Abstract
Background
Anhedonia, or loss of interest or pleasure, is a feature of depression and transdiagnostic construct in psychopathology. Theory and compelling evidence from preclinical models implicates stress-induced inflammation as a psychobiological pathway to anhedonic behavior; however, this pathway has not been tested in human models. Further, although anhedonia may reflect dysregulation in multiple dimensions of reward, the extent to which stress-induced inflammation alters these dimensions is unclear. Thus, the current experimental study used a standardized laboratory stressor task to elicit an inflammatory response and evaluate effects of stress-induced inflammation on multiple behavioral indices of reward processing.
Methods
Healthy young women (age 18–25) completed behavioral reward tasks assessing reward learning, motivation, and sensitivity and were randomized to undergo an acute psychosocial stressor (n = 37) or a nostress active control (n = 17). Tasks were re-administered 90–120min post-stress to coincide with the peak of the stress-induced inflammatory response. Blood samples were collected for assessment of the pro-inflammatory cytokine interleukin-6 (IL-6) at baseline and 90 and 120 min post stressor.
Results
Stress-induced IL-6 was associated with increased response bias during reward learning and increased motivation when probability of receiving a reward was low. Sensitivity to reward in the context of a motivation task was not altered in association with stress-induced IL-6.
Conclusions
Contrary to hypotheses, mild increases in IL-6 following acute stress were associated with increased reward responsiveness during reward learning and selective increases in motivation. Results contribute to an emerging and nuanced literature linking inflammation to reward processing, and demonstrate that behavioral effects of stress-induced inflammation may be detected in the laboratory setting.
Clinical trial registration
Keywords: Stress, Inflammation, Depression, Anhedonia, Reward motivation, Reward learning, Reward sensitivity, Reward responsiveness
1. Introduction
Anhedonia, or diminished interest or pleasure, is a transdiagnostic feature of psychopathology (Rømer Thomsen et al., 2015; Foti and Baskin-Sommers, 2015; Kashdan, 2004; Bedwell et al., 2014) and core diagnostic symptom of Major Depressive Disorder (American Psychiatric Association, 2013). Anhedonia is of particular interest in depression because it may precede and increase vulnerability for depression (Loas, 1996; Pizzagalli, 2014; Gotlib et al., 2010; Rawal et al., 2012) and predicts poor treatment response (Vrieze et al., 2013; Craske et al., 2016). Anhedonia is increasingly recognized as a multidimensional construct, reflecting deficits in reward motivation, learning, and/or sensitivity (Rømer Thomsen et al., 2015), each of which may warrant different pharmacological and behavioral therapeutic approaches (Craske et al., 2016; Nutt et al., 2006). However, the psychobiological mechanisms that give rise to dysregulation in the reward system have yet to be elucidated.
Stress is a well-established and robust predictor of depression onset and recurrence (Kendler et al., 2003; Monroe and Reid, 2009; Hammen, 2015) and is linked specifically with alterations in reward-related processes (Cavanagh et al., 2010; Porcelli et al., 2012; Treadway et al., 2013; Harrison et al., 2016; Harrison et al., 2015; Eisenberger et al., 2010; Capuron et al., 2012; Draper et al., 2018; Lasselin et al., 2016; Boyle et al., 2018). Stress also leads to increases in inflammation, which may be a critical pathway linking stress and reward dysregulation. Indeed, anhedonic behavior in animal models is reliably elicited by both chronic stress and inflammatory stimuli (Anisman et al., 2002; Yirmiya et al., 2015), and inflammation has been shown to mediate effects of chronic stress on sucrose preference in preclinical studies (Koo and Dumans, 2008). Consistent with animal research, chronic stress and acute laboratory stressors are associated with reduced neural and behavioral reward responsiveness in humans (Bogdan and Pizzagalli, 2006; Berghorst et al., 2013; Bogdan et al., 2007; Kogler et al., 2015; Cavanagh et al., 2010; Porcelli et al., 2012; Treadway et al., 2013), and peripherally induced inflammation decreases neural response to novel stimuli and anticipation or receipt of monetary reward (Harrison et al., 2016; Harrison et al., 2015; Eisenberger et al., 2010; Capuron et al., 2012). However, inflammation has shown mixed associations with reward in humans when assessment is behavioral. For example, participants who receive endotoxin have been shown to exhibit decreased (Draper et al., 2018) and increased (Lasselin et al., 2016) reward motivation, with no effects on reward sensitivity. Similarly, while one study found enhanced performance on a reward learning task in association with increases in inflammation following vaccination (Boyle et al., 2018), null effects have also been reported (Harrison et al., 2016). Thus, it is not yet clear if certain reward dimensions are more sensitive to fluctuations in inflammation, or the conditions under which such effects are facilitative or inhibitive.
Furthermore, experimental assessment of inflammation and reward in humans has only been studied in the context of an induced peripheral inflammatory response, typically through administration of endotoxin or the typhoid vaccine. Endotoxin elicits an acute and robust peripheral inflammatory response that far exceeds the normative physiological changes that occur in response to repeated or chronic stress (Eisenberger et al., 2010; Draper et al., 2018; Lasselin et al., 2016; Dooley et al., 2018). The inflammatory response to typhoid vaccination is far milder and more comparable in magnitude to the acute stress-evoked inflammatory response (Harrison et al., 2015), but does not reflect the neural and behavioral effects of stress. Stress elicits both a peripheral and a central inflammatory response (Johnson et al., 2005; Sugama et al., 2009), and anhedonic behavior is linked to increases in both peripheral (Ménard et al., 2017; Hodes et al., 2014) and central cytokines (Koo and Duman, 2008; Goshen et al., 2007) in preclinical models. A recent study in healthy women found that stress-induced increases in IL-6 were associated with changes in neural measures of reward responsiveness in a separate session (Treadway et al., 2017), but no previous studies have directly assessed effects of stress-induced inflammation on reward processing. However, this is feasible in the laboratory setting because acute laboratory stressors reliably elicit mild and delayed (90–120 min post stress) increases in the pro-inflammatory cytokine interleukin-6 (IL-6) (Marsland et al., 2017).
Thus, the goal of this study was to model a psychobiological pathway that is believed to be critical in understanding how stress may precipitate the transition to psychopathology by experimentally evaluating effects of stress-induced inflammation on behavioral measures of reward. To do so, we used a standardized acute psychosocial laboratory stressor, the Trier Social Stress Task (TSST) to elicit an inflammatory response and administered two well-established behavioral reward tasks, the Probabilistic Reward Task (PRT) (Tripp and Alsop, 1999; Pizzagalli et al., 2005) and the Effort Expenditure for Rewards Task (EEfRT) (Treadway et al., 2009) at the peak of this inflammatory response (90–120 min post-stress). Given the preponderance of evidence from preclinical models and neuroimaging studies, and mixed evidenced from behavioral studies, we hypothesized that stress-induced inflammation would lead to decreases in reward processing across three dimensions of reward in both tasks (i.e., learning, motivation, and sensitivity).
