Editor—The mainstay of treatment for severe bilateral pneumonia and acute respiratory distress syndrome (ARDS) caused by coronavirus disease 2019 (COVID-19) is positive-pressure mechanical ventilator support.1 However, ventilator shortages have occurred owing to overwhelming patient volumes coupled with prolonged durations of ventilator dependence.2, 3, 4 Potential solutions include bag-valve-mask ventilators and supporting two or more patients per ventilator (ventilator splitting).
The proof of concept of ventilator splitting was first demonstrated in 2006 by Neyman and Irvin5 as a method of ventilating multiple simulated lungs with a single ventilator. Subsequently, this strategy was validated on four sheep in 2008 for 12 h, and on two awake humans for 10 min.6 , 7 However, prolonged support of multiple patients per ventilator is challenging because of the inability to compensate for variability in patient size and pulmonary compliance, which can also vary over the course of the disease.8
COVID-19 patients typically require 10 days of mechanical ventilatory support, and the inability to individualise tidal volume during this time could lead to hyper- or hypoventilation. Therefore, numerous national societies have warned against splitting ventilators to support multiple patients.9 In order to safely ventilate multiple patients, systems must allow individualised control of patients' tidal volumes and ensure changes in one patient do not affect the other. We present a solution affording patient-specific peak inspiratory pressure (PIP) adjustment for multiple patients using a single ventilator. During the preparation of this manuscript, we became aware of the Pressure-Regulated Ventilator Splitting (PReVentS) group solution,10 which tackles some of these issues; similarities and differences are discussed.
In our design, an adjustable fixed-pressure regulator was added at the inspiratory limb of each simulated patient's breathing circuit. Critically, the regulators have adjustable diaphragms set relative to atmospheric pressure such that the pressure for each patient is fixed; adjustments to inspiratory pressure on the ventilator do not affect delivered PIP. Thus, airway pressures (and consequently tidal volumes) are modulated for each patient independently of one another. Both Dräger Apollo (Draeger Medical Inc., Telford, PA, USA) and Medtronic Puritan-Bennett 840 (Minneapolis, MN, United States) ventilators were used for testing; the data presented are from a Dräger Apollo (Draeger Medical Inc.).
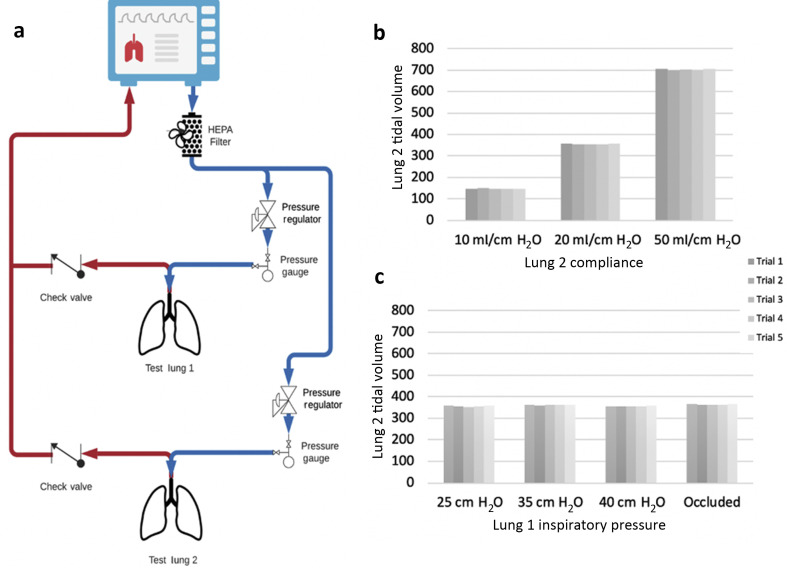
The ventilators were separately attached to two lung simulators using Y-pieces to split standard 60-inch ventilator circuits into parallel inspiratory configurations (Fig. 1 a). The PIP of each simulated lung was controlled by a 4116ANNKE Pneumatic Precision Low Pressure Regulator (Fairchild Industrial Products Company,Winston-Salem, NC, USA), and a pressure gauge was attached to the end of each inspiratory limb upstream of each simulated patient. This pressure regulator has a sensitivity of 0.127 cm H2O control. Connectors to the pressure regulator (at the inlet and outlet) were 3D-printed with biocompatible Stratasys MED610 (Stratasys, Rehovot, Israel). Inspiratory HEPA filters (SafeStar 55, Draeger Medical Inc., Telford, PA, USA) were placed proximal to the lungs to eliminate potential mixing of contaminated expiratory gas between patients. The ventilator was programmed in pressure-control mode with an inspiratory pressure of 40–50 mm Hg to ensure that sufficient pressures entered each regulator. Inspiratory time was set at 1 s and PEEP of 5 cm H2O. A Respironics NICO2 (model 7600; Respironics Inc., Murrysville, PA, USA) in-line sampler was used to obtain lung volume and pressure measurements. The test lungs (Ingmar Medical Quick Lung; Ingmar Medical, Pittsburgh, PA, USA) were set to a compliance of 20 mL cm H2O−1 during.
Fig. 1.
(a) Circuit regulating inspiratory pressure to individualise tidal volumes in a dual-patient, one ventilator system (MSK Octopus). Tidal volume of lung 2 when varying (b) lung 2 compliance and (c) lung 1 inspiratory pressure, as measured by Respironics NICO2.
