Abstract
Introduction:
Physical activity and sleep quality have been consistently associated with quality of life (QOL) in a number of clinical and non-clinical populations. However, mechanisms underlying this relationship are not well understood. The purpose of this study was to longitudinally test a model examining how changes in physical activity and sleep quality, predict physical, mental and social well-being and global QoL across a 6-month exercise trial in a sample of healthy older adults.
Methods:
Participants (N=247, mean age 65.4±4.6) wore an accelerometer to assess objective levels of physical activity and completed measures of sleep, physical and mental well-being, social well-being and QOL at baseline and following a 6-month physical activity intervention. Relationships among model constructs were examined over time using panel analysis within a covariance-modeling framework.
Results:
The hypothesized model provided a good model-data fit (χ2=58.77, df=41, p=.036); CFI=0.98; SRMR=0.05; RMSEA=0.04). At both time-points, physical activity and sleep quality were significantly correlated. Sleep quality indirectly influenced QOL via physical, mental and social well-being (QOL R2=.47, p<.001). These relationships were also supported across time at month 6 (QOL R2=.50, p<.001). Neither physical activity nor sleep quality directly influenced QOL.
Conclusion:
Our results support a novel sleep and QOL model that may inform the design of health interventions to promote sleep quality, and thereby influencing QOL by targeting physical activity and modifiable mediators of physical, mental and social health. Our findings may have significant implications for older adults as well as clinical populations that report compromised sleep, impaired health related and global QOL.
Keywords: Sleep quality, physical activity, health-related quality of life, global quality of life, older adults, actigraphy, daytime functioning, geriatric normal sleep
Introduction:
Quality of life (QOL) is a broad multidimensional concept that includes subjective evaluations of both positive and negative aspects of life (WHOQOL,1998). QoL concerns among older adults remain a research priority due to a rapidly growing older adult population facing the onset of comorbid diagnoses, disability, changes in lifestyle behavior, and the potential loss of independence (Chatterji, Byles, Cutler, Seeman, & Verdes, 2015). In 2014, 14.5% of the United States population was aged 65 years or older and is estimated to comprise 23.5% of the population by 2060 (Colby & Ortman, 2017). The concept of health-related quality of life (HRQoL) and its determinants have evolved since the 1980s to encompass those aspects of overall QoL that can be clearly shown to affect health (Centers for Disease Control and Prevention, 2018). Surveillance data indicate that approximately half of American adults have at least one chronic condition and over one-quarter have multiple conditions, which are known to lower HRQoL and increase risk of mortality with aging (Ward, Schiller, & Goodman, 2014). Conversely, studies have shown that older adults who report greater HRQoL also have reduced risk of short and long term morbidity and mortality (Brown, Thompson, Zack, Arnold, & Barile, 2015).
It is well-documented that modifiable lifestyle behaviors, particularly regular physical activity participation and good sleep hygiene, have positive influences on HRQoL in older adults (Vagetti et al., 2014). However, the majority of this evidence is derived from individual tests of physical activity and sleep’s effects on HRQoL outcomes (Awick et al., 2017; Schubert et al., 2002; Strine & Chapman, 2005), despite evidence indicating the need to test these behaviors in conjunction with each other (Ding et al., 2014; Murawski et al., 2018; Rayward, Duncan, Brown, Plotnikoff, & Burton, 2017). Specifically, the relationship between physical activity and sleep may be bidirectional (Chennaoui, Arnal, Sauvet, & Léger, 2015; Kline, 2014) indicating they may exert correlated influences on health outcomes. Studies have found that individuals with sleep-related problems are also more likely to be physically inactive (Strine & Chapman, 2005), while physical activity participation may improve sleep quality in aging adults (Kline et al., 2012; Yang, Ho, Chen, & Chien, 2012). However, the relationship between physical activity and sleep and their independent effects on HRQoL and QoL in older adults remain equivocal.
QoL is conceptualized as a global, distal outcome of behavioral exposures (Diener, Emmons, Larsen, & Griffin, 1985). As such, the effects of physical activity and sleep on QoL are likely to be multifactorial and mediated by other health outcomes (McAuley & Morris, 2007). A small number of empirical studies have provided some insight into the mechanisms of physical activity and sleep’s effects on QoL. Awick and colleagues (Awick et al., 2017) found that increases in moderate-to-vigorous physical activity (MVPA) among older adults participating in a 6-month aerobic exercise intervention predicted decreases in psychological distress (i.e., depression, anxiety, sleep dysfunction, stress), which, in turn, led to increases in global QoL. Earlier studies demonstrated that the effects of increased MVPA on global QoL in older adults may also be mediated by social cognitive, affective, and health-related factors, including self-efficacy, self-esteem, positive affect, and HRQoL (McAuley et al., 2006; White, Wójcicki, & McAuley, 2009). Likewise, cohort studies have linked sleep difficulties with HRQoL and other QoL-related concerns, including depressive symptoms and anxiety. In a very large study of nearly 200,000 adults aged 45 years and older, Ding and colleagues (Ding et al., 2014) observed a significant improvement in the predictive power of a lifestyle index (including physical activity, alcohol, diet, and smoking) on self-rated health, QoL, psychological distress, and physical function when sleep duration was added to the index. In another large study, Strine and Chapman (Strine & Chapman, 2005) found that adults with frequent sleep insufficiency tended to report worse health behaviors and poorer HRQoL outcomes, including physical and mental distress, depressive symptoms, and anxiety. Unsurprisingly, these findings have also been evident in older adult samples. In a study of 2,800 older adults, Schubert and colleagues (Schubert et al., 2002) found that HRQoL significantly decreased with increasing insomniac traits.
