Abstract
Microplastics (MPs) are ubiquitous in the environment and more abundant in the marine environment. Consequently, increasing focus has been put on MPs in oceans and seas, while little importance has been attached to their presence in freshwaters and soils. Therefore, this paper aimed to provide a comprehensive review of the occurrence, analysis and ecotoxicology of MPs. The abundance and distribution of MPs in several typical freshwater systems of China were summarized. It suggested that the surface water of Poyang Lake contained the highest concentration of 34 items/L MPs among all the 8 freshwater systems, and the content of MPs in sediments were higher than that of the surface water. Net-based zooplankton sampling methods are the most frequently utilized sampling methods for MPs, and density separation, elutriation and digestion are three major pretreatment methods. Fourier transform infrared spectroscopy, Raman spectroscopy and pyrolysis-gas chromatography coupled to mass spectrometry are often used to identify the polymer types of MPs. Besides, MPs might damage the digestive tract of various organisms and negatively inhibit their growth, feeding and reproduction. The ways of human exposure to MPs are by ingestion, inhalation and dermal exposure, digestive and respiratory system might be adversely influenced. However, potential health risks of MPs to humans are remained insufficiently researched. Overall, by showing the presence of MPs in freshwaters and soils as well as possible ecotoxicological effects on the environment and humans, this paper provided a framework for future research in this field.
Keywords: Microplastics, Analysis, Freshwater, Soil, Ecotoxicological impacts
Graphical abstract
1. Introduction
The increasing production of plastics in recent years has led to severe plastic pollution in the environment. Surveys show that the annual plastic production in 2017 has exceeded 348 million tons (PlasticsEurope, 2018), and it is estimated that the global annual production of plastics may be up to 33 billion tones by the year of 2050 (Cincinelli et al., 2019). Besides, the current outbreak of COVID-19 pandemic has resulted in a dramatic increase in personal protective equipment (e.g. gloves and masks) in which plastics and rubbers are two of the major components. It is reported that the accumulated medical waste in China from 20 January to 31 March in 2020 was about 207 kilotons (Klemes et al., 2020). This will lead to a severer plastic pollution in the environment.
Microplastics (MPs) that are plastics less than 5 mm in length are even more harmful than larger plastic items, and microplastic pollution (MP) has become an emerging environmental issue in the last two decades (Laskar and Kumar, 2019; Wright et al., 2013a).Studies have shown that 4.8 to 12.7 million tonnes of plastic waste entered the oceans and seas of 192 countries in 2010 and this number would increase by 22% by 2025 (Jahnke et al., 2017; Jambeck et al., 2015; Kühn et al., 2018; Thompson et al., 2004; Zarfl et al., 2011); however, MPs have also been detected in freshwater systems as well as terrestrial environment. Investigations of MPs in freshwaters and soils are far from adequate, as knowledge on MPs in freshwater and terrestrial environment is insufficient (Rillig, 2012).
Widely-recognized problems facing organisms are entanglement, suffocation and ingestion that are caused by MPs (Lo and Chan, 2018; Wu et al., 2018; Yang et al., 2015, 2018), and many studies have shown that MPs might pose threat to marine zooplanktons, fish, mussels and algae (Besseling et al., 2015; Desforges et al., 2015; Galloway et al., 2017; Lusher et al., 2015; Qin, 2012). Apart from MPs itself, manifold plastic additives are another hidden danger. For example, nonylphenol as an endocrine disrupting chemical is frequently added in polyvinylchloride (PVC) and high-density polyethylene (PE) to achieve high thermostability of plastics (Lam et al., 2018). Moreover, due to the small sizes and difficulty in degradation under natural condition, chances are that MPs can be ingested or respired by a wide range of organisms and therefore will cause harm to them (Wright et al., 2013b). Worse still, MPs have a high affinity for other pollutants. For example, MPs may adsorb heavy metals and hydrophobic organic chemicals on their surface and act as a vector for transporting these pollutants in the environment (Eerkes-Medrano et al., 2015; Fries et al., 2013; Koelmans et al., 2016; Lee et al., 2014, 2019; Lin et al., 2019; Mizukawa et al., 2013; Tang et al., 2020; Yang et al., 2005; Zhou et al., 2018).
Despite that information on the detection, identification and quantification of MPs can be found in a number of papers (Claessens et al., 2011; Lebreton et al., 2017; Martin et al., 2018; Reddy et al., 2006; Xiong et al., 2019; Zobkov and Esiukova, 2018), comprehensive studies on MPs which involve in their occurrence, distribution, sampling, pretreatment, characterization in freshwaters and sediments, as well as ecological influences on aquatic and soil organisms and humans are still needed.
Therefore, the review aimed to: (1) present the occurrence and abundance of MP in some typical freshwater systems in China; (2) summarize frequently used sampling, pretreatment and characterization methods for MPs in waters and sediments; (3) elucidate ecological impacts of MPs on aquatic organisms, soil biota and human health; (4) point out knowledge gaps in current studies of MPs and make corresponding recommendations for further research.
2. Methodology
A literature review was conducted on database of Web of Science and ScienceDirect for peer-reviewed publication from 2010 to February 2020. A combination of keywords was applied as the criteria such as “microplastics” OR “microplastic” AND “freshwater” OR “water”, “microplastics” OR “microplastic” AND “soil”, “microplastics” OR “microplastic” AND “effects”, and these keywords were retrieved in any topic, title or text. A total of 2126 papers were identified as candidate publications, and they were classified into several subtopics, namely, occurrence and distribution in major freshwater systems of China, sampling and pretreatment, identification, and ecotoxicology.
3. Abundance, sampling and analysis of MPs in waters and sediments
3.1. Abundance and distribution of MPs in some typical freshwater systems of China
Vast numbers of MPs are accumulated in oceans every year; however, they have also been widely detected in freshwater systems, which has sparked high-profile campaigns recently (Xu et al., 2020). It was reported that the annual plastics production in China was about 102.3 million tons in 2017, which accounted for 29.4% of the whole plastics production all over the world (PlasticsEurope, 2018). Moreover, mismanagement of waste plastics is a common phenomenon in China. For instance, the mismanagement rate of plastic wastes of China has reached 27.7% in 2010, which made China on the top among the listed 20 countries ranked by percentage of total mismanaged plastic wastes (Jambeck et al., 2015). Therefore, some freshwater systems of China were chosen as the typical example to analyze the occurrence and abundance of MPs in freshwaters.
According to a couple of studies (Di and Wang, 2018; Eerkes-Medrano et al., 2015; Kukulka et al., 2012; Wang et al., 2017b), factors such as human activity, climatic and hydrological conditions could have a direct effect on the abundance and distribution of MPs in freshwater environment. Di and Wang (2018) suggested that the concentration and distribution of MPs in freshwater systems were mainly influenced by human, agricultural, fisheries and industrial activities. For example, the study of Yin et al. (2020) suggested that the abundance of microplastics in the urban area sediment of Dongting Lake is lower than that of the rural area and the reason behind this phenomenon was that more environmental protection measures were taken by urban areas than rural areas.
Table 1 summarized the abundance of MPs in surface water and sediment of several typical freshwater systems in China. As shown in Table 1, among those major freshwater systems in China, Pearl Lake in Zhanjiang which is one of the developed industrial cities in Guangdong province was detected with the highest MPs concentration of 7924 items/m3. MPs were also found in lakes of rural areas such as Tibet, though the concentration of which was lower than those dense populated areas. However, how MPs were moved to rural areas remained ambiguous. Thus, more studies should be conducted to investigate the transportation and distribution of MPs in freshwater environment from a broad point of view.
Table 1.
Abundance of MPs in major freshwater systems in China (PE: polyethylene, PP: polypropylene, PS: polystyrene, PVC: polyvinyl chloride, PET: polyethylene terephthalate; PA: polyamide).
| Location | Sampling site | Abundance | Particle size (mm) | Dominant shape | Dominant polymer type | References |
|---|---|---|---|---|---|---|
| Dongting Lake, Hunan | surface water | 900-2800 items/m3 | <0.33 | fiber | PE, PP | Wang et al. (2018) |
| East Dongting Lake, Hunan | sediment | 180-693 items/kg | <0.5 | fiber | PET, PA | Yin et al. (2020) |
| Hong Lake, Hubei |
surface water | 1250-4650 items/m3 | <0.33 | fiber | PE, PP | Wang et al. (2018) |
| Pearl Lake, Guangdong | surface water | 379-7924 items/m3 | 0.02–2 | fiber | PE, PP | Lin et al. (2018) |
| sediment | 80-9597 items/kg | 0.02–1 | ||||
| Taihu Lake, Jiangsu |
surface water | 3.4–25.8 items/L | 0.1–1 | fiber | cellophane, PE | Su et al. (2016) |
| sediment | 11.0–234.6 items/kg | 0.1–1 | ||||
| Poyang Lake, Jiangxi | surface water | 5-34 items/L | <0.5 | fiber | PP, PE | Yuan et al. (2019) |
| sediment | 54-506 items/kg | <0.5 | ||||
| Lakes in Tibet | surface water | 8-563 items/m3 | 1–5 | fiber | PE, PP | Zhang et al. (2016) |
| Wei River, Shaanxi |
surface water | 3.67–10.7 items/L | <0.5 | fiber | PE, PVC | Ding et al. (2019) |
| sediment | 360-1320 items/kg | <0.5 | ||||
| Three Gorges Reservior, Chongqing | surface water | 1597-12611 items/m3 | <0.5 | fiber | PS, PP | Di and Wang (2018) |
| sediment | 25-300 items/kg | <0.5 |
In addition, it was noticeable that fiber was the most dominant polymer component in all listed references as presented in Table 1. Nowadays, synthetic textile fibers are widely applied, Napper and Thompson (2016) pointed out that domestic sewage derived from washing machines comprised high concentrations of fibers, which might be an important source of microfiber. Besides, fisheries activities could produce plenty of ageing fishing gears including fishing nets, thus it should be another source of microfiber (Su et al., 2016; Yuan et al., 2019). From Table 1, it could also be seen that PE, PP (polypropylene) and PS (polystyrene) were the most commonly detected plastic types, as the three kinds of polymers are frequently used in fishing tools, packaging and decorations (Wang et al., 2017a). Concerning the particle size of MPs detected in these freshwater systems, most of them were less than 1 mm. It was also reported that small-sized MPs were found more abundant in many investigations (Eriksen et al., 2013). However, the MPs concentrations in lakes of Tibet had larger particle size than that of other areas. This was probably owing to a fast dissipation of small plastic items by high ultraviolet radiation intensity and big temperature difference in Tibet (Zhang et al., 2016).
Because sediments were the final destination of high-density MPs, the concentration of MPs in sediments were higher than that of the surface water as seen in Table 1. Throughout the literature referred to, it could be found that different units were used to describe the concentration of MPs, which made it inconvenient to compare the abundance of MPs among different locations. For example, beside common unit of “items/m3” and “items/L″, less used unit such as “g/L” and “fibers/50 mL” were also seen in some researches (Baztan et al., 2014; Browne et al., 2011). Therefore, using unified units to measure the abundance of MPs would help the comparison and analysis of data.