2. Methods and Materials
2.1. Participants and procedure
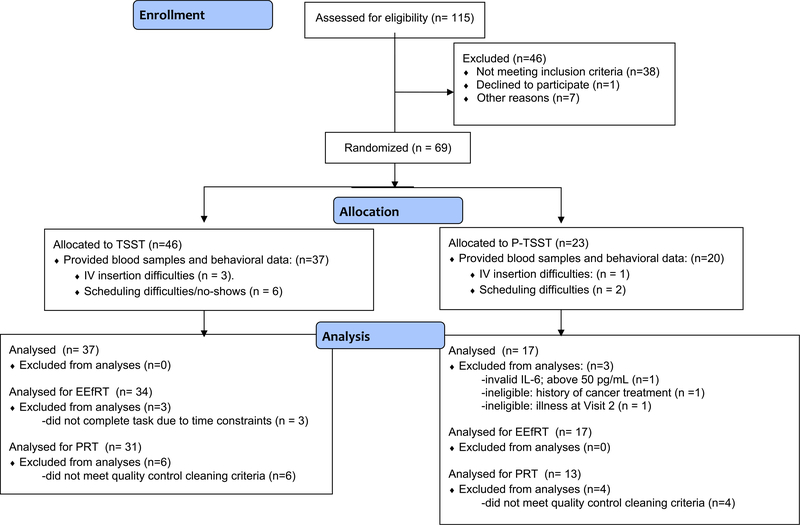
Fifty-four healthy young women at the University of California-Los Angeles (UCLA) were recruited May-December 2017 through flyers posted on the university campus and the psychology department participant pool. Inclusion criteria were English fluency, age 18–28, and female sex. We focused on women because there are gender differences in depression prevalence (Slavich and Irwin, 2014) and affective sensitivity to inflammatory challenge (Moieni et al., 2015). Exclusion criteria included current illness, major medical conditions, current/past alcohol use disorder, pregnancy, and use of tobacco or immune-altering medications. As shown in Fig. 1, 115 participants were assessed, 69 were eligible and randomized, and 54 provided valid blood samples and behavioral data. Of these, 51 completed the EEfRT (n = 34 stress) and 44 provided evaluable PRT data (n = 31 stress).
Fig. 1.
Consort diagram.
After providing informed consent, participants completed questionnaires and the EEfRT during a baseline laboratory visit (Visit 1) and were randomized 3:1 to the stress and control group via a computerized random number generator. Within approximately two weeks (for 85% of participants; range = 1–27 days), participants returned for Visit 2, which lasted 3.5–4 h and was scheduled in the afternoon (starting 1:00–1:30 pm) to control for diurnal variation in IL-6. Participants were instructed to refrain from exercising, eating, or drinking anything except water the hour prior to Visit 2. They received reminder emails and a text the day of the session and provided verbal confirmation of compliance. Upon arrival, a nurse inserted an intravenous catheter in the antecubital vein of the participant’s non-dominant arm. Participants first completed questionnaires and reported on recent health behaviors that could potentially influence levels of inflammation or the inflammatory response to stress (e.g., sleep, alcohol and caffeine use, time since last meal). Participants then completed the PRT and provided the first blood sample prior to undergoing the TSST (Kirschbaum et al., 1993) or a placebo control task (Placebo (P)-TSST) (Het et al., 2009). The TSST reliably activates the psychological and physiological stress response (Frisch et al., 2015) and involves a challenging 5-min speech and 5-min arithmetic task in front of two evaluators trained to remain impassive and provide negative non-verbal feedback (Eisenberger et al., 2010). The P-TSST has no evaluators and involves a 5-min speaking task on a neutral topic and a 5-min counting task (Het et al., 2009). After the TSST/P-TSST, participants watched a neutral movie until the PRT and EEfRT were re-administered at 90–120 min post-TSST/P-TSST. Blood samples were collected before, 90 and 120 min after the TSST/P-TSST. Participants were compensated with course credit or $50, and task performance was incentivized with money that participants received 4 months after Visit 21. All study procedures were approved by the UCLA Institutional Review Board (IRB); clinical trial registration NCT03828604.
2.2. Measures
2.2.1. Inflammation
Circulating concentrations of the pro-inflammatory cytokine IL-6 were used to measure inflammation because IL-6 increases following the TSST (Marsland et al., 2017), is elevated in individuals with depression (Haapakoski et al., 2015), and is associated with changes in reward processing and/or mood following an inflammatory stimulus (Lasselin et al., 2016; Boyle et al., 2018; Kuhlman et al., 2018). Blood samples were collected by venipuncture into ethylene diamine tetraacetic acid tubes, placed on ice, centrifuged for acquisition of plasma and stored at −80 °C. At study completion, samples were assayed for IL-6 using a high sensitivity enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, Minn). Samples were assayed in duplicate. Interand intra-assay coefficients of variation were < 6%. The lower limit of detection was 0.20 pg/mL, and there were no undetectable values.
2.2.2. Probabilistic reward task (PRT)
The PRT is a 15-min computerized task derived from signal detection theory which uses an asymmetric (3:1) pseudo-randomized reinforcement schedule to induce an implicit response bias towards one of two ambiguous stimuli (see Supplementary Materials for additional description of the PRT) (Tripp and Alsop, 1999; Pizzagalli et al., 2005). Because the development of this response bias relies both on reward learning (i.e., associating stimuli with rewards) and on reward sensitivity (i.e., immediate behavioral impact from reward feedback) (Huys et al., 2013), the total PRT response bias score indexes an overall reward responsiveness (e.g., Bogdan and Pizzagalli, 2006; Pechtel et al., 2015). In the current study the PRT was administered before and 90min post-TSST/P-TSST onset during Visit 2.
2.2.3. Effort expenditure for rewards task (EEfRT)
The EEfRT (Treadway et al., 2009) is a computerized task that assesses reward motivation and sensitivity to monetary reward. As previously described (Treadway et al., 2009; Treadway et al., 2012a,b), the EEfRT requires participants to choose between low and high effort trials. Low effort trials require 30 button presses using the dominant index finger in 7 s and are worth $1.00. High effort trials require 100 button presses with the pinky finger of the non-dominant hand in 21 s and the reward varies from $1.24$4.30. Participants are told the task lasts for the same amount of time regardless of the choices made, and that they will receive money for two successfully completed trials that are randomly selected and summed (range is $2.00 - $8.60). Participants are also told that only some trials will be rewarded, and each trial presents the probability (12%, 50%, 88%) that successful completion will be rewarded. If a choice is not made within 5-sec the trial is randomly assigned.
Lower motivation for reward on the EEfRT is operationalized as less willingness to exert greater effort for higher monetary reward and lower sensitivity to reward is operationalized as an attenuated association between the extent to which variations in potential monetary reward predict choice of high effort trials (Lasselin et al., 2016; Treadway et al., 2012). The EEfRT was shortened from 20 to 15 min in the current study and was administered at Visit 1 and 120 min post TSST/P-TSST onset during Visit 2. Of note, participants did not learn how much they had earned for either EEfRT administration until the 4-month follow-up.
