Abstract
Background
Endoplasmic reticulum (ER) stress is an essential response of epithelial and immune cells to inflammation in Crohn’s disease. The presence and mechanisms that might regulate the ER stress response in subepithelial myofibroblasts (SEMFs) and its role in the development of fibrosis in patients with Crohn’s disease have not been examined.
Methods
Subepithelial myofibroblasts were isolated from the affected ileum and normal ileum of patients with each Montreal phenotype of Crohn’s disease and from normal ileum in non-Crohn’s subjects. Binding of GRP78 to latent TGF-β1 and its subcellular trafficking was examined using proximity ligation-hybridization assay (PLA). The effects of XBP1 and ATF6 on TGF-β1 expression were measured using DNA-ChIP and luciferase reporter assay. Endoplasmic reticulum stress components, TGF-β1, and collagen levels were analyzed in SEMF transfected with siRNA-mediated knockdown of DNMT1 and GRP78 or with DNMT1 inhibitor 5-Azacytidine or with overexpression of miR-199a-5p.
Results
In SEMF of strictured ileum from patients with B2 Crohn’s disease, expression of ER stress sensors increased significantly. Tunicamycin elicited time-dependent increase in GRP78 protein levels, direct interaction with latent TGF-β1, and activated TGF-β1 signaling. The TGFB1 DNA-binding activity of ATF-6α and XBP1 were significantly increased and elicited increased TGFB1 transcription in SEMF-isolated from affected ileum. The levels of ER stress components, TGF-β1, and collagen expression in SEMF were significantly decreased following knockdown of DNMT1 or GRP78 by 5-Azacytidine treatment or overexpression of miR-199a-5p.
Conclusions
Endoplasmic reticulum stress is present in SEMF of patients susceptible to fibrostenotic Crohn’s disease and can contribute to development of fibrosis. Targeting ER stress may represent a novel therapeutic target to prevent fibrosis in patients with fibrostenotic Crohn’s disease.
Keywords: Crohn’s disease, endoplasmic reticulum stress, epigenetic regulation, subepithelial myofibroblasts, SEMF, intestinal fibrosis
INTRODUCTION
Endoplasmic reticulum (ER) stress response plays an important role in maintenance of cellular homeostasis by reducing the nascent and misfolded proteins that are produced under conditions of cellular stress including inflammation.1, 2 Endoplasmic reticulum stress and activation of the unfolded protein response (UPR) are associated with intestinal epithelial cell damage and apoptosis in Crohn’s disease.1, 2 Upon the onset of ER stress, the glucose-regulated protein 78 (GRP78), an ER stress master protein, dissociates from its binding partners: inositol-requiring enzyme 1α and β (IRE1α and β), activating transcription factor-6α (ATF-6α), and pancreatic ER kinase (PERK).1, 2 Dissociation of GRP78 from this complex activates the protective UPR. Unfolded protein response associated genes (eg, IRE1α, ATF6, and XBP1) have also been implicated in the genetic analysis of Crohn’s disease.3–6 Although ER stress and initiation of the UPR has been identified in intestinal epithelial cells and Treg cells in active Crohn’s disease, a potential role for ER stress and UPR in the response of activated subepithelial myofibroblast (SEMF) in Crohn’s disease has not been considered.
Our previous work has demonstrated increased expression of TGF-β1 and activation of the increased latent TGF-β1 in human intestinal smooth muscle cells by a nonproteolytic α Vβ 3 integrin-dependent mechanism.7 Oida et al demonstrated that latent TGF-β1 complexes with free GRP78 in the ER/Golgi of Foxp3 + regulatory T cells and translocates to the cell surface.8
Significant morbidity from bowel obstruction occurs in the up to 50% of Crohn’s disease patients with a Montreal B2 fibrostenotic phenotype.9, 10 Fibrostenotic Crohn’s disease is characterized by activation of intestinal mesenchymal cells, including SEMF and smooth muscle cells, and increased autocrine production of cytokines, including TGF-β1, that lead to excess production of extracellular matrix (ECM) proteins including collagen I and fibronectin.11–13 Due to its autocrine nature, once fibrosis is initiated, it progresses even in the absence of ongoing inflammation. Our current anti-inflammatory therapeutic strategy for Crohn’s disease does not inhibit development of fibrosis in patients with fibrostenotic Crohn’s disease.14, 15
MicroRNAs (miRs) are small noncoding (18–25 oligonucleotides) RNAs that play a role in the regulation of physiological and pathophysiological responses by post-transcriptional regulation of gene expression.16 A 6 to 8 nucleotide sequence of the microRNAs “seed sequence” binds to the 3’-untranslated region (UTR) of targeted mRNAs and causes translational repression or mRNA degradation.16 Previous studies have identified distinct tissue and plasma miRNA expression patterns in patients with Crohn’s disease.17–19 The TGF-β1 signaling pathway has numerous miRNA regulators, including a miR-199a-binding site in the 3’-UTR of Smad1 and Smad4 mRNA.20, 21 Hybridization analysis also reveals that the ER stress chaperone GRP78 and sensors ATF-6α and XBP1 are potential targets for regulation by miR-199a.22, 23
In this article, we provide evidence for the first time of the presence of ER stress and activation of the UPR in SEMF isolated from regions of fibrostenosis in the ileum of patients with fibrostenotic Crohn’s disease. Endoplasmic reticulum stress and UPR are not present in the normal ileum of the same patients or in patients with other phenotypes of Crohn’s disease.
METHODS
Isolation of SEMF From Patients With Crohn’s Disease
Intestine was obtained from patients undergoing ileal resection for Crohn’s disease (Table 1). All specimens in this analysis were obtained from patients with Montreal phenotype determined clinically, radiographically, and pathologically. Comparison is made between patients with solely inflammatory B1, fibrostenotic B2, and penetrating B3 disease. The Montreal classification is hierarchical, and subjects with combined phenotypes (ie, B2B3) were excluded from this analysis. The histologically normal ileum from non-Crohn’s subjects was used accordingly as a comparison.
TABLE 1.
