Abstract
Tuberculosis is a major cause of death and disability among children globally, yet children have been neglected in global tuberculosis control efforts. Historically, tuberculosis in children has been thought of as a family disease, and because of this, household contact tracing of children after identification of an adult tuberculosis case has been emphasised as the principal public health intervention. However, the population-level effect of household contact tracing is predicated on the assumption that most paediatric tuberculosis infections are acquired within the household. In this Personal View, we focus on accumulating scientific evidence indicating that the majority of Mycobacterium tuberculosis transmission to children in high-burden settings occurs in the community, outside of households in which a person has tuberculosis. We estimate the population-attributable fraction of M tuberculosis transmission to children due to household exposures to be between 10% and 30%. M tuberculosis transmission from the household was low (<30%) even in children younger than age 5 years. We propose that an effective public health response to childhood tuberculosis requires comprehensive, community-based interventions, such as active surveillance in select settings, rather than contact tracing alone. Importantly, the historical paradigm that most paediatric transmission occurs in households should be reconsidered on the basis of the scientific knowledge presented.
Introduction
Tuberculosis in children continues to pose a pressing public health challenge and remains one of the leading infectious causes of child morbidity and mortality globally.1,2 Most paediatric tuberculosis deaths occur in low-income and middle-income countries, predominantly among children younger than 5 years, who often die without being diagnosed with tuberculosis.1,3 Impressive strides have been made to address the tuberculosis epidemic in adults through mass implementation of directly observed therapy, development and adoption of novel diagnostics, such as GeneXpert, and the integration of tuberculosis and HIV care into health systems.4 However, progress in the prevention, early detection, and treatment of tuberculosis in young children has been more limited.
Historically, the global tuberculosis public health strategy has not addressed the disease burden in children for several reasons. Although children are more susceptible to primary progressive tuberculosis disease, they are considered to be relatively non-infectious since they are often unable to generate a forceful cough with sufficient bacteria to transmit infection, and therefore might not contribute substantially to ongoing transmission. This argument has made children with tuberculosis less important from a public health perspective. Because of poor sputum collection, paucibacillary disease, and nonspecific clinical presentation, paediatric tuberculosis is more difficult to diagnose than adult tuberculosis.5 Furthermore, most public health interventions designed to address adult tuberculosis are not translatable to children. Because of the lack of emphasis on children within the global tuberculosis strategy, the incidence of tuberculosis infection, disease, and death were previously largely unknown. The paediatric tuberculosis burden has only recently been examined and is estimated to be much higher than previously thought.1,6,7
In 2014, WHO published guidelines for National Tuberculosis Programs to manage children with or exposed to tuberculosis, and emphasised the primary strategy of tuberculosis contact tracing.8 Under this strategy, when an adult with active tuberculosis disease is diagnosed, health workers visit the household to examine any child for disease and, in some programmes, provide preventive therapy to children who are latently infected with tuberculosis. Alternatively, adults diagnosed with tuberculosis are asked whether there are any sick contacts in the family, and are asked to bring them to the hospital. This strategy is often of lower diagnostic yield. The potential of household contact tracing to affect the paediatric tuberculosis burden is predicated on conventional wisdom that Mycobacterium tuberculosis transmission to children occurs from people living within the household (and not by those living in the general community).9–13 This hypothesis is based on the traditional assumption that children spend the vast majority of their time in the household with limited exposure to other adults and, consequently, their social network structure includes predominantly household members. Several recent reviews10,11 and guidelines12,13 have stated that M tuberculosis transmission to children is largely attributable to exposures from within the household. However, accumulating epidemiological evidence suggests that this assumption might not be the case in high-burden settings and that children are more often infected by those living outside the household.
To address the crucial and unaddressed public health challenge of paediatric tuberculosis, an improved scientific understanding of the routes of transmission is urgently needed to inform a more effective global public health strategy. However, the ideal public health strategy that will maximise impact and cost-effectiveness remains subject to a key epidemiological question—what proportion of M tuberculosis transmission in children occurs in households and is therefore avertible by household contact-based strategies? In this Personal View, we provide a summary of scientific evidence on the route of M tuberculosis transmission to children, to bring insight to this important question and outline potential future public health steps necessary to address this vulnerable, at-risk paediatric population.
Epidemiological evidence: investigating where paediatric tuberculosis transmission occurs
Despite the complexity in quantifying where M tuberculosis transmission occurs in children at the population level, understanding this key question is essential to design appropriate and effective public health programmes to detect, diagnose, and treat children with tuberculosis. 13 studies14–26 published between 2003 and 2018 that made use of diverse methodologies and designs shed light on this topic. This description is specific to the type of study, and is thus described for each study type in later sections.27 The population-attributable fraction is defined in the figure. We find that the evidence is consistent across distinct study designs, settings, and diagnostic approaches; most tuberculosis cases in children probably result from transmission of M tuberculosis outside of the household (figure). In review of the literature, we define household transmission as any evidence of transmission occurring from an individual that lives in the same household, thereby targetable by household contact tracing. The definition of a household varies across settings and studies, which could alter inference about transmission within this unit. We accepted each study’s definition of a household (appendix); in some cases, no specific definition was provided. Despite this variability in definitions of what constitutes a household, we found consistent results in the 13 studies that we analysed that most transmission to children occurs outside the household (table 1 and figure).
Figure : Estimation of the population-attributable fraction of paediatric tuberculosis transmission due to exposure from the household15,16,18–23.
