Abstract
Objective:
To assess whether the relationship between hearing and depressive symptoms is present among older adults classified as normal hearing (≤25 dB).
Design:
Cross-sectional epidemiologic study embedded within a prospective cohort study (Hispanic Community Health Study)
Setting:
Multi-centered at 4 US communities (New York, Chicago, Miami, San Diego)
Participants:
Adults ≥50 years old (n=5,499) with normal hearing or hearing loss.
Measurements:
The primary exposure was hearing, defined continuously by the 4-frequency pure-tone average on audiometry (mean hearing threshold, in dB, at 500, 1000, 2000, and 4000 Hz pitch). Hearing was additionally categorized into normal hearing (≤25 dB) and hearing loss (>25 dB). The main outcome was depressive symptoms, measured with the Center for Epidemiologic Studies Depression Scale-10 (CESD-10). Depressive symptoms were defined both continuously and binarily (where CESD-10 ≥ 10 was categorized as clinically significant depressive symptoms). Multivariable linear, logistic, and generalized additive modeling (GAM) regressions were performed.
Results:
Worse hearing was related to higher depressive symptoms among those with normal hearing in GAM regression. Among those with normal hearing, the CESD-10 score increased by 1.04 points (95% CI = 0.70, 1.37) for every 10 dB decrease in hearing, adjusting for age, gender, education, cardiovascular disease, and hearing aid use. Among those with hearing loss, the CESD-10 score increased by 0.62 points (95% CI = 0.23, 1.01) for every 10 dB decrease in hearing, adjusting for the same confounders. Similar findings were noted when the outcome was clinically significant depressive symptoms (adjusted OR=1.28 [1.14, 1.44] in normal hearing versus 1.26 [1.11, 1.44] in HL). In certain sensitivity analyses, the relationship between hearing and depressive symptoms was significantly stronger among those with normal hearing than in those with hearing loss.
Conclusion:
The relationship between hearing and clinically significant depressive symptoms is present among older adults with normal hearing (<25 dB). We introduce the term subclinical hearing loss as imperfect hearing that is classically defined as normal (1–25 dB). The relationship between hearing and late life depressive symptoms may be more sensitive than previously recognized.
Keywords: age-related hearing loss, late life depressive symptoms
INTRODUCTION
Age-related hearing loss (HL) is the third most common disorder of later life.1 Nearly 80% of those over 80 years old are affected.2 Until recently, HL has been regarded as a mere nuisance with no broader health consequences. Growing evidence now links HL to a host of more serious age-related conditions, including depression3–6 and neurocognitive disorders.7–9 At the same time, treatment with hearing aids is universally uncommon, even in countries where they are covered by national healthcare.10 In the United States, fewer than 15% of adults with HL wear hearing aids.11 Given its high prevalence and low treatment levels, HL has thus gained interest as a potentially modifiable risk factor for mood12 and neurocognitive disorders.13 This interest has led to the recent passage of the Over the Counter Hearing Aid Act,14 which will greatly improve the accessibility of HL treatment.
The level at which HL begins to have a deleterious association with depressive symptoms is unknown. Hearing is defined continuously, with perfect hearing at 0 dB and larger numbers indicating worse hearing. In adults, hearing ≤25 dB is arbitrarily, but widely, defined as normal. Many, however, have suggested that these criteria are insufficiently strict15, 16. For example, in children the cutoff is typically defined at a stricter 20 dB, suggesting an unfair double standard.17 The definition of HL and recommendations of when to begin hearing aids are not evidence-based.
We have recently described an unexpected relationship between hearing and cognition among those with normal hearing (≤25 dB). We introduced the term subclinical HL, defined as imperfect hearing that is classically defined as normal (i.e., 1–25 dB).18 Whether associations exist between hearing and other neuropsychiatric conditions of older life among those with normal hearing has been unstudied.
Depression is a highly prevalent and disabling disorder in later life.2 Herein, we examine the relationship between the full spectrum of hearing and depressive symptoms. We focus on those considered to have normal hearing as defined by the historic ≤25 dB cutoff. We hypothesized that an association between worse hearing and worse depressive symptoms is present in those with normal hearing (≤25 dB).
METHODS
Study Cohort
The Hispanic Community Health Study/Study of Latinos (HCHS) is a community-based prospective study centered at four US sites (Bronx, NY; Chicago, IL; Miami, FL; and San Diego, CA). The majority of participants had both audiometry and a well-being/quality of life assessment that included the Center for Epidemiologic Studies Depression Scale-10 (CESD-10). Testing was conducted in Spanish or English based on preference. While the cohort is longitudinal, only the 2008–2011 wave of data has been released. A cross-sectional analysis was performed.
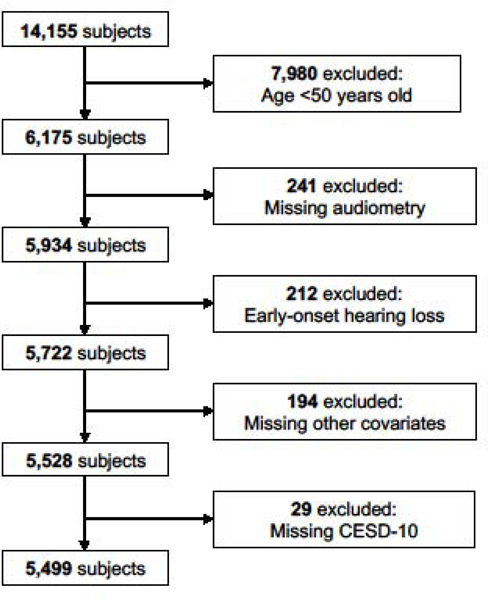
There were originally 14,155 participants. The analysis was restricted to those at risk for age-related HL, thus participants <50 years old (n=7,980) or with early-onset HL (n=212) were excluded. Participants with missing audiometry (n=241), covariate data (n=194), or CESD-10 (n=29) were also excluded. This left 5,499 participants for analysis. (Figure 1)
Figure 1.
Diagram of study subject inclusion and exclusion from the Hispanic Community Health Study (HCHS).