Subject Demographics
| Age (years) | Patient No. (% of total) |
|---|---|
| under 20 | 3 (10) |
| 20–29 | 11 (33) |
| 30–39 | 10 (30) |
| 40–49 | 5 (15) |
| 50–59 | 2 (6) |
| over 60 | 2 (6) |
| Sex | |
| Male | 13 (39) |
| Female | 20 (63) |
| Race | |
| White | 19 (58) |
| Black or African | 13 (39) |
| Other/unknown | 1 (3) |
| Non-Crohn’s Subjects | 8 |
| CD Subjects Montreal Phenotype | |
| B1-nonstricturing, non penetrating | 7 |
| B2-stricturing | 9 |
| L1-ileal | 6 (67%) |
| L3-ileo-colic | 3 (33%) |
| B3-Penetrating | 9 |
Subepithelial myofibroblasts were isolated based on a modification of a method previously described.24 Briefly, after obtaining a 1:2 to 1-inch strip of normal resection margin and affected ileum tissue from the patient with diagnosed Crohn’s disease, the epithelial layer was removed by shaking with 25 mL of 5 mM ethylenediaminetetraacetic acid in Hank’s balanced salt solution (HBSS, MilliporeSigma, St. Louis, MO, USA) at 37°C at 250 rpm for 15 minutes. After ethylenediaminetetraacetic acid treatment, mucosal samples were denuded of epithelial cells and rinsed with ice-cold phosphate buffered saline twice. Subsequently, the tissues were placed in a conical tube containing 20 mL of RPMI-5 (MilliporeSigma) complete medium supplemented with a final concentration of 10% fetal bovine serum, 2 mM of L-glutamine, 10mM of HEPES, pH 7.4, 1 mM of sodium pyruvate, 100 U/mL of penicillin, 100 µg/mL streptomycin, 20 U of dispase II (Thermo Fisher Scientific, Waltham, MA, USA), and 4000 of U collagenase D (MilliporeSigma) and shaken vertically at 250 rpm for 1 hour at room temperature. After the tissue was pelleted at 200 g for 5 minutes at 4°C and washed with ammonium-chloride-potassium lysis buffer once, the cells were resuspended with 10 mL of RPMI-5 complete medium and cultured at 37°C in a humidified 5% CO2 incubator. The following antibodies were used for the myofibroblast characterization: anti–α-smooth muscle actin (MilliporeSigma), anti-vimentin (Santa Cruz Biotechnology, Dallas, TX, USA), anti-desmin (Santa Cruz Biotechnology), and appropriate isotype-matched controls (MilliporeSigma).
Subepithelial myofibroblasts isolated from human intestine were used to prepare RNA, microRNA, whole cell lysates, or nuclear lysates or placed into primary cell culture as reported and validated previously.25, 26 Epithelial cells, endothelial cells, neurons, and interstitial cells of Cajal are not detected in cells isolated in this fashion. These cells possess a myofibroblasts phenotype: immunostaining for vimentin and α-smooth muscle actin markers but not smooth muscle cell markers, γ-enteric actin, or desmin.25, 27 Each characteristic is retained in primary culture, as are their epigenetic changes.25, 27 Cells were treated with 5 µg/mL of tunicamycin (Tocris Bioscience, Bristol, UK) for different time points and for different experimental purposes.
Ethical Considerations
Human studies were approved by the Virginia Commonwealth University Institutional Review Board. All patients gave informed consent and agreed to provide specimens.
Quantitative Real-time PCR
Quantitative real-time polymerase chain reaction (qRT-PCR) was used to measure RNA transcripts of TGFB1, Collagen IαI, and miR-199a-5p. Primers were used for human TGFB1, Hs00998133_m1; COLIAI, Hs00164004_m1; GAPDH, Hs03929097_g1; miR-199a-5p, 478231_mir, A25576; and RNU6B, snRNA 001093 (Applied Bio Systems, Foster City, CA, USA). Results were calculated using the 2-ΔΔCt method based on GAPDH for mRNA and U6 for miR amplification, which remained stable across the regions and phenotypes examined.
Transfection
Transfection of primary cultured SEMF was performed using X-tremeGENE siRNA (Roche) transfection reagent, mixed with pre-miR miRNA precursors for miR-199a-5p (Thermo Fisher Scientific), siRNA DNMT1, siRNA GRP78, or scrambled sense controls (Santa Cruz Biotechnology). For each transfection, 100 nM of siRNA or miR 199a-mimic was diluted in 250 μl Opti-MEM (Thermo Fisher Scientific). The transfected cells were incubated at 37°C at 85% humidity and 5% CO2 for 24~48 hours before RNA isolation or Western blot analysis.
Western Blot Analysis
Cell lysates were prepared as described previously from muscle cells isolated from strictured intestine and from normal proximal resection margin in the same patient or in cultured cells as described previously.7 The levels of GRP78, XBP1 (detecting both XBP1-S/U), ATF6α, DNMT1 (Cell Signaling Technologies, Danvers, MA, USA) and collagen 1A1 (Santa Cruz Biotechnology) in lysates were measured and normalized to β-actin (MilliporeSigma).
DNA-Chromatin Immunoprecipitation
DNA-chromatin immunoprecipitation (DNA-ChIP) assay was performed on nuclear extracts from freshly isolated cells from non-Crohn’s subjects and from the normal ileum and affected ileum of the same patient with fibrostenotic Crohn’s disease. Experiments were performed according to manufacturer’s protocol (QIAGEN, Germantown, MD, USA). The ChIP-grade antibodies for XBP1 and ATF6α (Cell Signaling) were used in addition to control rabbit IgG. Polymerase chain reaction was performed with primers specific for the promoter region of TGFB1. Results were calculated from input; PCR was performed with DNA isolated from nuclear extracts without immunoprecipitation. Real time PCR data analysis was performed according to the manufacturer’s protocol. Purified antibody-bound protein/DNA complexes were analyzed by PCR, with primers amplifying the human TGFB1 promoter fragment containing the CCACG-box (forward, 5’-GTCTGAGCAAGGCAGCTTCT-3’ and reverse, 5’-GCCAGGTGCCTGAATA AAGA-3’), generating a 211-bp product. Polymerase chain reaction products were visualized in 1% agarose gels. The acetyl histone H3 antibody as a positive control and normal rabbit IgG as a negative control were included. Aliquots of chromatin samples without the treatment with antibodies were used as an input control.
Confocal Microscopy
Seven-µm cryosections of human intestine were used for microscopy. Digital images were obtained using a Leica TCS-SP2 AOBS Confocal Laser Scanning Microscope.
Proximity Ligation Assay
Protein-protein interaction between LAP-β1 and GRP78 was determined using in situ hybridization proximity ligation assay (PLA) reported previously.7 Histologic sections or primary cultures of intestinal SEMF were incubated with 1:50 mouse anti-human LAP-β1 (R&D Systems, Minneapolis, MN) and 1:50 goat anti-GRP78 (Santa Cruz Biotechnology) overnight at 4°C, and ligation-hybridization was performed according to manufacturer’s directions (Olink Bioscience, Uppsala, Sweden). Images were obtained by indirect immunofluorescence using a Zeiss AxioImager Z1 Microscope and AxioVision 4.6.3-SP1 software (Carl Zeiss Microscopy GmBH, Jena, Germany). Images were analyzed with Duolink-II Imaging Software (Ver 1.0.1.2, Olink Bioscience, Uppsala, Sweden). Results were reported as blobs per cell minus background for 10 SEMF counted in 10 successive high power fields by a blinded observer.