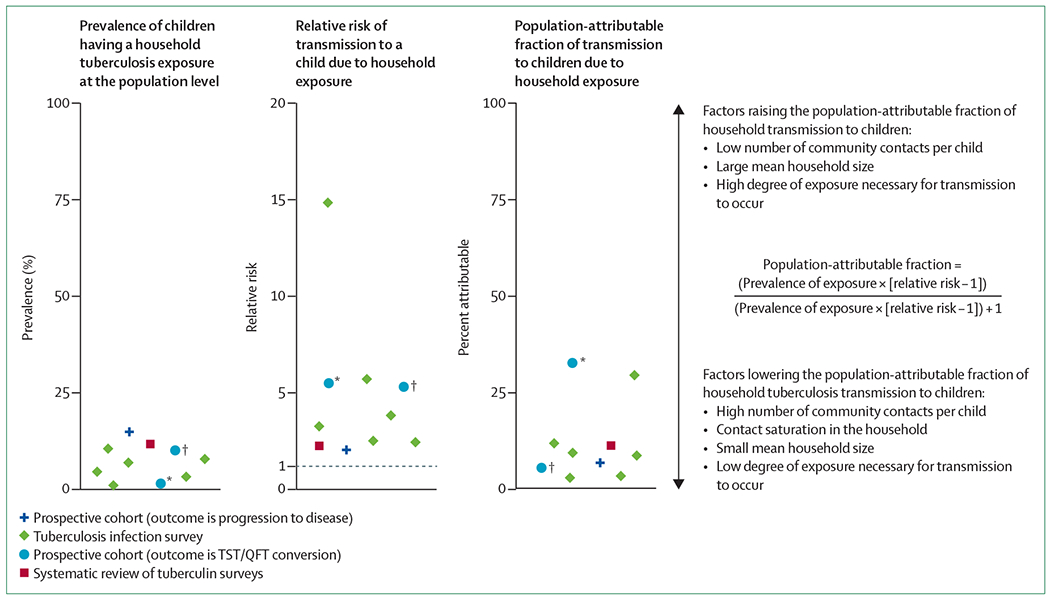
We included studies with information available on the prevalence of exposure to tuberculosis within the household at the population level and the relative risk of tuberculosis infection in children exposed to a tuberculosis case in a household compared with children not exposed in the household. Molecular epidemiology, mathematical modelling studies, and studies in which all the components of the population-attributable fraction formula were not extractable are included in table 1, but not included here in this figure. The systematic review on tuberculin surveys includes ten studies28–37 but is represented as a pooled value herein, as presented in Martinez and colleagues.18 We also include two data points for Khan and colleagues,16 2018. The population-attributable fraction was taken from the Rockhill and colleagues.27 *Uses any exposure at less than 200 m from the participant’s household (including household exposures) as the parameter. †Uses exposures that occurred only directly inside the participant’s household.
Table 1:
Summary of the evidence concerning the proportion of paediatric tuberculosis infections acquired at the population level through household and community exposures
| Years | Age, years | Setting | Study design (sample size)* | Conclusions | |
|---|---|---|---|---|---|
| Conversion studies | |||||
| Martinez et al (2018)15 | 2012–17 | 0–5 | South Africa | Prospective cohort (n=915), tuberculin conversion | 11% of conversions occurred in households with a tuberculosis case in the past year |
| Andrews et al (2017)14 | 2009–12 | 0–2 | South Africa | Prospective cohort (n=2512), QuantiFERON conversion | 19% of conversions occurred in households with a tuberculosis case |
| Khan et al (2018)16 | 2012–15 | <6 | Malawi | Prospective cohort (n=3066), tuberculin conversion | 11% of conversions occurred in children living at a distance of less than 200 m from a known tuberculosis case |
| Progression to tuberculosis disease studies | |||||
| Martinez et al (2018)15 | 2012–17 | 0–5 | South Africa | Prospective cohort (n=915) | 19% of children who developed tuberculosis were exposed to tuberculosis in the home |
| Nachman et al (2011)17 | 2004–08 | 0–3 | South Africa | Prospective trial data of infants exposed to mothers with HIV (n=1329) | 45 children developed tuberculosis; 28·8% were exposed to tuberculosis in the home† |
| Tuberculosis infection surveys | |||||
| Martinez et al (2017)18 | 1931–2015 | <15 | 12 countries | Systematic review of tuberculin surveys (n=170 615) | Household exposure at the population level was low; less than 25% of transmission occurred in households |
| Khan et al (2016)19 | 2012 | 2–4 | Malawi | Tuberculin survey (n=3170) | Less than 10% of transmission occurred in households with a tuberculosis case |
| Dorjee et al (2018)22 | 2017–18 | 13‡ | Tibet | Tuberculin survey (n=5234) | Less than 10% of childhood infections were attributable to recent household exposure |
| Cranmer et al (2014)20 | 1999–2002 | 0·5 | Kenya | T-SPOT.TB survey (n=128) | 14% of infants with a positive T-SPOT.TB were exposed to a parent with active tuberculosis |
| Lule et al (2015)21 | 2002–05 | 5 | Uganda | T-SPOT.TB survey (n=886) | 20% of children with a T-SPOT.TB-positive test were exposed to a tuberculosis case |
| Ganmaa et al (2018)23 | 2015–17 | 6–13 | Mongolia | QuantiFERON-TB survey (n=9810) | 13·1% of tuberculosis infections were attributable to household tuberculosis exposure |
| Molecular studies | |||||
| Schaaf et al (2003)24 | 1993–1998 | ≤15 | South Africa | Population based; all culture-confirmed paediatric cases (n=35) | 34% of children with tuberculosis were part of a cluster with a household tuberculosis case |
| Guthrie et al (2018)25 | 2005–14 | <18 | British Colombia | Population-based; all culture-confirmed paediatric cases (n=49) | 70% of transmission occurred either in a foreign country or locally but outside the household |
| Mathematical modelling | |||||
| Andrews et al (2014)26 | 2009 | <15 | South Africa | Modelling with support from empirical social network interaction data | 25–30% of new tuberculosis infections in children occurred in households |
Definitions in appendix.
Few children were excluded at baseline because of exposure to a known source case (personal communication, Dr Sharon Nachman).
Median age of the cohort.