3.2. Sampling from waters and sediments
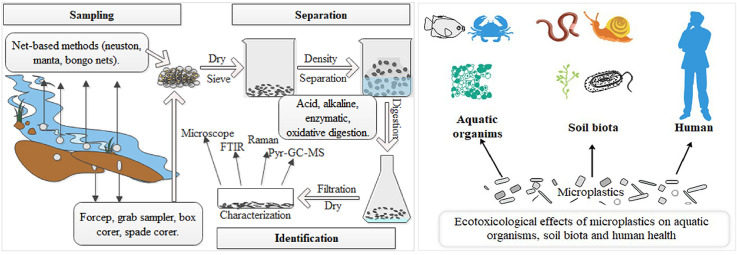
To date, various methods have been employed to sample and detect MPs in waters; however, standardized methods have not been proposed (Besley et al., 2017; Hanvey et al., 2017; Miller et al., 2017). One of the commonly used tools for sampling MPs from waters is net-based zooplankton sampling methods (Lattin et al., 2004). In order to make the samples more representative, large volumes of water should be collected. Nets with different mesh sizes are used to estimate the concentrations of MPs in open water bodies. Three kinds of zooplankton nets including manta trawl, neuston and bongo net are wildly used.
Manta trawl net and neuston net are suitable for sampling MPs that float on the surface of the water bodies (Cincinelli et al., 2017; Collignon et al., 2012; Mauro et al., 2017). Neuston net can be used under strong wind and wave condition while manta net is suitable for calm water (Frias et al., 2014; Lima et al., 2014). Common sizes of neuston and manta trawl nets range from 50 μm to 3000 μm, and the mesh size around 300 μm is the most frequently used one (Hidalgo-Ruz et al., 2012). Advantages of both nets are that large volumes of water can be sampled in a rather short time (Ruiz-Orejón et al., 2016), but this may underestimate the actual concentrations of MPs in seawater, for MPs with particle sizes smaller than 50 μm that are more toxicologically significant will be readily escaped (Doyle et al., 2011). Because small-mesh-size nets are easily blocked, nets whose mesh size are less than 300 μm are rarely studied.
Bongo net is usually used for collecting water-column samples, and it can sample on sea surface as well as at mid-ocean depths. Bongo net is a reliable single unit tow platform for sampling in shallow water (Wang et al., 2017a). One of the unique features of bongo net is its opening and closing mechanism that allows discrete known-depth sampling (Mauro et al., 2017). The most widely used mesh sizes of bongo net are also about 300 μm (Cózar et al., 2014). For instance, Mauro et al. (2017) deployed a bongo net with a diameter of 60 cm fitted with 335 μm mesh size in an oblique tow from a depth of 15 m to collect MPs samples from Northern Gulf of Mexico; in the experiments of Lattin et al. (2004), paired 61 cm diameter bongo nets with a length of 3 m and the mesh size of 333 μm were used to sample MPs in the southern California shore. Detailed information about neuston, manta and bongo net is presented in Table 2 .
Table 2.
A comparison of neuston, manta trawl and bongo net.
| Instrument | Mesh size (μm) | Tow length (km) | Location | Sample | Average concentration (±SD) | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|---|---|
| Neuston net | 200 | 0.93 | Faafu Atoll, Maldives | surface water | 0.32 ± 0.15 particles/m3 | large sample volumes, easy operation, short sampling time | expensive, underestimating plastic concentrations, causing potential pollution | Saliu et al., 2018 |
| Manta trawl | 333 | 1.94 | Mediterranean Sea | surface water | 43000 particles/km2 | efficient in sampling the sea surface microlayer | expensive, only suitable for calm water |
Eriksen et al. (2013); Karlsson et al. (2019) |
| Bongo net | 335 | – | Northern Gulf of Mexico | surface water | 10.7 ± 4.4 particles/m3 | suitable for sea surface and mid-ocean depth | cannot capture small-sized particles | Mauro et al. (2017) |
As sediments in coastal beaches or lakefront are more convenient to be sampled, samples are usually taken from these places when studying MPs in sediments. Commonly used tools for collecting MPs on beaches are forceps, sieves, grab samplers, box corers and spade corers (Tsang et al., 2017; Vianello et al., 2013). Because of the properties of MPs and various environmental factors, the distribution of MPs in sediments is not even. Therefore, when sampling MPs from sediments, the selecting of tide line, sampling depth and sampling volumes matters (Bergmann et al., 2017). In general, the main sampling areas are in the high tide line where more MPs may be accumulated (Claessens et al., 2011). The sampling depths in sediments are the top 10 cm, and a sampling area of 25 cm2 is often chosen (Dekiff et al., 2014; Turner and Holmes, 2015).
3.3. Pretreatment of MPs samples
Before characterizations being carried out, isolation and extraction processes are needed because organic matters (OMs), algae and other impurities are mixed with MPs samples (Zhao et al., 2017). The separation of MPs samples involves in two major categories: physical methods such as density separation, filtration and sieving, and biochemical methods including acidic/alkaline digestion, oxidization and enzymatic degradation (Qiu et al., 2016).
The density of MPs varies depending on their polymer types and manufacturing processes, and the specific density values usually range from 0.8 to 1.4 g/cm3 (Hidalgo-Ruz et al., 2012). Density separation is based on the differences in the density of MPs. As the typical density of sediments is about 2.7 g/cm3 which is heavier than most MPs, the lighter items can be separated from the heavier ones by mixing with saturated salt solutions and shaking for a certain period of time (Shim et al., 2016). After that, less dense MPs will float on the surface of the brine solutions while denser sediments will sink at the bottom. Different brine solutions are applied to separating different MPs, and the density of brine solutions that are used for density separation often should be higher than 1.4 g/cm3 (Zhang et al., 2018). Saturated sodium chloride (NaCl, density of 1.2 g/cm3) solution is one of the most commonly used flotation agents owing to its cheap price, high availability and environmental benignity (Nuelle et al., 2014). However, saturated NaCl solutions are not suitable for separating MPs whose density is heavier than 1.2 g/cm3 such as PVC and PET (polyethylene terephthalate) and the recovery rates of which are less than 90% (Quinn et al., 2016). In addition, sediment samples need to be washed for three times when using NaCl as separation solutions. Other frequently used brine solutions are zinc chloride (ZnCl2), zinc bromide (ZnBr2), and sodium iodide (NaI) (Hidalgo-Ruz et al., 2012; Nuelle et al., 2014). Quinn et al. (2016) showed that NaI and ZnBr2 were effective to separate MPs with particle sizes of 200–400 μm, and both of them had good recovery rates (>95%), and it only required a single wash of sediments. MPs that can be separated by NaI are similar to environmental samples, while ZnBr2 solution is more suitable for separating samples of single type (Rocha-Santos and Duarte, 2015). Moreover, ZnBr2 is harmful to the environment and may lead to a secondary pollution while using NaI is environmentally safe (Prata et al., 2019a). Therefore, NaI is a recommended brine solution for the separation of MPs from sediments. However, NaI should not be used with cellulose filters, as it can react with them. Sodium polytungstate (Na6O39W12) solution is also used for density separation, but the recovery rate test of which is scarce and its price is relatively high. More specific information about the density values of different polymers and commonly used brine solutions are provided in Table 3 . Although shaking time and settling time of density separation is often about 30 s to 2 h, 2 min to 6 h, respectively, it actually requires less time in both process (Hidalgo-Ruz et al., 2012). As shown in Table 3, it took 3–10 min for mixing MPs samples and brine solutions and 5–15 min for separation of MPs in most study. In addition, besides salt solution separation, hydrocyclonic separation technology is also applied in MPs separation (Yuan et al., 2015).
Table 3.
Information about five common types of polymers and possible separation salt solutions (PE: polyethylene, PP: polypropylene, PS: polystyrene, PVC: polyvinyl chloride, PET: polyethylene terephthalate).
| Polymer type | Chemical structure | Polymer density (g/cm3) | Commonly used saturated salt solution for density separation | Shaking time | Settling time | Recovery rate | References |
|---|---|---|---|---|---|---|---|
| PE | 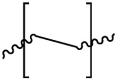 |
0.92-0.97 | NaCl (1.2 g/cm) | 3 min | 10 min | > 85.0% | Quinn et al. (2016) |
| PP | 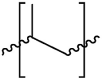 |
0.90-0.91 | ZnCl2 (1.5 g/cm) | 10 min | 15 min | > 95.0% | Rodrigues et al. (2020) |
| PS | 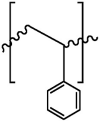 |
1.04-1.10 | ZnCl2 (1.5 g/cm) | 5 min stirring + 5 min rest + 3 short stirring bursts | overnight | 96.3% | Vermeiren et al. (2020) |
| PVC | 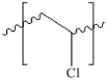 |
1.16-1.58 | ZnCl2 (1.5 g/cm) | 5 min stirring + 5 min rest + 3 short stirring bursts | overnight | 82.8% | Vermeiren et al. (2020) |
| PET | 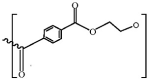 |
1.37-1.45 | ZnBr2 (1.7 g/cm3) | 3 min | 10 min | > 95.0% | Quinn et al. (2016) |
Elutriation is another separation method for extracting MPs samples by injecting some fluid such as water at the bottom of a column so that the buoyant MPs can be isolated from the settling OMs and sediments (Kedzierski et al., 2017). Filtration and sieving are also employed to separate MPs from water and sediment samples that are obtained from the density separation process (Prata et al., 2019a). The plastic particles will be filtered from the supernatant by a filter which is usually assisted by a vacuum (Ng and Obbard, 2006). In order to sort out larger particles before the filtration step, water samples can firstly pass through a sieve whose mesh size ranges from 0.038 to 0.475 mm (Andrady, 2011).
Another category for processing MPs samples with the purpose of removing OMs is digestion. There are four major types of digestion methods namely acidic, alkaline, enzymatic and oxidative digestion. For acidic digestion, nitric acid (HNO3), hydrofluoric acid (HF) and hydrochloric acid (HCl) are most frequently used. For example, Davidson and Dudas (2016) used 69–71% of HNO3 to separate MPs from clam tissues; Naidoo et al. (2017) found that 55% of HNO3 could fasten the digestion process when heating; Dubaish and Liebezeit (2013) utilized HF to digest OMs. Although HCl is reported to have relatively low digestion efficiency, studies using HCl to extract MPs can also be found (Cole et al., 2014; Desforges et al., 2014; Karami et al., 2016). Many types of plastics such as nylon and PET can be easily degraded by acid at high acid concentration and high processing temperature, and acidic digestion may also underestimate the effects of MPs on the environment (Qiu et al., 2016). Thus, acidic digestion should be used with caution. The effects of acid on the integrity of MPs have also been reported. For example, Prata et al. (2019a) summarized that when using HNO3 as acid digestion reagent, it may cause loss of some types of polymers such as PS and PET. Moreover, as heating is often required to assist the digestion process, polymers that have low resistance to acid may be more easily degraded at high temperature.
Alkaline digestion which serves as an alternative to acidic digestion has also been widely used in the separation of MPs. Among those available literature, sodium hydroxide (NaOH) and potassium hydroxide (KOH) are two commonly used alkaline solutions (Maes et al., 2017). Cole et al. (2014) found that 10 mol/L of NaOH solution was the optimal alkaline concentration among the tested ones. As for KOH, a concentration of 10% is often used when extracting MPs (Dehaut et al., 2016; Foekema et al., 2013). Alkaline digestion has high digestion efficiency of OMs and good recovery rate of MPs; however, it may damage the structure of plastics and causing discoloration to PE, PVC and nylon (Cole et al., 2014).