2.2.4. Psychosocial measures
Affect and fatigue were assessed using items from the Positive and Negative Affect Schedule (Thompson, 2007; Watson and Clark, 1994) and the fatigue subscale from the Profile of Mood States (McNair and Lorr, 1971) pre- and post-TSST/P-TSST and with each blood draw during Visit 2. Depressive symptoms and perceived stress over the past week were assessed at the beginning of Visit 2 using the 20-item Center for Epidemiologic Studies-Depression scale (Radloff, 1977) and the 10-item Perceived Stress Scale (Cohen et al., 1983). Additional information on psychosocial measures is available in Supplementary Materials.
2.3. Analytic approach
Analyses were conducted using Stata version 13.1. IL-6 values were skewed and log transformed. Independent samples t-tests were used to test for baseline differences between the TSST and P-TSST groups. Multiple regression was used to verify group differences in affect following the TSST/P-TSST.
2.3.1. Mediation
Mediation analysis was used to evaluate effects of stress-induced inflammation on behavioral measures of reward processing. The predictor of interest was group assignment (0 = P-TSST, 1 = TSST), the mediator was change in IL-6 (IL-6 at 120 min minus IL-6 at baseline), and the outcome was reward task performance (post-TSST/P-TSST performance minus baseline performance). This analytic approach was selected because it allowed us to model the experimental manipulation and parsimoniously examine multiple components of the psychobiological pathway. Specifically, the mediation analysis yielded coefficients representing the extent to which group assignment predicted change in IL-6, the extent to which changes in IL-6 predicted changes in reward task performance, and the mediated effect itself (i.e., whether IL-6 mediated the effect of group assignment on reward task performance).
Single mediation analyses were conducted separately for the PRT and the EEfRT. The significance of each mediated effect was tested using a non-parametric bootstrap approach (n = 10,000 samples) implemented using the STATA paramed module. This resampling method generates a coefficient for the mediated effect and bias-corrected bootstrap confidence intervals; the mediated effect is deemed significant if the confidence intervals do not include zero.
2.3.2. Task analyses: PRT
Consistent with prior work and current recommendations (e.g., Vrieze et al., 2013; Bogdan and Pizzagalli, 2006; Boyle et al., 2018; Pizzagalli et al., 2005; Pechtel et al., 2015), PRT data was cleaned using the following established inclusion criteria for evaluable data: accuracy greater than 50%; ratio of rewards received greater than 2.4; at least 80% trials within valid range (150 ms-2500 ms); < 16 outliers (after log transformation, trials with reaction times falling outside the mean +/− 3 standard deviations were considered outliers). Ten participants were excluded (18.5% of the sample) leaving a total of 44 participants (n = 31 stress).
Assessment of change in reward responsiveness with the PRT
Reward responsiveness was operationalized as the total response bias score calculated across the 200 trials at each administration of the task (see Supplementary Material for formulas). Change in response bias was calculated by subtracting the total response bias score at pre-TSST/PTSST from the total response bias score at post-TSST/P-TSST. This change score was the outcome variable for mediation analysis.
2.3.3. Task analyses: EEfRT
EEfRT trials that a participant did not choose within the 5-sec time limit were excluded (0.22% of all trials). Three participants did not complete the EEfRT due to time constraints. On average, participants successfully completed 96% of all trials that were chosen. Six participants completed < 79% of trials but were retained in analyses because incomplete trials were not due to low effort, as indicated by at least 80 out of 100 button presses on incomplete hard trials.
Assessment of change in reward motivation with the EEfRT
The proportion of high-effort trials chosen was calculated for the baseline assessment and the post-TSST assessment, consistent with past work (Lasselin et al., 2016; Treadway et al., 2009). A change score was calculated by subtracting the proportions chosen at Visit 1 from proportions chosen postTSST/P-TSST at Visit 2; this change score was the outcome variable for the mediation model. Also consistent with prior studies, we calculated change scores at each of three levels of probability (low, medium, and high).
Assessment of change in reward sensitivity with the EEfRT
To evaluate effects of stress-induced inflammation on reward sensitivity on the EEfRT, generalized estimating equations (GEEs) with a binary logistic model and exchangeable working correlation structure were conducted within the stress group on EEfRT data collected at 120 min post-TSST. GEEs account for correlated data, are appropriate for a binary dependent variable (i.e. likelihood of choosing high-effort trials), and are a standard approach for analyzing EEfRT performance on a trial by trial basis (Treadway et al., 2009). The predictor of interest was a 2-way interaction term between reward magnitude and change in IL-6; this term allowed us to assess whether increases in reward magnitude predicted increased choice of high effort trials less robustly in the context of greater increases in IL-6.
2.3.4. Sample size calculation
Specifying an α value of 0.05, a sample size of 57 was required to provide 80% power to detect a significant indirect effect in the mediation model. Sample size estimates come from Monte Carlo power analysis (Schoemann et al., 2017). Given the absence of previous work on stress-induced inflammation and behavioral measures of reward, estimates for standard deviations and correlations among the predictor, mediator and outcome variable were derived from pilot data in our lab (unpublished) as well as our prior work on peripheral IL-6 following vaccination and PRT performance (Boyle et al., 2018). We assumed a moderate correlation (r = 0.5) between our predictor (TSST vs. P-TSST) and mediator (change in IL-6).
3. Results
3.1. Participant characteristics
Participants were on average 20 years old and of Latina, Asian, or Non-Hispanic white ethnicity. Less than half reported current use of hormonal contraception (n = 15; 28%), most estimated they were in the luteal phase of their menstrual cycle (n = 35; 67%) and reported a typical range for cycle length (i.e., 28–32 days). Depressive symptoms and perceived stress were comparable to previous studies with female undergraduates and young adults (Wilson et al., 2014; Hamarat et al., 2001). Table 1 provides descriptive statistics for demographic and psychosocial data. There was a baseline imbalance between groups on several variables. Participants randomized to the P-TSST reported lower fatigue and negative affect upon arrival at Visit 2 (p’s < 0.029; in reference to their current affective state), and lower levels of perceived stress (p = .037; in reference to the past week). These three variables were included as covariates in all models. Consistent with best practices (Sugama et al., 2009), analyses with inflammatory biomarkers controlled for BMI, as well as age and ethnicity. The control group was slightly older than the stress group, t(52) = 1.816, p = .075, and age was negatively correlated with baseline IL-6, r = −0.329, p = .015. Non-Hispanic white participants exhibited a greater increase in IL-6 compared to other ethnicities, t(52) = −2.716, p = .009.
Table 1.
