Abstract
This study aims to evaluate the impacts of COVID-19 on cognitive functions in recovered patients and its relationship with inflammatory profiles. Twenty-nine patients recovered from COVID-19 as confirmed by negative nucleic tests for two consecutive times were recruited. A total of 29 age-, gender- and education-matched healthy controls were also recruited. The cognitive functions of all subjects were evaluated by the iPad-based online neuropsychological tests, including the Trail Making Test (TMT), Sign Coding Test (SCT), Continuous Performance Test (CPT), and Digital Span Test (DST). Blood samples from all patients were collected for examining inflammatory profiles, including interleukin-2 (IL-2), IL-4, IL-6, IL-10, tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), and C-reactive protein (CRP). The relationship between cognitive functions and inflammatory profiles were analyzed by Pearson correlation. In results, although no significant differences were found in TMT, SCT, and DST between the two groups, patients with COVID-19 scored lower in the correct number of the second and third parts of CPT, they also scored higher in the missing number of the third part of CPT (all P < 0.05). In patients with COVID-19, there was a trend of significant difference for lower reaction time in the first and second parts of CPT (P = 0.050, and 0.051, respectively), as well as the lower correct number of the second part of CPT (P = 0.050). Correlation analysis showed that the reaction time for the first and second parts of CPT was positively correlated with the CRP levels (r = 0.557 and 0.410, P < 0.05). In conclusion, our findings indicated that cognitive impairments exist even in patients recovered from COVID-19, and might be possibly linked to the underlying inflammatory processes.
Keywords: COVID-19, Cognitive function, Inflammation, CRP
1. Introduction
Since December 2019, a novel coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), has emerged in Wuhan, Hubei Province, China. The outbreak of this infection caused by SARS-CoV-2 was officially named as Coronavirus Disease 2019 (COVID-19) by the World Health Organization (WHO) (Lai et al., 2020). Plenty of studies have suggested human-to-human transmission of SARS-CoV-2 through droplets or direct contact (Carlos et al., 2020; Chang et al., 2020; Wang et al., 2020). Due to the SARS-CoV-2 infection surges in the public and associated substantial burdens worldwide, the WHO declared a global public health emergency (Liu et al., 2020). Almost all medical and social resources in China were devoted to mitigating the public health crisis. Although the epidemiological and clinical features of COVID-19 patients are currently well characterized, little attention has been paid to the psychological impact of SARS-CoV-2, such as cognitive function.
Public health emergencies, including influenza A (H1N1), Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS), are likely to cause adverse neuropsychiatric impacts. It has been reported that most of SARS patients had the common complaints, such as poor concentration, declined memory, and insomnia, as well as anxiety and depression symptoms, indicating cognitive impairments after SARS infection (Sheng et al., 2005a). These psychiatric morbidities might be still significant even when patients infected with SARS recovered physically (Tsang et al., 2004). Several clinical symptoms of COVID-19 patients were similar with SARS and MERS (Yap et al., 2018). Interestingly, a recent study has reported that COVID-19 patients might also suffer from anxiety, fear, and other mental health problems (Xu et al., 2020). Moreover, fever, cough, fatigue and gastrointestinal symptoms might worsen psychological distress as well (Zhang et al., 2020). In patients with depression or anxiety disorder, the clinical manifestations were associated with cognitive deficits as well (Culpepper et al., 2017). However, it remains unknown whether the SARS-CoV-2 infection is associated with cognitive dysfunction.
It has been revealed that inflammatory cytokines and CRP played an important role in the development of SARS manifestations (Sheng et al., 2005b). Moreover, inflammation may persistently exist after viral clearance (Peiris et al., 2003). As expected, SARS-CoV-2 particles spread through the respiratory mucosa and infected other cells, causing changes in peripheral immune cells and eliciting a cascade of immune responses, finally resulting a detrimental cytokine storm (Chen et al., 2020). Recent evidence has suggested that clinical symptoms were associated with inflammatory factor storms and elevated CRP levels in COVID-19 patients (Huang et al., 2020). Plenty of studies have also implied that inflammation activation is inextricably linked to cognitive dysfunction (Chakrabarty et al., 2019). A prior study conducted by Paulo de O. Duarte (2017) showed that higher levels of inflammation were common in demented older subjects with worse cognitive function (Duarte et al., 2017). In addition, the involvement of inflammatory molecules in cognitive development has been previously demonstrated (Magalhaes et al., 2018). Several studies have shown that a critical role of inflammation was involved in the pathological process of mild cognitive impairment (Shen et al., 2019). Therefore, a potential linkage between inflammatory status and cognitive function in patients with COVID-19 is worth investigating.