The purpose of the present study was to examine associations among physical activity, sleep quality, and QoL at baseline and across a 6-month period in older adults (N=247) enrolled in a 6-month exercise trial. Following theoretical frameworks of lifestyle behaviors and QoL (McAuley & Morris, 2007), we hypothesized that baseline levels of physical activity and sleep would be correlated and have independent, direct effects on HRQoL indices (i.e., physical, mental, and social well-being), which, in turn, would directly influence global QoL. We proposed the same hypothesis among changes in physical activity, sleep, HRQoL, and QoL across the 6-month intervention.
Methods:
Procedures
Healthy, low active older adults (N=247) were recruited to participate in a 6-month exercise- and nutritional supplement-based lifestyle intervention trial (see Clinical Trials: NCT01472744) at the University of Illinois at Urbana-Champaign. Recruitment efforts included local media (e.g., newspaper, radio, television advertisements), mailed postcards, local university e-newsletter, and family/friend referral. Older adults were eligible for participation if they were: between the ages of 60 and 79 years; English speaking; right handed; low active as defined as ≤2 days/week of 20+ minutes of MVPA during the past 6 months; willing to be randomized; local to the study location; capable of exercise per their primary care physician; and not currently enrolled in another exercise trial. The primary objective of the study was to examine the effects of exercise on cognition and brain health (Voss et al., 2018). Data presented herein represent secondary analyses of behavioral and psychosocial outcomes measured at baseline and end of the 6-month intervention.
Primary results, detailed procedures and descriptions of the interventions, including a CONSORT statement have been published elsewhere (Ehlers et al., 2017; Voss et al., 2018). Briefly, participants were randomized to one of four intervention groups: aerobic dance, aerobic walking, aerobic walking + nutritional supplement and a stretching, strengthening and stability group that served as an active control condition. All groups met three times per week for approximately one hour over the 6-month period and were led by trained exercise leaders. Each session began with approximately 5 minutes of a walking warm-up and ended with a stretching cool-down. The aerobic dance group completed social dancing similar to folk line-dancing. The aerobic walking group walked around a track at 50–60% of their maximal heart rate (as assessed via graded maximal exercise test) for the first 6 weeks at which point they were encouraged to increase this range to 60–75% for the remainder of the intervention. The stretching, strengthening and stability group engaged in 10–12 resistance band exercises per session focused on all major muscle groups with the goal of improving strength and balance. Each group was progressive in nature both within and across months, thus increasing in intensity. Total program adherence, defined as the ratio of classes attended to total classes held, was quite high (77.6%) and did not differ across groups (p=0.84) (Awick et al., 2017). The intervention was conducted in four waves from October 2011 to November 2014. All procedures were approved by the University of Illinois at Urbana-Champaign Institutional Review Board (ethics committee), and all participants provided written informed consent prior to study participation.
Measures
Demographics
Basic demographic information including marital status, education, age, sex, and household income was assessed via questionnaire at baseline. The following physical activity and psychosocial measures were administered at baseline and after the last week of the 6-month intervention.
Physical Activity
Physical activity was assessed using Actigraph accelerometers (Actigraph, Pensacola, FL: model GT1M or GT3X) at baseline and post-6-month intervention. Participants were instructed to wear the accelerometer on their non-dominant hip for seven consecutive days during waking hours and were provided with a log to record the wear times. For data reduction, the following criteria were applied to the raw data recorded by each monitor: wear time validation criterion of ≥10 hours of wear time per day for at least 3 days and an interruption period of 60 minutes (Troiano et al., 2008). These data were downloaded as activity counts, which represent raw accelerations summed over a specific epoch length (e.g., 1 second) and subsequently processed into activity intensities in ActiLife software package (Version 6; Actigraph, Pensacola, FL). We used older adult-specific cut-points (Copeland & Esliger, 2009) as follows: sedentary (<50 counts/minute), light (50–1040 counts/minute), and moderate-to-vigorous physical activity (MVPA; ≥1041 counts/minute). Each minute of wear was classified as sedentary, light, or MVPA according to these intensity cut-points. Estimated average daily minutes spent in each intensity category were calculated by dividing the number of minutes spent in each intensity category by the total number of valid days worn per participant. The average time per day spent in MVPA was used for data analysis.
Sleep Quality
Sleep quality was measured by the Pittsburgh Sleep Quality Index [PSQI; (Buysse, Reynolds III, Monk, Berman, & Kupfer, 1989)]. The PSQI measures sleep quality over the past month (past 4 weeks) and assesses the domains of Subjective Sleep Quality, Sleep Latency, Sleep Duration, Sleep Efficiency, Sleep Disturbances, Sleep Drug use, and Daytime Impairments. Since its introduction in 1989 the PSQI has gained widespread acceptance as a useful measure of sleep quality in different patient groups. The self-administered scale contains 15 multiple-choice items that inquire about frequency of sleep disturbances and subjective sleep quality and 4 write-in items that inquire about typical bedtime, wake-up time, sleep latency, and sleep duration. The component scores are summed to produce a global score (range of 0–21). A PSQI global score >5 is considered to be an indicator of sleep disturbance (Buysse et al., 1991). The Cronbach’s alpha was .77 and .72 at baseline and month 6 respectively.