Enzymatic digestion is a promising digestion method for the removal of OMs since it is less hazardous to both the environment and plastic structures (Courtene-Jones et al., 2017). Proteinase K, tripsin, collagenase and papain are used for digesting OMs; a sequential use of enzymes (protease, cellulase, chitinase) has achieved high removal efficiency of OMs (Löder et al., 2015). Enzymatic digestion usually has no effect on the integrity of MPs and obtains high recovery rates (Cole et al., 2014). However, despite the eco-friendly property of enzymatic digestion, the utilization of enzymes in the separation of MPs is limited by the high prices, long digestion time and small application scales (Crichton et al., 2017). But as the industrial enzymes including industrial proteases, lipases and Corolase 7089 are relatively affordable, they can be used to efficiently digest soft tissues and have good prospect in application (Catarino et al., 2017).
Oxidative digestion may digest OMs more efficiently than acidic/alkaline digestion. For example, Nuelle et al. (2014) showed that 35% of hydrogen peroxide (H2O2) was the ideal solution to digest OMs and it achieved higher digestion efficiency than 37% of HCl and 20, 30, 40 and 50% of NaOH. Different concentrations of H2O2 are often employed as oxidant to remove OMs. Zhao et al. (2017) used 15% of H2O2 to digest labile background organic matter in marine snow samples; Shim et al. (2016) found that 30% of H2O2 had higher digestion efficiency than other concentrations; Nuelle et al. (2014) also reported that incubation using 35% of H2O2 at room temperature for 7 days could only remove 25% of biogenic material. Therefore, the efficiency of oxidative digestion by H2O2 varies with its concentration and is related to the incubation temperature. A combined use of H2O2 oxidization and enzymatic digestion has been shown in the study of Karlsson et al. (2017). Nevertheless, degradation and color change of MPs and the generation of foam after being treated by H2O2 seem unavoidable according to Avio et al. (2015b). Fenton's reagent is an advanced oxidant as well as a potential alternative to H2O2. The U.S. National Oceanic and Atmospheric Administration Marine Debris Program has made a recommendation that 30% of H2O2 is used in the presence of an iron (Fe(II)) catalyst for extracting MPs from water and sediment samples (Li et al., 2009; Masura et al., 2015; He et al., 2018). When comparing with H2O2, the Fenton digestion can effectively destroy organic components and inorganic compounds that cannot be easily digested by H2O2 (Tagg et al., 2017). Besides, it takes shorter time to finish the whole reaction and makes lower demand on reaction temperature, but the pH of the Fenton's reagent must be adjusted (to 3.0–5.0) to optimize the reaction (Hermosilla et al., 2012).
3.4. Identification and characterization
The diverse components, shapes, sizes and sources of MPs make the characterization of MPs an important but arduous work. In addition, the characterization of MPs is particularly vital as it allows obtaining more information about the sources and chemical composition of them. A comparison among different characterization methods is presented in Table 4 , where the requirement for samples as well as advantages and disadvantages of each method was shown. Direct observation of MPs by visual inspection is adopted in most previous studies (Lares et al., 2018; Wagner et al., 2016). Microscopes including ordinary optical microscope, stereoscope, fluorescent microscope and scanning electron microscope can be used to record the classification and abundance of large MPs with distinctive colors or morphologies (Politikos et al., 2017; Vermeiren et al., 2020; Wang et al., 2017c). However, visual inspection is a subjective method for the identification of MPs because different observation results may be obtained by different observers, and a considerable amount of time is needed to conduct this work (Prata et al., 2019a). Furthermore, it is rather difficult to distinct those MPs from other organic or inorganic matters whose particle sizes are similar to MPs (Fries et al., 2013). Thus, spectroscopic approaches such as Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy and pyrolysis-gas chromatography coupled to mass spectrometry (Pyr-GC-MS) serve as more accurate methods to identify the polymer types of MPs. Owing to the attributes of cost, efficiency and reliability, these methods are widely used and highly recommended by many researches (Cauwenberghe and Janssen, 2014; Cho et al., 2019; Dümichen et al., 2014, 2017; Li et al., 2015; Ruiz-Orejón et al., 2016; Thushari et al., 2017).
Table 4.
Basic information of frequently-used characterization instruments of MPs (ATR-FTIR: attenuated total reflectance-FTIR; μ-FTIR: FTIR microspectroscopy; SRS: stimulated Raman scattering; μ-Raman: micro-Raman spectroscopy; Pyr-GC-MS: pyrolysis-gas chromatography + mass spectrometry; TED-GC-MS: thermo-extraction and desorption + GC-MS; HPLC-UV: high performance liquid chromatography + UV).
| Instrument | Measured size range | Sample amount | Requirement for MPs samples | Advantages/Limitations | References | |
|---|---|---|---|---|---|---|
| Ordinary microscope | 1–5 mm | – | distinctive colors or morphologies | Low cost, easy operation; time-consuming, inaccurate observation. | Politikos et al. (2017); Ruiz-Orejón et al. (2016); | |
| FTIR | ATR-FTIR | >500 μm | 1 mg | thin/thick fibers/films, powder; irregularly shaped |
Non-destructive to samples, reliable, fast; samples <20 μm cannot be detected, expensive. | Cho et al. (2019); Claessens et al. (2011); Dekiff et al. (2014); Li et al. (2015). |
| μ-FTIR | down to 20 μm | thick and opaque; regularly shaped |
||||
| Raman Spectroscopy | SRS | 1–20 μm | 1 μg | no requirement for sample thickness; | Able to analyze very small samples with high spatial resolution, possible to analyze opaque and dark samples, fast measurement; easily being interfered, requiring pre-selection of samples, expensive. | Cauwenberghe and Janssen (2014); Karami et al. (2016); Lenz et al. (2015); Thushari et al. (2017). |
| μ-Raman | >1 μm | |||||
| Pyr-GC-MS | >500 μm | 0.5 mg | identical samples under different pyrolysis temperature | Samples can be analyzed with organic plastic additives, sensitive and reliable; destructive to samples. |
Fries et al. (2013); Nuelle et al. (2014). |
|
| TED-GC-MS | – | 100 mg | no limitation to the size and purity of decomposition products | Be able to analyze large sample amount, fast and reliable; | Dümichen et al. (2014); Dümichen et al. (2017). | |
| HPLC-UV | – | 20–100 μL | aqueous and hydrocarbon-based | high recovery rate for certain polymers; only assessing small amount of samples per run. | Elert et al. (2017); Hintersteiner et al. (2015). | |
The polymer types of MPs can be identified by comparing the FTIR spectra with known spectra. FTIR can identify MPs with a minimum particle size of 20 μm while Raman spectroscopy allows the characterization of MPs whose particle size is smaller than 20 μm (Dekiff et al., 2014). Multiple FTIR techniques have been applied to analyzing MPs. For instance, FTIR microspectroscopy (μ-FTIR) can detect smaller MPs (20–300 μm) and provide high-resolution map (Harrison et al., 2012; Löder et al., 2015; Rocha-Santos and Duarte, 2015); attenuated total reflectance-FTIR (ATR-FTIR) is capable of identifying thick or opaque MPs (Nor and Obbard, 2014; Shim et al., 2017; Prata et al., 2019a). Stimulated Raman scattering (SRS) is suitable to characterize MPs on low Raman background filter membrane when no pretreatment processes is conducted (Zada et al., 2018), but the cost of SRS is relatively high. As Raman spectroscopy (including micro-Raman spectroscopy (μ-Raman)) can identify extremely small-sized MPs, it is always used together with FTIR spectroscopy (Ghosal et al., 2018; Karami et al., 2016). However, the accuracy of Raman spectroscopy would be affected by colored matters, additives and other pollutants that are attached to the MPs (Lenz et al., 2015).
Pyr-GC-MS has also been applied to analyzing the chemical composition and structural characteristics of higher molecular weight polymers by detecting their thermal degradation products (Nuelle et al., 2014). Fries et al. (2013) used sequential Pyr-GC-MS to simultaneously identify polymer types of MPs and analyze the content of organic plastic additives. This method could provide a more complete overview of the sample and could more accurately evaluate the actual chemical property of the sample (Tianniam et al., 2010). Nevertheless, wrong results may be obtained by this method for different polymers may produce similar thermal degradation products (Nuelle et al., 2014). Besides, Pyr-GC-MS is destructive to samples and cannot provide any information about the sizes, shapes and concentrations of MPs (Fabbri et al., 2000). Another deficiency of this method is that it can only analyze a very small number of simple and homogenous samples (0.5 mg) per run (Dümichen et al., 2017). Comparing with Pyr-GC-MS, thermo-extraction and desorption coupled to gas chromatography-mass spectroscopy (TED-GC-MS) has been used to measure relatively large numbers of sample masses (100 mg), and it is able to analyze complex and heterogeneous samples (Dümichen et al., 2014). Apart from that, pretreatment of standard MPs particles is not required when employing TED-GC-MS to characterize MPs (Elert et al., 2017).
Liquid chromatography is a less frequently used characterization method of MPs, and it is usually applied with the assist of UV detection. Elert et al. (2017) used size exclusion chromatography analyze PS and PET, and they found that changes in the molecular mass could be observed. Based on the different solubility of MPs, polymers can be dissolved by appropriate eluents. The advantage of this method is that larger numbers of samples can be analyzed and thus it can improve the representativeness of the characterization, but it is also destructive to samples and only shows the chemical composition of the samples. Different molecular weight distribution can be measured by high performance liquid chromatography (HPLC) coupled with size exclusion systems. As shown in the studies of Hintersteiner et al. (2015), HPLC-UV and HPLC-Orbitrap-MS have high recoveries of selected polymers, although this method cannot determine the particle size of the samples and can only analyze certain types of polymers such as PS and PE. Therefore, more investigations are required to verify this method for MPs identification.
Several less frequently used methods for the characterization of MPs have been reported in some studies. For instance, Ceccarini et al. (2018) used gel permeation chromatography to determine the molecular weight and molecular weight distribution of the polymeric fractions; Turner (2017) applied a portable X-ray fluorescence spectrometer to analyzing the elemental compositions of polymers; Shim et al. (2016) has reported a simple staining method by using Nile Red to stain highly hydrophobic polymers, and which could differentiate fragmented polypropylene particles from sand particles. MPs have complicated chemical characterizations and are potential carriers for other contaminants such as heavy metals and persistent organic pollutants. Moreover, MPs may also undergo complex natural processes and dramatic morphological change in the environment (Fu et al., 2020). Therefore, it is urgent to develop new analytical technologies or combine existed methods to obtain more reliable characterization data of MPs.
4. Ecotoxicological effects of MPs on the environment
4.1. Effects of MPs on aquatic organisms
The aforementioned section showed the abundance of MPs in several typical freshwater systems in China, which proved that MPs had wide distribution in the environment. As a result, MPs may pose potential threat to the environment even humans; however, little is known about their actual effects on organisms and human health. It is noticeable that increasing attention has been paid to the ecotoxicology of MPs, and escalating studies have investigated possible adverse effects of MPs on different organisms in recent years. Toxicological experiments usually used known polymer types of MPs to study the according impacts. Therefore, it is necessary to study the ecotoxicological effects of MPs after polymer types and physic-chemical properties of MPs were analyzed.
Nowadays, research focus of the impact of MPs on aquatic organisms is on marine organisms. It was reported that plankton, bivalves, fish and mammals could be affected by the presence of MPs (Besseling et al., 2015; Cauwenberghe and Janssen, 2014; Desforges et al., 2015; Foekema et al., 2013). Evidence has shown that MPs exist in manifold variety of organisms, which suggests the transport of MPs along food chain and food web (Besseling et al., 2017). Besides, as ingested MPs mainly accumulate in the gastrointestinal tract, it could be deduced that one of the major risks that MPs might cause to organisms is physical and chemical damage to their digestive process (Davidson and Dudas, 2016; Wright et al., 2013b).