In this context, the aim of this study was to evaluate the cognitive function in recovered COVID-19 patients and to investigate the potential relationship of serum levels of inflammatory factors and CRP with cognitive function. Even though a full neuropsychological evaluation is required to characterize cognitive strengths and weaknesses, simpler approaches may also be adequate in detecting cognitive decline (Brouillette et al., 2019). With the recent advancements in internet technology, the valid Web-based cognitive assessment tool can be slightly modified and validated to effectively screen for cognitive impairment in clinical settings (Hafiz et al., 2019). Given that human-to-human transmission of SARS-CoV-2 is confirmed, we conducted iPad-based online neuropsychological tests to minimize the contact between medical staff and patients with COVID-19. The tests evaluated attention, memory, executive functioning, information processing speed, visuo-spatial processing, and psychomotor function. We chose recently recovered patients as participants to diminish the adverse impacts of their regular treatment process on cognitive function. In addition, those who were severely illed were unable to finish this cognitive evaluation.
2. Methods
2.1. Participants
This study included patients with COVID-19 who met the diagnostic criteria in The First Affiliated Hospital, Zhejiang University School of Medicine. A total of 29 patients aged from 30 to 64 years were recruited, including 18-male and 11-female patients. The inclusion criteria were as follows: 1) cases met the set diagnostic standard of COVID-19 of testing negative at least twice for the nucleic acid tests; 2) education level ≥9 years (completed at least a junior middle school level of education); 3) Han ethnicity; 4) right-handedness; and 5) can complete the test content independently. A total of 29 age-, gender-, and education-matched healthy volunteers were recruited from the Sixth Hospital of Peking University. The general exclusion criteria for all participants included: 1) a history of mental disorders or current treatment for mental illnesses, such as taking antipsychotics, antidepressants, mood stabilizers, antiepileptics, benzodiazepines and other drugs that may interfere with the assessment; 2) severe physical illnesses that may interfere with assessment; 3) a history of drug abuse or drug dependence; 4) have serious suicide thoughts; 5) pregnant or lactating women; and 6) have hearing or visual impairments.
The study was approved by the Ethics Committee of The First Affiliated Hospital, Zhejiang University School of Medicine. All the subjects voluntarily participated in the study and were briefed on the aims, methods, and potential risks. Moreover, the subjects were informed they were at liberty to quit the study at any time, with an additional examination.
2.2. Demographic and clinical assessments
The tests were completed for all subjects by fully trained psychiatrists with consistent training courses in the First Affiliated Hospital, Zhejiang University School of Medicine. Demographic and clinical data were collected using a questionnaire designed for all participants. The severity of anxiety and depression symptoms were respectively self-reported by the Anxiety Screening Scale (GAD-7) and the Patient Health Questionnaire-9 (PHQ-9).
2.3. Neuropsychological tests
Most neuropsychological assessments generate measurements of function in several cognitive domains, including attention and memory function, processing speed, executive function, and perceptual abilities. An iPad-based online tool used in the study included the Trail Making Test (TMT), Sign Coding Test (SCT), Continuous Performance Test (CPT), and Digital Span Test (DST). This tool was developed by the Sixth Hospital of Peking University, and the tests contained therein have been verified for reliability in the Chinese population (Shi et al., 2015). The patients and healthy controls took the Neuropsychological tests last, after finishing all the other items.
-
1.