Sample Demographic and Psychosocial Characteristics.
| Total (n = 54) |
Stress (n = 37) |
Control (n = 17) |
|
|---|---|---|---|
| Age, M (range† | 20.09 (18–25) | 19.81 (18–25) | 20.71 (18–25) |
| Ethnicity, N (%) | |||
| White | 12 (22.2) | 7 (19.0) | 5 (29.4) |
| East Asian | 13 (24.1) | 10 (27.0) | 3 (17.6) |
| Latina | 18 (33.3) | 11 (29.7) | 7 (41.2) |
| Black | 4 (7.4) | 3 (8) | 1 (5.9) |
| Other | 7 (13) | 6 (16.3) | 1 (5.9) |
| Menstrual phase, N (%) | |||
| Menses | 4 (8) | 3 (8) | 1 (7) |
| Follicular | 13 (25) | 10 (27) | 3 (20) |
| Luteal | 35 (67) | 24 (65) | 11 (73) |
| Use of hormonal contraception, N(%) | 15 (28) | 12 (32) | 3 (18) |
| Academic Status, N (%) | |||
| Freshman | 8 (14.8) | 5 (13.5) | 3 (17.7) |
| Sophomore | 16 (29.6) | 14 (37.8) | 2 (11.7) |
| Junior | 8 (14.8) | 7 (18.9) | 1 (5.9) |
| Senior | 18 (33.4) | 9 (24.3) | 9 (53) |
| Post-graduate | 4(7.4) | 2 (5.5) | 2(11.7) |
| Parental Education, N (%) | |||
| Less than college | 20 (37) | 13 (35) | 7 (41) |
| College or more | 34 (63) | 24(65) | 10(59) |
| BMI, M (range) | 23.05 (17.5–30.4) | 23.26 (17.5–30.4) | 22.61 (17.8–27.5) |
| Depressive symptoms, M (range) | 13.41 (0–38) | 14.84 (1–38) | 10.29 (0–23) |
| Perceived stress, M (range)* | 18.39 (4–36) | 19.78 (5–36) | 15.35 (4–25) |
Note. M = mean; BMI = body mass index. Menstrual phase is missing for two participants and was assessed by asking participants to indicate the first day of their last menstrual cycle.
p < .05 independent samples t-test significant group difference.
p < .08 independent samples t-test significant group difference.
3.2. Manipulation check
As expected, negative affect was higher, and positive affect was lower, following the TSST compared to the P-TSST (p’s < 0.038; see Table 2). Analyses controlled for baseline affect, fatigue and perceived stress. There were no group differences in affect or fatigue at 90 or 120 min post-TSST/P-TSST (see Table 1S).
Table 2.
Stress and Control Group Differences in Negative Affect, Positive Affect, and Fatigue After the TSST/P-TSST.
| Outcome (post-TSST/P-TSST) |
Predictor |
B |
ß |
SE |
t |
p-value |
|---|---|---|---|---|---|---|
| Negative Affect | ||||||
| Group | 3.66 | 0.26 | 1.71 | 2.14 | 0.038 | |
| Negative Affect (entry) | 0.59 | 0.32 | 0.26 | 2.27 | 0.028 | |
| Fatigue (entry) | −1.54 | −0.17 | 1.37 | −1.12 | 0.268 | |
| Perceived stress | 3.03 | 0.34 | 1.44 | 2.10 | 0.041 | |
| Intercept | 5.88 | 2.80 | 2.10 | 0.041 | ||
| Positive Affect | ||||||
| Group | −4.40 | −0.29 | 1.87 | −2.35 | 0.023 | |
| Negative Affect (entry) | −0.13 | −0.06 | 0.29 | −0.45 | 0.655 | |
| Positive Affect (entry) | 0.48 | 0.45 | 0.15 | 3.22 | 0.002 | |
| Fatigue (entry) | 2.42 | 0.26 | 1.51 | 1.60 | 0.116 | |
| Perceived stress | −1.45 | −0.15 | 1.61 | −0.90 | 0.371 | |
| Intercept | 10.99 | 6.55 | 1.68 | 0.100 | ||
| Fatigue | ||||||
| Group | 0.29 | 0.14 | 0.21 | 1.41 | 0.165 | |
| Negative Affect (entry) | 0.04 | 0.13 | 0.03 | 1.15 | 0.254 | |
| Fatigue (entry) | 0.45 | 0.34 | 0.17 | 2.73 | 0.009 | |
| Perceived stress | 0.47 | 0.34 | 0.17 | 2.67 | 0.010 | |
| Intercept | 0.39 | 0.34 | 1.14 | 0.261 |
Note. Group (0 = control; 1 = stress).
3.3. Effect of stress on ΔIL-6
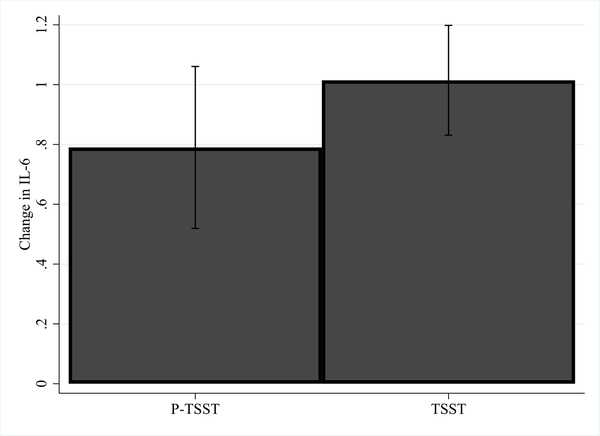
We next verified that the TSST induced greater increases in IL-6 than the P-TSST within the mediation model framework. As hypothesized, the effect of group (TSST/P-TSST) on ΔIL-6 was positive and significant in all mediation models, indicating that the stress group had a significantly greater increase in IL-6 than the control group, controlling for ethnicity, age, BMI, perceived stress and baseline negative affect and fatigue2 (see Tables 3, 4, 2S and Figs. 2 and 3).
Table 3.
Mediation Models for Response Bias on the PRT: Inflammation as a Mediator of the Relationship Between Stress and Reward Responsiveness.
| Outcome | Indirect effect (ab path) b(BS SE) [CI] | a path b(SE) | b path b(SE) | Direct effect(c’ path) b(BS SE) [CI] | Total effect (c path) b(BS SE) [CI] |
|---|---|---|---|---|---|
| △Total Response Bias | 0.190(0.134) CI [0.0002, 0.559] |
0.512(0.202)* | 0.370(0.203)** | 0.048(0.246) CI [−0.391, 0.573] |
0.238(0.224) CI [−0.154, 0.734] |
Note. In mediation analyses, the relationships among the group (stress vs. control), mediator (△IL-6), and outcome (△reward motivation) can be characterized by several paths. The c path represents the total effect, or the effect of group on the outcome. The c’ path represents the direct effect, or the effect of group on the outcome that is not transmitted by the mediator. The a path is the group effect on the mediator, the b path is the effect of the mediator on the outcome, and the product of the a and b paths (ab) represents the indirect, or mediated effect. The indirect/mediated, direct, and total effect are deemed significant if the confidence intervals do not include zero.
Abbreviations: BS SE, Bootstrapped standard errors; CI, Bootstrapped bias corrected 95% confidence intervals; SE, standard errors; IL-6, Interleukin-6
= significant at p < .05
= significant at p < .08.
Table 4.