Tuberculin skin test and interferon-γ release assay conversion: prospective cohort studies
In the past 5 years, three population-based cohort studies investigating new tuberculosis infection in young children have been completed.14–16 Tuberculin or interferon-γ release assay conversion studies provide a unique design to identify documented recent transmission events in children, independent of progressive disease data. These studies also document the prevalence of tuberculosis exposure to help understand the proportion of children with incident tuberculosis infections who have a household exposure. In these three cohort studies14–16 from settings with high tuberculosis burden, the estimated proportion of M tuberculosis transmission to children occurring because of household exposure was between 11% and 19%.
First, in the MVA85A tuberculosis vaccine trial14 that incorporated a prospective conversion design, infants who were HIV negative (aged 18–24 weeks) were tested with QuantiFERON-TB Gold (QFT) for tuberculosis infection. 2512 infants who were HIV and QFT negative were then followed for 6–24 months for QFT conversion and household tuberculosis exposure. In this study, 177 (7%) of 2512 children had a documented QFT conversion, of whom only 34 (19%) had known household tuberculosis exposure. Second, in a prospective birth cohort study15 in Cape Town, South Africa, 915 mother-infant pairs were followed from birth until age 5 years for tuberculin conversion and primary progressive tuberculosis. In this study, only 11% of the children with skin test conversion from ages 0–5 years were known to be exposed to a patient with tuberculosis in their household in the past 1 year. Third, in a prospective cohort study from Malawi,16 3066 children younger than age 6 years were tuberculin skin tested at baseline and then retested after 1–2 years. Among children with skin test conversion, few (11%) lived less than 200 m from a person known to have infectious tuberculosis. Additionally, most (98%) children who showed a conversion on the skin prick test did not have a known household member with infectious tuberculosis.
Although tuberculin and QuantiFERON conversion studies are the gold standard for measuring M tuberculosis transmission, they are not devoid of limitations. QuantiFERON and tuberculin reversion has been documented.38–40 Some of these studies are based on currently diagnosed household tuberculosis. Therefore, undiagnosed household tuberculosis might be missed, which might underestimate household exposures. In two hospital-based studies,41,42 10–15% of adults or mothers accompanying children admitted to a hospital for suspected tuberculosis were themselves diagnosed with tuberculosis when screened. However, even accounting for some underdiagnosis, most transmission cannot be explained by household exposures. Despite these limitations, these three studies14–16 had remarkably consistent findings showing that, among children with documented conversion, only 11–19% probably sustained M tuberculosis infection from household transmission in settings with high tuberculosis burden.
Progression of tuberculosis disease: prospective cohort studies
The development of disease in infants or young children implies infection that occurred recently (because infants have only been alive for up to 1 year). Two South African cohort studies have considered paediatric tuberculosis progression as the primary outcome.15,17,43 These studies have some advantages over conversion studies because they are not reliant on the QuantiFERON or tuberculin skin test, which might have diagnostic deficiencies in measuring tuberculosis infection.
First, in a birth cohort of 915 mother-child pairs, 81 young children developed primary progressive tuberculosis over 2737 child-years of follow-up (incidence of 2·9 per 100 child-years, 95% CI 2·4–3·7).15 A minority (19%) of children that developed progressive tuberculosis disease had a known exposure to an individual with tuberculosis in the household in the year before enrolment (figure). Second, in the IMPAACT P1041 preventive therapy trial,43 1329 infants exposed to HIV (525 infants infected with HIV and 804 exposed to HIV but uninfected) were followed for progression to tuberculosis starting at age 3–4 months. Children with a history of tuberculosis exposure or previous or current treatment for tuberculosis infection or disease were excluded from enrolment; few children were excluded (personal communication, Sharon Nachman).44 Intensive searching for sources of children who progressed to disease were done.17 After 96 weeks of follow-up, 45 infants were diagnosed with probable or definite tuberculosis, of which only 13 (28·8%) had an identifiable household tuberculosis exposure.
Similar to conversion studies, tuberculosis in households might be underdiagnosed in these disease development cohort studies, thereby underestimating household transmission. In some settings, several different families might stay in one house sharing the same amenities and living space; one family might not be aware of diseases in the other families. However, these were prospective cohort studies in which field teams and investigators were closely involved in the lives of the family and did robust household surveillance for several years. Therefore, missed diagnoses are possible, but are likely to be minimal. In addition, the proportion of transmission attributable to the household might be overestimated if children who were household contacts of people with tuberculosis were screened and followed more rigorously than children unexposed in the household, which is often the case. This bias is typically not present in tuberculin and QuantiFERON conversion studies or tuberculosis infection surveys, in which all children are given the same tests, irrespective of exposure status.
Tuberculosis infection surveys
Latent tuberculosis prevalence among young children is often used to assess transmission patterns. Community-based tuberculin surveys might be used to estimate both transmission and the population-attributable fraction of household and community exposures in a population. A systematic review and meta-analysis of these studies was done, including ten studies from 12 countries encompassing 6131 household contacts of tuberculosis cases and 164484 community controls with no household exposure.18 This review found that although children exposed to an individual with tuberculosis in their household were at higher individual risk of transmission than those without such exposure, household exposure to tuberculosis at the population level was rare. The mean proportion of household exposure to tuberculosis was 13% among the entire source population and 29% among contacts who were infected. The population-attributable fraction of household exposure of all new tuberculosis infections among studies in this review was consistently less than 25%. In these studies, community exposures contributed to population-level paediatric infections more than household exposures (figure). For example, in a tuberculin survey from a Peruvian shantytown,28 children exposed to people in the household with tuberculosis were 64% more likely to have a tuberculosis infection than unexposed controls; however, due to the higher number of total community exposures to children, only 17% of all paediatric tuberculosis infections were estimated to occur from the home.