Methylation PCR of miR-199a
Genomic DNA was converted with bisulfite using EZ DNA methylation-lighting kit (Zymo Research, Irvine, CA, USA) according to the manufacturer’s protocol. Bisulfite-converted DNA was then used for real-time PCR using a pair of custom-made TaqMan probes (Applied Biosystems, Foster City, CA, USA) specific for either the methylated (M) or unmethylated (U) region of the CpG island in the promoter of miR-199a. Sequences of the probes are as follows: M, 6FAM-5’-TGCGTTGTGTCGTTGGAGAGATCG-3’-MGBNFQ; U, VIC-5’-TGTGTTGTGTTGTTGGAGAGATTGTTA-3’-G-MGBNFQ. Methylation of miR-199a was calculated by Cmeth = 100 / (1 + 2(CtCG − CtTG))%, where CtCG and CtTG are the threshold cycles of methylated (FAM channel) and unmethylated (VIC channel) detectors, respectively.28 Conditions for methylation PCR analysis were initial denaturation at 95°C for 10 minutes before cycling conditions, which include 40 cycles of denaturation at 95°C for 30 seconds, annealing 55°C for 30 seconds, extension at 72°C for 30 seconds, and final extension at 72°C for 7 minutes.
Measurement of TGF-β1 by ELISA
The TGF-β1 levels were measured using isoform-specific, enzyme-linked immunosorbent assay (ELISA) (R&D Systems) in SEMF of normal ileum from patients with fibrostenotic Crohn’s disease. Subepithelial myofibroblasts were subject to indicated treatment before cell lysis for ELISA. Active TGF-β1 (already active in the SEMF) was measured in samples without acid activation. Total TGF-β1 was measured in SEMFs treated with acid activation and represented TGF-β1 already active in the SEMF including LAP-β1 (that was activated by the acid treatment). Absorbance was read using a Wallac Victor2 1420 Multilabel counter (Perkin Elmer Life Sciences, Waltham, MA). Wavelength correction was calculated by subtracting readings at 570 nm from the reading at 450 nm to eliminate background noise in the ELISA plate. The TGF-β1 assay has no appreciable cross reactivity with TGF-β2 or TGF-β3, activins, inhibins, or bone morphogenetic proteins. Results were calculated as in picograms per milliliter of protein.
Statistical Analysis
Values represent means ± SE of n experiments, where n represents the number of experiments on cells derived from separate subjects or animals. Statistical significance was tested by Student t test for either paired or unpaired data as appropriated. Comparison between multiple groups was made using the ANOVA (>2 groups), with a Turkey test for post hoc comparisons. Significance was assumed for P < 0.05.
RESULTS
Endoplasmic Reticulum Stress Increases in Fibrostenotic Crohn’s Disease
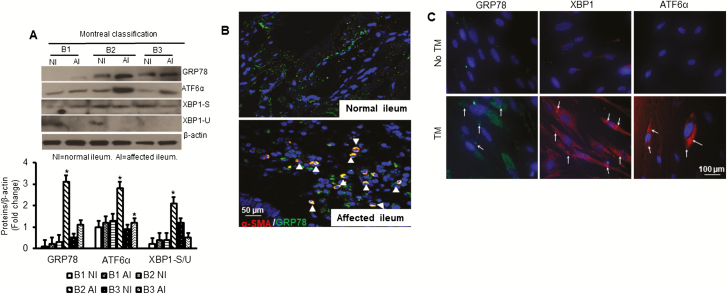
We first measured the protein expression patterns of several ER stress markers in cultured SEMF from different phenotypic CD and corresponding normal ileum. Western blot analysis demonstrated that GRP78 and ATF-6α protein levels were significantly increased by 3.1 ± 0.2-fold and 2.8 ± 0.1-fold respectively, in SEMFs that were isolated from affected ileum compared with normal ileum in the same patient with B2 fibrostenotic Crohn’s disease and compared with that of other phenotypes including inflammatory B1 and penetrating B3 (Fig. 1A). The ratio between XBP1-S and XBP1-U, a reflection of the activation of ER stress, was significantly increased by 2.1 ± 0.1-fold in SEMFs that were isolated from affected ileum compared with normal ileum in the same patient with B2 fibrostenotic phenotype and with that in other phenotypes (Fig. 1A).
FIGURE 1.
ER stress increases in fibrostenotic Crohn’s disease. A, GRP78, ATF-6α, and XBP-1 (spliced & unspliced) protein expressions were measured in SEMFs that were isolated from patient’s ileum with each Crohn’s disease phenotype, Montreal B1, B2, and B3 indicated conditions (n = 3–5 patients for each group). B, Representative histologic sections from normal ileum and fibrostenotic ileum in the same patient. Colocalization of α-smooth muscle actin and GRP78 is increased in vivo in the fibrostenotic ileum compared with normal ileum. Histologic sections were costained using antibodies recognizing α-smooth muscle actin and GRP78. C, Representative immunofluorescent staining of primary cultures of SEMF of normal ileum after treatment of SEMF from normal ileum with tunicamycin (5 µg/mL) for 8 hours. Expression of the ER stress proteins GRP-76, XBP1, and ATF6α was increased. Each image is representative of 5 independent experiments (n = 5). Results are expressed as mean ± SEM. *Denotes P < 0.05 vs normal ileum SEMF.
These in vitro findings were confirmed in co-immunofluorescent staining of histologic sections of normal ileum and affected ileum from the same patients with antibodies against GRP-78 and α-smooth muscle actin (α-SMA), a protein marker for identification of mesenchymal cells. The GRP78 protein expression was significantly increased in affected ileum compared with normal ileum in the same patient with B2 fibrostenotic Crohn’s disease (Fig. 1B).
Tunicamycin stimulates intracellular ER stress through inhibition of N-linked glycosylation in proteins and the generation of misfolded proteins.29 The increases in protein levels of GRP78, XBP1, and ATF6α that were seen in SEMF of strictured ileum could be reproduced in SEMF of normal ileum from the same patient by induction of ER stress with tunicamycin (5 µg/mL) for 8 hours (Fig. 1C). Taken together, these data indicate the presence of ER stress in ileal SEMF, a key cell type for the development of fibrosis in patients with Crohn’s disease.