Hearing (Exposure)
Hearing was measured objectively with pure tone audiometry. Hearing thresholds (in dB, decibel hearing level), were measured from 500 (low pitch) to 8,000 Hz (high pitch). Worse hearing is indicated by higher dB thresholds. Hearing, the main exposure variable, was operationally defined as the four-frequency pure tone average (PTA), which is the mean threshold (dB) at 500, 1000, 2000, and 4000 Hz.19
The widely used PTA cutoff of 25 dB20 was used to divide participants into classically-defined normal hearing (PTA ≤25) and HL (PTA >25 dB). Subclinical HL, a term we introduced,21 is defined as imperfect hearing that is classically defined as normal (PTA 1–25 dB). An alternative, more stringent cutoff of PTA 15 dB was used for part of the analysis to divide participants into strict normal hearing (PTA ≤15 dB) and strict HL (PTA >15 dB).
Depressive Symptoms (Outcome)
Depressive symptoms were ascertained with the 10-item Center for Epidemiologic Studies Depression Rating Scale (CESD-10).22 Both the continuous CESD-10 and a binarized CESD-10 were used. The binary outcome was created because it is clinically useful to define a cut-point for clinically significant depressive symptoms (CSDS). This cut-point was ≥10, which has been associated with greater adverse health outcomes and disability in older adults.23, 24 CESD-10 ≥ 10 has good predictive accuracy compared to the standard CESD-20 cut-point of ≥ 16.25
Alternative outcomes were used in sensitivity analyses. The CESD-10 cutpoint for CSDS is less rigidly defined than for the CESD-20. We thus used an alternative definition of a CESD-10 cut-point of ≥16.26 We also used antidepressant use (yes/no by documented prescription of any antidepressant class plus self-declared use in the past 4 weeks). This might capture subjects with previously diagnosed depression or depressive symptoms who currently have lower CESD-10 scores because of treatment.
Covariates
Variables that might confound the association between HL and depressive symptoms were added to the multivariable (also sometimes termed multivariate) models. These included age (years), hearing aid use (yes/no), gender (man/woman, self-reported), education (years), and cardiovascular disease. Cardiovascular disease is a potential confounder because it could cause both HL and depressive symptoms. A composite cardiovascular disease variable aggregating several risk factors was created to avoid multicollinearity.27 A point was added for each of 3 risk factors including coronary artery disease (any history of electrocardiogram with old/possible old myocardial infarction, angina, heart attack, angioplasty, or cardiac surgery), hypertension (blood pressure ≥140/90 or on hypertensive medications), and/or history of transient ischemic attack/stroke (self-reported). In addition, 1 additional point was added for borderline diabetes (fasting glucose 100–125 mg/dL or post-oral glucose tolerance test 140–199 mg/dL or HbA1C 5.7–6.5%) or 2 additional points for diabetes (fasting glucose ≥126 mg/dL or non-fasting glucose ≥200 mg/dL or post-oral glucose tolerance test ≥200 mg/dL or HbA1C ≥6.5%). The score range was 0 (no cardiovascular risk factors) to 5 (all cardiovascular risk factors).28 In a sensitivity analysis, lab-measured C-reactive protein, an inflammatory marker, was added as a covariate.
Statistical Analysis
Continuous variables were described using means and standard deviations. Categorical variables were described using frequencies and proportions.
Three regression modelling strategies were used to flexibly examine associations between exposure (hearing) and outcome (depressive symptoms):
(1a). Linear regression,
which assumes that the average score on the continuous CESD-10 correlates linearly with hearing. The univariable model is given by: CESD– 10 Score = β0 + β 1 Hearing + ε, where β0 is the intercept, β1is the change in average CESD-10 score for a 10 dB decrease in hearing (i.e., 10 dB increase in PTA). While this model is simple and easily interpreted, it does not encompass non-linear relationships.
(1b). Logistic regression.
This model is similar to (1a) except that logistic regression is used along with the binarized CESD-10 outcome.
(2). Generalized additive modeling (GAM)29, assuming a smooth, possibly non-linear effect of hearing on depressive symptoms.
This technique creates a relationship between hearing and continuous CESD-10 that is determined by the data rather than being pre-specified. In other words, GAM does not assume a particular a priori functional relationship (such as linearity) between hearing and depressive symptoms. Resultingly, it may allow a better fit than linear regression. The univariable GAM is given by: CESD– 10 Score = β0 + g (Hearing) + ε, where β0 and ε are as above, and g (∙) is a smooth, non-linear function that is estimated using penalized regression splines.30 A smoothing parameter controls the smoothness of the estimate for g (∙), selected to minimize the generalized cross validation score, a measure of model fit.30 In contrast to linear regression, GAMs do not produce point estimates. Instead, the interpretation of the model comes from a visual plot.
(3a). Separate linear regressions for those with and those without HL.
Linear regression models are fit such that the linear effect of hearing on continuous CESD-10 depends on whether a person has normal hearing or HL. These models were created to summarize the GAM regression via linear models, which are more familiar to most investigators. The model is given CESD– 10 Score = β0 +β1Hearing + β2Group + β3Hearing ∗ Group + ε, where Group = 1 if a subject has HL, or 0 if he/she has normal hearing. The other components are similar to those of model type (1a), above. The threshold between normal hearing and HL was the commonly-employed 25 dB cutoff,20 supported a posteriori from GAM modelling. An alternative, stricter HL cutpoint of 15 dB was also used.31 Testing β3 = 0 allows us to determine if the linear effect of hearing on CESD-10 changes depending on whether the subject has HL. Because our research question specifically asked what the regressions slopes were among those with normal hearing, we planned to present this stratum’s data whether or not the interaction term (β3) is significant.
(3b). Separate logistic regressions for those with and those without HL.
This is the same as (3a), except logistic regression is used along with the binarized CESD-10 outcome.
For each of the 3 regression strategies, we fit univariable models with hearing as the sole predictor. We then fit similar multivariable models, adjusting for age, gender, education, cardiovascular disease score, and hearing aid use. Akaike information criteria (AIC) was used to determine model fits for a given outcome across different regression strategies (lower values are better).