Physical, Mental and Social Well-being
Physical and mental well-being was measured with the SF-12 Health Survey (Ware Jr, Kosinski, & Keller, 1996). The SF-12 is an abridged version of the Medical Outcomes Study SF-36 and has well-documented psychometric qualities across multiple populations. The SF-12 provides a summary assessment of both physical and mental health and scores can range from 0–100 with higher scores indicating better health status. The Social Provisions Scale [SPS, (Cutrona & Russell, 1987)] was used to assess social well-being. This is a 24-item scale assesses six relational provisions: attachment, or emotional support; social integration, or network support; reassurance of worth, or esteem support; reliable alliance, or tangible aid; guidance, or informational support; and the opportunity of nurturance, in which assisting others bolsters one’s own sense of self-worth. For each item on the SPS, participants indicate the extent to which each statement describes their current social relationships on a 4-point Likert scale, ranging from 1 (strongly disagree) to 4 (strongly agree). The total score was computed by summing the item responses on the 24 items. The Cronbach’s alpha was .80 and .78 at baseline and month 6 respectively.
Quality of Life
The Flourishing Scale is a brief 8-item summary measure of the respondent’s self-perceived success in important areas such as relationships, self-esteem, purpose, and optimism (Diener et al., 2010). Each item of the scale is answered on a 1–7 scale that ranges from strong disagreement to strong agreement. The scale provides a single psychological well-being score ranging from 8 to 56 with high scores signifying that respondents view themselves in positive terms in important areas of functioning. The measure has good psychometric properties and is strongly associated with other psychological well-being scales. The Cronbach’s alpha was .89 and .92 at baseline and month 6 respectively.
Data Analysis
The hypothesized pathways from physical activity and sleep quality to QoL were tested using a panel model within a covariance framework in Mplus Version 8 (Muthén & Muthén, 2007). Panel analysis is a useful analytical procedure for examining hypothetical relationships among constructs across time (Kessler, 2014). In order to estimate stability coefficients across time for each variable, the 6-month variables were regressed upon their baseline counterparts. Goodness of fit tests for all models reported herein include, Root Mean Square Error of the Approximation (RMSEA) <.08, Standardized Root Mean Residual (SRMR) < 0.08, Comparative Fit Index (CFI) > .90, a non-significant normal theory weighted chi-square (χ2) indicated good model-data fit (Hu & Bentler, 1999).
Prior to the main analyses, measures were examined for outliers and normality. Seven missing data patterns were observed with 2.83% of missing data. Subsequently, maximum likelihood robust estimators were used for missing data to avoid violation of the multivariate normality assumption. Preliminary analyses indicated no significant time by group effects on QoL. Thus, the sample was collapsed across conditions, and group assignment was included in the model as a covariate only. Both the walking and dance groups showed significant improvements in MVPA at follow-up (mean change of 11.87 and 5.88 minutes respectively) whereas the change in MVPA for strengthening and stability group was not significant (mean change 2.59 minutes). Therefore, intervention group was dummy coded as a binary variable such that the stretching, strengthening and stability group was coded as 1 and the active intervention conditions of dancing and walking were coded as 0. After including the key model variables, we included the covariates: age, sex, and intervention group. Covariates have been omitted from the figures for clarity.
Results
Participant characteristics
Participant demographics have been described previously (Awick et al., 2017; Ehlers et al., 2017; Voss et al., 2018). Briefly, our sample comprised of primarily females (68.4%), Caucasian (83.8%), with a majority being married (59.1%) with a college education (58.7%). The average BMI of the sample was 30.98 (±5.58) indicating borderline obesity. Means and standard deviations for the model variables at each of the two time points are detailed in Table 1. Paired samples t-test showed a significant difference on two model variables between baseline and month 6 time points: average MVPA per day (p<.001) and social well-being (p=.001). We observed a time x group effect for MVPA whereby the walking and dance groups showed increased MVPA at follow-up (p<.001 and p=.06, respectively). Change in MVPA for the strengthening and stability group was not significant (p=.39). On the other hand, change in SPS scores was highest in the stretching, strengthening and stability group (84.17 to 86.03) compared to the walking (83.02 to 84.72) and dance (82.18 to 83.73) groups, although the group differences were not significant.
Table 1:
Means and standard deviations for each of the model variables at baseline and month 6.
| p value | ||||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| 46.45 | 30.49 | 55.30 | 28.51 | <.001 |
| 6.06 | 3.52 | 5.86 | 3.41 | .612 |
| 47.08 | 9.04 | 47.97 | 8.99 | .322 |
| 52.37 | 8.91 | 53.56 | 7.81 | .207 |
| 83.11 | 9.67 | 84.82 | 9.13 | .001 |
| 47.43 | 6.11 | 47.69 | 6.53 | .833 |
Note: MVPA: moderate to vigorous physical activity; PSQI: Pittsburgh Sleep Quality Inventory; SF-12: Short Form 12 item; SPS: Social Provisions Scale; QOL: Quality of Life
Correlations among model variables
Prior to testing the panel model, Pearson’s product moment correlation coefficients were computed for both time points. As seen in Table 2 all model variables were correlated across both timepoints. Age, sex and intervention group were included as covariates in the hypothesized path model. At baseline, age was positively associated with mental wellbeing (β=.18, p=.003) and negatively associated with MVPA (β=-0.30, p<.001). Females reported greater social wellbeing (β=.16, p=.01) and poorer sleep quality (β=.15, p=.01), and engaged in lower levels of MVPA compared to males (β=-.16, p=.02). At month 6, age was associated with lower levels of MVPA (β=-.22, p<.001) and poor sleep quality (β=.15, p=.04). Across the 6-month intervention, assignment to the strengthening, stretching, and stability intervention was positively associated with change in global QoL (β=.15, p<.001). No other effects of covariates on change in QoL outcomes were observed.