Five similar studies that were not included in this review18 also found similar results (table 1).19–23 A community-based survey of 3170 children from Malawi was done.19 Using mixture analysis of tuberculin data, the authors found that 1·1% of all children were infected. Only 19% of all children in the study were exposed to a person with tuberculosis within 200 m of their household. Among children with tuberculosis infection, less than 10% of infections were attributable to a person with known infectious tuberculosis inside their household (figure). Furthermore, fewer than 20% of childhood infections were attributable to a person with tuberculosis who lived within 200 m of their household. In a separate study, 128 infants born to mothers with HIV-1 were tested at age 6 months for tuberculosis infection with T-SPOT. TB assays.20 Consistent with the findings in the aforementioned systematic review,18 infants exposed to mothers with tuberculosis were at much higher individual risk of a positive T-Spot (odds ratio [OR] 15·5, 95% CI 1·3–184·1) than infants who were unexposed, but the number of children exposed was small. Only 14% of infants with a positive T-SPOT.TB test were exposed to a parent with active tuberculosis. Another cohort of 886 Ugandan children were tested with a T-SPOT.TB assay at age 5 years.21 Of the entire child cohort, 10% had a known household tuberculosis contact. Of 75 children with a positive T-SPOT.TB test, only 15 (20%) had a history of household tuberculosis exposure. In a large study in several Tibetan schools, 5234 children were screened with tuberculin skin tests.22 Only 156 (3%) of these students were exposed to a household tuberculosis case in the previous 2 years, indicating that the most new infections were community driven. Lastly, 9810 children between ages 6 years and 13 years were administered QFTs in Mongolia.23 Again, a history of household tuberculosis exposure was a risk factor for tuberculosis infection (adjusted OR 4·75, 95% CI 4·1–5·5), but only 4% of children had any history of household tuberculosis exposure. Because of this, only 13·1% of paediatric tuberculosis infections were attributable to household tuberculosis exposure (figure).
Unlike conversion studies, these population-based tuberculin surveys might be subject to temporality issues. Household exposures might have been present before the survey, and might therefore be unrecorded. However, when studies use a history of household tuberculosis exposure, rather than current exposure, results remained similar.
Molecular studies
Molecular epidemiological tools have enabled inference of M tuberculosis transmission events in patients with concordant genotypes.45 Several paediatric studies have estimated household transmission using molecular tools and epidemiological linkage in children and potential source cases inside and outside households.24,25,46 Similar to studies with other designs, these studies found that a majority of M tuberculosis transmission to children occurs outside of households.
First, in a prospective community-based study from 1993 to 1998, restriction fragment-length polymorphism (RFLP) analysis was done on M tuberculosis isolates from two communities in Cape Town, South Africa.24 Household transmission was assessed through interviews and evaluation of household members. Of 35 children with culture-positive disease, only 15 children formed part of a cluster and had a history of tuberculosis contact. In all, 12 children were part of a cluster with a household member with tuberculosis. Since RFLP clustering is a relatively crude metric of genetic closeness and therefore might overestimate transmission events, these results suggest an upper bound for household transmission to children in this study of 34%. Second, in a contact cohort of South African children, only two of six children with culture-confirmed disease had identical IS6110 DNA fingerprints to an adult with tuberculosis in their households.46 This finding suggests that in high-burden settings, even among children exposed in the household, community exposures are abundant and account for most infections. Third, in a population-based study from British Columbia, a setting with low tuberculosis burden, 49 paediatric cases were genotyped by mycobacterial interspersed repetitive units variable-number tandem repeats.25 Whole-genome sequencing was subsequently implemented in genotypically clustered cases. The researchers found that more than two-thirds of paediatric cases acquired tuberculosis outside of British Colombia, and therefore household contact tracing would have limited effectiveness.
These studies are not without limitations. Molecular studies elucidate transmission dynamics of disease only from microbiologically confirmed cases. Because of the paucibacillary nature of childhood tuberculosis, many children with tuberculosis are effectively excluded from these studies. Although it is possible that children with known household source cases might be diagnosed earlier and are less likely to have positive bacteriology than those without known source cases, this effect might be counterbalanced by the increased scrutiny of children in households with an adult case, which could lead to over-representation of household transmission cases in molecular samples. Which effect dominates is unclear. As with conventional epidemiologic studies, underdiagnosis of adult cases might lead to underestimation of exposures in the household.
Mathematical modelling studies
Data on indoor social contact have been used to further understand M tuberculosis transmission.26 In a study from South Africa,47 study participants used wearable CO2 recorders, which also measured time spent in various locations, and mapped extensive social networks in a community in Cape Town to estimate where transmission occurred. Among individuals of any age, only 16% of M tuberculosis transmission was estimated to occur in households, and this proportion was only slightly higher (25–30%) in children younger than 15 years.26 This low proportion was consistent among children younger than 5 years, those aged 5–9 years, and those aged 10–14 years. Most of the time during the day was spent in one’s own household, however this low proportion of estimated household transmission was driven by contact patterns. Social network analysis of children in this setting found that only 15–25% of all indoor contacts were in households. Community contacts were substantially more common, driving overall transmission events in children. Other than in the household, transmission events in children occurred in transit (about 20%), school (about 20%), and other households (5–20%, depending on the child’s age). In children younger than 5 years, a small proportion of transmission events (about 5%) also occurred in the workplace, possibly transmitted from parents.
Is household transmission more common for the youngest children?
On the basis of empirical evidence, we estimate that a minority of children with tuberculosis have household contact-based transmission. However, a key question remains whether the youngest children (aged <5 years) are more likely to acquire transmission from inside the household than older children who are more likely to go to school and acquire community exposures. Although this hypothesis remains plausible,18,48 our data suggest that, even among these infants and young children, community transmission is more common than household transmission. When broadly stratifying the data by age group, we find no substantial differences in proportion of household transmission between younger (aged <5 years) and older children. Specifically, when examining studies reporting on younger age groups (age 0–5 years), the evidence suggests that only 10–30% of children with infection or disease had a household member with tuberculosis. For example, in the three previously discussed conversion studies (two among infants and one in children aged <6 years) that were done in South Africa and Malawi,14–16 a small percentage (<20%) of children who converted their QuantiFERON or tuberculin skin test had household exposure. A mathematical modelling study found that only 25% of tuberculosis infections in children younger than 5 years were acquired in their own household.26 This overall finding among young children is seen in all study designs including conversion cohort studies, tuberculosis disease development cohort studies, molecular studies, and mathematical modelling.