Mediation Models for Reward Motivation: Inflammation as a Mediator of the Relationship Between Stress and Reward Motivation for Overall, Low, Medium, and High Probability Trials on the EEfRT.
| Outcome | Indirect effect (ab path) b(BS SE) [CI] | a path b(SE) | b path b(SE) | Direct effect (c’ path) b(BS SE) [CI] | Total effect (c path) b(BS SE) [CI] |
|---|---|---|---|---|---|
| △Overall Trials | 0.032(0.022)* CI[−0.001, 0.086] |
0.555(0.184)* | 0.058(0.033) | −0.002(0.041) CI [−0.080, 0.082] |
0.030(0.044) CI [−0.055, 0.118] |
| △12% Trials (Low prob) | 0.037(0.024)* CI [0.0005, 0.095] |
0.555 (0.184)* | 0.067(0.032)* | −0.007(0.037) CI [−0.074, 0.072] |
0.030(0.040) CI [−0.040, 0.120] |
| △50% Trials (Med prob) | 0.035(0.033) CI [−0.023, 0.106] |
0.555 (0.184)* | 0.063(0.058) | −0.054(0.084) CI [−0.212, 0.113] |
−0.019(0.084) CI [−0.184, 0.145] |
| △88% Trials (High prob) | 0.024(0.032) CI [−0.024, 0.106] |
0.555 (0.184)* | 0.044(0.049) | 0.056 (0.062) CI [−0.070, 0.175] |
0.080(0.055) CI [−0.030, 0.184] |
= significant at p < .05.
Note. In mediation analyses, the relationships among the group (stress vs. control), mediator (△IL-6), and outcome (△reward motivation) can be characterized by several paths. The c path represents the total effect, or the effect of group on the outcome. The c’ path represents the direct effect, or the effect of group on the outcome that is not transmitted by the mediator. The a path is the group effect on the mediator, the b path is the effect of the mediator on the outcome, and the product of the a and b paths (ab) represents the indirect, or mediated effect. The indirect/mediated, direct, and total effect are deemed significant if the confidence intervals do not include zero.
Abbreviations. BS SE = Bootstrapped standard errors; CI = Bootstrapped bias corrected 95% confidence intervals; SE = standard errors; IL-6 = Interleukin-6.
Fig. 2.
Adjusted means for change in IL-6 (log transformed) from baseline to 120 min post P-TSST/TSST.
Fig. 3.
Raw IL-6 values at pre and 120min post the P-TSST and TSST for all participants.
3.4. Effect of stress-induced ΔIL-6 on reward task performance
3.4.1. PRT reward responsiveness
Mediation analysis was conducted to test the hypothesis that stress-induced inflammation would be associated with blunted reward responsiveness, as indicated by a decrease in the PRT response bias score from pre-to 90 min post-TSST/P-TSST. For this mediation model, the indirect effect was significant, (b = 0.190, SEB = 0.134, CIBS [0.0002, 0.559]), indicating significant mediation. However, contrary to hypotheses, greater stress-induced inflammation was associated with an increase in response bias, indicating an increase in reward responsiveness (see Table 3).
3.4.2. EEfRT reward motivation
Mediation analysis was conducted to test the hypothesis that stress-induced inflammation would decrease reward motivation on the EEfRT, operationalized as a global reduction in the proportion of high effort trials chosen from Visit 1 to 120 min post-TSST/P-TSST at Visit 2. The indirect effect did not reach significance, (b = 0.032, SEB = 0.022, CIBS [−0.001; 0.086]), although the relationship between increases in IL-6 and change in the proportion of high effort trials, controlling for group assignment, approached significance (b = 0.058, p = .086; see Table 4), such that greater increases in IL-6 predicted increased choice of high effort trials. We next evaluated effects of stress-induced inflammation on change in the proportion of high effort trials chosen at each of the three levels of probability (low, medium, high). There was no evidence that stress-induced inflammation was associated with altered motivation for high or medium probability trials; however, as shown in Table 4, there was significant mediation for low probability trials, such that greater stress-induced inflammation was associated with an increase in high effort trial choice for low probability trials (indirect effect: b = 0.037, SEB = 0.024, CIBS [0.0005, 0.095]).
3.4.3. EEfRT reward sensitivity
A GEE model was conducted to test the hypothesis that greater stress-induced inflammation would be associated with lower reward sensitivity on the EEfRT. This analysis was conducted within the stress group on EEfRT data collected 120 min post-TSST. The predictor of interest was the interaction term between reward magnitude and ΔIL-6. Covariates included task specific variables (trial number, probability, reward magnitude), BMI, age, and ethnicity. As shown in Table 5, the interaction term was not significant (b = 0.028, SE = 0.126, p = .821), indicating that changes in reward magnitude did not predict high effort trial choice differently as a function of greater ΔIL-6. Thus, there was no evidence for a reduction in reward sensitivity using this metric.
Table 5.
Stress-Induced Inflammation and Reward Sensitivity on the EEfRT using GEE.
| Variable | b | SE | z | p-value | CI (lower) | CI (upper) |
|---|---|---|---|---|---|---|
| △IL-6 | 0.53 | 0.41 | 1.28 | 0.202 | −0.28 | 1.34 |
| Reward Magnitude | 1.39 | 0.14 | 9.90 | < 0.001 | 1.12 | 1.67 |
| △IL-6 X Reward Magnitude | 0.03 | 0.10 | 0.25 | 0.803 | −0.17 | 0.22 |
| Probability Probability (low vs. medium) |
1.64 | 0.19 | 8.67 | < 0.001 | 1.27 | 2.00 |
| Probability (low vs. high) | 2.73 | 0.20 | 13.62 | < 0.001 | 2.34 | 3.12 |
| Trial number | −0.03 | 0.005 | −6.69 | < 0.001 | −0.04 | −0.02 |
| Ethnicity | −0.99 | 0.38 | −2.57 | 0.010 | −1.74 | −0.24 |
| Age | 0.05 | 0.09 | 0.52 | 0.604 | −0.14 | 0.24 |
| BMI | 0.10 | 0.05 | 1.85 | 0.065 | −0.006 | 0.20 |
| Intercept | −9.62 | 2.52 | −3.82 | < 0.001 | −14.55 | −4.69 |
Note. Analysis includes 34 participants who underwent stress using EEfRT data collected 120 min post-TSST. Low probability = 12%; medium probability = 50%; high probability = 88%. △IL-6 refers to change in IL-6 from study entry to 120 min post-TSST. Ethnicity (0 = Non-Hispanic White; 1 = Asian/Latina/Black/Other).
Abbreviations. BMI = body mass index; CI = Confidence intervals; IL-6 = Interleukin-6; SE = standard error.
4. Discussion
The purpose of this study was to interrogate the effects of stress-induced inflammation on three dimensions of reward processing in healthy young women. Based on empirical evidence from animal models (Koo and Duman, 2008) and neuroimaging studies (Harrison et al., 2016; Harrison et al., 2015; Eisenberger et al., 2010; Capuron et al., 2012), we hypothesized that increases in inflammation following acute psychosocial stress would be associated with decreased performance on two well-establised behavioral reward tasks. However, contrary to hypotheses, we found that stress-induced inflammation increased reward responsiveness on the PRT and selectively increased reward motivation (but not reward sensitivity) on the EEfRT.
