Abstract
“The EVICT study was the first study to demonstrate a step-wise approach on how to safely screen EVD survivors for cataract surgery, providing evidence that vision restoration though surgical management was safe and feasible in this cohort of EVD survivors”
Keywords: cataract surgery, chronic infections, developing countries, Ebola, Ebola virus disease, neglected diseases, uveitis, viral persistence
The Ebola Virus Disease (EVD) outbreak in West Africa from 2013 to 2016 brought awareness to the international community regarding the ophthalmic sequelae of EVD, as up to 40% of affected eyes developed severe vision impairment or blindness, often due to uveitis and structural damage from untreated ocular inflammation [1]. Vision loss due to EVD is complex, involving a spectrum of ophthalmic manifestations including a range of sight-threatening uveitis and visually significant cataract [2]. While the pathogenesis of uveitis in EVD survivors likely involves an interplay of Ebola virus (EBOV) persistence and inflammation, addressing cataract surgery in EVD survivors includes multiple considerations. These considerations include the potential for EBOV persistence, possible EBOV transmission, appropriate personal protective equipment and postoperative management, raising additional questions that remain under investigation. Herein, we summarize our current understanding of cataract development in EVD survivors, EBOV persistence in ocular fluid and the relationship with other immune-privileged sites, as well as our experience with the surgical management of cataracts in EVD survivors.
Cataracts in EVD survivors: clinical features & epidemiology
Vision impairment from cataracts can create significant economic and social implications for EVD survivors from a global health perspective. Over 90% of the population afflicted with blindness resides in developing countries, with cataracts accounting for the primary cause of blindness worldwide [3]. Studies have shown that vision impairment leads to reduced quality of life, increased mortality and diminished economic productivity [4]. The majority of EVD survivors live in resource-limited countries where access of care is a challenge, and the burden of cataracts can leave considerable socioeconomic consequences for all individuals of Ebola-affected countries. Given that other systemic sequelae including arthritis/arthralgias, abdominal pain, psychosocial stressors and stigmata are observed with high frequency in EVD survivors, the high burden of ocular disease, vision impairment and challenges to socioeconomic productivity present additional challenges.
While the pathophysiology underlying the development of cataract in EVD patients has not been completely understood, it is known that EVD survivors can develop different forms of cataracts other than the classic age-related nuclear sclerotic cataract, commonly observed in the elderly population. In a cohort of 46 EVD survivors with visually significant cataracts in the Ebola Virus Persistence in Ocular Tissues and Fluids (EVICT) study, Shantha et al. found that the most frequent type of cataract seen was a uveitic white cataract (54.3%) followed by a posterior subcapsular cataract (17.4%) [2]. The other forms of cataracts found in order of decreasing frequency included a uveitic white cataract with anterior capsular fibrosis (8.7%), combined nuclear sclerotic/posterior subcapsular (8.7%), combined anterior subcapsular/posterior subcapsular (6.5%) and age-related nuclear sclerotic cataract (4.3%, Figure 1). It was also interesting to note that the median age of the EVD survivors enrolled in the study was 24 years, highlighting the young age demographic affected by visually significant cataracts in this patient population. Cataract formation in this cohort was likely secondary to a number of factors including untreated intraocular inflammation, possible prior EBOV persistence in cases with white uveitic cataracts and prolonged corticosteroid use in patients with posterior subcapsular cataract subtype [2].
Figure 1. Slit lamp photograph shows a dense white cataract with posterior synechiae (i.e., adhesion between iris and lens), which are indicative of prior uveitis.