A comprehensive public health strategy: looking forward
There is a growing consensus that new public health strategies are needed to address the global burden of paediatric tuberculosis. Policy discussions have focused on household contact tracing on the basis of the assumption that it has a high population-level yield (because of the idea of predominant household routes of transmission) and since the home represents a defined infrastructure that can be visited by health-care workers.8,49,50 We support that household contact tracing is an efficient approach to detect individual children with active tuberculosis, with a comparatively low number needed to test to identify a case, and that children recently exposed to tuberculosis are at high risk of progression, such that they should be prioritised for preventive therapy.51 However, we estimate that these household contact-based approaches will only reach 10–30% of all children with tuberculosis in high-burden settings on the basis of diverse scientific studies under optimistic assumptions for coverage of household contact tracing. To reach the broader at-risk paediatric population, we argue that there is a need for a comprehensive approach, including a range of community-based public health strategies in addition to household contact tracing.
Given the historical focus on household-based M tuberculosis transmission to children, few community-based interventions have been considered or empirically evaluated for control of paediatric tuberculosis. The principal approaches could include routine mass or targeted screening for active tuberculosis and targeted preventive therapy or environmental interventions. For each public health strategy, key points include a target age group, a delivery platform, a diagnostic tool with associated limitations, integration opportunities within existing health services (eg, QuantiFERON or tuberculin skin testing during routine infant health or immunisation visits), and public health goals. Potential examples of these strategies are outlined in table 2. Generally, all potential strategies will require substantial operation research and cost-effectiveness analyses to identify effective strategies that can be scalable within cost-constrained health systems.
Table 2:
Potential public health screening and intervention strategies for paediatric tuberculosis in high-burden settings
| Target population | Delivery platform | Diagnostic testing options | Pros | Cons | |
|---|---|---|---|---|---|
| Screening and intervention strategies | |||||
| Mass routine screening | Children (aged 1–5 years or 1–15 years) | Community-based platforms (eg, community health workers, schools, and churches)16,22,26 | Clinical assessment; chest radiography, smear, and culture; QFT/TST | Highest averted disease burden52 | Research is needed to establish the optimal delivery platform; a high-cost intervention; cost-effectiveness analyses are needed |
| Routine screening of young children at vaccination visits | Children (aged <2 years) | Infant health and immunisation visits; expanded programme of immunisation53 | Symptomatology and QFT/TST followed by diagnostics for active tuberculosis if positive | Potentially high-coverage screening for at-risk groups; good feasibility with the opportunity for integration; QFT tests have higher predictive value among young children than other types of tests; opportunity for targeted preventive therapy | Symptom screens, QFT, and TST are imperfect prognostic tests; TST has limited predictive value when BCG vaccines are administered; a second visit is required for TST; QFT is expensive; cost-effectiveness analyses are needed |
| Active screening in health-care facilities | Children (aged 1–15 years) in health-care settings | Health clinics and hospitals; increased training to diagnose paediatric tuberculosis among health-care workers54 | Clinical assessment; chest radiography, smear, and culture; QFT/TST | Higher predictive value for screening in a high-risk paediatric group*54 | The yield of all paediatric tuberculosis this would identify needs further study; integration and coverage might be challenging; tests only a subset of the at-risk population |
| Future approaches | |||||
| Prognostic assays to identify incipient tuberculosis | Children | Health-care facilities; mass screening in communities | Gene expression assays and other types of tests | Gene expression assays have shown promising prognostic value in adolescents and adults52,55 | More research is needed to establish efficacy and cost-effectiveness |
| Indoor environmental modifications | Children and adults | Community-based platforms but targeted to specific high-risk settings (eg, hospitals, clinics, public transport, and churches) | NA | No additional demand on health-care workers; sustainable intervention that might have benefits for several years | Operational research is needed on effectiveness; changes need acceptance from the community and leaders; expense is unclear and might vary by setting |
We outline potential approaches to detecting paediatric tuberculosis in community settings, including opportunity for case detection and preventive therapy. Generally, all potential strategies will require operational research and cost-effectiveness analyses before implementation. QFT=QuantiFERON Gold In-Tube test. TST=tuberculin skin test. TB=tuberculosis. NA=not applicable
Given the high incidence of paediatric tuberculosis in high-burden settings, active surveillance programmes that screen the entire at-risk paediatric community could potentially yield impressive public health gains, although investigations of optimal screening approaches and cost-effectiveness will be needed. Available studies provide insight into possible strategies for community-based programmes. As one example, a community-wide, symptom-based approach was evaluated in a large paediatric South African cohort and found that this approach was effective for identifying paediatric tuberculosis, especially in children without HIV.52
There remain key operational concerns for development of a public health strategy, including defining the diagnostic tool, delivery strategy and platform, and opportunities for integration within existing health-care systems. Diagnostic tests for screening could include traditional diagnostics for disease, such as symptom screening, radiographic examinations, and molecular diagnostics, or tests for infection, such as tuberculin skin tests or interferon-γ release assays, which have higher predictive value among young children than among adults.14 Overdiagnosis of paediatric tuberculosis might be a concern and should be considered when evaluating these diagnostics. New prognostics, including gene expression assays, hold promise for improved predictive accuracy, reducing the number needed to provide prophylaxis to prevent a case of tuberculosis.55,58 For delivery platforms and integration with existing healthcare systems, population-level paediatric tuberculosis screening could be integrated with existing programmes, such as routine infant immunisation or health clinics. With the Expanded Program of Immunization,53 which often achieves high coverage, screening could be done in key at-risk age groups, such as younger children (aged <1 year). Several studies have shown that lower respiratory tract infections and tuberculosis might be risk factors for each other (or share risk factors) in settings in which both infections are endemic.15,56,57 Therefore, screening for tuberculosis at the time of a lower respiratory tract infection in the clinical setting might be effective to detect childhood tuberculosis cases; integration of childhood pneumonia and tuberculosis programmes might be effective in areas of high prevalence. A before-and-after implementation study from Uganda evaluated the effect of strengthening diagnosis, treatment, and prevention of paediatric tuberculosis at peripheral health facilities (partnered with household contact tracing).54 After implementation, a 140% increase in paediatric case notification was recorded, almost entirely driven by health-care facility interventions indicating that healthcare training might be an effective intervention to increase childhood case detection among children with disease who are difficult to find. Lastly, given predominance of community-driven M tuberculosis transmission, certain social settings have been identified as drivers of M tuberculosis transmission in children.16,22,26 Studies have implicated church attendance, mini-bus transportation, and schools as key locations for paediatric conversion events.16,22,26 Intuitively, these locations might be ideal locations to focus health programmes that provide screening for children. Although the individual-level risk of tuberculosis is likely to be lower in these settings, the total number of people exposed to a tuberculosis case is likely to be higher because the number of unique individuals in this setting is much greater.16,19 Importantly, settings will differ by tuberculosis epidemiology, transmission patterns, health-care access, and other factors that will affect the optimal tuberculosis strategy for a given setting. Future guidelines development will need to balance the complexity of setting-specific heterogeneity with the need for generally applicable recommendations.