Logistic regression (1b and 3b, above) was used for the binary outcome because the existing literature on the hearing loss-depression association overwhelming uses logistic regression, thus allowing comparisons with results from previous studies.3–6, 32 Since logistic regression does not directly estimate prevalence ratios, and the odds ratio does not approximate the prevalence ratio when the outcome is common (as in our sample), we additionally fit Poisson regression (with a log-link function) models using the same outcome and predictors as the logistic models in a sensitivity analysis.
Estimates are presented with 95% confidence intervals (CI) unless otherwise indicated. Data analysis was performed in R 3.6.0 (R Foundation for Statistical Computing) with RStudio 1.2.1335 (RStudio, Inc, Boston, MA). GAM modeling was performed with the mgcv package.30, 33 Statistical significance was considered at the p<0.05 level (two-tailed).
RESULTS
Baseline Characteristics
Study subject characteristics are detailed in Table 1. There were 5,499 subjects, 4,517 with normal hearing (≤25 dB) and 982 with HL (>25 dB). The mean age was 58.6 years (±6.3 SD). Men comprised 38.5% of subjects. Hearing aid use was only 0.9% overall and 4% among those with HL. Hearing aid use was kept as a model covariate, despite its low use, because it could theoretically have a meaningful effect.
Table 1.
Subject Characteristics Stratified by Hearing Loss Status; Hispanic Community Health Study (HCHS)
| Total | Normal Hearing (≤25 dB) | Hearing Loss (>25 dB) | Test Statistica | DF | p | |
|---|---|---|---|---|---|---|
| No. | 5499 | 4517 | 982 | N/A | N/A | N/A |
| Age, Mean ± SD | 58.6 ± 6.3 | 57.8 ± 5.9 | 62.2 ± 6.7 | t = −19.0 | 1335 | <0.001 |
| Men, No. (%) | 2115 (38.5) | 1599 (35.4) | 516 (52.5) | X2 = 99.5 | 1 | <0.001 |
| Hearing Aid Use, No. (%) | 48 (0.9) | 9 (0.2) | 39 (4.0) | X2 = 128.3 | 1 | <0.001 |
| Education, Years, Mean ± SD | 10.3 ± 4.8 | 10.6 ± 4.7 | 9.1 ± 4.7 | t = 8.8 | 1450 | <0.001 |
| Antidepressant Use, No (%) | 553 (10.1) | 437 (9.7) | 116 (11.8) | X2 = 3.8 | 1 | <0.05 |
| Cardiovascular Disease Scoreb, Mean ± SD | 1.7 ± 1.1 | 1.6 ± 1.1 | 1.9 ± 1.1 | t = −8.3 | 1413 | <0.001 |
| Cardiovascular Disease Score Components, No. (%) | ||||||
| Coronary artery disease | 640 (11.6) | 480 (10.6) | 160 (16.3) | X2 = 24.6 | 1 | <0.001 |
| Hypertension | 2652 (48.2) | 2095 (46.4) | 557 (56.7) | X2 = 34.1 | 1 | <0.001 |
| Stroke | 145 (2.6) | 105 (2.3) | 40 (4.1) | X2 = 8.9 | 1 | <0.01 |
| Diabetes | ||||||
| Impaired glucose tolerance | 2728 (49.6) | 2254 (49.9) | 474 (48.3) | |||
| Diabetes | 1539 (28.0) | 1200 (26.6) | 339 (34.5) | X2 = 33.1 | 2 | <0.001 |
| CESD-10 Score, Mean ± SD | 7.8 ± 6.5 | 7.7 ± 6.4 | 8.2 ± 6.7 | t = −2.1 | 1397 | <0.05 |
| Clinically Significant Depressive Symptoms (CESD-10 ≥ 10), No. (%) | 1836 (33.4) | 1469 (32.5) | 367 (37.4) | X2 = 8.3 | 1 | <0.01 |
| Clinically Significant Depressive Symptoms (CESD-10 ≥ 16), No. (%) | 728 (13.2) | 581 (12.9) | 147 (15.0) | X2 = 2.9 | 1 | 0.09 |
CESD-10 = Center for Epidemiologic Studies Depression Scale-10, HL = hearing loss, SD = standard deviation. DF = degrees of freedom
The test used is indicated by the test statistic. The chi-squared test was used for categorical variables, the t-test was used for continuous variables
Cardiovascular disease score ranges from 0 (lowest) to 5 (highest). Points were assigned for each prevalent component (1 for coronary artery disease, 1 for hypertension, 1 for stroke; 1 for impaired glucose tolerance, 2 for diabetes).
Regression Analyses
(1a). Linear regression.
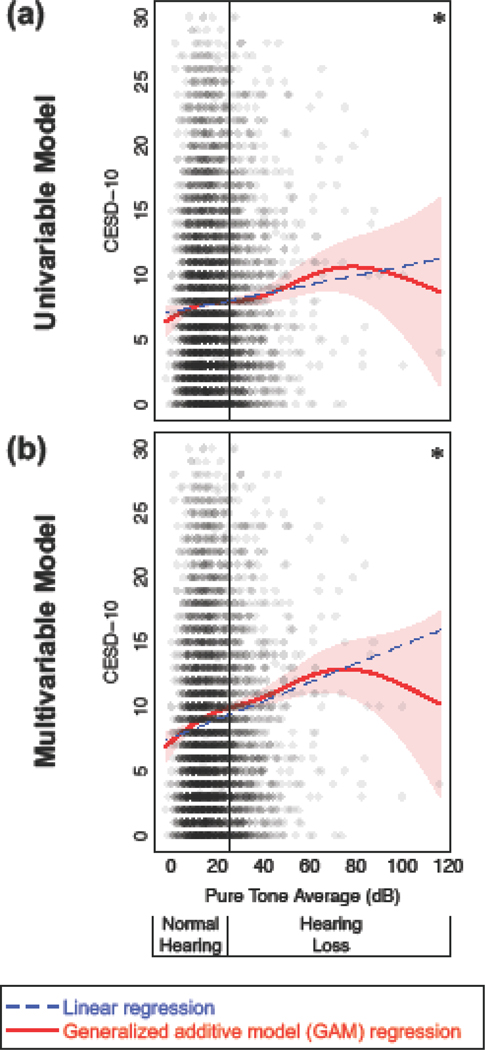
Assumptions of linear regression were assessed and met. In simple, univariable linear regression, depressive symptoms increased as hearing decreased (Figure 2a; Table 2, Model 1). For every 10 dB decrease in hearing, the CESD-10 score increased by 0.35 points (95% CI = 0.19, 0.51). A 10 dB decrease in hearing is approximately equal to a half-category decrease (i.e., across categories of normal, mild, moderate, moderately-severe, severe, and profound HL). Associations were strengthened in multivariable regression, adjusting for confounders (Figure 2b; Table 2, Model 2). For every 10 dB decrease in hearing, the CESD-10 score increased by 0.71 points (0.54, 0.89), adjusting for age, gender, education level, cardiovascular disease, and hearing aid use.