In preclinical models, inducing an inflammatory response consistently produces anhedonic behavior, and, while far less studied, similar patterns have been observed in human neuroimaging studies following endotoxin, typhoid vaccine, and interferon-alpha therapy (Harrison et al., 2016; Eisenberger et al., 2010; Capuron et al., 2012). Furthermore, among individuals with depression, elevated inflammation has been linked to dysregulation in reward neural circuitry (Felger et al., 2015). Given this background, enhanced reward responsiveness and motivation in association with the stress-induced inflammatory response was unexpected. The rapidly expanding literature on inflammation and reward suggests a complicated relationship that may vary as a function of reward dimension (e.g., motivation vs. sensitivity; Draper et al., 2018; Lasselin et al., 2016; Dantzer et al., 2014), reward type (e.g., monetary vs social; Eisenberger et al., 2010; Inagaki et al., 2015), level of analysis (e.g., neuroimaging vs. behavior; 26), and the magnitude of the inflammatory response (e.g., endotoxin vs. vaccination; Lasselin et al., 2016; Boyle et al., 2018). In addition, effects of stress and inflammation on reward in human models have previously been examined in isolation, but stress-induced inflammation involves a different psychological and physiological experience. Psychological stress not only initiates a cascade of physiological reactions, including the delayed peripheral release of pro-inflammatory cytokines, but also induces inflammation in the brain (O’Connor et al., 2003). Whether or how this central and peripheral co-activation alters behavior is unknown. Perhaps psychosocial stress alters neural sensitivity to peripheral inflammatory signaling, for example (Anisman et al., 2002). Indeed, in a sample of healthy young men, negative mood immediately following an acute laboratory stressor was higher among participants who had received typhoid vaccine (compared to placebo) 30-min earlier (Brydon et al., 2009). Thus, source of inflammation (e.g., peripherally vs. centrally induced) could be yet another moderator of the relationship between inflammation and reward. Accumulating evidence will allow more specific and targeted hypotheses in the future regarding how inflammation shapes reward processing.
Although small, the literature consistently suggests that motivational processes are sensitive to inflammatory challenges; however, how this alteration in motivation manifests behaviorally is not well understood. Three studies have used the EEfRT and all found a different pattern. The current study found stress-induced inflammation increased motivation for low probability trials, endotoxin has been shown to increase motivation for high probability trials (Lasselin et al., 2016) and influenza vaccination was associated with a global decrease in motivation regardless of probability (Boyle et al., 2018). Using an ‘effort-stake task’ that does not alter probability, Draper and colleagues (2018) found a reduction in motivation following endotoxin in a sample of healthy men. As noted previously, these varied findings may be attributable to differences in the magnitude of the inflammatory stimulus, source of inflammation, task specific variables (e.g., presence of probability cues), or the intersection of these moderators. Participant sex is also an important variable to further interrogate given that stress (Lighthall et al., 2012) and inflammation (Moieni et al., 2015) have both been shown to alter behavior differently in women compared to men.
There was no evidence that stress-induced inflammation altered sensitivity to reward on the EEfRT. This finding aligns with all three studies that have evaluated inflammation and reward sensitivity in the context of a reward motivation task (Draper et al., 2018; Lasselin et al., 2016; Boyle et al., 2018), such that sensitivity to increases in monetary reward magnitude remained preserved while motivation was altered. This suggests a fairly consistent picture, at least for monetary reward and on a behavioral level. By contrast, an inflammatory challenge has been shown to increase reactivity in reward-related brain regions in response to social reward (Inagaki et al., 2015; Muscatell et al., 2016) and decrease response to novel images (Harrison et al., 2015). Whether similar effects would be seen for non-monetary rewards on a behavioral level has yet to be tested.
While behavioral effects of stress-induced inflammation have not previously been investigated, there is a literature on stress-induced cortisol release that may inform the current results. Specifically, when behavioral learning and decision-making tasks are administered 30-min after stress, increased sensitivity to reward cues is apparent (and/or decreased sensitivity to punishment cues; Cavanagh et al., 2010; Lighthall et al., 2013; Petzold et al., 2010). This increase may result from dopamine release within the reward system in response to elevated glucocorticoids (Lighthall et al., 2012). Greater increases in cortisol following stress are correlated with greater increases in striatal dopamine release (Vaessen et al., 2015), which could enhance the salience of reinforcement cues during learning. Indeed, relevant to the current results, the development of PRT response bias is associated with extrastriatal dopaminergic release (Vrieze et al., 2013; Santesso et al., 2009). Furthermore, increased dopaminergic activity increased choice of low probability trials on the EEfRT, which was the pattern observed in the current study (Wardle et al., 2011). Thus, increased reward responsiveness on the PRT and increased low probability choice on the EEfRT could both be driven by the downstream consequence of the cortisol response to stress, or, given that endotoxin administration also increases central dopaminergic activity (Anisman et al., 2002), the inflammatory response to stress. Additional work characterizing the time course of dopamine release in association with the stress-induced inflammatory response will be required to evaluate this possibility further.
Regarding study limitations, there was a chance imbalance on several variables at baseline; however, these were controlled in analyses. Monetary compensation for the EEfRT and the PRT was not immediate, which may have reduced the salience of the reward at task administration and contributed to the loss of participant data, particularly on the PRT. This left the study underpowered to implement computational analyses (Huys et al., 2013) that would have allowed us to parse the contribution of learning rate and reward sensitivity parameters to the total PRT response bias score. Our control group was small and demonstrated an increase in IL-6 in response to the P-TSST and laboratory environment, which may have precluded detection of significant associations between stress-induced inflammation and reward sensitivity on the EEfRT. Finally, assessment of health behaviors and status was by self-report with no independent diagnostic verification (e.g., toxicology test).
Delineating the psychobiological mechanisms of reward dysregulation has the potential to inform treatment and prevention of MDD, and is also relevant for other clinical conditions that involve impaired reward processing and dysregulated inflammatory biology, such as Alzheimer’s disease, schizophrenia, and post-traumatic stress disorder. Consistent with other emerging work, our results highlight the need to assess multiple reward dimensions, dysregulation of which may warrant different pharmacological and behavioral therapeutic approaches. It will be particularly important to continue to explore whether some reward processes, such as motivation, are more sensitive to fluctuations in inflammation than others, such as sensitivity. Examination of diverse reward stimuli beyond monetary incentive, particularly social reward (Inagaki et al., 2015), is needed. Finally, our demonstration of detectable behavioral changes in response to the delayed inflammatory response to stress indicates this is a feasible method that can be added to the repertoire for studying affective and behavioral responses to inflammation. This method should be further interrogated given that it may capture the normative physiological changes that repeatedly occur across the lifetime in response to stressors and potentially precipitate the transition to psychopathology.
Supplementary Material
Acknowledgments
This study received funding and support from the UCLA Clinical and Translational Science Institute Grant # UL1TR001881 and a seed grant award from the Norman Cousins Center for Psychoneuroimmunology, UCLA Semel Institute (JEB, CCB). CCB received support as a postdoctoral fellow at the Norman Cousins Center for Psychoneuroimmunology. Additional support came from the 2017 UCLA Mautner Fellowship, the 2017 Society for Health Psychology Graduate Student Award, and the UCLA Dissertation Year Fellowship (CCB). The behavioral computer tasks were programmed by Daniel J. Neufeldt with Inquisit Millisecond Software. Data from this study were previously presented at the PsychoNeuroImmunology Research Society meeting (2018, June).
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbi.2019.09.023.
The IRB did not allow immediate compensation.