Ultimately, the most promising strategies will need further study and field validation. A growing body of evidence shows the need for a comprehensive strategy that combines community-based interventions with contact tracing. What is clear is that there is no single ideal solution to improve the control of paediatric tuberculosis; no single intervention, even one as efficient as household contact tracing, will effectively address paediatric tuberculosis at the population-level, especially with known heterogeneities between settings. A modelling study suggests that only 16% of paediatric tuberculosis cases would be prevented by full global implementation of household contact tracing.59 Only with a global paediatric tuberculosis strategy that includes a comprehensive package of interventions customised to individual settings that target transmission to children and diagnose undetected disease will we adequately reduce the childhood tuberculosis burden in sub-Saharan Africa and other low-income settings.
Conclusion
Over the past 10 years, the field of paediatric tuberculosis has moved towards household contact tracing because of its pragmatic nature and the belief that most paediatric tuberculosis infections occur in the household. Although we support household contact investigations as a component of the global strategy to address paediatric tuberculosis, a strategy primarily focused on this intervention will probably continue to miss most tuberculosis infections and cases among children. We believe that a comprehensive approach that combines a set of public health, community-based interventions, in combination with contact tracing, will be required. Importantly, the historical paradigm that the majority of paediatric transmission occurs in the household should be reconsidered based on the existing scientific knowledge base.
Supplementary Material
Key messages.
The public health strategy of household contact tracing of children after identification of an adult tuberculosis case has been emphasised as the principal public health intervention for paediatric tuberculosis, and is predicated on the hypothesis that most children are infected with tuberculosis through a household contact
We estimate that the population-attributable fraction of paediatric tuberculosis transmission due to household exposure is between 10% and 30%, which is substantially lower than previously thought
At the population level, transmission from the household was low (<30%) even in children younger than 5 years
This suggests that household contact tracing is unlikely to reach the majority of children with tuberculosis
We propose that new public health strategies are necessary to address childhood tuberculosis and will require comprehensive, community-based interventions in addition to household contact tracing
Search strategy and selection criteria.
We first searched for any previous narrative or systematic review that attempted to quantify the population-attributable fraction of tuberculosis transmission to children caused by household exposure. None were found. We did find several review articles attempting to quantify the percentage of adult tuberculosis transmission attributable to household exposure. All of these studies excluded children. We then searched MEDLINE and Google Scholar for articles published before Dec 1, 2018. We used the search terms “child”, ‘tuberculosis’, “conversion”, “transmission”, “community”, “pediatric”, “paediatric”, and “household”, amongst others. We also reviewed reference lists, bibliographies, our personal files, and other narrative reviews on tuberculosis transmission for additional relevant articles. We read abstracts in any language if relevant.
Acknowledgments
We thank James Seddon for his comments and thoughts on previous drafts of this manuscript. We also thank Ben Marais for several discussions on this topic. Lastly, we thank Dr Sharon Nachman, one of the principal investigators of the IMPAACT P1041 preventive therapy trial, for providing additional details regarding the study design and results of their study. LM was supported by the Ruth L Kirschstein National Research Service Award. NCL is supported by the Medical Scientist Training Program (Stanford University School of Medicine).
Footnotes
Declaration of interests
We declare no competing interests.