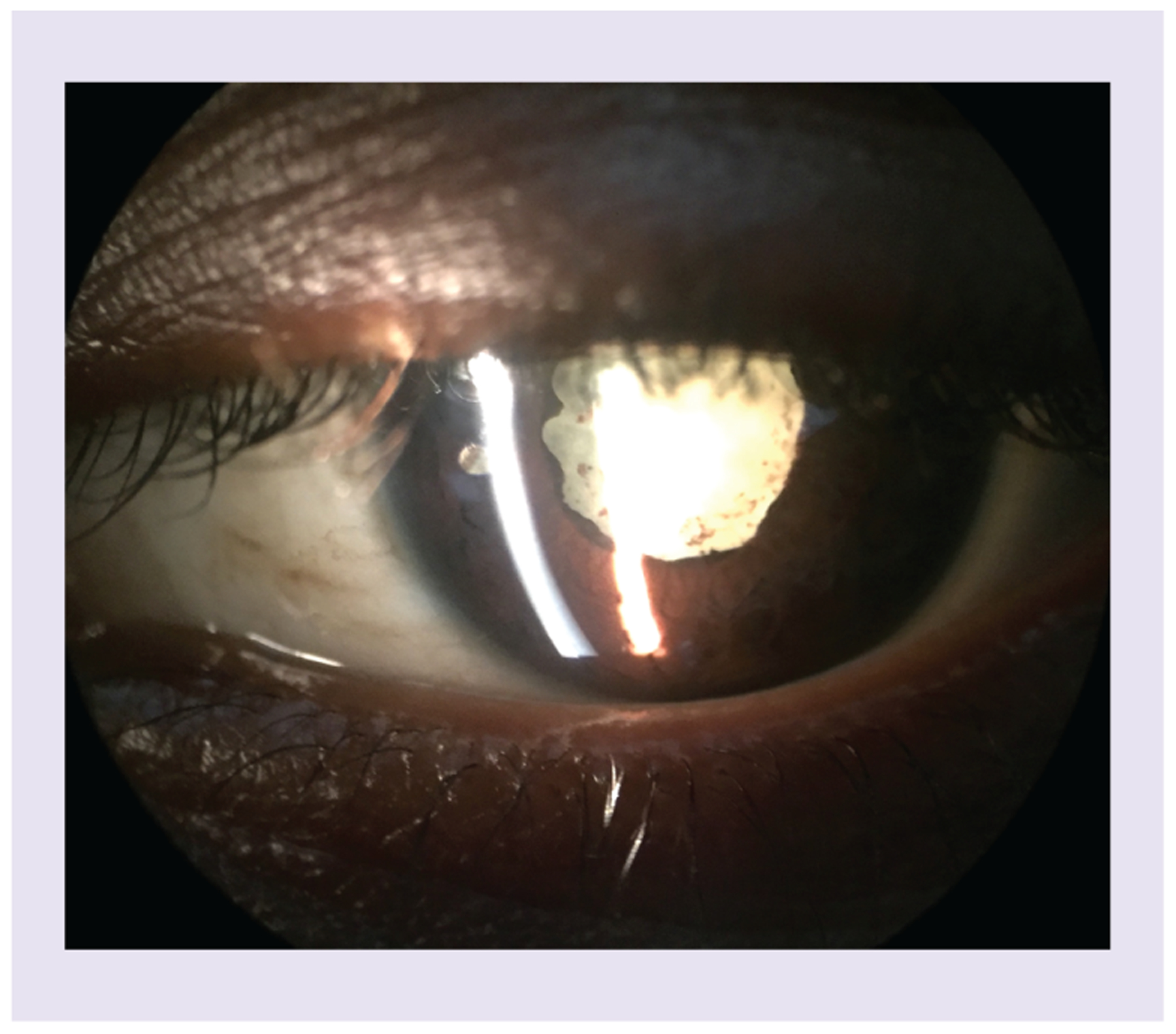
There is pigment on the lens capsule indicating prior inflammation with liberation of pigment and deposits on the anterior lens capsule.
Regarding the prevalence of cataracts in EVD survivors, Shantha et al. reported that 10% of the 96 patients from the cohort in Monrovia, Liberia, were found to have cataracts. In this cross-sectional study, there was no significant difference in the frequency of cataracts in patients with or without uveitis (p > 0.05) [5]. From a cohort of 341 EVD survivors in Guinea, Hebert et al. observed a cataract prevalence of 6.7% [6]. A total of 46 patients of the 341 studied presented with uveitis and of these 46 patients, 11 (23.9%) presented with subcapsular cataract, 8 (17.4%) were opaque, leading to decreased visual acuity of less than 6/60. Mattia et al. also found a similar finding of early cataract in 10% of 50 patients diagnosed with uveitis among EVD survivors in Port Loko, Sierra Leone [7].