Figure 2.
Depressive symptoms (Center for Epidemiologic Studies Depression Scale, 10-item score; CESD-10) versus hearing assessed with general additive models (GAMs; solid red lines ± 1 pointwise standard error confidence intervals in light red shading). Linear regression models (dashed blue lines) are used for comparison. (a) Univariable models. (b) Multivariable models, adjusting for age, gender, education level, cardiovascular disease, and hearing aid use. *p<0.001 for linear and GAM regression. Normal hearing defined by pure tone average ≤25 dB; hearing loss defined by pure tone average >25 dB.
Table 2.
Regression Models for Depressive Symptoms Based on Hearing Loss; Hispanic Community Health Study (HCHS)
| Linear Regression | Logistic Regression | |||||||
|---|---|---|---|---|---|---|---|---|
| Model | CESD-10 Score Difference Per 10 dB Decrease in Hearing (95% CI) | t | DF | p | OR of Clinically Significant Depressive Symptomsa Per 10 dB Decrease in Hearing (95% CI) | Wald X2 | DF | p |
| 1. Univariable | 0.35 (0.19, 0.51) | 4.30 | 5497 | <0.001 | 1.13 (1.07, 1.19) | 21.8 | 1 | <0.001 |
| 2. Multivariable | 0.71 (0.54, 0.89) | 8.08 | 5492 | <0.001 | 1.24 (1.17, 1.31) | 49.4 | 1 | <0.001 |
| 3. Multivariable, Restricted to Normal Hearing (≤25 dB) | 1.04 (0.70, 1.37) | 6.04 | 4510 | <0.001 | 1.28 (1.14, 1.44) | 17.1 | 1 | <0.001 |
| 4. Multivariable, Restricted to Hearing Loss (>25 dB) | 0.62 (0.23, 1.01) | 3.11 | 975 | <0.001 | 1.26 (1.11, 1.44) | 12.3 | 1 | <0.001 |
| 5. Multivariable, Restricted to Strict Normal Hearing (≤15 dB) | 1.38 (0.67, 2.09) | 3.83 | 2626 | <0.001 | 1.54 (1.20, 1.99) | 11.5 | 1 | <0.001 |
| 6. Multivariable, Restricted to Strict Hearing Loss (>15 dB) | 0.54 (0.29, 0.78) | 4.23 | 2859 | <0.01 | 1.22 (1.12, 1.33) | 21.7 | 1 | <0.01 |
Clinically significant depressive symptoms defined by CESD-10 score ≥ 10
HCHS (n=5,499), HCHS restricted to normal hearing (n=4,517) or HL (n=982), HCHS restricted to strict normal hearing (n=2,633) or strict HL (n=2,866)
Univariable models contain only hearing loss as a predictor. Multivariable models contain hearing loss and adjust for age, gender, education level, cardiovascular disease, and hearing aid use.
(1b). Logistic regression.
Similar findings were noted when we used logistic regression where the outcome, CSDS, was binary. In the univariable model, for every 10 dB decrease in hearing, the odds of CSDS increased 1.13 times (1.07, 1.19). The findings were strengthened in the multivariable model. For every 10 dB decrease in hearing, the odds of CSDS increased 1.24 times (1.17, 1.31), adjusting for age, gender, education level, cardiovascular disease, and hearing aid use.
(2). GAM regression.
Smooth effect curves were created to explore the relationships between hearing and CESD-10 without the constraints of a linear model. As hearing decreased, CESD-10 increased, both for the univariable (Figure 2a) and multivariable (Figure 2b) model. In the multivariable model, the relationship was stronger. All relationships were significant at p<0.001 (the smooth effect curve was non-constant).
Notably the relationship appeared as strong, or possibly stronger, among those with normal hearing compared to those with HL. Furthermore, the 95% confidence interval for the GAM regression did not overlap the linear regression line for the majority of subjects with normal hearing. Akaike information criterion (AIC) values indicated improved goodness-of-fit in GAM models versus linear regression models (Supplemental Table 1).
(3a). Separate linear regressions for those with and those without HL.
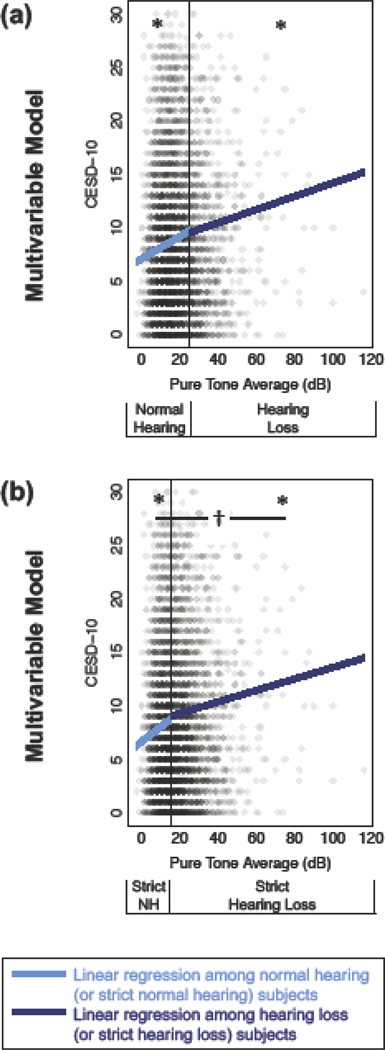
Because the GAM regression illustrated a relationship between hearing and depressive symptoms among those with normal hearing, we decided to examine this group specifically. Two separate groups of multivariable linear regression models were created: among those with normal hearing (≤25 dB) and those with HL (>25 dB). In both groups, the adjusted relationship between hearing and depressive symptoms was significant (p<0.01) (Figure 3; Table 2, Models 3 and 4)
Figure 3.