Table 2:
Correlations between the model variables at baseline and month 6.
| Age | MVPA | PSQI | Physical Wellbeing | Mental Wellbeing | Social Wellbeing | QoL | |
|---|---|---|---|---|---|---|---|
| Age | 1 | −.38** | .07 | −.18** | .09 | .06 | .00 |
| MVPA | −.29** | .71** | −.16* | .15* | .06 | −.04 | −.04 |
| PSQI | −.03 | −.16* | .62** | −.20** | −.29** | −.13 | −.22** |
| Physical Wellbeing | −.09 | .14* | −.23** | .59** | −.15* | .08 | .14* |
| Mental Wellbeing | .16* | .07 | −.36** | −.12 | .61** | .35** | .38** |
| Social Wellbeing | .07 | .05 | −.18** | .09 | .35** | .79** | .52** |
| QoL | .06 | .09 | −.31** | .14 | .53** | .58** | .58** |
Note: Baseline correlations are below the diagonal, Month 6 correlations are above the diagonal, values along the diagonal are correlations between the baseline and month 6 scores for each variable;
indicates p <.05;
indicates p <.005;
MVPA = moderate to vigorous physical activity; PSQI = Pittsburgh Sleep Quality Index; QoL = Quality of Life
Physical Activity-Sleep-QOL model
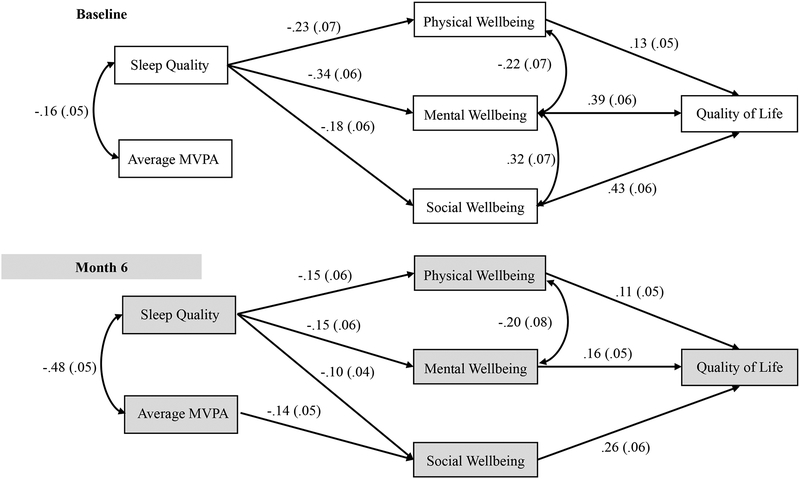
The results of the panel model analysis with standardized regression coefficients are presented in Figure 1. The model had a good fit with a chi-square = 58.77 (df=41, p =.04), RMSEA = .04, SRMR =.05 and CFI =.98. Stability coefficients for MVPA and sleep quality were satisfactory (MVPA β=.73, p<.001; PSQI β=.65, p<.001). As seen in Figure 1, average minutes of daily MVPA and sleep quality were significantly correlated such that greater MVPA was correlated with better sleep quality. At baseline, sleep quality directly affected physical, mental and social well-being, which ultimately predicted global QoL. No direct effects of baseline MVPA on HRQOL outcomes were observed (all p>.13). The indirect paths from sleep to global QoL through physical (β=-.03, p=.05), mental (β=-.13, p<.001), and social well-being (β=-.08, p=.02) were significant.
Figure 1: The Physical Activity-Sleep-Quality of Life Model tested at Baseline and month 6 (shaded) time-points.
Note: MVPA = Moderate to vigorous physical activity; standardized regression coefficients and standard error (in parenthesis) are indicated for the paths
Across the 6-month intervention, improvements in sleep quality significantly predicted increases in physical, mental, and social well-being, which predicted increased global QoL. Alternately, increases in MVPA across the intervention predicted decreases in social well-being, but was not predictive of changes in physical or mental well-being (both p>.22). Indirect effects of improved sleep quality on improved global QoL were observed through mental (β=-.02, p=.04) and social well-being (β=-.03, p=.04). An indirect effect of increased MVPA on decreased QoL through social well-being (β=-.04, p=.01) was also observed. Change in MVPA was not associated with change in global QoL through mental well-being (p=.25), and neither change in MVPA nor sleep quality indirectly influenced change in global QoL through physical well-being (both p>.10). Neither MVPA nor sleep quality directly predicted global QoL at either time point (all p>.15). R-squares indicated that the model explained 47% of the variance in QoL at baseline and 50% at month 6.