Negative effects of MPs on different aquatic organisms such as growth inhibition and low reproduction rate were found in some studies. Effects of MPs on three classifications including plankton, invertebrate and vertebrate were summarized in Table 5 . From Table 5, it could be seen that PS is the most frequently used material to investigate the toxicity of MPs on plankton. Another characteristic of the effects of MPs on plankton is that small-sized MPs are more easily to exert detrimental influence. One of the possible reasons might be that extremely small-sized MPs could act as the substitute of nutrients that plankton needed, resulting in a sustained loss of energy inputs, and consequently leading to death of organisms (Botterell et al., 2019). Impacts of MPs on phytoplankton were seen on microalgae. Some studies have suggested that the growth rate, chlorophyll content and photosynthesis activity of microalgae were negatively affected by the presence of MPs (Prata et al., 2019a; Xu et al., 2020). Moreover, according to the investigation of Prata et al. (2019b), the disturbing effects of MPs could decrease available nutrients that microalgae needed by functioning as substrates, which revealed a potential assimilation metabolism of MPs by microalgae.
Table 5.
Ecotoxicological effects of MPs on aquatic organisms (PE: polyethylene; PS: polystyrene).
| Organism | Species | MPs type | MPs size (μm) | Exposure concentration | Exposure time | Ecotoxicological effects | References |
|---|---|---|---|---|---|---|---|
| plankton | Scenedesmus obliquus | PS | 0.07 | 1000 mg/L | 72 h | 2.5% growth inhibition; 50% decrease in chlorophyll content |
Besseling et al. (2014) |
| Chlorella sp. | PS | 0.02 | 6.5 mg/L | 65 h | 33.3% increase reactive oxygen species | Bhattacharya et al. (2010) | |
| Chaetoceros neogracile | PS | 2 | 3.95 μg/L | 30 d | aggregate sinking rate was 18 times lower than the control group | Long et al. (2017) | |
| Centropages typicus | PS | 20.6 | 0.1% v/v | 24 h | 25% decrease in herbivory | Cole et al. (2013) | |
| invertebrate | Gammarus fossarum | PS | 1.6 | 12500 beads/L | 28 d | 22% decrease in assimilation efficiency | Blarer and Burkhardt-Holm (2016) |
| Mytilus galloprovincialis | PE PS |
<100 | 20 g/L | 7 d | accumulated in digestive tissues: 3.6–6.5 folds higher than the control | Avio et al. (2015a) | |
| Corbicula fluminea | unknown | 5 | 0.13 mg/L | 8 d | accumulated in gills damage: 2 folds higher than the control | Oliveira et al. (2018) | |
| Eriocheir sinensis | PS | 5 | 40000 mg/L | 7 d | accumulated in liver: 1.66 μg/mg of tissue dry weight | Yu et al. (2018) | |
| vertebrate | Danio rerio | PS | 1 | 0.750–0.047 mg/L | 10 d | 39% decrease in predatory performance | Prokić et al. (2019) |
| Pomatoschistus microps | PE | 1–5 | 1 mg/L | 7 d | oxidative damage; |
Ferreira et al. (2016); Oliveira et al. (2013) |
As surface waters contain relatively high abundance of MPs than deep water, zooplankton which predominately feeds in this area is more easily to encounter and take in them (Cózar et al., 2014). Known effects of MPs on zooplankton include but not limited to inhibition of growth and feeding, reproduction and life span (Botterell et al., 2019). For example, Cole et al. (2014) demonstrated that MPs could influence the feeding of copepod, and the ingestion rates of copepod Centropages typicus were testified to have a significant dose-response relationship with MPs concentration and total algal ingestion rates. Lee et al. (2013) showed that the fecundity of copepod Tigriopus japonicas was decreased by exposing to different concentrations of PS microbead. Lo and Chan (2018) found that gastropod Crepidula onyx could ingest PS microbead, which led to slow growth.
Ecotoxicologic experiments that investigate the effects of MPs on invertebrate often utilize Mytilus galloprovincialis as a bioindicator, by which the quality of marine environments can be evaluated and monitored (Capillo et al., 2018). As invertebrate usually have no specific enzymes to digest synthetic polymers, and thus once MPs are ingested by these organisms, they will not be digested or absorbed (Andrady, 2011). However, the ingestion of MPs might cause a series of problems to creatures. Some investigations showed that the main adverse effect of MPs is cellular damage resulting from oxidative stress (Avio et al., 2015a; Pagano et al., 2016; Sureda et al., 2018). These studies demonstrated that MPs could reduce the energy input and further damage the immune functions, and continuous accumulation of MPs could also exert chronic effects on the life span of invertebrates.
The ecotoxicological effects of MPs on vertebrate are represented by the influences that MPs could have on fishes, for fishes are typical vertebrates in aquatic environments. As shown in some studies, major impacts of MPs on fishes include intestinal damage, oxidative damage and reduction of predatory activity (Ferreira et al., 2016; Prokić et al., 2019). Because fishes are important food for humans, chances are that individual fitness and population fitness would be decreased when MPs-polluted fishes are consumed by human. Another nonnegligible fact is that MPs could change the bioavailability and ways of adsorbing metal contaminants, which indicate that other pollutants such as heavy metals could co-exist with MPs, and might cause certain co-effect to aquatic organisms (Guzzetti et al., 2018).
Mammals such as whales were also reported to have MPs ingestion and bioaccumulation. Fossi et al. (2016) showed that organochlorine compounds were found in fin whales suggesting that MPs might have potential effects on them. Nevertheless, the metabolism mechanisms and effect mechanisms of MPs on aquatic organisms are still remained to be explored.
4.2. Effects of MPs on soil biota
The investigation of Ng et al. (2018) has showed that 79% of global plastic wastes is accumulated in terrestrial environment. Nevertheless, compared with the aquatic environments, the studies of MPs in soils are relatively insufficient. MPs can change the structure and function of soils and can also affect microbial diversity (Rillig, 2012). Whether soils are health or not determines food quality as well as food safety, which will ultimately threaten human health (Murugan et al., 2014). On the other hand, complex terrestrial environment is also able to impact the ecotoxicology of MPs, and interaction between MPs and soils may lead to unpredictable effects on the environmental behavior of other contaminants, which could result in more complicated ecotoxicological impact on soil (Guo et al., 2020).
The effects of of MPs on terrestrial plants are scarcely reported, and only limited plants are investigated. The study of Jassby et al. (2019) showed that MPs might be taken in by agricultural plants such as wheat and then further entered into food chain. The experiments of Qi et al. (2018) demonstrated that the presence of MPs had negative impacts on wheat growth tissue elemental composition at both vegetative and reproductive stages. Furthermore, leaf and root traits together with total biomass of Allium fistulosum were reported to be affected by three kinds of MPs, namely, microfiber, microbead and micro-fragment (de Souza Machado et al., 2019). This study found that all three kinds of MPs would significantly change the biomass, tissue elemental composition (e.g. water content and leaf nitrogen content), root traits (e.g. root length, diameter, density) and root symbioses (de Souza Machado et al., 2019). Moreover, several potential positive and negative mechanisms that MPs might work on plants were proposed by Rillig et al. (2019), and their study showed that the effects of MPs would vary in terms of plant species. However, these mechanistic pathways should be tested in the near future.
MPs could exert direct and indirect influence on soil animals. MPs may adhere to the surface of some soil animals so that their movement will be hindered (Kim and An, 2019). If MPs are mistakenly ingested by soil organism, reduce in carbon biomass ingestion and further energy depletion would happen (Cole et al., 2013). Earthworms are the most commonly researched targets when studying the toxic effects of MPs on soil animals. Wang et al. (2019a) found that MPs at low concentrations (<20% (w/w)) had no significant impacts on growth and death rates of earthworms (Eisenia fetida); whereas the growth of Oligochaeta lumbricidae was significantly inhibited when the concentrations of MPs were higher than 28% (Huerta Lwanga et al., 2016). In addition to earthworms, other small soil invertebrates including springtails, nematodes and snails could also ingest MPs. For instance, MPs could decrease the gut microbial community, reproduction and avoidance behaviors of springtails (Kim and An, 2019; Ju et al., 2019); MPs could disrupt energy metabolism, lower down the locomotor behavior and decrease the body length of nematodes (Caenorhabditis elegans) (Kim et al., 2019; Lei et al., 2018); MPs inhibited food intake and excretion of snails (Achatina fulica) and affected oxidative stress (Song et al., 2019). The indirect effects of MPs mainly refer to their adsorption and combination of other pollutants such as heavy metals and organic contaminants, which will aggravates soil pollution and amplify the risks posed to soil organisms and humans (Hüffer et al., 2019; Wang et al., 2017a). Results from Hodson et al. (2017) suggested that the bioavailability of zinc increased by adsorbed onto MPs, and earthworms ingesting zinc-adsorbed MPs accumulated more zinc in their guts. On the contrary, high concentration of MPs in soils might reduce the accumulation of polycyclic aromatic hydrocarbons and polychlorobiphenyls as reported by Wang et al. (2019b). Therefore, the adsorption and desorption mechanisms of MPs to other environmental pollutants need to be further studied.
Microorganisms as the primary decomposers are one of the good indicators of the soil health condition, but current studies of the effects of MPs on soil microorganisms are limited. Soil properties are closely related to soil microbial activity. It was held that the transport of MPs in soils might change soil microbial community (Li et al., 2020). Changes in soil aggregation by MPs could affect microbial evolution, and MPs could provide a novel ecological habitat for soil microorganisms (Rillig et al., 2018; Wang et al., 2019b). On the other hand, MPs could also change soil porosity and soil moisture, which would affect the relative distribution of anaerobic and aerobic microorganisms in soils (Rubol et al., 2013). Changes in soil pores may result in loss of microhabitat and extinction of indigenous microorganisms (Veresoglou et al., 2015). Bacteria, fungi and soil enzymes are three parameters to assess the impacts of MPs on soil microbiota. For example, some bacteria (Vibrionaceaeor and Pseudoalteromonadaceae) were detected in large amounts on the surface of microplastic debris, while they were seldom found in natural soil surroundings (Tender et al., 2015). Bläsing and Amelung (2018) reported that fungus Zalerion maritimum could biodegrade PE and promote their growth. However, MPs were also found accumulated in fungi such as yeasts and filamentous fungi (Chae and An, 2018). As for soil enzymes, both positive and negative effects of MPs were showed. Liu et al. (2017) demonstrated that enzymatic activity was enhanced by the addition of MPs, benefiting the input of C, N and P into soils. Whereas adding small polyacrylic and PE particles would have negative effects on the enzymatic activity of fluorescein diacetate hydrolase, as shown in the study of de Souza Machado et al. (2018). To sum up, how the presence of MPs in soil affected microorganisms still remained unknown, and more efforts should be made to address issues in this field.
4.3. Effects of MPs on human health
MPs are ubiquitous in the environment, which makes it unavoidable for humans to be exposed. Nevertheless, currently there are inadequate studies on health risks that MPs may cause to human. Similar to other pollutants, humans contact MPs mainly via three main pathways, namely ingestion, inhalation and dermal exposure. Correspondingly, the effects of MPs on human health are embodied in these aspects, but dermal contact was rarely reported to have significant influence on human health., as only particle with sizes <100 nm could transverse dermal barrier (Revel et al., 2018). Overall, MPs exposure to humans may result in particle toxicity, with oxidative stress, inflammatory lesions and increased uptake or translocation, and as the immune system cannot remove MPs, it may cause chronic inflammation and increase risk of neoplasia (Prata et al., 2020).