The TMT examines the trajectories of visual scanning and visual movement, reflecting the information processing speed of the subjects. Participants were required to quickly draw lines to connect consecutively numbered circles in ascending order. The task completion was measured in seconds. On the iPad. Test duration is set at 300 s, and the number of errors, as well as time taken to complete the test, are recorded. Any subject who stops midway is requested to continue the test for the remainder of the test duration. If not completed within the time limit, it is recorded as an error.
-
2.
The SCT is used to investigate visual perception, visual scanning, eye movement, and memory. This test requires participants to compare the standard template, select numbers that are paired with different symbols and fill in the spaces. The test score is computed by subtracting the number of errors from the number of correct entries filled in within 90 s. The numbers are filled on the iPad within 90 s, and the results are recorded. Each correct entry is awarded a point, and the maximum score is 110 points.
-
3.
The CPT measures continuous and selective attention, and impulse. It is divided into 3 parts. First (CPT 1), the subject is shown a series of animal pictures and is expected to click the “Same” button if two successive images are similar. In the second part (CPT 2), a pair of animal pictures is displayed and the subject is expected to click the “Same” button if two successive image pairs are similar. In part three (CPT 3), the number of animal pictures displayed per view is three, and test instructions are similar to the previous parts. This test measures the correct detection rate, error detection rate, missed detection rate, and the average response time of the three parts. The results are recorded on the iPad.
-
4.
The DST focuses on concentration, instantaneous memory, and resistance to information interference. These include the forward and backward count tests, where an iPad reads out a series of numbers to the test participant. Participants are then required to repeat these numbers in the same order or in the reverse order. A point is awarded for each test passed, and zero for a failed test. The highest scores in the forward count test and backward count test are 16 and 14 points, respectively. Therefore, the highest total score is 30 points.
2.4. Serum sample collection and analysis
Venous blood sample (3 ml) was collected from each patient the next morning after admission. The blood samples were centrifuged at 3500 r/min for 15 min, and serum extracted for analysis. The IL-2/IL-4/IL-6/IL-10/TNF-α/IFN-γ detection kit from ACEA was used to detect the expression of cytokines in blood samples. The kits contain capture microspheres with different fluorescence intensities. It can be specifically combined with human cytokines, and the cytokines can be distinguished by analyzing the fluorescence intensity of the captured microspheres by the flow cytometer platform. At the same time, the fluorescence intensity of each cytokine complex can be analyzed separately to obtain the content of each cytokine in the tested sample. Determination of CRP was done using either Roche cobasc701 automatic biochemical analyzer, or CRP detection kit (latex enhanced turbidimetric method, batch number: 201502400432). This kit uses latex to enhance immune turbidimetry.
2.5. Statistics
All analyses were carried out using the Statistical Package for the Social Sciences (SPSS), version 24 (IBM Corp., Armonk, NY). All data are presented as mean ± standard deviation. Group differences in demographic data and neuropsychological variables were evaluated using the independent sample t-tests. The Mann–Whitney U test was used as a nonparametric equivalent of the Student's t-test for non-normally distributed data. Correlations between inflammatory levels and the clinical characteristics were assessed using the Pearson correlation analysis. All results were quoted as 2-tailed P values, with statistical significance set at P < 0.05.
3. Results
3.1. Demographic characteristics
The demographic and clinical characteristics of the 29 patients (47.00 ± 10.54 years; 18 males and 11 females) and 29 healthy controls (42.48 ± 6.94 years; 12 males and 17 females) were outlined in Table 1 . There were no significant differences with regard to age, gender, and education levels between the two groups. In the patient group, the self-reported severity of depression and anxiety as evaluated by the PHQ-9 and GAD-7 was 4.20 ± 3.98 and 4.28 ± 4.05, respectively.
Table 1.
Demographic and baseline clinical characteristics.
| COVID-19 patients (N = 29) | Control (N = 29) | t/χ2 | p | |
|---|---|---|---|---|
| Age (years) | 47.00 ± 10.54 | 42.48 ± 6.94 | 1.927 | 0.060a |
| Gender (male/female) | 18/11 | 12/17 | 2.486 | 0.115b |
| Education (years) | 12.59 ± 2.78 | 12.38 ± 3.14 | 0.265 | 0.792a |
| PHQ-9 | 4.20 ± 3.98 | |||
| GAD-7 | 4.28 ± 4.05 | |||
| CRP | 19.98 ± 25.29 |
aThe P-values were obtained by two-sample t-tests.
bThe P-value was obtained by a chi-square test.