Discussion
Insufficient physical activity and low-quality sleep have consistent, independent negative effects on QoL and HRQoL across the lifespan (Duncan et al., 2014). These behaviors can be expected to operate bidirectionally, such that greater physical activity should support better sleep hygiene, while sufficient sleep quality better supports physical activity participation across the following day (Murawski et al., 2018). The purpose of this study was to provide an initial examination of whether sleep quality and physical activity independently affect HRQoL indices, which are expected to influence global QoL.
Consistent with our hypothesis, we found an association between sleep and physical activity at baseline and month 6, a finding consistent with previous research (Kline, 2014; Lopresti, Hood, & Drummond, 2013). As hypothesized, our results also identified a significant effect of self-reported sleep quality on proximal HRQoL indices. Specifically, better sleep quality was significantly associated with better mental, physical, and social well-being at baseline, and improvements in sleep quality were associated with improved HRQoL across the 6-month period. Consistent with the conceptualization of global QoL as a distal outcome of health behaviors, HRQoL improvements were, in turn, associated with improvements in global QoL (Diener et al., 1985; Rejeski & Mihalko, 2001). Contrary to our prediction, physical activity at both time-points had more limited effects on HRQoL and global QoL.
Our data suggest that older adults who participated in greater levels of MVPA tended to report better sleep quality, and those reporting better sleep quality tended to engage in more MVPA. Contrary to previous literature (McAuley et al., 2006; Vagetti et al., 2014; White et al., 2009), MVPA showed no association with physical or mental well-being. These previous studies did not examine the role of sleep, and it is plausible that physical activity demonstrates a significant role in the absence of a sleep construct. In our models, sleep quality emerged as the important player in leading to downstream effects on HRQoL and global QoL, although it clearly exhibited a strong correlation with MVPA. Given the significance of improving QoL among older adults, this central role of sleep quality and its relationship with physical activity has implications for the design of physical activity behavior change trials (Kline, 2014; Murawski et al., 2018). Whereas our results pertain to relationships between sleep, HRQoL, and QoL at a more global level, emerging evidence suggests that even one night of poor sleep affects daily participation in health behaviors and key psychosocial states. For instance, poor nightly sleep quality is associated with fluctuations in affective states and perceptions of fatigue and stress across the following day (“More Sleep Would Make Most Americans Happier, Healthier and Safer,” 2014). These transient effects are associated with lower levels of daily physical activity (Dunton, 2017), and can be expected to negatively influence indices of HRQoL. Indeed, sleep dysfunction is common in old age and has the capacity to impair all domains of HRQoL in older adults, including mental, physical, and social health (Schubert et al., 2002; Strine & Chapman, 2005). Thus, to maximize positive HRQoL changes, attention should be given to assuring that physical activity participation directly enhances sleep and reduces sitting time. In summary, future research should examine the role of sleep on QoL and HRQoL independent of and in conjunction with physical activity.
Intervening on sleep and activity concurrently is a promising area for future research, with no data published from randomized controlled trials to the authors’ knowledge. Promisingly, Murawski and colleagues (2018) recently published the Synergy Study protocol, which will utilize an mHealth platform to deliver a theory-based multicomponent intervention targeting sleep health, resistance training, and aerobic exercise. Consistent with the 24-hour activity cycle approach (Rosenberger, Buman, Haskell, McConnell, & Carstensen, 2016) - emphasizing the finite number of minutes in a given day in which a person can choose to sleep, sit, or engage in activity - each additional minute of physical activity participation must replace one minute of sitting or sleeping. To reduce the likelihood that increased activity participation harms sleep health, the intervention should be structured so as not to impair sleep quality or incentivize reduced sleep (e.g., should not occur in the early morning or late in the day), and knowledge-based content should include discussion on the importance of sleep for QoL and lasting behavior change. A more intensive approach may also include initial screening and additional intervention for more pressing sleep concerns, such as sleep apnea and insomnia. Fortunately, contemporary popular technologies provide a feasible method for administering such intervention tools. For instance, Ritterband and colleagues have demonstrated the efficacy of automated, internet-delivered cognitive behavioral therapy for insomnia across several randomized controlled trials (Ritterband et al., 2009, 2017). This type of automated toolset could be readily integrated into many physical activity intervention frameworks with little additional cost or researcher burden.
Finally, our results showed a significant inverse relationship between changes in MVPA and social well-being at month 6. Our data show that older adults, on average, reported significant increases in social well-being over the 6-month period (see Table 1 for SPS means). This is not surprising as the context of the exercise intervention provided opportunities for social interaction and engagement across the sample. However, it appears that the social environment of the structured exercise program elicited changes in social well-being independent of changes in MVPA. This is evidenced in our stretching, strengthening and stability group that showed the largest increase in the SPS score at follow-up but demonstrated the smallest change in MVPA. This suggests that social well-being can be increased through group-based exercise programs even in the absence of increases in MVPA. Previous research has suggested that factors other than mode of exercise may explain relationships among social support, stress, and loneliness (Ehlers et al., 2017; Mackenzie, Chung, Zuniga, Woods, & McAuley, 2014). Other social characteristics of the intervention groups, such as group cohesion and dynamics with the group exercise leader could have contributed to more favorable changes in social well-being within the strengthening and stability vs. other intervention groups.