Ingestion is regarded as the major way that humans expose to MPs (Galloway et al., 2017). As MPs were detected in some food such as fish and table salt, the consumption of these stuffs might lead to possible ingestion of MPs by humans (Barboza et al., 2018; Karami et al., 2017). MPs could enter into the gastrointestinal system once MPs-polluted foodstuff are taken up by human, specialized M-cells in the intestine might absorb small microplastic particles, and consequently covering intestine lymphoid tissues, causing inflammatory response and changing gut microbe composition and metabolism (Ensign et al., 2012; Salim et al., 2013). Powell et al. (2007) suggested there was a possibility that insoluble MPs might penetrate intestinal mucus to get solubility increased. Impacts including gene expression, inhibited cell viability, induced pro-inflammatory responses and morphological changes were recorded in the research of Forte et al. (2016), when MPs were internalized by human gastric adenocarcinoma cells. Although food and drinking water are widely contaminated by MPs making MPs ingestion unavoidable by humans, how MPs negatively affect human health is little known for relative studies are limited.
Because MPs can release into air from various sources such as synthetic textiles and car tires, humans might also inhale MPs in the environment. Dris et al. (2017) showed that MPs concentrations in outdoor air ranging from 0.3 to 1.5 particles/m3. Moreover, the study of Prata (2018) estimated that average MPs concentration inhaled by individuals was 26–130 MPs/d. Respiratory symptoms such as dyspnea and interstitial inflammatory responses might be induced when considerable amount of MPs were inhaled by susceptible individuals, especially those who exposed to microplastic environments (industrial workers) for a long time (Prata, 2018). A couple of studies showed that interstitial lung disease occurred in synthetic textile, flock, vinyl chloride and PVC industries (Atis et al., 2005; Pimentel et al., 1975; Xu et al., 2004). Studnicka et al. (1995) reported that vinyl chloride and PVC industries workers who exposed for 10 years had diseases like pneumoconiosis, narrowing of bronchial segments, foreign body granulomas, and fibrotic changes of the alveolar wall. In addition, cancer risk may be increased when chronically exposing to synthetic fiber dust, though more confirmed evidences were not founded (Mastrangelo et al., 2002).
Studies of MPs adverse effects on human health are rather limited. Nonetheless, as the accumulation of MPs along tropic chain, the potential health risks that MPs may cause to humans should not be ignored. Moreover, measurement of human exposure to MPs should be more precisely conducted by using diagnostic tools including MPs identification and toxicity determination. Further studies focusing on human exposure to MPs are urgently needed to fully understand the risks of MPs to humans.
5. Conclusions
This work presented a comprehensive review on the status quo of abundance, distribution, sampling and analyses of MPs in both waters and soils. Moreover, potential ecological risks that MPs may pose to the environments were also elucidated. Four key conclusions were made as follows:
-
(1)
Freshwater environments in cities with dense population and large agricultural, fishery and industrial scales contained higher MPs concentrations, but MPs were also detected in rural freshwater systems. MPs with particle size less than 1 mm were most abundant and microfiber was a common polymer shape in surface waters and sediments of several typical freshwater systems in China. Besides, among all the detected MPs samples, polymer types of PE and PP were the most common two.
-
(2)
Zooplankton nets were the major tools for sampling MPs from waters, and forceps, sieves, grab samplers, box corers and spade corers were often used to collect MPs from sediments. However, deficiencies including limited sampling volumes and non-uniform sampling methods have hindered quantification of MPs in the environment.
-
(3)
Pretreatment methods including density separation, filtration, acidic/alkaline digestion, oxidization and enzymatic digestion could efficiently remove impurities from MPs samples. Advanced characterization technologies such as FTIR, Raman and Pyr-GC-MS were frequently employed to analyze the chemical composition and other properties of MPs.
-
(4)
MPs have been reported to have ecological impacts on aquatic organisms, soil biota and human health. Inhibition in growth and decrease in assimilation efficiency were seen in both aquatic and terrestrial organisms. Digestive and respiratory damages to humans might be caused by the ingestion and inhalation of MPs. Nevertheless, metabolic mechanisms of MPs in organisms and humans were generally lacking.
Although the studies of MPs have been springing up in the last decade, research gaps still existed and should be filled in the future. Suggestions were proposed on the grounds of the current knowledge of MPs:
-
(1)
Current studies provided limited perspectives and information about MPs in freshwaters and soils due to that the research of MPs is first focused on marine environment. The study of MPs including their occurrence, fate and toxicity in freshwater and terrestrial environment are still insufficient. In addition, freshwater as the source of drinking water and soil as the matrix of cereals and vegetables, both of them are closely related to human health. Thus, more studies should be conducted to better understand how MPs distribute and influence freshwater and soil health.
-
(2)
Different sampling and analytical methods will lead to different results of MPs concentration and chemical properties. It is known that MPs can act as carriers of other contaminants including heavy metals and persistent organic pollutants. Furthermore, MPs may undergo complicated environmental processes such as solar radiation, mechanical abrasion and biodegradation, which will hamper the characterization and quantification of MPs. As a result, it is necessary to establish a standard and reliable procedure to sample, separate, purify and identify MPs. Noval characterization tools or a proper combination use of existed technologies are needed to analyze MPs in complex environmental matrix.
-
(3)
The detrimental effects of MPs on soil biota and humans are inadequately researched compared with aquatic organisms, so that future investigations should concentration on the two aspects. Besides, the mechanism that MPs interact with different organisms in nature is remained to be revealed. Realistic MPs samples in the environment are rather complicated, and thus complex composition of MPs and actual exposure conditions should be considered when carrying on ecotoxicological experiments in laboratory.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No.: 51978178, 51478172 and 51521006), the Department of Science and Technology of Guangdong Province of China (Contract No.: 2019A1515012044), Maoming Municipal Bureau of Science and Technology of Guangdong Province of China (Contract No.: 2018S0011), the International S&T Cooperation Program of China (Contract No.: 2015DFG92750)
References
- Andrady A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011;62:1596–1605. doi: 10.1016/j.marpolbul.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Atis S., Tutluoglu B., Levent E., Ozturk C., Tunaci A., Sahin K., Saral A., Oktay I., Kanik A., Nemery B. The respiratory effects of occupational polypropylene flock exposure. Eur. Respir. J. 2005;25:110–117. doi: 10.1183/09031936.04.00138403. [DOI] [PubMed] [Google Scholar]
- Avio C.G., Gorbi S., Milan M., Benedetti M., Fattorini D., D’ Errico G., Pauletto M., Bargelloni L., Regoli F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015;198:211–222. doi: 10.1016/j.envpol.2014.12.021. [DOI] [PubMed] [Google Scholar]
- Avio C.G., Gorbi S., Regoli F. Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: first observations in commercial species from Adriatic Sea. Mar. Environ. Res. 2015;111:18–26. doi: 10.1016/j.marenvres.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Barboza L.G.A., Vethaak A.D., Lavorante B.R.B.O., Lundebye A.K., Guilhermino L. Marine microplastic debris: an emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018;133:336–348. doi: 10.1016/j.marpolbul.2018.05.047. [DOI] [PubMed] [Google Scholar]
- Baztan J., Carrasco A., Chouinard O., Cleaud M., Gabaldon J.E., Huck T., Jaffrès L., Jorgensen B., Miguelez A., Paillard C., Vanderlinden J.P. Protected areas in the Atlantic facing the hazards of micro-plastic pollution: first diagnosis of three islands in the Canary Current. Mar. Pollut. Bull. 2014;80:302–311. doi: 10.1016/j.marpolbul.2013.12.052. [DOI] [PubMed] [Google Scholar]
- Bergmann M., Wirzberger V., Krumpen T., Lorenz C., Primpke S., Tekman M.B., Gerdts G. High quantities of microplastic in Arctic deep-sea sediments from the HAUSGARTEN observatory. Environ. Sci. Technol. 2017;51:11000–11010. doi: 10.1021/acs.est.7b03331. [DOI] [PubMed] [Google Scholar]
- Besley A., Vijver M.G., Behrens P., Bosker T. A standardized method for sampling and extraction methods for quantifying microplastics in beach sand. Mar. Pollut. Bull. 2017;114:77–83. doi: 10.1016/j.marpolbul.2016.08.055. [DOI] [PubMed] [Google Scholar]
- Besseling E., Foekema E.M., Franeker J.A.V., Leopold M.F., Kühn S., Rebolledo E.L.B., Heße E., Mielke L., Ijzer J., Kamminga P. Microplastic in a macro filter feeder: humpback whale Megaptera novaeangliae. Mar. Pollut. Bull. 2015;95:248–252. doi: 10.1016/j.marpolbul.2015.04.007. [DOI] [PubMed] [Google Scholar]
- Besseling E., Quik J.T.K., Sun M., Koelmans A.A. Fate of nano- and microplastic in freshwater systems: a modeling study. Environ. Pollut. 2017;220:540–548. doi: 10.1016/j.envpol.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Besseling E., Wang B., Lürling M., Koelmans A.A. Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environ. Sci. Technol. 2014;48:12336–12343. doi: 10.1021/es503001d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya P., Lin S., Turner J.P., Ke P.C. Physical adsorption of charges plastic nanoparticles affect algal photosynthesis. J. Phys. Chem. C. 2010;114:16556–16561. [Google Scholar]
- Blarer P., Burkhardt-Holm P. Microplastics affect assimilation efficiency in the freshwater amphipod Gammarus fossarum. Environ. Sci. Pollut. Control Ser. 2016;23:23522–23532. doi: 10.1007/s11356-016-7584-2. [DOI] [PubMed] [Google Scholar]