Persistence of EBOV in ocular fluid & other immune-privileged sites
The safety of performing cataract surgery in EVD patients is a subject of concern due to the potential persistence of EBOV in ocular fluid. The World Health Organization Clinical Care for survivors of EVD: Interim Guidance initially advised refraining from elective surgery within immune privileged sites (e.g., cataract surgery) until further data were available. The eye is known to be a site of immune privilege due to multiple factors including the anatomy of the blood ocular barrier, which may allow a variety of viral pathogens to persist despite serologic clearance (e.g., Chikungunya, Ebola, Marburg virus) [6]. Varkey et al. described the detection of viable EBOV in aqueous humor of a patient during the convalescent stage of disease 9 weeks after viremic clearance [8]. The patient presented with an acute unilateral hypertensive anterior uveitis that evolved into a panuveitis 14 weeks after initial symptom onset, prompting an anterior chamber paracentesis, which revealed the presence of viable EBOV at a cycle threshold value of 18.7. This low cycle threshold indicated that there was a high burden of virus present that was likely undergoing active replication. The mechanism behind ocular EBOV persistence has not been well defined; however, experimental studies with animal models and tissue cultures have reported that the retinal pigment epithelium and macrophages are potential reservoirs for EBOV persistence [9,10].
There are significant public safety implications associated with EBOV persistence in immune-privileged sites [11]. Besides ocular fluid, it has been reported that EBOV has been detected in semen 18.5 months after discharge from the Ebola Treatment Unit [12]. Sexual transmission of EBOV 470 and 179 days after symptom onset has been confirmed in cases in Guinea and Liberia, respectively [13,14]. The CNS has also been implicated as a site of persistence of EBOV, as the virus was detected at high levels in the cerebrospinal fluid of a patient who developed meningoencephalitis 9 months after discharge from initial EBOV treatment unit [15]. These reports highlight the need to ensure clearance of EBOV from immune-privileged sites even during times of convalescence to avoid transmission of EBOV.
Surgical management of cataracts in EVD survivors
The question of safety and feasibility of elective ophthalmic surgeries in EVD survivors in the context of public health concern regarding persistence of EBOV in immune-privileged sites such as the eye prompted the development of the EVICT study [2]. This investigation was the first cross-sectional study to systematically demonstrate how cataract surgery could be safely implemented in EVD survivors [2]. As a part of the cataract evaluation, retina fellowship-trained specialists performed ocular fluid sampling in the form of an anterior chamber paracentesis or a vitreous tap in addition to conjunctival swab sampling before and after intraocular fluid sampling. These procedures were performed in full personal protective equipment in a uniquely designed ophthalmic procedure room compliant with infection prevention measures based on the WHO and the Emory University Serious Communicable Disease Unit guidelines. EBOV reverse transcription PCR (RT-PCR) was then performed on these ocular samples, and patients that tested negative for EBOV RT-PCR were given the opportunity to proceed with manual small-incision cataract surgery with intraocular lens implantation.
A total of 50 patients were enrolled into the EVICT study, of whom 49 patients (98%) underwent an anterior chamber paracentesis and one patient underwent a vitreous tap [2]. All 50 intraocular samples and all conjunctival swab specimens tested negative for EBOV RNA by RT-PCR. The median time between the time of EVD diagnosis and ocular fluid sampling was 19 months in Phase I (June 2016) of the study and 34 months for Phase II (July and August 2017). Regarding visual outcomes, 27 (79%) of the 34 patients achieved a gain in visual acuity of at least three Snellen eye chart lines by their last examination, with more than 60% attaining a final visual acuity of 20/40 or better. The EVICT study was the first study to demonstrate a step-wise approach on how to safely screen EVD survivors for cataract surgery, providing evidence that vision restoration though surgical management was safe and feasible in this cohort of EVD survivors.
Preoperative, intraoperative & postoperative considerations: uveitis patients & EVD survivors
In addition to ocular fluid sampling proposed by the EVICT study, careful preoperative testing is critical in the evaluation of cataracts in an EVD survivor to determine if the cause of vision loss is primarily due to cataracts or if there is other underlying vision-limiting pathology. Ocular co-morbidities such as glaucoma, optic atrophy, macular edema, macular ischemia and retinal detachment may limit the final postoperative visual acuity [16]. B-scan ultrasonography is a useful instrument used in situations in which a white uveitic cataract precludes a view to the posterior segment. In the EVICT study, B-scan ultrasound was used preoperatively in 72% of the patients due to an inadequate view to the fundus to rule out underlying vitreoretinal pathology [2]. Preoperative testing is thus crucial in weighing the risks and benefits of cataract surgery and to help determine if a patient will benefit from a surgically invasive procedure.
