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Klar J, Khalfallah A, Arzoo PS, Gazda HT, Dahl N. Recurrent GATA1 mutations in Diamond-Blackfan anaemia. Br J Haematol. 2014;166(6):949–951. [DOI] [PubMed] [Google Scholar]
- 102.Cheng Z, Mugler CF, Keskin A, et al. Small and Large Ribosomal Subunit Deficiencies Lead to Distinct Gene Expression Signatures that Reflect Cellular Growth Rate. Mol Cell. 2019;73(1):36-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chennupati V, Veiga DF, Maslowski KM, et al. Ribonuclease inhibitor 1 regulates erythropoiesis by controlling GATA1 translation. J Clin Invest. 2018;128(4):1597–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Quigley JG, Yang Z, Worthington MT, et al. Identification of a human heme exporter that is essential for erythropoiesis. Cell. 2004;118(6):757–766. [DOI] [PubMed] [Google Scholar]
- 105.Mercurio S, Petrillo S, Chiabrando D, et al. The heme exporter Flvcr1 regulates expansion and differentiation of committed erythroid progenitors by controlling intracellular heme accumulation. Haematologica. 2015;100(6):720–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rio S, Gastou M, Karboul N, et al. Regulation of globin-heme balance in Diamond-Blackfan anemia by HSP70/GATA1. Blood. 2019;133(12):1358–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Doty RT, Yan X, Lausted C, et al. Single-cell analyses demonstrate that a heme-GATA1 feedback loop regulates red cell differentiation. Blood. 2019;133(5):457–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gastou M, Rio S, Dussiot M, et al. The severe phenotype of Diamond-Blackfan anemia is modulated by heat shock protein 70. Blood Adv. 2017;1(22):1959–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.O’Brien KA, Farrar JE, Vlachos A, et al. Molecular convergence in ex vivo models of Diamond-Blackfan anemia. Blood. 2017;129(23):3111–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li Z, Godinho FJ, Klusmann JH, Garriga-Canut M, Yu C, Orkin SH. Developmental stage-selective effect of somatically mutated leukemogenic transcription factor GATA1. Nat Genet. 2005;37(6):613–619. [DOI] [PubMed] [Google Scholar]
- 111.Kobayashi E, Shimizu R, Kikuchi Y, Takahashi S, Yamamoto M. Loss of the Gata1 gene IE exon leads to variant transcript expression and the production of a GATA1 protein lacking the N-terminal domain. J Biol Chem. 2010;285(1):773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shivdasani RA, Fujiwara Y, McDevitt MA, Orkin SH. A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte growth and platelet development. EMBO J. 1997;16(13):3965–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shimizu R, Kuroha T, Ohneda O, et al. Leukemogenesis caused by incapacitated GATA-1 function. Mol Cell Biol. 2004;24(24):10814–10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kratz CP, Niemeyer CM, Karow A, Volz-Fleckenstein M, Schmitt-Graff A, Strahm B. Congenital transfusion-dependent anemia and thrombocytopenia with myelodysplasia due to a recurrent GATA1(G208R) germline mutation. Leukemia. 2008;22(2):432–434. [DOI] [PubMed] [Google Scholar]
- 115.Del Vecchio GC, Giordani L, De Santis A, De Mattia D. Dyserythropoietic anemia and thrombocytopenia due to a novel mutation in GATA-1. Acta Haematol. 2005;114(2):113–116. [DOI] [PubMed] [Google Scholar]
- 116.Astrom M, Hahn-Stromberg V, Zetterberg E, Vedin I, Merup M, Palmblad J. X-linked thrombocytopenia with thalassemia displays bone marrow reticulin fibrosis and enhanced angiogenesis: comparisons with primary myelofibrosis. Am J Hematol. 2015;90(3):E44–48. [DOI] [PubMed] [Google Scholar]
- 117.Hughan SC, Senis Y, Best D, et al. Selective impairment of platelet activation to collagen in the absence of GATA1. Blood. 2005;105(11):4369–4376. [DOI] [PubMed] [Google Scholar]
- 118.Balduini CL, Pecci A, Loffredo G, et al. Effects of the R216Q mutation of GATA-1 on erythropoiesis and megakaryocytopoiesis. Thromb Haemost. 2004;91(1):129–140. [DOI] [PubMed] [Google Scholar]
- 119.Phillips JD, Steensma DP, Pulsipher MA, Spangrude GJ, Kushner JP. Congenital erythropoietic porphyria due to a mutation in GATA1: the first trans-acting mutation causative for a human porphyria. Blood. 2007;109(6):2618–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Freson K, Devriendt K, Matthijs G, et al. Platelet characteristics in patients with X-linked macrothrombocytopenia because of a novel GATA1 mutation. Blood. 2001;98(1):85–92. [DOI] [PubMed] [Google Scholar]
- 121.Freson K, Matthijs G, Thys C, et al. Different substitutions at residue D218 of the X-linked transcription factor GATA1 lead to altered clinical severity of macrothrombocytopenia and anemia and are associated with variable skewed X inactivation. Hum Mol Genet. 2002;11(2):147–152. [DOI] [PubMed] [Google Scholar]
- 122.Hermans C, De Waele L, Van Geet C, Freson K. Novel GATA1 mutation in residue D218 leads to macrothrombocytopenia and clinical bleeding problems. Platelets. 2014;25(4):305–307. [DOI] [PubMed] [Google Scholar]
- 123.Zucker J, Temm C, Czader M, Nalepa G. A Novel Splice Site Mutation in the 5 ‘ UTR of GATA1 resulting in Abnormal Hematopoiesis. Blood. 2015;126(23). [Google Scholar]