- Bläsing M., Amelung W. Plastics in soil: analytical methods and possible sources. Sci. Total Environ. 2018;612:422–435. doi: 10.1016/j.scitotenv.2017.08.086. [DOI] [PubMed] [Google Scholar]
- Botterell Z.L.R., Beaumont N., Dorrington T., Steinke M., Thompson R.C., Lindeque P.K. Bioavailability and effects of microplastics on marine zooplankton: a review. Environ. Pollut. 2019;245:98–110. doi: 10.1016/j.envpol.2018.10.065. [DOI] [PubMed] [Google Scholar]
- Browne M.A., Phillip C., Niven S.J., Emma T., Andrew T., Tamara G., Richard T. Accumulation of microplastic on shorelines worldwide: sources and sinks. Environ. Sci. Technol. 2011;45:9175–9179. doi: 10.1021/es201811s. [DOI] [PubMed] [Google Scholar]
- Capillo G., Silvestro S., Sanfilippo M., Fiorino E., Giangrosso G., Ferrantelli V., Vazzana I., Faggio C. Assessment of electrolytes and metals profile of the faro lake (capo peloro lagoon, sicily, Italy) and its impact on Mytilus galloprovincialis. Chem. Biodivers. 2018;15:1800044–1800053. doi: 10.1002/cbdv.201800044. [DOI] [PubMed] [Google Scholar]
- Catarino A.I., Thompson R., Sanderson W., Henry T.B. Development and optimization of a standard method for extraction of microplastics in mussels by enzyme digestion of soft tissues. Environ. Toxicol. Chem. 2017;36:947–951. doi: 10.1002/etc.3608. [DOI] [PubMed] [Google Scholar]
- Cauwenberghe L.V., Janssen C.R. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 2014;193:65–70. doi: 10.1016/j.envpol.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Ceccarini A., Corti A., Erba F., Modugno F., Nasa J.L., Bianchi S., Castelvetro V. The hidden microplastics: new insights and figures from the thorough separation and characterization of microplastics and of their degradation byproducts in coastal sediments. Environ. Sci. Technol. 2018;52:5634–5643. doi: 10.1021/acs.est.8b01487. [DOI] [PubMed] [Google Scholar]
- Chae Y., An Y.J. Current research trends on plastic pollution and ecological impacts on the soil ecosystem: a review. Environ. Pollut. 2018;240:387–395. doi: 10.1016/j.envpol.2018.05.008. [DOI] [PubMed] [Google Scholar]
- Cho Y., Shim W.J., Jang M., Han G.M., Hong S.H. Abundance and characteristics of microplastics in market bivalves from South Korea. Environ. Pollut. 2019;245:1107–1116. doi: 10.1016/j.envpol.2018.11.091. [DOI] [PubMed] [Google Scholar]
- Cincinelli A., Martellini T., Guerranti C., Scopetani C., Chelazzi D., Giarrizzo T. A potpourri of microplastics in the sea surface and water column of the Mediterranean Sea. Trac. Trends Anal. Chem. 2019;110:321–326. [Google Scholar]
- Cincinelli A., Scopetani C., Chelazzi D., Lombardini E., Martellini T., Katsoyiannis A., Fossi M.C., Corsolini S. Microplastic in the surface waters of the Ross Sea (Antarctica): occurrence, distribution and characterization by FTIR. Chemosphere. 2017;175:391–400. doi: 10.1016/j.chemosphere.2017.02.024. [DOI] [PubMed] [Google Scholar]
- Claessens M., Meester S.D., Landuyt L.V., Clerck K.D., Janssen C.R. Occurrence and distribution of microplastics in marine sediments along the Belgian coast. Mar. Pollut. Bull. 2011;62:2199–2204. doi: 10.1016/j.marpolbul.2011.06.030. [DOI] [PubMed] [Google Scholar]
- Cole M., Lindeque P., Fileman E., Halsband C., Goodhead R., Moger J., Galloway T.S. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013;47:6646–6655. doi: 10.1021/es400663f. [DOI] [PubMed] [Google Scholar]
- Cole M., Webb H., Lindeque P.K., Fileman E.S., Halsband C., Galloway T.S. Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci. Rep. 2014;4:4528–4535. doi: 10.1038/srep04528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collignon A., Hecq J.H., Glagani F., Voisin P., Collard F., Goffart A. Neustonic microplastic and zooplankton in the north western mediterranean sea. Mar. Pollut. Bull. 2012;64:861–864. doi: 10.1016/j.marpolbul.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Courtene-Jones W., Quinn B., Murphy F., Gary S.F., Narayanaswamy B.E. Optimisation of enzymatic digestion and validation of specimen preservation methods for the analysis of ingested microplastics. Analytical Methods. 2017;9:1437–1445. [Google Scholar]
- Cózar A., Echevarría F., González-Gordillo J.I., Irigoien X., Ubeda B., Hernández-León S., Palma A.T., Navarro S., García-De-Lomas J., Ruiz A., Fernández-de-Puellesh M.L., Duartei C.M. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. Unit. States Am. 2014;111:10239–10244. doi: 10.1073/pnas.1314705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton E.M., Noël M., Gies E.A., Ross P.S. A novel, density-independent and FTIR-compatible approach for the rapid extraction of microplastics from aquatic sediments. Analytical Methods. 2017;9:1419–1428. [Google Scholar]
- Davidson K., Dudas S.E. Microplastic ingestion by wild and cultured manila clams (Venerupis philippinarum ) from baynes sound, British columbia. Arch. Environ. Contam. Toxicol. 2016;71:147–156. doi: 10.1007/s00244-016-0286-4. [DOI] [PubMed] [Google Scholar]
- de Souza Machado A.A., Lau C.W., Kloas W., Bergmann J., Bachelier J.B., Faltin E., Becker R., Görlich A.S., Rillig M.C. Microplastics can change soil properties and affect plant performance. Environ. Sci. Technol. 2019;53:6044–6052. doi: 10.1021/acs.est.9b01339. [DOI] [PubMed] [Google Scholar]
- de Souza Machado A.A., Lau C.W., Till J., Kloas W., Lehmann A., Becker R., Rillig M.C. Impacts of microplastics on the soil biophysical environment. Environ. Sci. Technol. 2018;52:9656–9665. doi: 10.1021/acs.est.8b02212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaut A., Cassone A.L., Frère L., Hermabessiere L., Himber C., Rinnert E., Rivière G., Lambert C., Soudant P., Huvet A., Duflos G., Paul-Pont I. Microplastics in seafood: benchmark protocol for their extraction and characterization. Environ. Pollut. 2016;215:223–233. doi: 10.1016/j.envpol.2016.05.018. [DOI] [PubMed] [Google Scholar]
- Dekiff J.H., Remy D., Klasmeier J., Fries E. Occurrence and spatial distribution of microplastics in sediments from Norderney. Environ. Pollut. 2014;186:248–256. doi: 10.1016/j.envpol.2013.11.019. [DOI] [PubMed] [Google Scholar]
- Desforges J.P.W., Galbraith M., Dangerfield N., Ross P.S. Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean. Mar. Pollut. Bull. 2014;79:94–99. doi: 10.1016/j.marpolbul.2013.12.035. [DOI] [PubMed] [Google Scholar]
- Desforges J.P.W., Galbraith M., Ross P.S. Ingestion of microplastics by zooplankton in the northeast pacific ocean. Arch. Environ. Contam. Toxicol. 2015;69:320–330. doi: 10.1007/s00244-015-0172-5. [DOI] [PubMed] [Google Scholar]
- Di X.M., Wang J. Microplastics in surface waters and sediments of the three gorges reservoir, China. Sci. Total Environ. 2018;616–617:1620–1627. doi: 10.1016/j.scitotenv.2017.10.150. [DOI] [PubMed] [Google Scholar]
- Ding L., Mao R.F., Guo X.T., Yang X.M., Zhang Q., Yang C. Microplastics in surface waters and sediments of the Wei River, in the northwest of China. Sci. Total Environ. 2019;667:427–434. doi: 10.1016/j.scitotenv.2019.02.332. [DOI] [PubMed] [Google Scholar]
- Doyle M.J., Watson W., Bowlin N.M., Sheavly S.B. Plastic particles in coastal pelagic ecosystems of the Northeast Pacific ocean. Mar. Environ. Res. 2011;71:41–52. doi: 10.1016/j.marenvres.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Dris R., Gasperi J., Mirande C., Mandin C., Guerrouache M., Langlois V., Tassin B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 2017;221:453–458. doi: 10.1016/j.envpol.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Dubaish F., Liebezeit G. Suspended microplastics and black carbon particles in the jade system, Southern North Sea. Water, Air. & Soil Pollution. 2013;224:1352–1359. [Google Scholar]
- Dümichen E., Braun U., Senz R., Fabian G., Sturm H. Assessment of a new method for the analysis of decomposition gases of polymers by a combining thermogravimetric solid-phase extraction and thermal desorption gas chromatography mass spectrometry. J. Chromatogr. A. 2014;1354:117–128. doi: 10.1016/j.chroma.2014.05.057. [DOI] [PubMed] [Google Scholar]
- Dümichen E., Eisentraut P., Bannick C.G., Barthel A.K., Senz R., Braun U. Fast identification of microplastics in complex environmental samples by a thermal degradation method. Chemosphere. 2017;174:572–584. doi: 10.1016/j.chemosphere.2017.02.010. [DOI] [PubMed] [Google Scholar]
- Eerkes-Medrano D., Thompson R.C., Aldridge D.C. Microplastics in freshwater systems: a review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015;75:63–82. doi: 10.1016/j.watres.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Elert A.M., Becker R., Duemichen E., Eisentraut P., Falkenhagen J., Sturm H., Braun U. Comparison of different methods for microplastic detection: what can we learn from them, and why asking the right question before measurements matters? Environ. Pollut. 2017;231:1256–1264. doi: 10.1016/j.envpol.2017.08.074. [DOI] [PubMed] [Google Scholar]
- Ensign L.M., Cone R., Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012;64:557–570. doi: 10.1016/j.addr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen M., Mason S., Wilson S., Box C., Zellers A., Edwards W., Farley H., Amato S. Microplastic pollution in the surface waters of the laurentian great lakes. Mar. Pollut. Bull. 2013;77:177–182. doi: 10.1016/j.marpolbul.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Fabbri D., Tartari D., Trombini C. Analysis of poly (vinyl chloride) and other polymers in sediments and suspended matter of a coastal lagoon by pyrolysis-gas chromatography-mass spectrometry. Anal. Chim. Acta. 2000;413:3–11. [Google Scholar]
- Ferreira P., Fonte E., Soares M.E., Carvalho F., Guilhermino L. Effects of multi-stressors on juveniles of the marine fish Pomatoschistus microps: gold nanoparticles, microplastics and temperature. Aquat. Toxicol. 2016;170:89–103. doi: 10.1016/j.aquatox.2015.11.011. [DOI] [PubMed] [Google Scholar]
- Foekema E.M., De Gruijter C., Mergia M.T., Van Franeker J.A., Murk A.J., Koelmans A.A. Plastic in north sea fish. Environ. Sci. Technol. 2013;47:8818–8824. doi: 10.1021/es400931b. [DOI] [PubMed] [Google Scholar]
- Forte M., Iachetta G., Tussellino M., Carotenuto R., Prisco M., De Falco M., Laforgia V., Valiante S. Polystyrene nanoparticles internalization in human gastric adenocarcinoma cells. Toxicol. Vitro. 2016;31:126–136. doi: 10.1016/j.tiv.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Fossi M.C., Marsili L., Baini M., Giannetti M., Coppola D., Guarranti C., Caliani I., Minutoli R., Lauriano G., Finoia M.G., Rubegni F., Panigada S., Bérubé M., Ramirez J.U., Panti C. Fin whales and microplastics: the Mediterranean Sea and the sea of Cortez scenarios. Environ. Pollut. 2016;209:68–78. doi: 10.1016/j.envpol.2015.11.022. [DOI] [PubMed] [Google Scholar]