References
- 1.Dodd PJ, Yuen CM, Sismanidis C, Seddon JA, Jenkins HE. The global burden of tuberculosis mortality in children: a mathematical modelling study. Lancet Glob Health 2017; 5: e898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012; 379: 2151–61. [DOI] [PubMed] [Google Scholar]
- 3.Jenkins HE, Yuen CM, Rodriguez CA, et al. Mortality in children diagnosed with tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 2017; 17: 285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lonnroth K, Castro KG, Chakaya JM, et al. Tuberculosis control and elimination 2010–50: cure, care, and social development. Lancet 2010; 375: 1814–29. [DOI] [PubMed] [Google Scholar]
- 5.Nicol MP, Zar HJ. New specimens and laboratory diagnostics for childhood pulmonary tb: progress and prospects. Paediatr Respir Rev 2011; 12: 16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenkins HE, Tolman AW, Yuen CM, et al. Incidence of multidrug-resistant tuberculosis disease in children: systematic review and global estimates. Lancet 2014; 383: 1572–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dodd PJ, Gardiner E, Coghlan R, Seddon JA. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modelling study. Lancet Glob Health 2014; 2: e453–9. [DOI] [PubMed] [Google Scholar]
- 8.WHO. Guidance for national tuberculosis programmes on the management of tuberculosis in children. Geneva: World Health Organization, 2014. [PubMed] [Google Scholar]
- 9.Hsu KH. Contact investigation: a practical approach to tuberculosis eradication. Am J Public Health 1963; 53: 1761–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yates TA, Khan PY, Knight GM, et al. The transmission of mycobacterium tuberculosis in high burden settings. Lancet Infect Dis 2016; 16: 227–38. [DOI] [PubMed] [Google Scholar]
- 11.Khan PY, Yates TA, Osman M, et al. Transmission of drug-resistant tuberculosis in HIV-endemic settings. Lancet Infect Dis 2018; 9: e77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO Stop TB Partnership, Childhood TB Subgroup. Guidance for national tuberculosis programmes on the management of tuberculosis in children. Int J Tuberc Lung Dis 2006; 10: 1091–97 [PubMed] [Google Scholar]
- 13.Kenyan Ministry of Health, Division of Leprosy, Tuberculosis, and Lung Disease. National guidelines on management of tuberculosis in children, 2nd ed Nairobi: Kenya Ministry of Health, 2013. [Google Scholar]
- 14.Andrews JR, Nemes E, Tameris M, et al. Serial QuantiFERON testing and tuberculosis disease risk among young children: an observational cohort study. Lancet Respir Med 2017; 5: 282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez L, le Roux DM, Barnett W, Stadler A, Nicol MP, Zar HJ. Tuberculin skin test conversion and primary progressive tuberculosis disease in the first five years of life: a birth cohort study from Cape Town, South Africa. Lancet Child Adolesc Health 2018; 2: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan PY. Investigating Mycobacterium Tuberculosis Transmission in Rural Malawi. Doctoral dissertation. London: London School of Hygiene & Tropical Medicine, 2018. [Google Scholar]
- 17.Nachman S, Zeldow B, Dittmer S, et al. Lack of identification of adult TB contacts in infants with microbiologically confirmed or clinically presumed TB (MCCP TB) in clinical trial P1041. 6th International AIDS Society Conference on HIV Pathogenesis and Treatment; Rome; 2011. WEPDB0201. [Google Scholar]
- 18.Martinez L, Shen Y, Mupere E, Kizza A, Hill PC, Whalen CC. Transmission of Mycobacterium tuberculosis in households and the community: a systematic review and meta-analysis. Am J Epidemiol 2017; 185: 1327–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan PY, Glynn JR, Fielding KL, et al. Risk factors for Mycobacterium tuberculosis infection in 2–4 year olds in a rural HIV-prevalent setting. Int J Tubercul Lung Dis 2016; 20: 342–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cranmer LM, Kanyugo M, Jonnalagadda SR, et al. High prevalence of tuberculosis infection in HIV-1 exposed kenyan infants. Pediatr Infect Dis J 2014; 33: 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lule SA, Mawa PA, Nkurunungi G, et al. Factors associated with tuberculosis infection, and with anti-mycobacterial immune responses, among five year olds BCG-immunised at birth in Entebbe, Uganda. Vaccine 2015; 33: 796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorjee K, Topgyal S, Dorjee C, et al. High prevalence of active and latent tuberculosis in children and adolescents in Tibetan schools in India: the zero TB kids initiative in Tibetan refugee children. Clin Infect Dis 2018; published online Nov 20. DOI: 10.1093/cid/ciy987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganmaa D, Khudyakov P, Buyanjargal U, et al. Prevalence and determinants of QuantiFERON-diagnosed tuberculosis infection in 9 810 Mongolian schoolchildren. Clin Infect Dis 2018; published online Nov 27. DOI: 10.1093/cid/ciy975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaaf HS, Michaelis IA, Richardson M, et al. Adult-to-child transmission of tuberculosis: household or community contact? Int J Tubercul Lung Dis 2003; 7: 426–31. [PubMed] [Google Scholar]
- 25.Guthrie JL, Delli Pizzi A, Roth D, et al. Genotyping and whole-genome sequencing to identify tuberculosis transmission to pediatric patients in British Columbia, Canada, 2005–2014. J Infect Dis 2018; 40: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrews JR, Morrow C, Walensky RP, Wood R. Integrating social contact and environmental data in evaluating tuberculosis transmission in a South African township. J Infect Dis 2014; 210: 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Pub Health 1998; 88: 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madico G, Gilman RH, Cabrera L, et al. Community infection ratio as an indicator for tuberculosis control. Lancet 1995; 345: 416–19. [DOI] [PubMed] [Google Scholar]
- 29.Dow DJ, Lloyd WE. The incidence of tuberculous infection and its relation to contagion in children under 15. Br Med J 1931; 2: 183–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roelsgaard E, Iversen E, Blocher C. Tuberculosis in tropical Africa. An epidemiological study. Bull World Health Organ 1964; 30: 459–518. [PMC free article] [PubMed] [Google Scholar]