The effect of group on ΔIL-6 was also significant when covariates were limited to ethnicity, age, and BMI (PRT model: p = .047; EEfRT models: p =.016).
References
- Anisman H, Kokkinidis L, Merali Z, 2002. Further evidence for the depressive effects of cytokines: Anhedonia and neurochemical changes. Brain Behav. Immun. 16, 544–556. [DOI] [PubMed] [Google Scholar]
- Association AP (2013): Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Pub. [Google Scholar]
- Bedwell JS, Gooding DC, Chan CC, Trachik BJ, 2014. Anhedonia in the age of RDoC. Schizophr. Res. 160, 226–227. [DOI] [PubMed] [Google Scholar]
- Berghorst LH, Bogdan R, Frank MJ, Pizzagalli DA, 2013. Acute stress selectively reduces reward sensitivity. Front Hum Neurosci. 7 10.3389/fnhum.2013.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Pizzagalli DA, 2006. Acute stress reduces reward responsiveness: Implications for depression. Biol. Psychiatry 60, 1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Pizzagalli DA, Ratner KG, Jahn AL, 2007. Increased perceived stress is associated with blunted hedonic capacity: Potential implications for depression research. Behav. Res. Ther. 45, 2742–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle CC, Kuhlman KR, Dooley LN, Haydon MD, Robles TF, Ang Y-S, et al. , 2018. Inflammation and dimensions of reward processing following exposure to the influenza vaccine. Psychoneuroendocrinology. 102, 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L, Walker C, Wawrzyniak A, Whitehead D, Okamura H, Yajima J, et al. , 2009. February): Synergistic effects of psychological and immune stressors on inflammatory cytokine and sickness responses in humans. Brain Behav. Immun. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, et al. , 2012. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry. 69, 1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ, Allen JJB, 2010. Social stress reactivity alters reward and punishment learning. Soc. Cognit. Affect. Neurosci. 6 nsq041–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R, 1983. A global measure of perceived stress. J. Health Soc. Behav. 24, 385. [PubMed] [Google Scholar]
- Craske MG, Meuret AE, Ritz T, Treanor M, Dour HJ, 2016. Treatment for anhedonia: A neuroscience driven approach. Depression and anxiety. 33, 927–938. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Vichaya EG, Hunt SC, 2014. Lipopolysaccharide reduces incentive motivation while boosting preference for high reward in mice. Neuropsychopharmacology 39, 2884–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley LN, Kuhlman KR, Robles TF, Eisenberger NI, Craske MG, Bower JE, 2018. The role of inflammation in core features of depression_ Insights from paradigms using exogenously-induced inflammation. Neurosci. Biobehav. Rev. 94, 219–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper Amelia, Koch, Rebecca M, van der Meer, Jos WM, Apps AJ, Matthew, Pickkers Peter, Husain Masud, van der Schaaf, Marieke E, 2018. Effort but not reward sensitivity is altered by acute sickness induced by experimental endotoxemia in humans. Neuropsychopharmacologu 43, 1107–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Way BM, Slavich GM, Slavich GM, Taylor SE, Way BM, et al. , 2010. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proc. Natl. Acad. Sci. 107, 14817–14822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Irwin MR, Inagaki TK, Berkman ET, Rameson LT, Mashal NM, 2010. Inflammation-induced anhedonia: Endotoxin reduces ventral striatum responses to reward. Biol. Psychiatry 68, 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, Miller AH, 2015. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatry 21, 1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Baskin-Sommers AR, 2015. Abnormal reward functioning across substance use disorders and major depressive disorder: Considering reward as a transdiagnostic mechanism. Int. J. Psychophysiol. 98, 227–239. [DOI] [PubMed] [Google Scholar]
- Frisch JU, Häusser JA, Mojzisch A, 2015. The Trier Social Stress Test as a paradigm to study how people respond to threat in social interactions. Front. Psychol. 6 10.3389/fpsyg.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R, 2007. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol. Psychiatry 13, 717–728. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joormann J, 2010. Neural processing of reward and loss in girls at risk for major depression. Arch. Gen. Psychiatry 67, 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimäki M, 2015. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav. Immun. 49, 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamarat Errol, Thompson Dennis, Karen M, 2001. Perceived stress and coping resource availability as predictors of life satisfaction in young, middle-aged, and older adults. Exp. Aging Res. 27 (2), 181–196. [DOI] [PubMed] [Google Scholar]
- Hammen CL, 2015. Stress and depression: old questions, new approaches. Curr. Opin. Psychol. 4, 80–85. [Google Scholar]
- Harrison NA, Cercignani M, Voon V, Critchley HD Effects of inflammation on hippocampus and substantia nigra responses to novelty in healthy human participants Neuropsychopharmacol. Off. Publicat. Am. Coll. Neuropsychopharmacol. 40 2015. 831 838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Voon V, Cercignani M, Cooper EA, Pessiglione M, Critchley HD, 2016. A neurocomputational account of how inflammation enhances sensitivity to punishments versus rewards. Biol. Psychiatry 80, 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Het S, Rohleder N, Schoofs D, Kirschbaum C, Wolf OT, 2009. Neuroendocrine and psychometric evaluation of a placebo version of the “Trier Social Stress Test”. Psychoneuroendocrinology 34, 1075–1086. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, et al. , 2014. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl. Acad. Sci. 111, 16136–16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huys QJ, Pizzagalli DA, Bogdan R, Dayan P, 2013. Mapping anhedonia onto reinforcement learning: a behavioural meta-analysis. Biol Mood Anxiety Disord. 3, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Duman RS, 2008. IL-1 is an essential mediator of the antineurogenic and anhedonic effects of stress. Proceed. Natil. Acad. Sci. 105, 751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki TK, Muscatell KA, Irwin MR, Moieni M, Dutcher JM, Jevtic I, et al. , 2015. The role of the ventral striatum in inflammatory-induced approach toward support figures. Brain Behav. Immun. 44, 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M, 2005. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience 135, 1295–1307. [DOI] [PubMed] [Google Scholar]
- Kashdan TB, 2004. The neglected relationship between social interaction anxiety and hedonic deficits: Differentiation from depressive symptoms. J. Anxiety Disord. 18, 719–730. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Hettema JM, Butera F, Gardner CO, Prescott CA, 2003. Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. Arch. Gen. Psychiat. 60, 789–796. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH, 1993. The “Trier Social Stress Test”–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. [DOI] [PubMed] [Google Scholar]