Social relations and social support are psychosocial factors of importance for health and well-being of individuals (Cobb, 1976; Colby & Ortman, 2017). There is evidence for health-promoting effects of social relationships (Fratiglioni, Paillard-Borg, & Winblad, 2004) and deficits in social support have been associated with a wide variety of adverse health outcomes in older age including poor physical health and depression (Uchino, 2006). A recent systematic review suggested that older adults with greater social support for physical activity are more likely to engage in leisure time physical activity but not MVPA (Lindsay Smith, Banting, Eime, O’Sullivan, & van Uffelen, 2017). This, in addition to the fact that our measure of social well-being assessed the function of social relationships rather than social support for physical activity specifically, may explain the relationship observed in our analyses.
It should be noted that the current study sample was comprised of highly educated, healthy, and predominantly White older adults, limiting generalizability. However, these effects were evident in our healthy sample, and it might be expected that the magnitude of the effects and benefits would be greater among individuals demonstrating compromised HRQoL or sleep disorders. It will be important to replicate these findings in more heterogeneous samples, including those with insomnia, physical illness, or depression. Our sample also reported on average 46 minutes of MVPA at baseline, however it should also be noted that we employed the Copeland and Eslinger cut-points (≥1042) which are lenient in estimating MVPA as compared to the NHANES criterion (≥ 2020 counts/min). Additionally, this study focused on the effects of average self-reported sleep quality and MVPA. Examining the day-to-day relationships between nightly sleep quality and daily physical activity via ecological momentary assessment (Maher, Rebar, & Dunton, 2018; Parsey & Schmitter-Edgecombe, 2019; Shiffman, Stone, & Hufford, 2008) and use of objective sleep reports using actigraphy may provide a richer understanding of the QoL effects we observed. This is of particular interest, as there is at present limited evidence on the optimal structure of physical activity for promoting healthy sleep (Rosenberger et al., 2016). For instance, does sustained movement across the day (Fanning et al., 2018), which reduces sustained bouts of sitting, promote more restful sleep? Is participating in structured vigorous bouts of activity more beneficial? If so, is there an optimal time of day for such an activity? Methods such as ecological momentary assessment that allow for the study of these intensive temporal dynamics will be particularly valuable in this line of research with older adults. Lastly, we did not test the mediating role of negative psychological constructs, such as depression or anxiety, which may be associated with sleep, physical activity, and QoL; however, the dimensions implicated as important factors of QoL in old age are wide and varied making it nearly impossible for researchers to simultaneously test each one (Rejeski & Mihalko, 2001).
Despite these limitations, our findings replicate and extend previous work attempting to clarify some of the mechanisms driving the effects of lifestyle behaviors on QoL in older adulthood. Maintaining and enhancing QoL will remain an important public health goal as the size of the older adult population continues to grow, and trials designed to address the dynamic interplay between sleep and physical activity represent an exciting frontier for research in the behavioral sciences.
Impact Statement:
Quality of life (QoL) concerns among older adults remain a research priority due to a rapidly growing older adult population facing the onset of comorbid diagnoses, disability, changes in lifestyle behavior, and the potential loss of independence. Data presented in this manuscript highlight the role of two modifiable lifestyle behaviors – sleep and physical activity in improving health related and global QoL for older adults. Based on our study findings, trials designed to address the dynamic interplay between sleep and physical activity represent an exciting frontier for research in the behavioral sciences.
Acknowledgements:
Thank you to all research participants who volunteered for this study and the research staff who contributed to the data collection process. Special thanks to the project coordinators, Susan Houseworth and Anya Knecht.
Funding:
This work was supported by grants from the National Institutes of Health, National Institute on Aging (R37 AG025667) and the Abbott Nutrition through the Center for Nutrition, Learning, and Memory at the University of Illinois at Urbana-Champaign. The trial is registered with United States ClinicalTrials.gov (identifier: NCT01472744).
Footnotes
Related Paper Presentation:
Preliminary analyses of these data were presented at the 15th International Congress of Behavioral Medicine in November 2018 at Santiago, Chile.
References
- Awick EA, Ehlers DK, Aguiñaga S, Daugherty AM, Kramer AF, & McAuley E (2017). Effects of a randomized exercise trial on physical activity, psychological distress and quality of life in older adults. General Hospital Psychiatry, 49, 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DS, Thompson WW, Zack MM, Arnold SE, & Barile JP (2015). Associations between health-related quality of life and mortality in older adults. Prevention Science : The Official Journal of the Society for Prevention Research, 16(1), 21–30. 10.1007/s11121-013-0437-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF III, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF III, Monk TH, Hoch CC, Yeager AL, & Kupfer DJ (1991). Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI). Sleep, 14(4), 331–338. [PubMed] [Google Scholar]
- Chatterji S, Byles J, Cutler D, Seeman T, & Verdes E (2015). Health, functioning, and disability in older adults--present status and future implications. Lancet (London, England), 385(9967), 563–575. 10.1016/S0140-6736(14)61462-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chennaoui M, Arnal PJ, Sauvet F, & Léger D (2015). Sleep and exercise: a reciprocal issue? Sleep Medicine Reviews, 20, 59–72. [DOI] [PubMed] [Google Scholar]
- Cobb S (1976). Social support as a moderator of life stress Psychosomatic Medicine. US: Lippincott Williams & Wilkins; 10.1097/00006842-197609000-00003 [DOI] [PubMed] [Google Scholar]
- Colby SL, & Ortman JM (2017). Projections of the size and composition of the US population: 2014 to 2060: Population estimates and projections.