- Frias J.P.G.L., Otero V., Sobral P. Evidence of microplastics in samples of zooplankton from Portuguese coastal waters. Mar. Environ. Res. 2014;95:89–95. doi: 10.1016/j.marenvres.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Fries E., Dekiff J.H., Willmeyer J., Nuelle M.T., Ebert M., Remy D. Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environmental Science-Processes & Impacts. 2013;15:1949–1956. doi: 10.1039/c3em00214d. [DOI] [PubMed] [Google Scholar]
- Fu W.Y., Min J.C., Jiang W.Y., Li Y., Zhang W. Separation, characterization and identification of microplastics and nanoplastics in the environment. Sci. Total Environ. 2020;721:137561–137586. doi: 10.1016/j.scitotenv.2020.137561. [DOI] [PubMed] [Google Scholar]
- Galloway T.S., Cole M., Lewis C. Interactions of microplastic debris throughout the marine ecosystem. Nature Ecology & Evolution. 2017;1:116–123. doi: 10.1038/s41559-017-0116. [DOI] [PubMed] [Google Scholar]
- Ghosal S., Chen M., Wagner J., Wang Z.M., Wall S. Molecular identification of polymers and anthropogenic particles extracted from oceanic water and fish stomach - a Raman micro-spectroscopy study. Environ. Pollut. 2018;233:1113–1124. doi: 10.1016/j.envpol.2017.10.014. [DOI] [PubMed] [Google Scholar]
- Guo J.J., Huang X.P., Xiang L., Wang Y.Z., Li Y.W., Li H., Cai Q.Y., Mo C.H., Wong M.H. Source, migration and toxicology of microplastics in soil. Environ. Int. 2020;137:105263–105275. doi: 10.1016/j.envint.2019.105263. [DOI] [PubMed] [Google Scholar]
- Guzzetti E., Sureda A., Tejada S., Faggio C. Microplastic in marine organism: environmental and toxicological effects. Environ. Toxicol. Pharmacol. 2018;64:164–171. doi: 10.1016/j.etap.2018.10.009. [DOI] [PubMed] [Google Scholar]
- Hanvey J., Lewis P., Lavers J., Crosbie N., Posa K., Clarke B. A review of analytical techniques for quantifying microplastics in sediments. Analytical Methods. 2017;9:1369–1383. [Google Scholar]
- Harrison J.P., Ojeda J.J., Romero-González M.E. The applicability of reflectance micro-Fourier-transform infrared spectroscopy for the detection of synthetic microplastics in marine sediments. Sci. Total Environ. 2012;416:455–463. doi: 10.1016/j.scitotenv.2011.11.078. [DOI] [PubMed] [Google Scholar]
- He H.J., Zhang X.M., Yang C.P., Zeng G.M., Li H.R., Chen Y.J. Treatment of organic wastewater containing high concentration of sulfate by crystallization-Fenton-SBR. J. Environ. Eng. 2018;144:4018041–4018048. [Google Scholar]
- Hermosilla D., Merayo N., Ordóñez R., Blanco Á. Optimization of conventional Fenton and ultraviolet-assisted oxidation processes for the treatment of reverse osmosis retentate from a paper mill. Waste Manag. 2012;32:1236–1243. doi: 10.1016/j.wasman.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Ruz V., Gutow L., Thompson R.C., Thiel M. Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ. Sci. Technol. 2012;46:3060–3075. doi: 10.1021/es2031505. [DOI] [PubMed] [Google Scholar]
- Hintersteiner I., Himmelsbach M., Buchberger W.W. Characterization and quantitation of polyolefin microplastics in personal-care products using high-temperature gel-permeation chromatography. Anal. Bioanal. Chem. 2015;407:1253–1259. doi: 10.1007/s00216-014-8318-2. [DOI] [PubMed] [Google Scholar]
- Hodson M.E., Duffus-Hodson C.A., Clark A., Prendergast-Miller M.T., Thorpe K.L. Plastic bag derived-microplastics as a vector for metal exposure in terrestrial invertebrates. Environ. Sci. Technol. 2017;51:4714–4721. doi: 10.1021/acs.est.7b00635. [DOI] [PubMed] [Google Scholar]
- Huerta Lwanga E., Gertsen H., Gooren H., Peters P., Salanki T., van der Ploeg M., Besseling E., Koelmans A.A., Geissen V. Microplastics in the terrestrial ecosystem: implications for lumbricus terrestris (Oligochaeta, lumbricidae) Sci. Total Environ. 2016;50:2685–2691. doi: 10.1021/acs.est.5b05478. [DOI] [PubMed] [Google Scholar]
- Hüffer T., Metzelder F., Sigmund G., Slawek S., Schmidt T.C., Hofmann T. Polyethylene microplastics influence the transport of organic contaminants in soil. Sci. Total Environ. 2019;657:242–247. doi: 10.1016/j.scitotenv.2018.12.047. [DOI] [PubMed] [Google Scholar]
- Jahnke A., Arp H.P., Escher B.I., Gewert B., Gorokhova E., Kühnel D., Ogonowski M., Potthoff A., Rummel C.D., Schmitt-Jansen M. Reducing uncertainty and confronting ignorance about the possible impacts of weathering plastic in the marine environment. Environ. Sci. Technol. Lett. 2017;4:85–90. [Google Scholar]
- Jambeck J.R., Geyer R., Wilcox C., Siegler T.R., Perryman M., Andrady A., Narayan R., Law K.L. Marine pollution. Plastic waste inputs from land into the ocean. Science. 2015;347:768–771. doi: 10.1126/science.1260352. [DOI] [PubMed] [Google Scholar]
- Jassby D., Su Y., Kim C., Ashworth V., Adeleye A.S., Rolshausen P., Roper C., White J. Delivery, uptake, fate, and transport of engineered nanoparticles in plants: a critical review and data analysis. Environmental Science Nano. 2019;6:2311–2331. [Google Scholar]
- Ju H., Zhu D., Qiao M. Effects of polyethylene microplastics on the gut microbial community, reproduction and avoidance behaviors of the soil springtail, Folsomia candida. Environ. Pollut. 2019;247:890–897. doi: 10.1016/j.envpol.2019.01.097. [DOI] [PubMed] [Google Scholar]
- Karami A., Golieskardi A., Cheng K.C., Romano N., Yu B.H., Salamatinia B. A high-performance protocol for extraction of microplastics in fish. Sci. Total Environ. 2016;578:485–494. doi: 10.1016/j.scitotenv.2016.10.213. [DOI] [PubMed] [Google Scholar]
- Karami A., Golieskardi A., Choo C.K., Larat V., Galloway T.S., Salamatinia B. The presence of microplastics in commercial salts from different countries. Sci. Rep. 2017;7:46173–46181. doi: 10.1038/srep46173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson T.M., Kärrman A., Rotander A., Hassellöv M. Comparison between manta trawl and in situ pump filtration methods, and guidance for visual identification of microplastics in surface waters. Environ. Sci. Pollut. Res. 2019;27:5559–5571. doi: 10.1007/s11356-019-07274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson T.M., Vethaak A.D., Almroth B.C., Ariese F., Van V.M., Hassellöv M., Leslie H.A. Screening for microplastics in sediment, water, marine invertebrates and fish: method development and microplastic accumulation. Mar. Pollut. Bull. 2017;122:403–408. doi: 10.1016/j.marpolbul.2017.06.081. [DOI] [PubMed] [Google Scholar]
- Kedzierski M., Tilly V.L., César G., Sire O., Bruzaud S. Efficient microplastics extraction from sand. A cost effective methodology based on sodium iodide recycling. Mar. Pollut. Bull. 2017;115:120–129. doi: 10.1016/j.marpolbul.2016.12.002. [DOI] [PubMed] [Google Scholar]
- Kim H.M., Lee D.K., Long N.P., Kwon S.W., Park J.H. Uptake of nanopolystyrene particles induces distinct metabolic profiles and toxic effects in Caenorhabditis elegans. Environ. Pollut. 2019;246:578–586. doi: 10.1016/j.envpol.2018.12.043. [DOI] [PubMed] [Google Scholar]
- Kim S.W., An Y.J. Soil microplastics inhibit the movement of springtail species. Environ. Int. 2019;126:699–706. doi: 10.1016/j.envint.2019.02.067. [DOI] [PubMed] [Google Scholar]
- Klemes J.J., Fan Y.V., Tan R.R., Jiang P. Minimising the present and future plastic waste, energy and environmental footprints related to COVID-19. Renew. Sustain. Energy Rev. 2020;127:109883–109889. doi: 10.1016/j.rser.2020.109883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelmans A.A., Bakir A., Burton G.A., Janssen C.R. Microplastic as a vector for chemicals in the aquatic environment: critical review and model-supported re-interpretation of empirical studies. Environ. Sci. Technol. 2016;50:3315–3326. doi: 10.1021/acs.est.5b06069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S., Van Oyen A., Booth A., Meijboom A., Van Franeker I.A. Marine microplastic: preparation of relevant test materials for laboratory assessment of ecosystem impacts. Chemosphere. 2018;213:103–113. doi: 10.1016/j.chemosphere.2018.09.032. [DOI] [PubMed] [Google Scholar]
- Kukulka T., Proskurowski G., Morét-Ferguson S., Meyer D.W., Law K.L. The effect of wind mixing on the vertical distribution of buoyant plastic debris. Geophys. Res. Lett. 2012;39:7601–7606. [Google Scholar]
- Lam C.S., Ramanathan S., Carbery M., Grey K., Vanka K.S., Maurin C., Bush R., Palanisami T. A comprehensive analysis of plastics and microplastic legislation worldwide. Water Air Soil Pollut. 2018;229:345–363. [Google Scholar]
- Lares M., Ncibi M.C., Sillanpää M., Sillanpää M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018;133:236–246. doi: 10.1016/j.watres.2018.01.049. [DOI] [PubMed] [Google Scholar]
- Laskar N., Kumar U. Plastics and microplastics: a threat to environment. Environ. Technol. Innovation. 2019;14:100352–100359. [Google Scholar]
- Lattin G.L., Moore C.J., Zellers A.F., Moore S.L., Weisberg S.B. A comparison of neustonic plastic and zooplankton at different depths near the southern California shore. Mar. Pollut. Bull. 2004;49:291–294. doi: 10.1016/j.marpolbul.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Lebreton L.C.M., Zwet J.V.D., Damsteeg J.W., Slat B., Andrady A., Reisser J. River plastic emissions to the world's oceans. Nat. Commun. 2017;8:15611–15620. doi: 10.1038/ncomms15611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Lee H.-J., Kwon J.-H. Estimating microplastic-bound intake of hydrophobic organic chemicals by fish using measured desorption rates to artificial gut fluid. Sci. Total Environ. 2019;651:162–170. doi: 10.1016/j.scitotenv.2018.09.068. [DOI] [PubMed] [Google Scholar]
- Lee H., Shim W.J., Kwon J.H. Sorption capacity of plastic debris for hydrophobic organic chemicals. Sci. Total Environ. 2014;470–471:1545–1552. doi: 10.1016/j.scitotenv.2013.08.023. [DOI] [PubMed] [Google Scholar]
- Lee K.W., Shim W.J., Kwon O.Y., Kang J.H. Size-dependent effects of micropolystyrene particles in the marine copepod Tigriopus japonicus. Sci. Total Environ. 2013;47:11278–11283. doi: 10.1021/es401932b. [DOI] [PubMed] [Google Scholar]
- Lei L., Liu M., Song Y., Lu S., Hu J., Cao C., Xie B., Shi H., He D. Polystyrene (nano)microplastics cause size-dependent neurotoxicity, oxidative damage and other adverse effects in Caenorhabditis elegans. Environ. Sci. J. Integr. Environ. Res.: Nano. 2018;5:2009–2020. [Google Scholar]
- Lenz R., Enders K., Stedmon C.A., Mackenzie D.M.A., Nielsen T.G. A critical assessment of visual identification of marine microplastic using Raman spectroscopy for analysis improvement. Mar. Pollut. Bull. 2015;100:82–91. doi: 10.1016/j.marpolbul.2015.09.026. [DOI] [PubMed] [Google Scholar]