Depressive symptoms (Center for Epidemiologic Studies Depression Scale, 10-item score; CESD-10) versus hearing assessed with separate multivariable linear regressions among normal hearing (light blue) or hearing loss (dark blue) subjects. All models are multivariable and adjust for age, gender, education level, cardiovascular disease, and hearing aid use. (a) Classic definition of normal hearing (≤25 dB) and hearing loss (>25 dB). (b) Strict definition of normal hearing (≤15 dB) and hearing loss (>15 dB). *p<0.05 for a hearing stratum. †p<0.05 for a difference between the two hearing strata.
Among those with normal hearing, the CESD-10 score increased by 1.04 points (0.70, 1.37) for every 10 dB decrease in hearing, adjusting for the same confounders. Among those with HL, the CESD-10 score increased by 0.62 point (0.23, 1.01) for every 10 dB decrease in hearing, adjusting for confounders. (Figure 3a; Table 2, Models 3 and 4)
Significant differences of the adjusted relationship between hearing and depressive symptoms between stratified groups (i.e., normal hearing vs HL) were then tested by creating a single multivariable regression model with an interaction term between continuous and binary hearing. There was no significant difference between normal hearing and HL (interaction term p=0.09)
Because the relationship between hearing and depressive symptoms was consistently, and unexpectedly, seen among those with normal hearing, we hypothesized that the cutpoint for defining normal hearing was not strict enough. The analysis was then performed again using a more stringent cutpoint of 15 dB31 to create groups of strict normal hearing (PTA ≤15 dB) and strict HL (PTA >15 dB). Among those with strict normal hearing, the CESD-10 score increased by 1.38 points (0.67, 2.09) for every 10 dB decrease in hearing. Among those with strict HL, the CESD-10 score increased by 0.54 point (0.29, 0.78) for every 10 dB decrease in hearing. (Figure 3b; Table 2, Models 5 and 6)
Significant differences of the adjusted hearing-depressive symptoms relationship between stratified strict groups (i.e., strict normal vs strict HL) were then tested by through a single multivariable regression model with an interaction term between continuous and binary hearing. There was a significantly stronger relationship between hearing and depressive symptoms among those with strict normal hearing compare to those with strict HL (interaction term p=0.03).
(3b). Separate logistic regressions for those with and those without HL.
Similar findings were noted when we used logistic regression, where the outcome, CSDS, was binary. Among those with normal hearing, the odds of CSDS increased 1.28 times (1.14, 1.44) for every 10 dB decrease in hearing. Among those with HL, the odds increased 1.26 (1.11, 1.44) for every 10 dB decrease in hearing. (Table 2, Models 3 and 4). There was no significant difference in associations between normal hearing and HL (interaction term p=0.96).
The analysis was then performed again using the more stringent cutpoint of 15 dB31 to create groups of strict normal hearing (PTA ≤15 dB) and strict HL (PTA >15 dB). Similar to the linear regression analysis, the association was strengthened in logistic regression. Among those with strict normal hearing, the odds of CSDS increased 1.54 times (1.20, 1.99) for every 10 dB decrease in hearing. Among those with HL, the odds increased 1.22 times (1.12, 1.33) for every 10 dB decrease in hearing. (Table 2, Models 5 and 6) There was no significant difference in associations between strict normal hearing and strict HL (interaction term p=0.15).
Sensitivity Analyses
In a sensitivity analysis, we used alternative definitions to define binary depressive symptoms. First, we used an alternative definition for CSDS of CESD-10 ≥ 16.26 The relationship between hearing and CSDS remained significant among those with both normal hearing and strict normal hearing. Second, we defined depressive symptoms by use of antidepressants (yes/no). Similar findings were noted. (Supplemental Table 2) For both of these alternative outcomes, the relationship was significantly stronger among those with normal hearing (regardless of the cutpoint) than HL (interaction terms p<0.05).34
In an additional sensitivity analysis, C-reactive protein, a measure of inflammation, was added to the main models as a potential confounder. The hearing-depressive symptoms relationship was minimally attenuated (e.g., coefficient change from 1.04 [0.70, 1.37] to 1.03 [0.70, 1.37] among those with normal hearing.)
Finally, Poisson regression (with a log-link function) was performed ins order to directly estimate prevalence ratios. In the fully adjusted model, the prevalence of clinically significant depressive symptoms increased 1.17 (1.07, 1.27) times for every 10 dB decrease in hearing among those with normal hearing compared to 1.12 (1.03, 1.21) times among those with HL. (Supplemental Table 3)
DISCUSSION
An association was seen between worse hearing and higher depressive symptoms among those with normal hearing, as traditionally defined by a pure tone average ≤25 dB, even after adjustment for various confounders, including age, gender, education, cardiovascular disease, and hearing aid use. We observed similar findings regardless of whether depressive symptoms were defined continuously or binarily as CSDS. This follows a recent parallel finding that worse hearing is associated with worse cognition among those with normal hearing.18
Some have proposed stricter definitions of HL in adults. In children, 20 dB is often employed. This suggests an unequitable double standard whereby hearing is valued more in children than it is in adults. In adults, 15 dB has rarely been used.17, 31 We performed the analysis using a stricter HL cutoff of 15 dB, yet this cutoff only strengthened the previously observed patterns. In fact, the relationship between hearing and depressive symptoms (defined continuously) was stronger among those with strict HL than those with strict normal hearing.