- Copeland JL, & Esliger DW (2009). Accelerometer Assessment of Physical Activity in Active, Healthy Older Adults. Journal of Aging and Physical Activity, 17(1), 17–30. 10.1123/japa.17.1.17 [DOI] [PubMed] [Google Scholar]
- Cutrona CE, & Russell DW (1987). The provisions of social relationships and adaptation to stress. Advances in Personal Relationships, 1(1), 37–67. [Google Scholar]
- Diener E, Emmons RA, Larsen RJ, & Griffin S (1985). The Satisfaction With Life Scale. Journal of Personality Assessment, 49(1), 71–75. 10.1207/s15327752jpa4901_13 [DOI] [PubMed] [Google Scholar]
- Diener E, Wirtz D, Tov W, Kim-Prieto C, Choi D, Oishi S, & Biswas-Diener R (2010). New well-being measures: Short scales to assess flourishing and positive and negative feelings. Social Indicators Research, 97(2), 143–156. [Google Scholar]
- Ding D, Rogers K, Macniven R, Kamalesh V, Kritharides L, Chalmers J, & Bauman A (2014). Revisiting lifestyle risk index assessment in a large Australian sample: Should sedentary behavior and sleep be included as additional risk factors? Preventive Medicine, 60, 102–106. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Kline CE, Vandelanotte C, Sargent C, Rogers NL, & Di Milia L (2014). Cross-Sectional Associations between Multiple Lifestyle Behaviors and Health-Related Quality of Life in the 10,000 Steps Cohort. PLoS ONE, 9(4), e94184 10.1371/journal.pone.0094184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunton GF (2017). Ecological Momentary Assessment in Physical Activity Research. Exercise and Sport Sciences Reviews, 45(1), 48–54. 10.1249/JES.0000000000000092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers DK, Daugherty AM, Burzynska AZ, Fanning J, Awick EA, Chaddock-Heyman L, … McAuley E (2017). Regional brain volumes moderate, but do not mediate, the effects of group-based exercise training on reductions in loneliness in older adults. Frontiers in Aging Neuroscience, 9, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning J, Opina MT, Leng I, Lyles MF, Nicklas BJ, & Rejeski WJ (2018). Empowered with Movement to Prevent Obesity & Weight Regain (EMPOWER): Design and Methods. Contemporary Clinical Trials, 72, 35–42. 10.1016/J.CCT.2018.07.010 [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Paillard-Borg S, & Winblad B (2004). An active and socially integrated lifestyle in late life might protect against dementia. The Lancet. Neurology, 3(6), 343–353. 10.1016/S1474-4422(04)00767-7 [DOI] [PubMed] [Google Scholar]
- Health-Related Quality of Life (HRQOL). (n.d.). Retrieved from https://www.cdc.gov/hrqol/concept.htm
- Hu L, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6(1), 1–55. [Google Scholar]
- Kessler RC (2014). Linear panel analysis: Models of quantitative change. Elsevier. [Google Scholar]
- Kline CE (2014). The Bidirectional Relationship Between Exercise and Sleep. American Journal of Lifestyle Medicine, 8(6), 375–379. 10.1177/1559827614544437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline CE, Sui X, Hall MH, Youngstedt SD, Blair SN, Earnest CP, & Church TS (2012). Dose–response effects of exercise training on the subjective sleep quality of postmenopausal women: exploratory analyses of a randomised controlled trial. BMJ Open, 2(4). Retrieved from http://bmjopen.bmj.com/content/2/4/e001044.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay Smith G, Banting L, Eime R, O’Sullivan G, & van Uffelen JGZ (2017). The association between social support and physical activity in older adults: a systematic review. The International Journal of Behavioral Nutrition and Physical Activity, 14(1), 56 10.1186/s12966-017-0509-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopresti AL, Hood SD, & Drummond PD (2013). A review of lifestyle factors that contribute to important pathways associated with major depression: Diet, sleep and exercise. Journal of Affective Disorders, 148(1), 12–27. 10.1016/j.jad.2013.01.014 [DOI] [PubMed] [Google Scholar]
- Mackenzie MJ, Chung HD, Zuniga KE, Woods JA, & McAuley E (2014). Multilevel modeling of exercise effects on loneliness in older adults. In Annals of Behavioral Medicine (Vol. 47, pp. S178–S178). [Google Scholar]
- Maher JP, Rebar AL, & Dunton GF (2018). Ecological Momentary Assessment Is a Feasible and Valid Methodological Tool to Measure Older Adults’ Physical Activity and Sedentary Behavior. Frontiers in Psychology, 9, 1485 10.3389/fpsyg.2018.01485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley E, Konopack JF, Motl RW, Morris KS, Doerksen SE, & Rosengren KR (2006). Physical activity and quality of life in older adults: Influence of health status and self-efficacy. Annals of Behavioral Medicine, 31(1), 99–103. 10.1207/s15324796abm3101_14 [DOI] [PubMed] [Google Scholar]
- McAuley E, & Morris KS (2007). State of the Art Review: Advances in Physical Activity and Mental Health: Quality of Life. American Journal of Lifestyle Medicine, 1(5), 389–396. 10.1177/1559827607303243 [DOI] [Google Scholar]
- More Sleep Would Make Most Americans Happier, Healthier and Safer. (2014). Retrieved December 12, 2018, from https://www.apa.org/research/action/sleep-deprivation.aspx