ER Stress Protein GRP-78 Associates With Latent TGF-β1
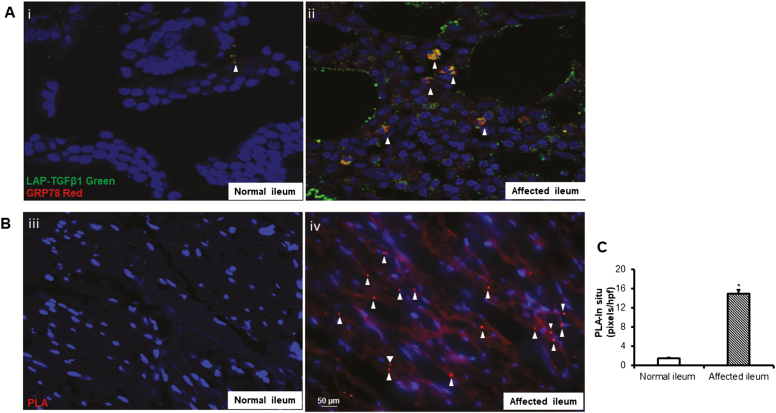
The role of ER stress in the inflammation-induced immune response and intestinal epithelial cell damage in Crohn’s disease is well known.30–32 However, whether ER stress plays a role in the development of TGF-β1-dependent intestinal fibrosis is unknown. Latent TGF-β1 forms a complex with free GRP78 that translocates to the cell surface in regulatory T cells.8 Our previous work demonstrated that latent TGF-β1 is activated by a nonproteolytic α Vβ 3 integrin-dependent mechanism at the RGD-binding domain of the extracellular integrin β 3 receptor in mesenchymal cells. This process is increased in fibrostenotic ileum compared with normal ileum in the same patient and results in excess activated TGF-β1.7 To examine whether GRP78 interacts with latent TGF-β1 in vivo in SEMF of affected ileum of patients with fibrostenotic Crohn’s disease, histologic sections were immunostained using antibodies against GRP78 and latent TGF-β1. There was significant association of GRP78 and latent TGF-β1 in fibrostenotic ileum compared with a minimum level in normal ileum. (Fig. 2A, i and ii). The direct interaction, within 15 nm, of GRP78 and latent TGF-β1 was confirmed using proximity ligation-hybridization assay, which detects direct protein-protein interaction. (Fig. 2B, iii and iv). In situ hybridization pixels are in red, and DAPI counterstained nuclei are in blue. Quantification data of in situ hybridization are expressed as pixels per high power field in each of 10 consecutive high power fields with similar numbers of cells (Fig. 2C).
FIGURE 2.
Complex formation between latent TGF-β1 and GRP78 is increased in SEMF of fibrostenotic ileum in vivo compared with normal ileum in the same patient. A, Co-immunostaining of GRP78 and latent TGF-β1 in affected ileum compared with histologically normal ileum (i and ii). B, Direct protein-protein interaction between latent TGF-β1 and GRP78 is increased in affected ileum compared with normal ileum in the same patient with fibrostenotic Crohn’s disease. Proximity ligation hybridization assay was used to demonstrate direct protein interaction within 15 nm as indicated by the red chromogen. Images are representative of the normal and fibrostenotic ileum in 5 different patients. C, Data are expressed as pixels per high power field in each of 10 consecutive high power fields with similar numbers of cells. *Denotes P < 0.05 between normal ileum and affected ileum. Results are expressed as mean ± SEM of 5 patients.
ER Stress Components Induce TGF-β1 Activation
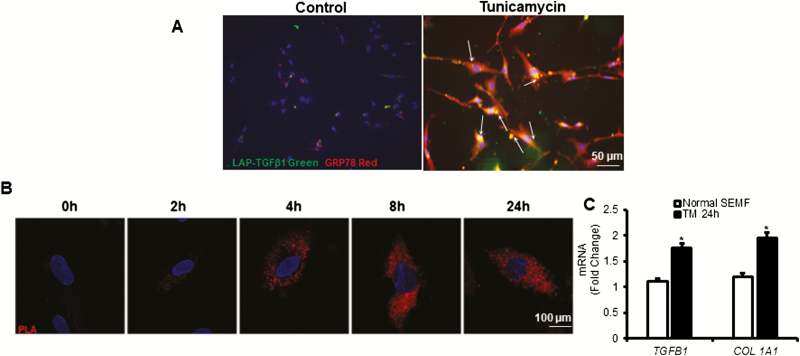
When compared with vehicle-treated SEMF of normal ileum, treatment with tunicamycin increased protein interaction between GRP78 and latent TGF-β1 as determined by immunofluorescent staining, which is similar to that seen in vivo in fibrostenotic ileum (Fig. 3A). Intestinal mesenchymal cells were treated with tunicamycin (5 µg/mL) for increasing periods of times (Fig.3B). Tunicamycin-induced ER stress in SEMF resulted in a time-dependent increase in association of GRP78, and latent TGF-β1 within 2 hours was maximal by 8 hours and sustained for up to 24 hours (Fig. 3B). In tunicamycin-treated SEMF, RT-PCR analysis showed that transcript levels of both TGFB1 and COL 1A1 were significantly increased (Fig. 3C). Taken together, these findings suggest that ER stress plays a role in latent TGF-β1 activation signaling in SEMF.
FIGURE 3.
Endoplasmic reticulum stress induced by tunicamycin causes time-dependent association of latent TGF-β1 and GRP78 and increased TGF-β1 and collagen expression. A, Representative images showing that the interaction between latent TGF-β1 and GRP78 is increased after 24 hours of treatment of SEMF from normal ileum after induction of ER stress with tunicamycin (5 µg/mL). Co-immunofluorescent staining was performed using antibodies recognizing latent TGF-β1 and GRP78. Images represent the results of experiments using SEMF isolated from normal ileum in 5 patients with fibrostenotic Crohn’s disease. B, Representative images showing that the interaction of latent TGF-β1 and GRP increases in time-dependent fashion in SEMF of normal ileum after induction of ER stress with tunicamycin (5 µg/mL). Images are of a single cell from 10 different locations on the same slide and are representative of the response of SEMF of normal ileum isolated from 5 different patients. C, Expression of TGFB1 and COL 1A1 increased in normal SEMF after treatment with tunicamycin (TM) for 24 hours. Transcript levels were measured by RT-PCR and normalized to the expression of GAPDH. Results are expressed as mean ± SEM. *Denotes P < 0.05 vs vehicle-treated cells.
ER Stress Sensors XBP1 and ATF-6α Regulate TGFB1 Gene Transcription
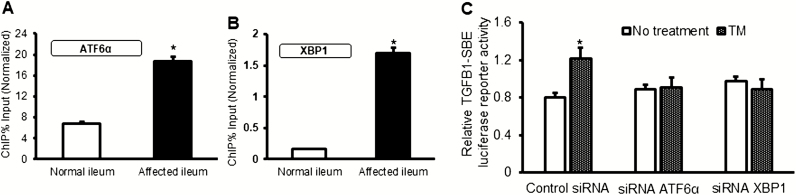
ATF-6α and XBP1 are transcription factors that bind to the ER stress responsive element 1 (ERSE1) in the promoters of UPR-responsive genes.33 We therefore tested the notion that ATF-6α and XBP1 might regulate transcription of the TGFB1 gene. First, ChIP assays were performed in SEMF of normal and strictured ileum to measure the DNA-binding affinity of both XBP1 and ATF6α transcription factors for the TGFB1 promoter. DNA-binding affinity was increased in SEMF of fibrostenotic ileum compared with SEMF of normal ileum in the same patient with Montreal B2 fibrostenotic Crohn’s disease (Fig. 4A and 4B). These DNA-ChIP data suggest that ER stress is involved in the regulation of TGFB1 gene expression through enhanced DNA binding of ATF-6α and XBP1 to the TGFB1 promoter region. This notion was confirmed after siRNA-mediated knockdown of ATF6α or XBP1 in normal SEMF treated with tunicamycin or vehicle. Tunicamycin-induced upregulation of TGFB1 transcription was lost in the absence of ATF6α or XBP1 using TGFB1 luciferase reporter assay (Fig. 4C). These results showed that, in addition to GRP78, transcription factors ATF6α and XBP1 are also involved in the ER stress-mediated response in SEMF that contributes to TGF-β1-mediated fibrosis.