Biologically, hearing is a continuum and there is no absolute threshold defining normal and abnormal. The value of 0 dB hearing level is artificially set based on historic, normative data from organizations such as the American National Standards Institute (ANSI).17 Technically, any value above this (>0 dB) is worse, on average, than these normative data. In clinical practice, however, more lenient cutoffs are used so that half the population is not labeled with HL. We propose the term subclinical HL, defined as imperfect hearing (>0 dB) but within the currently accepted normal range (≤25 dB for adults). For clinical purposes, it may be prudent to recommend a more patient-friendly term for the upper range of subclinical HL, such as borderline HL, for 16–25 dB. The term borderline is sometimes used today among children, so it would be familiar.17 Future research is needed to validate the use of these terms.
To our knowledge, no prior study has looked at whether the association between HL and depressive symptoms is present among those with normal hearing. The majority of studies observing an association between hearing and depression (or depressive symptoms) have used crude, subjective hearing measures,35–37 which are inadequately sensitive and subject to bias.34 Studies that included objective audiometry usually categorize HL, using the entire normal category as a reference.4, 6, 38, 39 Studies looking at audiometric hearing continuously are rare and have not specifically examined the relationship among those with normal hearing.6
The observed relationships between HL and depressive symptoms were clinically meaningful. Changes in outcomes were expressed in terms of a relatively small 10 dB unit decrease in hearing, which is approximately a half-category hearing drop (where categories are normal, mild, moderate, moderately-severe, severe, and profound). It may be more intuitive to think of results in terms of a change in hearing from perfect (0 dB) to the low range of normal (25 dB). Thus, the CESD-10 score dropped approximately 2.6 points (95% CI = 1.8, 3.4) as hearing decreased from perfect to the low range of normal, adjusting for potential confounders. Similarly, the odds of clinically significant depressive symptoms nearly doubled (OR = 1.9; 95% CI = 1.4, 2.5) as hearing decreased from perfect to the low range of normal.
The relationships between HL and depressive symptoms could be explained by confounders or mediators. Confounders cause both HL and depressive symptoms, refuting a causal pathway between HL and depressive symptoms. In contrast, mediators would act as an intermediary on a causal pathway between hearing and depressive symptoms. A number of key confounders were adjusted for in this study, including age, sex, education, cardiovascular disease, and inflammation. Socialization and impaired cognition may act as a mediators of a mechanistic relationship between hearing and depressive symptoms. Decreased hearing might increase social isolation40, 41 and loneliness42, 43 which, in turn, might increase the risk of depressive symptoms. Encouragingly, the pathway may be modifiable as loneliness has improved with certain treatments for HL.42, 44 In one randomized controlled trial, hearing aids improved both social function and depressive symptoms.45 Decreased hearing might lead to impaired cognition, which, in turn, could lead to depressive symptoms.6
Reverse causation, where depressive symptoms cause HL, cannot be definitively ruled out in this cross-sectional design. For example, depressed individuals may be less likely to protect against noise exposure, which can result in noise-induced HL. However, we rarely observed HL centered at 4000 Hz, the hallmark of noise damage. In addition, subjects with depressive symptoms may concentrate less during hearing testing, resulting in worse scores. While impossible to fully discount, test examiners were certified in conducting audiometry, which includes checking for reliability and consistency. Audiometry has a test-retest reliability of nearly 99%.46
Why would the relationship between hearing and depressive symptoms be present among those with normal hearing? Perhaps hearing should not be thought of as a disability with a cutpoint below which there is a deleterious effect. Rather, hearing may be an ability, where more is simply better. If healthy socialization protects against depressive symptoms, then any biologic advantage that enhances socialization may reduce the risk of depressive symptoms. Hearing well, or even better than average, may therefore confer extra advantage. For example, many normal hearing individuals have difficulty conversing in noisy restaurants. Those with superior hearing may be at a unique advantage and have, on average, more meaningful social connections than those who struggle to follow conversations. Moreover, those who have greater ease communicating in social settings may tend to seek out these settings in the future, creating a positive loop. Subclinical HL has also been recently association with impaired cognition.21 It is possible that superior hearing facilitates social engagement, which in turn promotes cognitively stimulating input, thereby improving cognitive function, which in turn reduces the risk for depressive symptoms.
One could also speculate on an analogy between HL and hypertension. Hypertension used to be considered physiologic and was rarely treated.47 Over time, hypertension was defined as pathologic and treated at relatively high levels. The definition has since become stricter. Today, a blood pressure of 120/80 is considered high.48, 49 These changes resulted from sequential evidence showing a benefit of increasingly aggressive hypertension treatment. This level of evidence is lacking for HL and illustrates the need for randomized controlled trials.
In a sensitivity analysis, the relationship between hearing and alternative binary definitions of depressive symptoms held or became stronger among those with normal hearing. The relationship between hearing and CSDS defined by an alternative CESD-10 cutpoint of 16 was stronger among those with normal hearing than those with HL. The same was noted when depressive symptoms were defined by antidepressant use.
This study has limitations. Analysis was cross-sectional, which does not allow causal inference. While other studies have shown that HL predicts later depressive symptoms,50 we cannot show this temporality. It is possible that early declines in both hearing and mood are related to common aging-related processes. We adjusted for major potential confounders. However, it is possible that unmeasured or unknown factors confound the relationship between hearing and depressive symptoms. Because HCHS is community-based, few individuals had severe to profound HL, resulting in greater uncertainty among these individuals in regression models. Oversampling these individuals could be considered in future epidemiologic studies.
This study has strengths. We used a large, multicentered national study with a high-quality audiometric hearing measure, rare among studies of hearing and depressive symptoms (or depression).51 We also studied Hispanics, a group that despite growing in the US52 has historically been neglected in research. Results were robust to four different outcomes, two types of hearing categorization, and several types of regression. This includes GAM regression, which does not assume any particular (for example, linear) relationship between hearing and depressive symptoms.