- Kogler L, Müller VI, Chang A, Eickhoff SB, Fox PT, Gur RC, Derntl B, 2015. Psychosocial versus physiological stress — Meta-analyses on deactivations and activations of the neural correlates of stress reactions. NeuroImage 119, 235–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Robles TF, Dooley LN, Boyle CC, Haydon MD, Bower JE, 2018. Within-subject associations between inflammation and features of depression: Using the flu vaccine as a mild inflammatory stimulus. Brain Behav. Immun. 69, 540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasselin J, Treadway MT, Lacourt TE, Soop A, Olsson MJ, Karshikoff B, et al. , 2016. Lipopolysaccharide alters motivated behavior in a monetary reward task: a randomized trial. Neuropsychopharmacol. Off. Publicat. Am. Coll. Neuropsychopharmacol. 42, 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighthall NR, Sakaki M, Vasunilashorn S, Nga L, Somayajula S, Chen EY, et al. , 2012. Gender differences in reward-related decision processing under stress. Soc. Cognit. Affect. Neurosci. 7, 476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighthall NR, Gorlick MA, Schoeke A, Frank MJ, Mather M, 2013. Stress modulates reinforcement learning in younger and older adults. Psychol. Aging. 28, 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loas G, 1996. Vulnerability to depression: A model centered on anhedonia. J. Affect. Disord. 41, 39–53. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Walsh C, Lockwood K, John-Henderson NA, 2017. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behav. Immun. 64, 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF Profile of mood states. Educational and Industrial Testing Service, 1971. [Google Scholar]
- Ménard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, et al. , 2017. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moieni Mona, Irwin, Jevtic Michael R, Ivana Olmstead, Richard Breen, Eisenberger Elizabeth C, Naomi I, 2015. Sex differences in depressive and socioemotional responses to an inflammatory challenge: implications for sex differences in depression. Neuropsychopharmacol. Off. Publicat. Am. Coll. Neuropsychopharmacol. 40 (7), 1709–1716. 10.1038/npp.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SM, Reid MW, 2009. Life stress and major depression. Curr. Direct. Psychol. Sci. 18, 68–72. [Google Scholar]
- Muscatell KA, Moieni M, Inagaki TK, Dutcher JM, Jevtic I, Breen EC, et al. , 2016. Exposure to an inflammatory challenge enhances neural sensitivity to negative and positive social feedback. Brain Behav. Immun. 57, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D, Demyttenaere K, Janka Z, Aarre T, Bourin M, Canonico PL, et al. , 2006. The other face of depression, reduced positive affect: the role of catecholamines in causation and cure. J. Psychopharmacol. 21, 461–471. [DOI] [PubMed] [Google Scholar]
- O’Connor KA, Johnson JD, Hansen MK, Wieseler Frank JL, Maksimova E, Watkins LR, Maier SF, 2003. Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Res. 991, 123–132. [DOI] [PubMed] [Google Scholar]
- Pechtel P, Huys QJ, Webb CA, Pizzagalli DA, Dillon DG, Goer FK, et al. , 2015. Neural correlates of three promising endophenotypes of depression: evidence from the EMBARC study. Neuropsychopharmacology 41, 454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold A, Plessow F, Goschke T, Kirschbaum C, 2010. Stress reduces use of negative feedback in a feedback-based learning task. Behav. Neurosci. 124, 248–255. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, 2014. Depression, stress, and anhedonia: Toward a synthesis and integrated model. Annu Rev Clin Psychol. 10, 393–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O’Shea JP, 2005. Toward an objective characterization of an anhedonic phenotype: A signal-detection approach. Biol. Psychiatry 57, 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcelli AJ, Lewis AH, Delgado MR Acute Stress Influences Neural Circuits of Reward Processing 2012. 6 doi: 10.3389/fnins.2012.00157. [DOI] [PMC free article] [PubMed]
- Radloff LS, 1977. The CES-D Scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1, 385–401. [Google Scholar]
- Rawal A, Collishaw S, Collishaw S, Thapar A, Thapar A, Rice F, Rice F, 2012. “The risks of playing it safe”: A prospective longitudinal study of response to reward in the adolescent offspring of depressed parents. Psychol. Med. 43, 27–38. [DOI] [PubMed] [Google Scholar]
- Rømer Thomsen K, Whybrow PC, Kringelbach ML, 2015. Reconceptualizing anhedonia: Novel perspectives on balancing the pleasure networks in the human brain. Front. Behav. Neurosci. 9, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesso DL, Evins AE, Frank MJ, Cowman Schetter EM, Bogdan R, Pizzagalli DA, 2009. Single dose of a dopamine agonist impairs reinforcement learning in humans: evidence from event-related potentials and computational modeling of striatal-cortical function. Hum. Brain Mapp. 30, 1963–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoemann AM, Boulton AJ, Short SD, 2017. Determining power and sample size for simple and complex mediation models. Soc. Psychol. Personal. Sci. 8, 379–386. [Google Scholar]
- Slavich GM, Irwin MR, 2014. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol. Bull. 140, 774–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugama S, Takenouchi T, Fujita M, Conti B, Hashimoto M, 2009. Differential microglial activation between acute stress and lipopolysaccharide treatment. J. Neuroimmunol. 207, 24–31. [DOI] [PubMed] [Google Scholar]
- Thompson ER, 2007. Development and Validation of an Internationally Reliable ShortForm of the Positive and Negative Affect Schedule (PANAS). J. Cross Cult. Psychol. 38, 227–242. [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH, 2009. Worth the “EEfRT?” The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS ONE 4 e6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH, Schwartzman AN, Buckholtz JW, Treadway MT, Buckholtz JW, et al. , 2012a. Dopaminergic Mechanisms of Individual Differences in Human Effort-Based Decision-Making. J. Neurosci. 32, 6170–6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Bossaller NA, Shelton RC, Zald DH, 2012b. Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J. Abnorm. Psychol. 121, 553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Zald D, 2013. Perceived stress predicts altered reward and loss feedback processing in medial prefrontal cortex. Front. Hum. Neurosci. 7, 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Admon R, Arulpragasam AR, Mehta M, Douglas S, Vitaliano G, et al. , 2017. Association Between Interleukin-6 and Striatal Prediction-Error Signals Following Acute Stress in Healthy Female Participants. Biol. Psychiatry 82, 570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp G, Alsop B, 1999. Sensitivity to reward frequency in boys with attention deficit hyperactivity disorder. J. Clin. Child Psychol. 28, 366–375. [DOI] [PubMed] [Google Scholar]
- Vaessen T, Hernaus D, Myin-Germeys I, van Amelsvoort T, 2015. The dopaminergic response to acute stress in health and psychopathology: a systematic review. Neurosci. Biobehav. Rev. 56, 241–251. [DOI] [PubMed] [Google Scholar]
- Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, De Boer P, et al. , 2013. Reduced reward learning predicts outcome in major depressive misorder. Biol. Psychiatry 73, 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze E, Ceccarini J, Pizzagalli DA, Bormans G, Vandenbulcke M, Demyttenaere K, et al. , 2013. Measuring extrastriatal dopamine release during a reward learning task. Hum. Brain Mapp. 34, 575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, Treadway MT, Mayo LM, Zald DH, de Wit H, 2011. Amping up effort: effects of d-amphetamine on human effort-based decision-making. J. Neurosci. 31, 16597–16602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, 1994. Positive and negative affect schedule-expanded version. PsycTESTS Dataset. 10.1037/t04754-000. [DOI] [Google Scholar]
- Wilson KT, Bohnert AE, Ambrose A, Davis DY, Jones DM, Magee MJ, 2014. Social, behavioral, and sleep characteristics associated with depression symptoms among undergraduate students at a women’s college: A cross-sectional depression survey, 2012. BMC Women’s Health 14, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Rimmerman N, Reshef R, 2015. Depression as a microglial disease. Trends Neurosci. 38, 637–658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.