- 31.Narain R, Nair SS, Rao GR, et al. Distribution of tuberculous infection and disease among households in a rural community. Bull World Health Organ 1966; 34: 639–54. [PMC free article] [PubMed] [Google Scholar]
- 32.Olender S, Saito M, Apgar J, et al. Low prevalence and increased household clustering of Mycobacterium tuberculosis infection in high altitude villages in Peru. Am J Trop Med Hyg 2003; 68: 721–27 [PubMed] [Google Scholar]
- 33.den Boon S, Verver S, Marais BJ, et al. Association between passive smoking and infection with Mycobacterium tuberculosis in children. Pediatrics 2007; 119: 734–39. [DOI] [PubMed] [Google Scholar]
- 34.Dogra S, Narang P,Mendiratta DK, et al. Comparison of a whole blood interferon-gamma assay with tuberculin skin testing for the detection of tuberculosis infection in hospitalized children in rural India. J Infect 2007; 54: 267–76. [DOI] [PubMed] [Google Scholar]
- 35.Radhakrishna S, Frieden TR, Subramani R, et al. Additional risk of developing TB for household members with a TB case at home at intake: a 15-year study. Int J Tuberc Lung Dis 2007; 11: 282–88. [PubMed] [Google Scholar]
- 36.Hoa NB, Cobelens FG, Sy DN, et al. First national tuberculin survey in Vietnam: characteristics and association with tuberculosis prevalence. Int J Tuberc Lung Dis 2013; 17: 738–44. [DOI] [PubMed] [Google Scholar]
- 37.Hossain S, Zaman K, Banu S, et al. Tuberculin survey in Bangladesh, 2007–2009: prevalence of tuberculous infection and implications for TB control. Int J Tuberc Lung Dis 2013; 17: 1267–72. [DOI] [PubMed] [Google Scholar]
- 38.Andrews JR, Hatherill M, Mahomed H, et al. The dynamics of QuantiFERON-TB Gold in-tube conversion and reversion in a cohort of South African adolescents. Am J Respir Critic Care Med 2015; 191: 584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slater M, DuBose A, Banaei N. False-positive quantiferon results at a large healthcare institution. Clin Infect Dis 2014; 58: 1641–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slater ML, Welland G, Pai M, Parsonnet J, Banaei N. Challenges with QuantiFERON-TB Gold assay for large-scale, routine screening of us healthcare workers. Am J Respir Critic Care Med 2013; 188: 1005–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muñoz FM, Ong LT, Seavy D, Medina D, Correa A, Starke JR. Tuberculosis among adult visitors of children with suspected tuberculosis and employees at a children’s hospital. Infect Control Hosp Epidemiol 2002; 23: 568–72. [DOI] [PubMed] [Google Scholar]
- 42.Schaaf HS, Donald PR, Scott F. Maternal chest radiography as supporting evidence for the diagnosis of tuberculosis in childhood. J Tropic Pediatr 1991; 37: 223–25. [DOI] [PubMed] [Google Scholar]
- 43.Madhi SA, Nachman S, Violari A, et al. Primary isoniazid prophylaxis against tuberculosis in HIV-exposed children. New Engl J Med 2011; 365: 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cotton MF, Schaaf HS, Lottering G, et al. Tuberculosis exposure in HIV-exposed infants in a high-prevalence setting. Int J Tuberc Lung Dis 2008; 12: 225–27. [PubMed] [Google Scholar]
- 45.Borgdorff MW, Van Soolingen D. The Re-emergence of tuberculosis: what have we learnt from molecular epidemiology? Clin Microbiol Infect 2013; 19: 889–901. [DOI] [PubMed] [Google Scholar]
- 46.Marais BJ, Hesseling AC, Schaaf HS, Gie RP, Van Helden PD, Warren RM. Mycobacterium tuberculosis transmission is not related to household genotype in a setting of high endemicity. J Clin Microbiol 2009; 47: 1338–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnstone-Robertson SP, Mark D, Morrow C, et al. Social mixing patterns within a South African township community: implications for respiratory disease transmission and control. Am J Epidemiol 2011; 174: 1246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vallejo JG, Ong LT, Starke JR. Clinical features, diagnosis, and treatment of tuberculosis in infants. Pediatrics 1994; 94: 1–7. [PubMed] [Google Scholar]
- 49.WHO. Roadmap towards ending TB in children and adolescents, 2nd ed Geneva: World Health Organization, 2018. [Google Scholar]
- 50.WHO. Global tuberculosis report 2018. Geneva: World Health Organization, 2018. [Google Scholar]
- 51.Martinez L, Shen Y, Handel A, et al. Effectiveness of WHO’s pragmatic screening algorithm for child contacts of tuberculosis cases in resource-constrained settings: a prospective cohort study in Uganda. Lancet Respir Med 2018; 6: 276–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marais BJ, Gie RP, Hesseling AC, et al. A refined symptom-based approach to diagnose pulmonary tuberculosis in Children. Pediatrics 2006; 118: e1350–59. [DOI] [PubMed] [Google Scholar]
- 53.Machingaidze S, Wiysonge CS, Hussey GD. Strengthening the expanded programme on immunization in Africa: looking beyond 2015. PLoS Med 2013; 10: e1001405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zawedde-Muyanja S, Nakanwagi A, Dongo JP, et al. Decentralisation of child tuberculosis services increases case finding and uptake of preventive therapy in Uganda. Int J Tubercul Lung Dis 2018; 22: 1314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Warsinske HC, Rao AM, Moreira FM, et al. Assessment of validity of a blood-based 3-gene signature score for progression and diagnosis of tuberculosis, disease severity, and treatment response. JAMA 2018; 1: e183779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zar HJ, Hanslo D, Tannenbaum E, et al. Aetiology and outcome of pneumonia in human immunodeficiency virus-infected children hospitalized in South Africa. Acta Paediatrica 2001; 90: 119–25. [PubMed] [Google Scholar]
- 57.Oliwa JN, Karumbi JM, Marais BJ, Madhi SA, Graham SM. Tuberculosis as a cause or comorbidity of childhood pneumonia in tuberculosis-endemic areas: a systematic review. Lancet Respir Med 2015; 3: 235–43. [DOI] [PubMed] [Google Scholar]
- 58.Zak DE, Penn-Nicholson A, Scriba TJ, et al. A blood RNA signature for tuberculosis disease risk: a prospective cohort study. Lancet 2016; 387: 2312–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dodd PJ, Yuen CM, Becerra MC, Revill P, Jenkins HE, Seddon JA. Potential effect of household contact management on childhood tuberculosis: a mathematical modelling study. Lancet Glob Health 2018; 6: e1329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