In conclusion, decreasing hearing was independently associated with CSDS among adults with subclinical HL. The effect of hearing on depressive symptom risk may begin earlier than previously recognized. Future studies examining whether treating HL can reduce depressive symptoms should consider a lower threshold for defining HL.
Supplementary Material
HIGHLIGHTS.
It is unknown whether the association between hearing and depressive symptoms already exists among older adults classified as having normal hearing.
Worse hearing was associated with worse depressive symptoms among those classified as having normal hearing, despite adjusting for confounders.
The relationship between age-related hearing loss and depressive symptoms is present at an earlier stage of hearing loss than previously recognized.
Acknowledgements:
The authors thank Nicholas S. Reed, AuD, Jay T. Rubinstein, MD, PhD, Ward R. Drennan PhD, Ilana Cellum, AuD, Jessica Galatioto, AuD, and Megan Kuhlmey, AuD for their insight on the definition of 0 dB hearing level as well as Daichi Shimbo, MD, for his discussion on parallels to hypertension.
Grant Support: Collaborative and Multidisciplinary Pilot Research Awards, Phase I and II (Columbia University Irving Institute for Clinical and Translational Research, JSG, KKB, AMB, AHK, BRR); National Institute on Aging K24AG045334 (JAL), K23AG057832, L30AG060513 (JSG).
Footnotes
Conflicts of Interest/Financial Disclosures: Justin S. Golub: travel expenses for industry-sponsored meetings (Cochlear, Advanced Bionics, Oticon Medical), consulting fees or honoraria (Oticon Medical, Auditory Insight, Optinose, Abbott, Decibel Therapeutics), department received unrestricted educational grants (Storz, Stryker, Acclarent, 3NT, Decibel Therapeutics). Ana H. Kim: industry-sponsored grant (Advanced Bionics). José A Luchsinger: editor-in-chief stipend (Alzheimer’s Disease & Associated Disorders) consulting fees (vTv Therapeutics)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Collins JG: Prevalence of selected chronic conditions: United States, 1990–1992. Vital Health Stat 10 1997; 1–89 [PubMed] [Google Scholar]
- 2.Goman AM,Lin FR: Prevalence of Hearing Loss by Severity in the United States. Am J Public Health 2016; 106:1820–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gopinath B, Wang JJ, Schneider J, et al. : Depressive symptoms in older adults with hearing impairments: the Blue Mountains Study. J Am Geriatr Soc 2009; 57:1306–1308 [DOI] [PubMed] [Google Scholar]
- 4.Lee AT, Tong MC, Yuen KC, et al. : Hearing impairment and depressive symptoms in an older chinese population. J Otolaryngol Head Neck Surg 2010; 39:498–503 [PubMed] [Google Scholar]
- 5.Mener DJ, Betz J, Genther DJ, et al. : Hearing loss and depression in older adults. J Am Geriatr Soc 2013; 61:1627–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golub JS, Brewster KK, Brickman AM, et al. : Association of Audiometric Age-Related Hearing Loss With Depressive Symptoms Among Hispanic Individuals. JAMA Otolaryngol Head Neck Surg 2019; 145:132–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deal JA, Betz J, Yaffe K, et al. : Hearing Impairment and Incident Dementia and Cognitive Decline in Older Adults: The Health ABC Study. J Gerontol A Biol Sci Med Sci 2017; 72:703–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golub JS: Brain changes associated with age-related hearing loss. Curr Opin Otolaryngol Head Neck Surg 2017; 25:347–352 [DOI] [PubMed] [Google Scholar]
- 9.Golub JS, Brickman AM, Ciarleglio AJ, et al. : Audiometric Age-Related Hearing Loss and Cognition in the Hispanic Community Health Study. J Gerontol A Biol Sci Med Sci 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis A, Smith P, Ferguson M, et al. : Acceptability, benefit and costs of early screening for hearing disability: a study of potential screening tests and models. Health Technol Assess 2007; 11:1–294 [DOI] [PubMed] [Google Scholar]
- 11.Chien W,Lin FR: Prevalence of hearing aid use among older adults in the United States. Arch Intern Med 2012; 172:292–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brewster KK, Stein A,AP, et al. : Hearing aids and depression: a methods study, Atlatna, GA, 2019 [Google Scholar]
- 13.Livingston G, Sommerlad A, Orgeta V, et al. : Dementia prevention, intervention, and care. The Lancet 2017; [DOI] [PubMed] [Google Scholar]
- 14.Warren E,Grassley C: Over-the-Counter Hearing Aids: The Path Forward. JAMA Intern Med 2017; 177:609–610 [DOI] [PubMed] [Google Scholar]
- 15.Martin FN,Champlin CA: Reconsidering the limits of normal hearing. J Am Acad Audiol 2000; 11:64–66 [PubMed] [Google Scholar]
- 16.Era P, Jokela J, Qvarnberg Y, et al. : Pure-tone thresholds, speech understanding, and their correlates in samples of men of different ages. Audiology 1986; 25:338–352 [DOI] [PubMed] [Google Scholar]
- 17.in Otolaryngology-Head & Neck Surgery: Clinical Reference Guide. Edited by Pasha R,Golub JS. San Diego, Plural Publishing, 2018, pp 355 [Google Scholar]
- 18.Golub JS, Brickman AM, Ciarleglio A, et al. : Association of subclinical hearing loss with cognitive performance. JAMA Otolaryngol Head Neck Surg 2019; 146:57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loughrey DG, Kelly ME, Kelley GA, et al. : Association of Age-Related Hearing Loss With Cognitive Function, Cognitive Impairment, and Dementia. JAMA Otolaryngology–Head & Neck Surgery 2018; 144: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO. World Health Organization (WHO). Grades of Hearing Impairment. Accessed January 14, 2019 Available http://www.who.int/pbd/deafness/hearing_impairment_grades/en/.