Preoperative steroid use is important in controlling inflammation prior to cataract surgery in uveitic patients to optimize visual outcomes and may similarly benefit EVD patients preoperatively. Although there is currently no consensus regarding a standard preoperative corticosteroid regimen, achieving inactive disease for at least 3 months prior to cataract surgery has been recommended for uveitis patients anticipating cataract surgery [17]. In a prospective, randomized, unmasked clinical trial of 50 patients with noninfectious uveitis requiring cataract surgery, Mora et al. found that perioperative topical corticosteroids alone were as effective as oral corticosteroid supplementation combined with topical corticosteroids in preventing the relapse of uveitis over a period of 6 months [18]. However, careful case selection and tailoring the pre- and post-operative corticosteroid regimen to the patient are important, as well as close postoperative follow-up. In EVD patients afflicted with uveitis, topical and systemic corticosteroids were used for treatment with improved visual outcomes. Consideration should be given to the addition of preoperative steroids in preparation for cataract surgery for EVD survivors [1].
The intraoperative surgical techniques for cataract in EVD survivors require instrumentation and techniques similar to the management in the uveitic population, and it is important to discuss potential complications with these patients prior to pursuing cataract surgery. Factors such as poor pupillary dilation due to posterior synechiae, zonular instability and pupillary membranes can make the surgery inherently more difficult to perform, necessitating additional procedures that can lead to further complications [17]. In one of the largest observational studies analyzing cataract surgery in uveitic patients, Chu et al. found that the uveitic group was associated with a smaller pupil size and a higher incidence of surgical pupil manipulation, which included procedures such as iris hooks, capsular tension rings and synechiolysis [19]. Compared with patients without uveitis, the uveitic group also underwent a greater number of combined procedures including pars plana vitrectomy and required the expertise of more senior consultant surgeons. While patients with uveitis overall experienced an improvement in visual acuity after cataract surgery, the final visual acuity in this subset of patients was worse than that of patients without uveitis at all measured time points. Patients with uveitis also have been reported to have a higher risk of postoperative complications such as cystoid macular edema, posterior capsular opacification, recurrent or postoperative inflammation, glaucoma, hypotony and intraocular lens dislocation [17].
Barriers to care & cataract surgery in a resource-limited setting
Performing cataract surgery in the context of a resource-limited setting should also be addressed, as the majority of EVD survivors reside in countries barriers to access of ophthalmic subspecialty care. One of the key limiting factors in providing access to cataract surgery is a dearth of eye surgeons in developing countries. It has been estimated that the prevalence of ophthalmologists is only one per 1 million people in sub-Saharan Africa. Within Sierra Leone, where the EVICT Study was conducted, there are currently five in-country ophthalmologist caring for over 6 million people [20]. In addition to a shortage of supply of ophthalmologists, the cost of cataract surgery and difficulty with transportation in rural areas may deter many patients afflicted with cataracts from pursuing treatment. Addressing issues of costs, insufficient numbers of ophthalmologists and eye care providers, as well as low government funding are necessary throughout much of western sub-Saharan Africa, where the age-standardized prevalence of cataract blindness in adults over 50 remains the highest, with a rate of 6% [20].
Conclusion
In summary, EVD survivors are at high risk of uveitis and related structural complications including cataract during disease convalescence. As such, cataract management remains a key issue in their long-term vision care. Results from the EVICT study showed that surgery can be safely performed to restore vision loss in EVD survivors, although a number of factors warrant consideration prior to surgery. Specifically, careful preoperative planning, ocular fluid sampling and infection control practices were paramount in the medical and surgical management of cataract in EVD survivors. As a consequence of the delayed timing of ocular fluid assessment of EVD survivors in the EVICT cohorts (i.e., median time of 19 and 34 months), the true prevalence of EBOV persistence in ocular fluid and dynamics of clearance require further investigation. Improved understanding in these areas will help to guide appropriate infection control measures in the medical and surgical management of ophthalmic sequelae of EVD. Given the unprecedented magnitude of survivors during the West Africa EVD outbreak, the opportunity to safely offer vision restoration in the form of cataract surgery has broad global health implications for the future of EVD survivors.