FIGURE 4.
Binding of ER stress sensors XBP1 and ATF-6α to the TGFB1 promoter regulates its expression. DNA-binding affinity of ATF6α (Panel A) and XBP1 (Panel B) for the TGFB1 promoter was increased in SEMF isolated from the affected fibrostenotic ileum compared with normal ileum in the same patient. Chromatin immunoprecipitation using genomic DNA extracted from SEMF was isolated from the normal and affected ileum in 5 patients with fibrostenotic Crohn’s disease. C, The transcriptional regulation of TGFB1 gene expression by ATF-6α or XBP1 was measured by TGFB1 luciferase reporter assay. Normal SEMFs were cotransfected with siRNA ATF-6α or XBP1 or control scramble siRNA and TGFB1 luciferase reporter for 24 hours, then further incubated with tunicamycin (5 µg/mL) for 16 hours (n = 3). Results are expressed as mean ± SEM of 5 experiments using different patient samples.*Denotes P < 0.05 for normal vs affected ileum, or vehicle vs tunicamycin (TM) treatment.
Silencing of miR-199a-5p Activates ER Stress Response in Fibrostenotic Crohn’s Disease
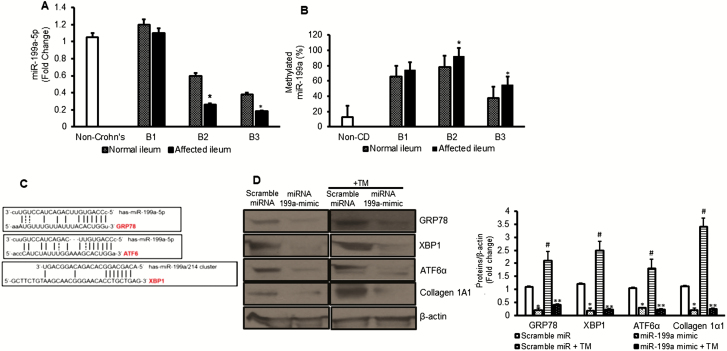
MicroRNAs can exert epigenetic regulation of gene expression by their ability to effect post-transcriptional gene expression. However, they are also subject to epigenetic regulation themselves. Hypermethylation and silencing of miR-199a-5p expression has been observed in the UPR in monocytes and the proliferative response of keloid fibroblasts through disinhibition of ER stress.22, 34 We hypothesized that silencing of miR-199a-5p causes disinhibition of ER stress in SEMF of fibrostenotic ileum in Crohn’s disease. The level of miR-199a-5p expression was significantly decreased in SEMFs that were isolated from the normal ileum in patients with B2 fibrostenotic and in B3 penetrating Crohn’s disease but not in B1 inflammatory disease (Fig. 5A). The expression of miR-199a-5p was further diminished in the SEMF of affected ileum in B2 and B3 patients. The methylation state of miR-199a-5p had the opposite pattern and was higher in SEMF of affected ileum in B2 and B3 patients compared with SEMF of normal ileum in the same patient (Fig. 5B). Hybridization analysis using TargetScan and results from others have shown that the ER stress chaperone GRP78 and the ER stress sensors ATF-6α, XBP1, and IRE1α were potential targets of regulation by miR-199a-5p (Fig. 5C).35, 36 A 7mer-m8 sequence was identified at position 345–351 of HSPA5 (GRP78), a 7mer-A1 sequence was identified at position 186–192 of ATF6 (ATF6α), and a 7mer-A1 sequence was identified at 3’UTR region of XBP1 promoter by bioinformatic analysis.35, 36
FIGURE 5.
Silencing of miR-199a-5p activates ER stress in fibrostenotic Crohn’s disease. A, Expression of miR-199a-5p in SEMFs that were isolated from normal ileum and affected ileum in the same patient with Montreal B1 inflammation, B2 stricturing, and B3 penetrating Crohn’s disease and from non-Crohn’s subjects (n = 3). B, Expression of methylated miR-199a-5p compared with unmethylated miR-199a-5p in SEMFs that were isolated from the same patient with different phenotypes of Crohn’s disease (n = 3). C, Hybridization complementary binding sequence between the promoter of ER stress genes GRP78, XBP1, and ATF6 and miR-199a-5p or miR-199a-5/214 cluster. D, Overexpression of miR-199a prevented tunicamycin-induced upregulation of ER stress response and collagen production. Representative Western blot and densitometric analysis for GRP78, XBP1, ATF6, and collagen 1A1 protein levels in SEMF of normal ileum in a patient with fibrostenosis after transfection of miR-199a-5p mimic or scrambled miR. After transfection, ER stress was induced by treatment with tunicamycin (5μg/mL) for 16 hours. The β-actin was used as a loading control. Results are expressed as the mean ± SEM for 5 experiments using cells from different patients. *Denotes P < 0.05 for normal vs affected ileum, or scramble miR vs miR199a-5p mimic in naïve cells, #Denotes P < 0.05 scrambled miR vs scramble miR + tunicamycin (TM), **Denotes P < 0.05 for scramble miR + TM vs miR-199a-5p mimic + TM.
Overexpression of miR-199a-5p in otherwise naïve SEMF isolated from fibrostenotic ileum inhibited endogenous ER stress response, with a decrease in the protein levels of GRP78, XBP1, and ATF6α (Fig. 5D). Overexpression of miR199–5p also prevented activation of tunicamycin-induced ER stress and the resultant increased collagen 1 production in SEMF isolated from normal ileum (Fig. 5D).