To ensure safety for a dual-patient system, it is necessary to verify that (a) the regulator settings are maintained under different operating conditions within one circuit and (b) changes in one lung circuit do not affect the other. To verify (a), we measured PIP as the same-circuit lung compliance was varied. To verify (b), we sampled lung pressure and tidal volume in one lung circuit while varying the pressure in the other circuit. In both tests, lung 2 parameters were measured. For (a), we varied the compliance of lung 2 with a fixed regulator PIP setting of 25 cm H2O. As compliance was reduced in the test lung, the observed pressure remained fixed at 25 cm H2O, whereas the tidal volume decreased accordingly. Figure 1b shows the observed tidal volumes as a function of compliance. For (b), lung 2 PIP was observed as we varied the PIP of lung 1. Figure 1c demonstrates that changing the inspiratory pressure conditions for lung 1 did not significantly change the tidal volume for lung 2. This was true even when lung 1 was completely and suddenly occluded, simulating cough or bronchospasm.
Using two lung simulators placed in parallel as described, we were able to adjust two inspiratory pressures independently and achieve physiologic tidal volumes in multiple model lungs of different compliances and sizes. Allowing the inspiratory pressure to be independently modified may permit two or more patients to be connected to a single ventilator without depending on matching of patients' lung compliances and sizes. By controlling the pressure in each circuit, appropriate tidal volumes can be achieved, whereas lung compliance varies between patients, changes over time, or both (e.g. with developing acute respiratory distress syndrome).
We have not used this circuit on patients. We recommend that patients be sedated and paralysed for utilisation of this method. In addition, we only tested the adjustment and regulation of inspiratory pressure in relation to tidal volume. Although individualised PEEP was not the focus of this test, expiratory limb 4116ANNKE regulators could achieve individual PEEP control. The PReVentS preprint discloses pressure-regulating valves on both the inspiratory and expiratory limbs to adjust PIP and PEEP. In their system, a pressure difference within the circuit will increase and decrease with varying input pressures. However, it may be advantageous to use regulators that allow a fixed absolute pressure setting relative to atmosphere, which does not change as other parameters are modified. Post-market modification of valves from AMBU bags (AMBU Inc, Columbia, MD, USA) may be more susceptible to failure during use compared with our use of commercial pressure regulators (produced under ISO standards).
Our data do not cover the full range of clinical parameters. For our studies, inspiratory times were kept fixed, although in actual patients, inspiratory times may be intermittently adjusted. Furthermore, this scheme is not intended as a permanent solution for ventilating multiple patients, and should be used only with hospital administration approval and acknowledgement of the unique ethical considerations during a crisis (such as the COVID-19 pandemic).11 , 12 Although the COVID-19 pandemic inspired our designs, it may have utility in other mass casualty scenarios such as natural disasters, terrorist attacks, and battlefield medicine. Future versions should aim to extend to more than two patients per ventilator.
Declarations of interest
GWF is a consultant for and on the speaker's bureau of Edwards LifeSciences. All other authors have no conflicts to declare.
Funding
US National Cancer Institute Cancer Center Support Grant P30CA008748 (partial support).
References
- 1.Sorbello M., El-Boghdadly K., Di Giacinto I., et al. The Italian coronavirus disease 2019 outbreak: recommendations from clinical practice. Anaesthesia. 2020;75:724–732. doi: 10.1111/anae.15049. [DOI] [PubMed] [Google Scholar]
- 2.Bhatraju P.K., Ghassemieh B.J., Nichols M., et al. Covid-19 in critically ill patients in the Seattle region — case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J Med Virol. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neyman G., Irvin C.B. A single ventilator for multiple simulated patients to meet disaster surge. Acad Emerg Med. 2006;13:1246–1249. doi: 10.1197/j.aem.2006.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paladino L., Silverberg M., Charchaflieh J.G., et al. Increasing ventilator surge capacity in disasters: ventilation of four adult-human-sized sheep on a single ventilator with a modified circuit. Resuscitation. 2008;77:121–126. doi: 10.1016/j.resuscitation.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Smith R., Brown J.M. Simultaneous ventilation of two healthy subjects with a single ventilator. Resuscitation. 2009;80:1087. doi: 10.1016/j.resuscitation.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Branson R.D., Blakeman T.C., Robinson B.R., Johannigman J.A. Use of a single ventilator to support 4 patients: laboratory evaluation of a limited concept. Respir Care. 2012;57:399–403. doi: 10.4187/respcare.01236. [DOI] [PubMed] [Google Scholar]
- 9.Society of Critical Care Medicine . American Society of Anesthesiologists; March 26, 2020. American association for respiratory care.https://www.sccm.org/getattachment/Disaster/Joint-Statement-on-Multiple-Patients-Per-Ventilato/Joint-Statement-Patients-Single-Ventilator.pdf?lang=en-US Anesthesia Patient Safety Foundation, American Association of Critical-Care Nurses, American College of Chest Physicians. Joint Statement on Multiple Patients Per Ventilator. Available from: [Google Scholar]
- 10.Niklason L., Raredon M.S.B., Fisher C. 2020. Pressure-regulated ventilator splitting (PReVentS) – a COVID-19 response paradigm from Yale University.www.medrxiv.org/content/10.1101/2020.04.03.20052217v1.full.pdf Available from: [Google Scholar]
- 11.Laffey J.G., Chikhani M., Bates D.G., Hardman J.G. Supporting more than one patient with a single mechanical ventilator: useful last resort or unjustifiable risk? Br J Anaesth Adv. 2020 doi: 10.1016/j.bja.2020.05.029. Access published on June 1, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook D.C. Implementing shared ventilation must be scientific and ethical, or it risks harm. Br J Anaesth. 2020;125:e181–e183. doi: 10.1016/j.bja.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]