*P < 0.05.
3.2. Neuropsychological testing results
Results were based on the four modules namely TMT, SCT, CPT and DST. For CPT, the COVID-19 patients had a lower correct number CPT 2 and CPT 3 compared with the controls (9.83 ± 1.93 vs 8.21 ± 1.90, P = 0.002). CPT 2 and CPT 3 also had a higher missing number in the patient group compared with the controls (41.55 ± 2.90 vs 39.59 ± 2.31, P = 0.028; 40.38 ± 3.10 vs 38.45 ± 2.13, P = 0.002). In addition, there was a trend of significant difference in the reaction time of CPT 1 and CPT 2 (774.59 ± 119.33 vs 843.22 ± 140.97, P = 0.050; 817.06 ± 114.53 vs 879.59 ± 123.87, P = 0.051) and correct number of CPT 2 (7.07 ± 2.4 vs 8.72 ± 1.79, P = 0.050). However, there was no significant difference between the two groups in TMT, SCT, or DST. The details are presented in Table 2 .
Table 2.
Results of neuropsychological tests in all participants.
| Measure | COVID-19 patients (N = 29) | Controls (N = 29) | t | p | |
|---|---|---|---|---|---|
| Trail Making Test | 47.82 ± 16.55 | 49.76 ± 21.53 | −0.383 | 0.703 | |
| Sign Coding Test | 32.14 ± 9.02 | 34.48 ± 13.31 | −0.784 | 0.432 | |
| Digital Span Test | 19.24 ± 5.36 | 18.97 ± 5.23 | 0.198 | 0.843 | |
| Continuous Performance Test | |||||
| CPT part 1 | |||||
| Correct Number | 9.83 ± 1.93 | 10.21 ± 2.62 | −0.627 | 0.533 | |
| Error Number | 0.41 ± 0.63 | 0.97 ± 2.61 | −1.106 | 0.273 | |
| Missing Number | 39.76 ± 1.96 | 38.83 ± 3.56 | 1.235 | 0.222 | |
| Reaction time | 774.59 ± 119.33 | 843.22 ± 140.97 | −2.001 | 0.050 | |
| CPT part 2 | |||||
| Correct Number | 7.07 ± 2.45 | 8.72 ± 1.79 | −2.938 | 0.050 | |
| Error Number | 1.38 ± 1.59 | 1.66 ± 2.19 | −0.549 | 0.586 | |
| Missing Number | 41.55 ± 2.90 | 39.59 ± 2.31 | 2.857 | 0.006* | |
| Reaction time | 817.06 ± 114.53 | 879.59 ± 123.87 | −1.996 | 0.051 | |
| CPT part 3 | |||||
| Correct Number | 6.34 ± 2.50 | 8.21 ± 1.90 | −3.198 | 0.002* | |
| Error Number | 3.28 ± 1.85 | 3.34 ± 2.32 | −0.125 | 0.901 | |
| Missing Number | 40.38 ± 3.10 | 38.45 ± 2.13 | 2.765 | 0.008* | |
| Reaction time | 868.24 ± 99.73 | 879.10 ± 197.08 | −0.265 | 0.792 | |
*P < 0.05.
3.3. Associations between inflammatory levels and neuropsychological tests
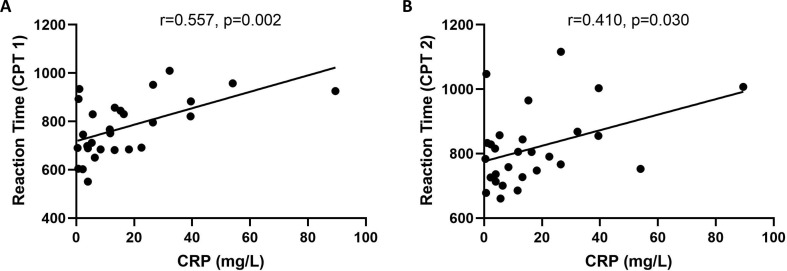
The average serum concentration of CRP was 19.98 ± 25.29 mg/L in the patient group. Further statistical tests revealed no statistically significant correlation between IL-6, IL-10, TNF-α, INF-γ and the CPT tests. Nevertheless, the correlation analyses showed that the reaction time of CPT 1 and CPT 2 was positively correlated with the CRP levels (Fig. 1 ).