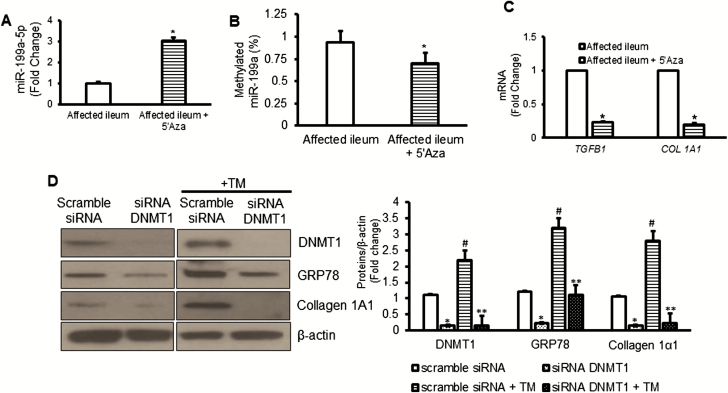
Hypermethylation of CpG islands identified within the pri-miR-199a gene are targets for DNMT1-mediated methylation and silencing.37 We have previously shown that DNMT1 levels are significantly increased in SEMF of fibrostenotic ileum compared with normal ileum.38 We examined whether the increase in DNMT1 resulted in promoter hypermethylation that could account for silencing of miR-199 in these cells. Treatment of SEMF of fibrostenotic ileum where the pri-miR-199 gene was hypermethylated and miR-199–5p expression was diminished (Fig. 5A and B) with the demethylating agent 5’azacytidine (5’Aza), restored miR-199a-5p expression (Fig. 6A). In these cells, methylation of pri-miR-199 was reduced by 5’Aza treatment (Fig. 6B). Restoration of miR-199–5p expression by demethylation of pri-miR-199 in SEMF of fibrostenotic ileum was accompanied by a decrease in TGFB1 and COL 1A1 expression (Fig. 6B and 6C). The role of DNMT1 in pre-miR-199a-5p methylation was confirmed by siRNA-mediated knockdown of DNMT1 in SEMF of normal ileum before induction of ER stress with tunicamycin (Fig. 6D). In SEMF where DNMT1 was knocked down, not only were DNMT1 levels diminished but the increases in GRP78 and collagen 1A1 protein levels seen after tunicamycin-induced ER stress were also abolished (Fig. 6D). In summary, these data support the notion that DNMT1-mediated hypermethylation and silencing miR-199a-5p in SEMF of fibrostenotic ileum prevent ER stress activation and the resultant increase in TGF-β1 and collagen 1 production.
FIGURE 6.
DNMT1 mediates the silencing of miR-199a-5p in fibrostenotic Crohn’s disease. A, Expression of miR-199a-5p increased in SEMF after treatment with the DNMT1 inhibitor, 5’-Azacytidine (5’Aza, 5 μg/mL) for 16 hours. B, The ratio of methylated/unmethylated miR-199a-5p decreased in SEMF from fibrostenotic ileum of patients treated with 5’Aza. C, Expression of TGFB1 and COL 1A1 RNA transcripts were measured by qRT-PCR and decreased in SEMF of fibrostenotic ileum after treatment with 5’Aza. D, Representative Western blot and densitometric analysis showing that in normal ileal SEMF from patients with fibrostenosis knockdown of DNMT1 resulted in lower GRP78 and collagen 1A1 production compared with SEMF transfected with scramble siRNA. Results represent the mean ± SEM of 5 to 6 experiments using normal SEMF isolated from 5 to 6 patients with fibrostenotic Crohn’s disease. *Denotes P < 0.05 between vehicle and 5’Aza treated cells or between scramble siRNA and siRNA DNMT1 transfected cells. #Denotes P < 0.05 between scramble siRNA and scramble siRNA + tunicamycin (TM). **Denotes P < 0.05 between scramble siRNA + TM and siRNA DNMT1 + TM.
Inhibition of GRP78 Reduces Profibrotic Factors in Vitro
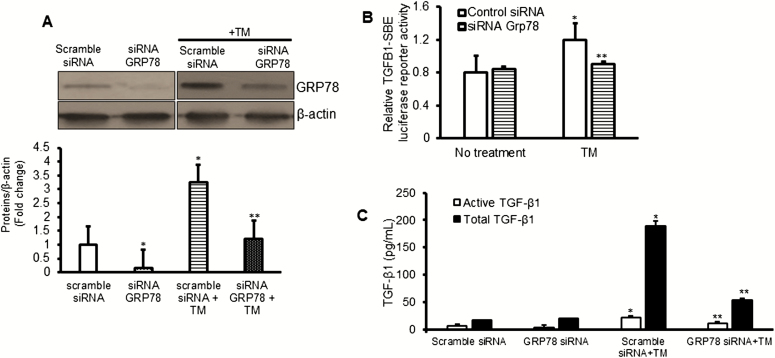
Endoplasmic reticulum chaperone proteins like GRP78 play a critical role in maintenance of intracellular homeostasis, protecting cells from ER stress–induced cell damage. The role of GRP78 in mesenchymal cells during the development of fibrosis is largely unknown. To investigate the role of GRP78 in regulating profibrotic TGF-β1, we used a siRNA-mediated knockdown of GRP78. Transfection with GRP78 siRNA decreased GRP78 levels in naïve cells and abolished the ability of tunicamycin-induced ER stress to increase GRP78 levels (Fig. 7A).
FIGURE 7.
Inhibition of GRP78 reduces TGFB1 transcription and TGF-β1 protein levels. A, Representative Western blot and densitometric analysis of GRP78 protein levels in SEMF of normal ileum after siRNA-mediated knockdown of GRP78 in the presence or absence of tunicamycin. B, Tunicamycin-induced increase in TGFB1 transcription is abolished by siRNA-mediated knockdown of GRP78. C, Tunicamycin-induced increase in active and total (active + latent) TGF-β1 is inhibited after siRNA-mediated knockdown of Grp78. TGFB1 transcriptional activity was measured by luciferase reporter assay. Protein levels were measured using Western blot or ELISA. Results represent the mean ± SEM of 5 to 6 experiments using normal SEMF isolated from 5 to 6 patients with fibrostenotic Crohn’s disease. *Denotes P < 0.05 between scramble siRNA and scramble siRNA + tunicamycin (TM). **Denotes P < 0.05 between scramble siRNA + tunicamycin (TM) and siRNA Grp78 + TM.
Our data showing the interaction between GRP78 and latent TGF-β1 and those of Oida8 led us to hypothesize whether this interaction was necessary for the activation of latent TGF-β1. Here, we show that the increased transcriptional activity of TGFB1 that resulted from ER stress was abolished in the cells transfected with siRNA GRP78 (Fig. 7B). Furthermore, knockdown of endogenous GRP78 in SEMF of normal ileum marginally diminished the levels of active TGF-β1 (Fig. 7C), whereas the levels of both active and total (active + inactive) TGF-β1 were abrogated in the cells after tunicamycin-induced ER stress response (Fig. 7C). Taken together, these findings indicate that induction of ER stress results in increased transcription of TGFB1 and increased activation of the latent TGF-β1, the product of the TGFB1 gene.