Financial & competing interests disclosure
This project was supported by unrestricted departmental grant from Research to Prevent Blindness, Inc. (NY, USA), NIH/NEI core grant P30-EY06360 (Department of Ophthalmology, Emory University School of Medicine), Building Interdisciplinary Research Careers in Women’s Health of the National Institutes of Health K12HD085850 (JG Shantha), an Alcon Research Institute Young Investigator Grant (S Yeh), Bayer Global Ophthalmology Awards Program (JG Shantha and S Yeh), Marcus Foundation Combating Childhood Illness Seed Grant to Emory Global Health Institute (S Yeh) and an unrestricted grant from Santen, Inc. (S Yeh and JG Shantha). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Shantha JG, Crozier I, Yeh S. An update on ocular complications of Ebola virus disease. Curr. Opin. Ophthalmol 28(6), 600–606 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shantha JG, Mattia JG, Goba A et al. Ebola Virus Persistence in Ocular Tissues and Fluids (EVICT) study: reverse transcription-polymerase chain reaction and cataract surgery outcomes of Ebola survivors in Sierra Leone. EBioMedicine 30, 217–224 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khanna R, Pujari S, Sangwan V. Cataract surgery in developing countries. Curr. Opin. Ophthalmol 22(1), 10–14 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Finger RP, Kupitz DG, Fenwick E et al. The impact of successful cataract surgery on quality of life, household income and social status in South India. PLoS ONE 7(8), 1–7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shantha JG, Crozier I, Hayek BR et al. Ophthalmic manifestations and causes of vision impairment in Ebola virus disease survivors in Monrovia, Liberia. Ophthalmology 124(2), 170–177 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hebert EH, Bah MO, Etard JF et al. Ocular complications in survivors of the Ebola outbreak in Guinea. Am. J. Ophthalmol 175, 114–121 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Mattia JG, Vandy MJ, Chang JC et al. Early clinical sequelae of Ebola virus disease in Sierra Leone: a cross-sectional study. Lancet Infect. Dis 16(3), 331–338 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varkey JB, Shantha JG, Crozier I et al. Persistence of Ebola virus in ocular fluid during convalescence. N. Engl. J. Med 372(25), 2423–2427 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng X, Blancett CD, Koistinen KA et al. Identification and pathological characterization of persistent asymptomatic Ebola virus infection in rhesus monkeys. Nat. Microbiol 2(7), 1–11 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Smith JR, Todd S, Ashander LM et al. Retinal pigment epithelial cells are a potential reservoir for Ebola virus in the human eye. Transl. Vis. Sci. Technol 6(4), 12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeh S, Shantha JG, Hayek B, Crozier I, Smith JR. Clinical manifestations and pathogenesis of uveitis in Ebola virus disease survivors. Ocul. Immunol. Inflamm 3948, 1–7 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Purpura LJ, Rogers E, Baller A et al. Ebola virus RNA in semen from an HIV-positive survivor of Ebola. Emerg. Infect. Dis 23(4), 714–715 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diallo B, Sissoko D, Loman NJ et al. Resurgence of Ebola virus disease in guinea linked to a survivor with virus persistence in seminal fluid for more than 500 days. Clin. Infect. Dis 63(10), 1353–1356 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mate SE, Kugelman JR, Nyenswah TG et al. Molecular evidence of sexual transmission of Ebola virus. N. Engl. J. Med 373(25), 2448–2454 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs M, Rodger A, Bell DJ et al. Late Ebola virus relapse causing meningoencephalitis: a case report. Lancet 388(10043), 498–503 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conway MD, Stern E, Enfield DB, Peyman GA. Management of cataract in uveitis patients. Curr. Opin. Ophthalmol 29(1), 69–74 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Llop SM, Papaliodis GN. Cataract surgery complications in uveitis patients: a review article. Semin. Ophthalmol 33(1), 64–69 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Mora P, Gonzales S, Ghirardini S et al. Perioperative prophylaxis to prevent recurrence following cataract surgery in uveitic patients: a two-centre, prospective, randomized trial. Acta Ophthalmol. 94(6), e390–e394 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Chu CJ, Dick AD, Johnston RL, Yang YC, Denniston AK. Cataract surgery in uveitis: a multicentre database study. Br. J. Ophthalmol 101(8), 1132–1137 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Lee CM, Afshari NA. The global state of cataract blindness. Curr. Opin. Ophthalmol 28(1), 98–103 (2017). [DOI] [PubMed] [Google Scholar]