- 21.Golub JS, Brickman AM, Ciarleglio AJ, et al. : Association of Subclinical Hearing Loss With Cognitive Performance. JAMA Otolaryngol Head Neck Surg 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radloff LS,Rae DS: Susceptibility and precipitating factors in depression: sex differences and similarities. J Abnorm Psychol 1979; 88:174–181 [DOI] [PubMed] [Google Scholar]
- 23.Brown PJ, Roose SP, Fieo R, et al. : Frailty and depression in older adults: a high-risk clinical population. Am J Geriatr Psychiatry 2014; 22:1083–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meeks TW, Vahia IV, Lavretsky H, et al. : A tune in “a minor” can “b major”: a review of epidemiology, illness course, and public health implications of subthreshold depression in older adults. J Affect Disord 2011; 129:126–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andresen EM, Malmgren JA, Carter WB, et al. : Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med 1994; 10:77–84 [PubMed] [Google Scholar]
- 26.Bjorgvinsson T, Kertz SJ, Bigda-Peyton JS, et al. : Psychometric properties of the CES-D-10 in a psychiatric sample. Assessment 2013; 20:429–436 [DOI] [PubMed] [Google Scholar]
- 27.Luchsinger JA, Reitz C, Honig LS, et al. : Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology 2005; 65:545–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.HCHS/SOL: Derived Variable Dictionary. 2014; 68 [Google Scholar]
- 29.Hastie T,Tibshirani R: Generalized additive models, New York NY, Chapman and Hall, 1990 [Google Scholar]
- 30.Wood SN: Generalized Additive Models: An Introduction with R, Boca Raton, FL, Chapman and Hall/CRC, 2006 [Google Scholar]
- 31.ASHA. Degrees of Hearing Loss. https://www.asha.org/public/hearing/degree-of-hearing-loss/
- 32.Contrera KJ, Betz J, Deal JA, et al. : Association of Hearing Impairment and Emotional Vitality in Older Adults. J Gerontol B Psychol Sci Soc Sci 2016; 71:400–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wood S mgcv: Mixed GAM Computation Vehicle with Automatic Smoothness Estimation. 2019. https://cran.r-project.org/web/packages/mgcv [Google Scholar]
- 34.Choi JE, Moon IJ, Baek SY, et al. : Discrepancies between self-reported hearing difficulty and hearing loss diagnosed by audiometry: prevalence and associated factors in a national survey. BMJ Open 2019; 9:e022440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cacciatore F, Napoli C, Abete P, et al. : Quality of life determinants and hearing function in an elderly population: Osservatorio Geriatrico Campano Study Group. Gerontology 1999; 45:323–328 [DOI] [PubMed] [Google Scholar]
- 36.Han JH, Lee HJ, Jung J, et al. : Effects of self-reported hearing or vision impairment on depressive symptoms: a population-based longitudinal study. Epidemiol Psychiatr Sci 2018; 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu A,Liljas AEM: The relationship between self-reported sensory impairments and psychosocial health in older adults: a 4-year follow-up study using the English Longitudinal Study of Ageing. Public Health 2019; 169:140–148 [DOI] [PubMed] [Google Scholar]
- 38.Lisan Q, van Sloten TT, Lemogne C, et al. : Association of Hearing Impairment with Incident Depressive Symptoms: A Community-Based Prospective Study. Am J Med 2019; [DOI] [PubMed] [Google Scholar]
- 39.Kim SY, Kim HJ, Park EK, et al. : Severe hearing impairment and risk of depression: A national cohort study. PLoS One 2017; 12:e0179973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoo M, Kim S, Kim BS, et al. : Moderate hearing loss is related with social frailty in a community-dwelling older adults: The Korean Frailty and Aging Cohort Study (KFACS). Arch Gerontol Geriatr 2019; 83:126–130 [DOI] [PubMed] [Google Scholar]
- 41.Resnick HE, Fries BE,Verbrugge LM: Windows to their world: the effect of sensory impairments on social engagement and activity time in nursing home residents. J Gerontol B Psychol Sci Soc Sci 1997; 52:S135–144 [DOI] [PubMed] [Google Scholar]
- 42.Contrera KJ, Sung YK, Betz J, et al. : Change in loneliness after intervention with cochlear implants or hearing aids. Laryngoscope 2017; 127:1885–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sung YK, Li L, Blake C, et al. : Association of Hearing Loss and Loneliness in Older Adults. J Aging Health 2015; [DOI] [PubMed] [Google Scholar]
- 44.Weinstein BE, Sirow LW,Moser S: Relating Hearing Aid Use to Social and Emotional Loneliness in Older Adults. Am J Audiol 2016; 25:54–61 [DOI] [PubMed] [Google Scholar]
- 45.Mulrow CD, Aguilar C, Endicott JE, et al. : Quality-of-life changes and hearing impairment. A randomized trial. Ann Intern Med 1990; 113:188–194 [DOI] [PubMed] [Google Scholar]
- 46.Schmuziger N, Probst R,Smurzynski J: Test-Retest Reliability of Pure-Tone Thresholds from 0.5 to 16 kHz using Sennheiser HDA 200 and Etymotic Research ER-2 Earphones. Ear and Hearing 2004; 25:127–132 [DOI] [PubMed] [Google Scholar]
- 47.Saklayen MG,Deshpande NV: Timeline of History of Hypertension Treatment. Front Cardiovasc Med 2016; 3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Group SR, Wright JT Jr., Williamson JD, et al. : A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med 2015; 373:2103–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whelton PK, Carey RM, Aronow WS, et al. : 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018; 138:e426–e483 [DOI] [PubMed] [Google Scholar]
- 50.Brewster KK, Ciarleglio A, Brown PJ, et al. : Age-Related Hearing Loss and Its Association with Depression in Later Life. Am J Geriatr Psychiatry 2018; 26:788–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lawrence BJ, Jayakody DMP, Bennett RJ, et al. : Hearing Loss and Depression in Older Adults: A Systematic Review and Meta-analysis. Gerontologist 2019; [DOI] [PubMed] [Google Scholar]
- 52.Flores A How the U.S. Hispanic population is changing. 2017. http://www.pewresearch.org/fact-tank/2017/09/18/how-the-u-s-hispanic-population-is-changing/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.