DISCUSSION
Endoplasmic reticulum stress compromises intestinal epithelial barrier function and activates SEMF through interaction between SEMF and epithelial cells and between luminal microbiota and surrounding immune cells.39 However, the role of ER stress in SEMF during the development of intestinal fibrosis in patients with Crohn’s disease has not been investigated. To the best of our knowledge, the current study is the first to provide evidence that ER stress in SEMF could result in activation of the fibrosis program in these cells. First, we show the presence of an increased ER stress response in SEMF isolated from affected ileum in patients with fibrostenotic Crohn’s disease. Secondly, we demonstrated that the ER stress sensor protein GRP78 interacts directly with latent TGF-β1 and increases its activation. Thirdly, ER stress proteins XBP1 and ATF-6α act as transcription factors, binding to the TGFB1 promoter to increase TGFB1 gene expression. We also demonstrated the epigenetic regulatory mechanism that facilitates activation of ER stress. The DNMT1-mediated hypermethylation of pre-miR199a-5p silences its expression, thereby disinhibiting the ER stress response. Ultimately, these processes contribute to in greater active TGF-β1 and collagen 1 production in SEMF of strictured ileum.
The mechanisms regulating translocation of GRP78, along with bound latent TGF-β1 from the cytosol to the cell membrane, are not fully understood. Conflicting evidence has been presented to suggest that O-linked glycosylation of GRP78 is necessary for translocation. One study has shown that GRP78 required glycosylation to translocate from the cytoplasm to the cell membrane.8 In contrast is evidence from other investigators that shows the translocation of GRP78 was not affected by mutation of the O-linked glycosylation site at the C-terminus of GRP78 in their cultured cell lines.39 What is known is that deletion of the ER retrieval signal, the C-terminal KDEL motif in GRP78, increased the amount of GRP78 on cell surface localization of GRP78 compared with intracellular protein, presumably by inhibiting GRP78 recycling.40
Epigenetic control of gene expression is exerted through modification of DNA regulatory elements or enhancers that induce transition of condensed heterochromatin, where gene transcription is inhibited by histone modifications and DNA methylation, to euchromatin, where genes are accessible to transcription factors and regulation of gene transcription. Nucleosome position also regulates this process. Noncoding RNA, both microRNA and long noncoding RNA, play a role in the regulation of physiological and pathophysiological responses by post-transcriptional regulation of gene expression. Epigenetic mechanisms are emerging as key mediators of the effects of both genetics and the environment on gene expression and disease. In addition to a set of inherited epigenetic marks, there are likely nonheritable epigenetic marks that are more dynamic and change in response to environmental stimuli. The role of epigenetics in the regulation of fibrosis is increasingly demonstrated in the studies from a variety of organs including lung, kidney, liver, skin, and intestine.41–44
In this study, we show that DNMT1 silences miR-199a-5p by promoter hypermethylation, thereby preventing it from inactivating the ER stress response. This has 2 reinforcing effects. First, as ATF6α and XBP1 are targets of miR-199a-5p, it allowed increased ATF6α-dependent and XBP1-dependent TGFB1 transcription and increased the resulting latent TGF-β1 expression. Second, it resulted in increased association of GRP78 with latent TGF-β1 and facilitated excess activation of TGF-β1. Reversal of miR-199a-5p silencing by the demethylating agent 5’Aza resulted in lower TGF-β1 and collagen 1α1. Studies in activated murine hepatic stellate cells have provided complementary results showing that connective tissue growth factor (CCN) 2, α-smooth muscle actin and collagen1A1 were suppressed by expression of a miR-199a-5p mimic.45
In summary, our study has provided evidence for the induction of ER stress in SEMF of fibrostenotic ileum in patients expressing a Montreal B2 phenotype of Crohn’s disease. Our results also indicate that the ER stress response in ileal SEMF is in part the result of DNMT1-dependent silencing of miR-199a-5p, which ultimately results in enhanced expression of TGF-β1 and collagen 1α1 in these cells. Both TGF-β1 and collagen 1α1 are key drivers of fibrosis in the intestine. Targeting this mechanism of ER stress activation in patients susceptible to fibrostenotic Crohn’s disease may represent a novel therapeutic approach for these patients.
Supported by: VCU’s CTSA (UL1TR000058 from the National Center for Advancing Translational Sciences) and the Center for Clinical and Translational Research (CCTR) Endowment Fund of Virginia Commonwealth University (to CL) and the Crohn’s & Colitis Foundation Ref. #550514 (to CL) and National Institute of Diabetes and Digestive and Kidney Diseases DK49691 (to JFK).
Conflicts of interest: The authors disclose no conflicts.
Presented at the Annual Meeting of Digestive Disease Week (USA, 2016), at the 2nd Annual Crohn’s & Colitis Congress (USA, 2018), and at the 14th Congress of European Crohn’s and Colitis Organisation (Denmark, 2019).
Author Contribution: CL had the initial concept, managed the study, collected data, and drafted, revised, and finalized the manuscript. JFK, JG, and KSM provided the experimental resources and space. JB, ER, and NW provided surgical tissue samples from the patients. CL, JFK, JG, and KSM were involved in revising the manuscript. All authors read and approved the final manuscript.
REFERENCES
- 1. Tomczak MF, Kaser A, Blumberg RS. ER stress in intestinal inflammatory disease. In: Agostinis P, Afshin S, eds. Endoplasmic Reticulum Stress in Health and Disease. Dordrecht: Springer; 2012:281–298. [Google Scholar]
- 2. Hooper KM, Barlow PG, Henderson P, et al. . Interactions between autophagy and the unfolded protein response: implications for inflammatory bowel disease. Inflamm Bowel Dis. 2019;25:661–671. [DOI] [PubMed] [Google Scholar]
- 3. Cao SS. Endoplasmic reticulum stress and unfolded protein response in inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:636–644. [DOI] [PubMed] [Google Scholar]
- 4. Cao SS. Epithelial ER stress in crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2016;22:984–993. [DOI] [PubMed] [Google Scholar]
- 5. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. [DOI] [PubMed] [Google Scholar]
- 6. Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. [DOI] [PubMed] [Google Scholar]
- 7. Li C, Flynn RS, Grider JR, et al. . Increased activation of latent TGF-β1 by αVβ3 in human Crohn’s disease and fibrosis in TNBS colitis can be prevented by cilengitide. Inflamm Bowel Dis. 2013;19:2829–2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oida T, Weiner HL. Overexpression of TGF-ß 1 gene induces cell surface localized glucose-regulated protein 78-associated latency-associated peptide/TGF-ß. J Immunol. 2010;185:3529–3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li C, Kuemmerle JF. Mechanisms that mediate the development of fibrosis in patients with Crohn’s disease. Inflamm Bowel Dis. 2014;20:1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reider F, Fiocchi C, Rogler G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology. 2017;152:340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Filidou E, Valatas V, Drygiannakis I, et al. . Cytokine receptor profiling in human colonic subepithelial myofibroblasts: a differential effect of th polarization-associated cytokines in intestinal fibrosis. Inflamm Bowel Dis. 2018;24:2224–2241. [DOI] [PubMed] [Google Scholar]