Fig. 1.
The correlation analyses revealed that serum CRP level was associated with the reaction time of CPT 1 (A), and CPT 2 (B), respectively.
4. Discussion
To the best of our knowledge, this is the first study on cognitive aspects of COVID-19 patients. The main findings in this study were that COVID-19 patients exhibited cognitive dysfunction in sustained attention domain as revealed by CPT. A potential correlation between serum CRP level and reaction time in CPT was also reported.
The present results are consistent with currently available data on patients with viral infection. Cognitive dysfunction in patients with viral infection has been commonly reported in prior studies. For example, in a study evaluating the effects of viral factors on neurocognitive performance in 347 Human Immunodeficiency Virus (HIV) positive and 395 seronegative adult Cameroonians found that individuals infected with HIV had impaired attention, learning, and memory functions (Kanmogne et al., 2020). Similarly, Another study showed that Zika virus (ZIKV) infection had a profound impact on long-lasting neurodevelopmental consequences, inducing cognitive deficits (Raper et al., 2020). Moreover, virus infection that involves the central nervous system (CNS) and cardiopulmonary failure may be associated with neurologic sequelae, delayed neurodevelopment, and reduced cognitive functioning (Chang et al., 2007). In the present study, only some parts of CPT tests showed significant group differences which might suggest that the cognitive impairments in patients with SARS-CoV-2 infection were mild and mainly located in sustained attention domain. However, this study evaluated the immediate effects of SARS-CoV-2 infection on cognitive function since the neuropsychological assessments were done during a short period after the COVID-19 patients recovered, usually two to three weeks after infection. Thus, longitudinal studies should be designed in this area to appraise the long-term influence of SARS-CoV-2 infection on cognitive function.
Further, we demonstrated that there is a significant correlation between continuous attention function changes and CRP levels at the time of admission in COVID-19 patients. This finding was in line with the result of a previous study that reported a significant association between hs-CRP concentration and long-term cognitive decline in a large sample from wave 7 (2014–2015) of the English Longitudinal Study of Ageing (Zheng and Xie, 2018). In light of studies indicating a significant correlation of CRP levels with verbal fluency and executive function (Vintimilla et al., 2019), our present finding is interesting. Besides, a separate study has identified high serum CRP level as a risk factor for depression related to Chronic Obstructive Pulmonary Disease (COPD) (Xu and Li, 2018). Collectively, these findings imply that several aspects of cognitive impairments could be associated with CRP. Although the underlying mechanisms of the CRP and cognitive impairment association is unclear, CRP is associated with the inflammatory process. Moreover, a previous study suggested that CRP has an early effect on frontal lobe functioning (Vintimilla et al., 2019), such as sustained attention (Han et al., 2019; Mitko et al., 2019). These findings could inform other researches seeking to predict the cognitive function of COVID-19 patients by measuring the CRP level. Further work is however needed to assess the long-term impact of the SARS-Cov-2 on cognitive function in COVID-19 patients.
The present study is an observational cross-sectional study which assessed the cognitive functions in patients with COVID-19, and its relationship with inflammatory profiles. However, the following limitations should be taken into account when interpreting our findings. Firstly, the study sample size was relatively small, thus we might have missed some subtle functional changes in cognitive functions. Secondly, the over 9 years education experience requirement of participants might have led to a selection bias as level of education is closely linked to cognitive function. Thirdly, we could not determine whether levels of CRP might constantly correlate with cognitive decline over time. Therefore, longitudinal observation on the changes in cognitive functions and inflammatory profiles should be explored further. Fourthly, the emotional status and inflammatory indexes of healthy controls were not examined. Finally, we did not assess the influence of antiviral therapy on cognitive function.