- Murawski B, Plotnikoff RC, Rayward AT, Vandelanotte C, Brown WJ, & Duncan MJ (2018). Randomised controlled trial using a theory-based m-health intervention to improve physical activity and sleep health in adults: the Synergy Study protocol. BMJ Open, 8(2), e018997 10.1136/bmjopen-2017-018997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (2007). Mplus User’s Guide. Statistical analysis with latent variables. Version (5th editio, Vol. 3). Los Angeles, CA. [Google Scholar]
- Parsey CM, & Schmitter-Edgecombe M (2019). Using Actigraphy to Predict the Ecological Momentary Assessment of Mood, Fatigue, and Cognition in Older Adulthood: Mixed-Methods Study. JMIR Aging, 2(1), e11331 10.2196/11331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayward AT, Duncan MJ, Brown WJ, Plotnikoff RC, & Burton NW (2017). A cross-sectional cluster analysis of the combined association of physical activity and sleep with sociodemographic and health characteristics in mid-aged and older adults. Maturitas, 102, 56–61. 10.1016/j.maturitas.2017.05.013 [DOI] [PubMed] [Google Scholar]
- Rejeski WJ, & Mihalko SL (2001). Physical Activity and Quality of Life in Older Adults. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 56(Supplement 2), 23–35. 10.1093/gerona/56.suppl_2.23 [DOI] [PubMed] [Google Scholar]
- Ritterband LM, Thorndike FP, Gonder-Frederick LA, Magee JC, Bailey ET, Saylor DK, & Morin CM (2009). Efficacy of an Internet-Based Behavioral Intervention for Adults With Insomnia. Archives of General Psychiatry, 66(7), 692 10.1001/archgenpsychiatry.2009.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritterband LM, Thorndike FP, Ingersoll KS, Lord HR, Gonder-Frederick L, Frederick C, … Morin CM (2017). Effect of a Web-Based Cognitive Behavior Therapy for Insomnia Intervention With 1-Year Follow-up. JAMA Psychiatry, 74(1), 68 10.1001/jamapsychiatry.2016.3249 [DOI] [PubMed] [Google Scholar]
- Rosenberger ME, Buman MP, Haskell WL, McConnell MV, & Carstensen LL (2016). Twenty-four Hours of Sleep, Sedentary Behavior, and Physical Activity with Nine Wearable Devices. Medicine and Science in Sports and Exercise, 48(3), 457–465. 10.1249/MSS.0000000000000778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert CR, Cruickshanks KJ, Dalton DS, Klein BEK, Klein R, & Nondahl DM (2002). Prevalence of sleep problems and quality of life in an older population. Sleep, 25(8), 48–52. [PubMed] [Google Scholar]
- Shiffman S, Stone AA, & Hufford MR (2008). Ecological Momentary Assessment. Annual Review of Clinical Psychology, 4(1), 1–32. 10.1146/annurev.clinpsy.3.022806.091415 [DOI] [PubMed] [Google Scholar]
- Strine TW, & Chapman DP (2005). Associations of frequent sleep insufficiency with health-related quality of life and health behaviors. Sleep Medicine, 6(1), 23–27. 10.1016/J.SLEEP.2004.06.003 [DOI] [PubMed] [Google Scholar]
- The World Health Organization Quality of Life Assessment (WHOQOL): development and general psychometric properties. (1998). Social Science & Medicine, 46(12), 1569–1585. [DOI] [PubMed] [Google Scholar]
- Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, & McDowell M (2008). Physical activity in the United States measured by accelerometer. Medicine and Science in Sports and Exercise, 40(1), 181–188. 10.1249/mss.0b013e31815a51b3 [DOI] [PubMed] [Google Scholar]
- Uchino BN (2006). Social support and health: a review of physiological processes potentially underlying links to disease outcomes. Journal of Behavioral Medicine, 29(4), 377–387. 10.1007/s10865-006-9056-5 [DOI] [PubMed] [Google Scholar]
- Vagetti GC, Barbosa Filho VC, Moreira NB, Oliveira V. de, Mazzardo O, & Campos W. de. (2014). Association between physical activity and quality of life in the elderly: a systematic review, 2000–2012. Revista Brasileira de Psiquiatria (Sao Paulo, Brazil : 1999), 36(1), 76–88. 10.1590/1516-4446-2012-0895 [DOI] [PubMed] [Google Scholar]
- Voss MW, Sutterer M, Weng TB, Burzynska AZ, Fanning J, Salerno E, … Kramer AF (2018). Nutritional supplementation boosts aerobic exercise effects on functional brain systems. Journal of Applied Physiology, japplphysiol.00917.2017. 10.1152/japplphysiol.00917.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BW, Schiller JS, & Goodman RA (2014). Multiple chronic conditions among US adults: a 2012 update. Preventing Chronic Disease, 11, E62 10.5888/pcd11.130389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE Jr, Kosinski M, & Keller SD (1996). A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care, 34(3), 220–233. [DOI] [PubMed] [Google Scholar]
- White SM, Wójcicki TR, & McAuley E (2009). Physical activity and quality of life in community dwelling older adults. Health and Quality of Life Outcomes, 7(1), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P-Y, Ho K-H, Chen H-C, & Chien M-Y (2012). Exercise training improves sleep quality in middle-aged and older adults with sleep problems: a systematic review. Journal of Physiotherapy, 58(3), 157–163. [DOI] [PubMed] [Google Scholar]