- 12. Drygiannakis I, Valatas V, Sfakianaki O, et al. . Proinflammatory cytokines induce crosstalk between colonic epithelial cells and subepithelial myofibroblasts: implication in intestinal fibrosis. J Crohns Colitis. 2013;7:286–300. [DOI] [PubMed] [Google Scholar]
- 13. Powell DW, Mifflin RC, Valentich JD, et al. . Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol. 1999;277:C183–C201. [DOI] [PubMed] [Google Scholar]
- 14. Rieder F, Zimmermann EM, Remzi FH, et al. . Crohn’s disease complicated by strictures: a systematic review. Gut. 2013;62:1072–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bettenworth D, Rieder F. Reversibility of stricturing crohn’s disease-fact or fiction? Inflamm Bowel Dis. 2016;22:241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Inui M, Martello G, Piccolo S. MicroRNA control of signal transduction. Nat Rev Mol Cell Biol. 2010;11:252–263. [DOI] [PubMed] [Google Scholar]
- 17. Kalla R, Ventham NT, Kennedy NA, et al. . MicroRNAs: new players in IBD. Gut. 2015;64:504–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li C. Intestinal fibrosis in inflammatory bowel disease: the role of microRNAs. Explor Res and Hypoth in Med. 2016;1:7–16. [Google Scholar]
- 19. Netz U, Carter J, Eichenberger MR, et al. . Plasma microRNA profile differentiates crohn’s colitis from ulcerative colitis. Inflamm Bowel Dis. 2017;24:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Butz H, Rácz K, Hunyady L, et al. . Crosstalk between TGF-β signaling and the microRNA machinery. Trends Pharmacol Sci. 2012;33:382–393. [DOI] [PubMed] [Google Scholar]
- 21. Lin EA, Kong L, Bai XH, et al. . miR-199a, a bone morphogenic protein 2-responsive MicroRNA, regulates chondrogenesis via direct targeting to Smad1. J Biol Chem. 2009;284:11326–11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hassan T, Carroll TP, Buckley PG, et al. . miR-199a-5p silencing regulates the unfolded protein response in chronic obstructive pulmonary disease and α1-antitrypsin deficiency. Am J Respir Crit Care Med. 2014;189:263–273. [DOI] [PubMed] [Google Scholar]
- 23. Duan Q, Wang X, Gong W, et al. . ER stress negatively modulates the expression of the miR-199a/214 cluster to regulates tumor survival and progression in human hepatocellular cancer. Plos One. 2012;7:e31518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hassan K, Nie W, Edwards RA, et al. . Isolation of primary myofibroblasts from mouse and human colon tissue. JoVE 2013;80:e50611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Flynn RS, Murthy KS, Grider JR, et al. . Endogenous IGF-I and alphaVbeta3 integrin ligands regulate increased smooth muscle hyperplasia in stricturing Crohn’s disease. Gastroenterology. 2010;138:285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuemmerle JF. Autocrine regulation of growth in cultured human intestinal muscle by growth factors. Gastroenterology. 1997;113:817–824. [DOI] [PubMed] [Google Scholar]
- 27. Teng B, Murthy KS, Kuemmerle JF, et al. . Expression of endothelial nitric oxide synthase in human and rabbit gastrointestinal smooth muscle cells. Am J Physiol. 1998;275:G342–G351. [DOI] [PubMed] [Google Scholar]
- 28. Eads CA, Danenberg KD, Kawakami K, et al. . MethyLight: a high-throughput assay to measure DNA methylation. Nucleic Acids Res. 2000;28:E32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yoshida T, Sakai T. Tunicamycin. In: Schwab M, ed. Encyclopedia of Cancer. Berlin, Heidelberg: Springer; 2011. [Google Scholar]
- 30. Kaser A, Lee AH, Franke A, et al. . XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cao SS, Zimmermann EM, Chuang BM, et al. . The unfolded protein response and chemical chaperones reduce protein misfolding and colitis in mice. Gastroenterology. 2013;144:989–1000.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shkoda A1, Ruiz PA, Daniel H, et al. . Interleukin-10 blocked endoplasmic reticulum stress in intestinal epithelial cells: impact on chronic inflammation. Gastroenterology. 2007;132:190–207. Epub 2006 Oct 21. [DOI] [PubMed] [Google Scholar]
- 33. Yamamoto K, Yoshida H, Kokame K, et al. . Differential contributions of ATF6 and XBP1 to the activation of endoplasmic reticulum stress-responsive cis-acting elements ERSE, UPRE and ERSE-II. J Biochem. 2004;136:343–350. [DOI] [PubMed] [Google Scholar]
- 34. Wu ZY, Lu L, Liang J, et al. . Keloid microRNA expression analysis and the influence of miR-199a-5p on the proliferation of keloid fibroblasts. Genet Mol Res. 2014;13:2727–2738. [DOI] [PubMed] [Google Scholar]
- 35. Dai BH, Geng L, Wang Y, et al. . microRNA-199a-5p protects hepatocytes from bile acid-induced sustained endoplasmic reticulum stress. Cell Death Dis. 2013;4:e604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lou Z, Gong YQ, Zhou X, et al. . Low expression of miR-199 in hepatocellular carcinoma contributes to tumor cell hyper-proliferation by negatively suppressing XBP1. Oncol Lett. 2018;16:6531–6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen BF, Gu S, Suen YK, et al. . microRNA-199a-3p, DNMT3A, and aberrant DNA methylation in testicular cancer. Epigenetics. 2014;9:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li C, Kuemmerle J. Epigenetic Silencing of SOCS3 expression contributes to fibrosis in crohn’s disease. Inflamm Bowel Dis. 2017:23:S97. doi.10.1097/01.MIB.0000512845.68840.de. [Google Scholar]
- 39. Kaser A, Flak MB, Tomczak MF, et al. . The unfolded protein response and its role in intestinal homeostasis and inflammation. Exp Cell Res. 2011;317: 2772–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang Y, Liu R, Ni M, et al. . Cell surface relocalization of the endoplasmic reticulum chaperone and unfolded protein response regulator GRP78/BiP. J Biol Chem. 2010;285:15065–15075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Neary R, Watson CJ, Baugh JA. Epigenetics and the overhealing wound: the role of DNA methylation in fibrosis. Fibrogenesis Tissue Repair. 2015;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Däbritz J, Menheniott TR. Linking immunity, epigenetics, and cancer in inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:1638–1654. [DOI] [PubMed] [Google Scholar]
- 43. Li C, Kuemmerle JF. Genetic and epigenetic regulation of intestinal fibrosis. United European Gastroenterol J. 2016;4:496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Robinson CM, Watson CJ, Baugh JA. Epigenetics within the matrix. A neo-regulator of fibrotic disease. Epigenetics. 2012;7:987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen L, Chen R, Velazquez VM, et al. . Fibrogenic signaling is suppressed in hepatic stellate cells through targeting of Connective Tissue Growth Factor (CCN2) by cellular or exosomal MicroRNA-199a-5p. Am J Pathol. 2016;186:2921–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]