5. Conclusions
Our findings indicate a potential cognitive dysfunction in patients with COVID-19. Specifically, sustained attention is correlated with the inflammatory level as indicated by CRP. Further studies should therefore investigate the long-term cognitive function dynamics in patients with COVID-19 and its relationship with inflammatory profiles.
Role of funding source
We are sincerely grateful for the support of funds from the Medical Science and Technology Project of Zhejiang Province (2019RC169 to Hetong Zhou), and the National Key Research and Development Program of China (2016YFC1307100 to Shaohua Hu).
CRediT authorship contribution statement
Hetong Zhou: Conceptualization, Formal analysis, Writing - original draft, Funding acquisition. Shaojia Lu: Formal analysis, Investigation. Jingkai Chen: Formal analysis. Ning Wei: Investigation. Dandan Wang: Investigation. Hailong Lyu: Investigation. Chuan Shi: Formal analysis, Methodology, Writing - review & editing. Shaohua Hu: Conceptualization, Writing - review & editing, Funding acquisition.
Declaration of competing interest
All authors declare that they have no conflict of interest.
Acknowledgements
The authors would like to thank all patients who participated in this study, and experts from Peking University Sixth Hospital for their technical assistance.
References
- Brouillette M.J., Fellows L.K., Finch L., Thomas R., Mayo N.E. Properties of a brief assessment tool for longitudinal measurement of cognition in people living with HIV. PloS One. 2019;14 doi: 10.1371/journal.pone.0213908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlos W.G., Dela Cruz C.S., Cao B., Pasnick S., Jamil S. Novel Wuhan (2019-nCoV) coronavirus. Am. J. Respir. Crit. Care Med. 2020;201:P7–P8. doi: 10.1164/rccm.2014P7. [DOI] [PubMed] [Google Scholar]
- Chakrabarty T., Torres I.J., Bond D.J., Yatham L.N. Inflammatory cytokines and cognitive functioning in early-stage bipolar I disorder. J. Affect. Disord. 2019;245:679–685. doi: 10.1016/j.jad.2018.11.018. [DOI] [PubMed] [Google Scholar]
- Chang Lin M., Wei L., Xie L., Zhu G., Dela Cruz C.S. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. Jama. 2020;323:1092–1093. doi: 10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L.Y., Huang L.M., Gau S.S., Wu Y.Y., Hsia S.H., Fan T.Y. Neurodevelopment and cognition in children after enterovirus 71 infection. N. Engl. J. Med. 2007;356:1226–1234. doi: 10.1056/NEJMoa065954. [DOI] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culpepper L., Lam R.W., McIntyre R.S. Cognitive impairment in patients with depression: awareness, assessment, and management. J. Clin. Psychiatr. 2017;78:1383–1394. doi: 10.4088/JCP.tk16043ah5c. [DOI] [PubMed] [Google Scholar]
- Duarte P.O., Duarte M.G.F., Pelichek A., Pfrimer K., Ferriolli E., Moriguti J.C. Cardiovascular risk factors and inflammatory activity among centenarians with and without dementia. Aging Clin. Exp. Res. 2017;29:411–417. doi: 10.1007/s40520-016-0603-9. [DOI] [PubMed] [Google Scholar]
- Hafiz P., Miskowiak K.W., Kessing L.V., Elleby Jespersen A., Obenhausen K., Gulyas L. The internet-based cognitive assessment tool: system design and feasibility study. JMIR formative. Res. 2019;3 doi: 10.2196/13898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H.B., Lee K.E., Choi J.H. Functional dissociation of theta oscillations in the frontal and visual cortices and their long-range network during sustained attention. eNeuro. 2019;6 doi: 10.1523/ENEURO.0248-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanmogne G.D., Fonsah J.Y., Umlauf A., Moul J., Doh R.F., Kengne A.M. Attention/working memory, learning and memory in adult Cameroonians: normative data, effects of HIV infection and viral genotype. J. Int. Neuropsychol. Soc. 2020;26:607–623. doi: 10.1017/S1355617720000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Xiao X., Wei X., Li J., Yang J., Tan H. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J. Med. Virol. 2020;92:595–601. doi: 10.1002/jmv.25726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes R.C., Pimenta L.P., Barbosa I.G., Moreira J.M., de Barros J., Teixeira A.L. Inflammatory molecules and neurotrophic factors as biomarkers of neuropsychomotor development in preterm neonates: a Systematic Review. Int. J. Dev. Neurosci: Offc. J. Int. Soc. Dev. Neurosci. 2018;65:29–37. doi: 10.1016/j.ijdevneu.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Mitko A., Rothlein D., Poole V., Robinson M., McGlinchey R., DeGutis J. Individual differences in sustained attention are associated with cortical thickness. Hum. Brain Mapp. 2019;40:3243–3253. doi: 10.1002/hbm.24594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J., Kovacs-Balint Z., Mavigner M., Gumber S., Burke M.W., Habib J. Long-term alterations in brain and behavior after postnatal Zika virus infection in infant macaques. Nat. Commun. 2020;11:2534. doi: 10.1038/s41467-020-16320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X.N., Niu L.D., Wang Y.J., Cao X.P., Liu Q., Tan L. Inflammatory markers in Alzheimer's disease and mild cognitive impairment: a meta-analysis and systematic review of 170 studies. J. Neurol. Neurosurg. Psychiatr. 2019;90:590–598. doi: 10.1136/jnnp-2018-319148. [DOI] [PubMed] [Google Scholar]
- Sheng B., Cheng S.K., Lau K.K., Li H.L., Chan E.L. The effects of disease severity, use of corticosteroids and social factors on neuropsychiatric complaints in severe acute respiratory syndrome (SARS) patients at acute and convalescent phases. Eur. Psychiatr. : J. Assoc. Eur. Psychiatr. 2005;20:236–242. doi: 10.1016/j.eurpsy.2004.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng W.H., Chiang B.L., Chang S.C., Ho H.N., Wang J.T., Chen Y.C. Clinical manifestations and inflammatory cytokine responses in patients with severe acute respiratory syndrome. J. Formosan. Med. Assoc. Taiwan Yi Zhi. 2005;104:715–723. [PubMed] [Google Scholar]
- Shi C., Kang L., Yao S., Ma Y., Li T., Liang Y. The matrics consensus cognitive battery (MCCB): Co-norming and standardization in China. Schizophr. Res. 2015;169:109–115. doi: 10.1016/j.schres.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang H.W., Scudds R.J., Chan E.Y. Psychosocial impact of SARS. Emerg. Infect. Dis. 2004;10:1326–1327. doi: 10.3201/eid1007.040090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vintimilla R., Hall J., Johnson L., O'Bryant S. The relationship of CRP and cognition in cognitively normal older Mexican Americans: a cross-sectional study of the HABLE cohort. Medicine. 2019;98 doi: 10.1097/MD.0000000000015605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Jama. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Cai H., Shen Y., Ni Q., Chen Y., Hu S. [Management of corona virus disease-19 (COVID-19): the Zhejiang experience] Zhejiang da xue xue bao Yi xue ban = J. Zhejiang. Univ. Med. Sci. 2020;49:147–157. doi: 10.3785/j.issn.1008-9292.2020.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Li X. Risk factors for depression in patients with chronic obstructive pulmonary disease. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2018;24:1417–1423. doi: 10.12659/MSM.904969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap Y.Y., Sathar J., Law K.B., Zulkurnain P.A.B., Edmund S.C., Chang K.M. Clinical characteristics and outcomes of thrombotic microangiopathy in Malaysia. Blood. Res. 2018;53:130–137. doi: 10.5045/br.2018.53.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q. Allergy; 2020. Clinical Characteristics of 140 Patients Infected with SARS-CoV-2 in Wuhan, China. [DOI] [PubMed] [Google Scholar]
- Zheng F., Xie W. High-sensitivity C-reactive protein and cognitive decline: the English longitudinal study of ageing. Psychol. Med. 2018;48:1381–1389. doi: 10.1017/S0033291717003130. [DOI] [PubMed] [Google Scholar]