- Li J., Song Y., Cai Y.B. Focus topics on microplastics in soil: analytical methods, occurrence, transport, and ecological risks. Environ. Pollut. 2020;257:113570–113581. doi: 10.1016/j.envpol.2019.113570. [DOI] [PubMed] [Google Scholar]
- Li J.N., Yang D.Q., Li L., Jabeen K., Shi H.H. Microplastics in commercial bivalves from China. Environ. Pollut. 2015;207:190–195. doi: 10.1016/j.envpol.2015.09.018. [DOI] [PubMed] [Google Scholar]
- Li R.X., Yang C.P., Hong C., Zeng G.M., Yu G.L., Guo J.Y. Removal of triazophos pesticide from wastewater with Fenton reagent. J. Hazard Mater. 2009;167:1028–1032. doi: 10.1016/j.jhazmat.2009.01.090. [DOI] [PubMed] [Google Scholar]
- Lima A.R.A., Costa M.F., Barletta M. Distribution patterns of microplastics within the plankton of a tropical estuary. Environ. Res. 2014;132:146–155. doi: 10.1016/j.envres.2014.03.031. [DOI] [PubMed] [Google Scholar]
- Lin L., Zuo L.Z., Peng J.P., Cai L.Q., Fok L., Yan Y., Li H.X., Xu X.R. Occurrence and distribution of microplastics in an urban river: a case study in the Pearl River along Guangzhou City, China. Sci. Total Environ. 2018;644:375–381. doi: 10.1016/j.scitotenv.2018.06.327. [DOI] [PubMed] [Google Scholar]
- Lin Y., Wu S.H., Yang C.P., Chen M., Li X. Preparation of size-controlled silver phosphate catalysts and their enhanced photocatalysis performance via synergetic effect with MWCNTs and PANI. Appl. Catal. B Environ. 2019;245:71–86. [Google Scholar]
- Liu H.F., Yang X.M., Liu G.B., Liang C.T., Xue S., Chen H., Ritsema C.J., Geissen V. Response of soil dissolved organic matter to microplastic addition in Chinese loess soil. Chemosphere. 2017;185:907–917. doi: 10.1016/j.chemosphere.2017.07.064. [DOI] [PubMed] [Google Scholar]
- Lo H.K.A., Chan K.Y.K. Negative effects of microplastic exposure on growth and development of Crepidulaonyx. Environ. Pollut. 2018;233:588–595. doi: 10.1016/j.envpol.2017.10.095. [DOI] [PubMed] [Google Scholar]
- Löder M.G.N.J., Kuczera M., Mintenig S., Lorenz C., Gerdts G. Focal plane array detector-based micro-Fourier-transform infrared imaging for the analysis of microplastics in environmental samples. Environ. Chem. 2015;12:563–581. [Google Scholar]
- Long M., Paul-Pont I., Hégaret H., Moriceau B., Lambert C., Huvet A., Soudant P. Interactions between polystyrene microplastics and marine phytoplankton lead to species-specific hetero-aggregation. Environ. Pollut. 2017;228:454–463. doi: 10.1016/j.envpol.2017.05.047. [DOI] [PubMed] [Google Scholar]
- Lusher A.L., Hernandez-Milian G., O'Brien J., Berrow S., O'Connor I., Officer R. Microplastic and macroplastic ingestion by a deep diving, oceanic cetacean: the True's beaked whale Mesoplodon mirus. Environ. Pollut. 2015;199:185–191. doi: 10.1016/j.envpol.2015.01.023. [DOI] [PubMed] [Google Scholar]
- Maes T., Jessop R., Wellner N., Haupt K., Mayes A.G. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci. Rep. 2017;7:44501–44510. doi: 10.1038/srep44501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Lenka C., Katerina N., Petra P., Tomas C., Vaclav J. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018;643:1644–1651. doi: 10.1016/j.scitotenv.2018.08.102. [DOI] [PubMed] [Google Scholar]
- Mastrangelo G., Fedeli U., Fadda E., Milan G., Lange J.H. Epidemiologic evidence of cancer risk in textile industry workers: a review and update. Toxicol. Ind. Health. 2002;18:171–181. doi: 10.1191/0748233702th139rr. [DOI] [PubMed] [Google Scholar]
- Masura J., Baker J., Foster G., Arthur C. Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles in Waters and Sediments. NOAA Technical Memorandum; 2015. Methods for the Analysis of Microplastics in Beach Samples; pp. 13–19. [Google Scholar]
- Mauro R.D., Kupchik M.J., Benfield M.C. Abundant plankton-sized microplastic particles in shelf waters of the northern Gulf of Mexico. Environ. Pollut. 2017;230:798–809. doi: 10.1016/j.envpol.2017.07.030. [DOI] [PubMed] [Google Scholar]
- Miller M.E., Kroon F.J., Motti C.A. Recovering microplastics from marine samples: a review of current practices. Mar. Pollut. Bull. 2017;123:6–18. doi: 10.1016/j.marpolbul.2017.08.058. [DOI] [PubMed] [Google Scholar]
- Mizukawa K., Takada H., Ito M., Geok Y.B., Hosoda J., Yamashita R., Saha M., Suzuki S., Miguez C., Frias J. Monitoring of a wide range of organic micropollutants on the Portuguese coast using plastic resin pellets. Mar. Pollut. Bull. 2013;70:296–302. doi: 10.1016/j.marpolbul.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Murugan R., Beggi F., Kumar S. Belowground carbon allocation by trees, understory vegetation and soil type alter microbial community composition and nutrient cycling in tropical Eucalyptus plantations. Soil Biol. Biochem. 2014;76:257–267. [Google Scholar]
- Naidoo T., Goordiyal K., Glassom D. Are nitric acid (HNO3) digestions efficient in isolating microplastics from juvenile fish? Water. Air, & Soil Pollution. 2017;228:470–480. [Google Scholar]
- Napper I.E., Thompson R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016;112:39–45. doi: 10.1016/j.marpolbul.2016.09.025. [DOI] [PubMed] [Google Scholar]
- Ng E.L., Lwanga E.H., Eldridge S.M., Johnston P., Hu H.W., Geissen V., Chen D. An overview of microplastic and nanoplastic pollution in agroecosystems. Sci. Total Environ. 2018;627:1377–1388. doi: 10.1016/j.scitotenv.2018.01.341. [DOI] [PubMed] [Google Scholar]
- Ng K.L., Obbard J.P. Prevalence of microplastics in Singapore's coastal marine environment. Mar. Pollut. Bull. 2006;52:761–767. doi: 10.1016/j.marpolbul.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Nor N.H.M., Obbard J.P. Microplastics in Singapore's coastal mangrove ecosystems. Mar. Pollut. Bull. 2014;79:278–283. doi: 10.1016/j.marpolbul.2013.11.025. [DOI] [PubMed] [Google Scholar]
- Nuelle M.T., Dekiff J.H., Remy D., Fries E. A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 2014;184:161–169. doi: 10.1016/j.envpol.2013.07.027. [DOI] [PubMed] [Google Scholar]
- Oliveira M., Ribeiro A., Hylland K., Guilhermino L. Single and combined effects of microplastics and pyrene on juveniles (0+ group) of the common goby Pomatoschistus microps (Teleostei, Gobiidae) Ecol. Indicat. 2013;34:641–647. [Google Scholar]
- Oliveira P., Barboza L.G.A., Branco V., Figueiredo N., Carvalho C., Guilhermino L. Effects of microplastics and mercury in the freshwater bivalve Corbicula fluminea (Müller, 1774): filtration rate, biochemical biomarkers and mercury bioconcentration. Ecotoxicol. Environ. Saf. 2018;164:155–163. doi: 10.1016/j.ecoenv.2018.07.062. [DOI] [PubMed] [Google Scholar]
- Pagano M., Capillo G., Sanfilippo M., Palato S., Trischitta F., Manganaro A., Faggio C. Evaluation of functionality and biological responses of Mytilus galloprovincialis after exposure to quaternium-15 (methenamine 3-chloroallylochloride) Molecules. 2016;21:144–155. doi: 10.3390/molecules21020144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel J.C., Avila R., Lourenço A.G. Respiratory disease caused by synthetic fibers: a new occupational disease. Thorax. 1975;30:204–219. doi: 10.1136/thx.30.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PlasticsEurope . Plastics Europe; Brussels: 2018. Plastics - The Facts 2018. An analysis of European Latest Plastics Production, Demand and Waste Data. [Google Scholar]
- Politikos D.V., Ioakeimidis C., Papatheodorou G., Tsiaras K. Modeling the fate and distribution of floating litter particles in the Aegean Sea (E. Mediterranean) Frontiers in Marine Science. 2017;4:191–208. [Google Scholar]
- Powell J.J., Thoree V., Pele L.C. Dietary microparticles and their impact on olerance and immune responsiveness of the gastrointestinal tract. Br. J. Nutr. 2007;98:S59–S63. doi: 10.1017/S0007114507832922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prata C.J. Airborne microplastics: consequences to human health? Environ. Pollut. 2018;234:115–126. doi: 10.1016/j.envpol.2017.11.043. [DOI] [PubMed] [Google Scholar]
- Prata J.C., da Costa J.P., Lopes I., Duarte A.C., Rocha-Santos T. Environmental exposure to microplastics: an overview on possible human health effects. Sci. Total Environ. 2020;702:134455–134463. doi: 10.1016/j.scitotenv.2019.134455. [DOI] [PubMed] [Google Scholar]
- Prata J.C., da Costa J.P., Duarte A.C., Rocha-Santos T. Methods for sampling and detection of microplastics in water and sediment: a critical review. Trac. Trends Anal. Chem. 2019;110:150–159. [Google Scholar]
- Prata J.C., da Costa J.P., Lopes I., Duarte A.C., Rocha-Santos T. Effects of microplastics on microalgae populations: a critical review. Sci. Total Environ. 2019;665:400–405. doi: 10.1016/j.scitotenv.2019.02.132. [DOI] [PubMed] [Google Scholar]
- Prokić M.D., Radovanović T.B., Gavrić J.P., Faggio C. Ecotoxicological effects of microplastics: examination of biomarkers, current state and future perspectives. Trac. Trends Anal. Chem. 2019;111:37–46. [Google Scholar]
- Qi Y., Yang X., Pelaez A.M., Huerta Lwanga E., Beriot N., Gertsen H., Garbeva P., Geissen V. Macro- and micro- plastics in soil-plant system: effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci. Total Environ. 2018;645:1048–1056. doi: 10.1016/j.scitotenv.2018.07.229. [DOI] [PubMed] [Google Scholar]
- Qin X.S. Assessing environmental risks through fuzzy parameterized probabilistic analysis. Stoch. Environ. Res. Risk Assess. 2012;26:43–58. [Google Scholar]
- Qiu Q.X., Tan Z., Wang J.D., Peng J.P., Li M.M., Zhang Z.W. Extraction, enumeration and identification methods for monitoring microplastics in the environment. Estuar. Coast Shelf Sci. 2016;176:102–109. [Google Scholar]
- Quinn B., Murphy F., Ewins C. Validation of density separation for the rapid recovery of microplastics from sediment. Analytical Methods. 2016;9:1491–1498. [Google Scholar]
- Reddy M.S., Basha S., Adimurthy S., Ramachandraiah G. Description of the small plastics fragments in marine sediments along the Alang-Sosiya ship-breaking yard, India. Estuarine. Coastal and Shelf Science. 2006;68:656–660. [Google Scholar]
- Revel M., Châtel A., Mouneyrac C. Micro(nano)plastics: a threat to human health? Curr. Opin. Environ. Sci. Health. 2018;1:17–23. [Google Scholar]
- Rillig M.C. Microplastic in terrestrial ecosystems and the soil? Environ. Sci. Technol. 2012;46:6453–6454. doi: 10.1021/es302011r. [DOI] [PubMed] [Google Scholar]
- Rillig M.C., Abel D.S.M.A., Lehmann A., Klümper U. Evolutionary implications of microplastics for soil biota. Environ. Chem. 2018;16:3–7. doi: 10.1071/EN18118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rillig M.C., Lehmann A., de Souza Machado A.A., Yang G.W. Microplastic effects on plants. New Phytol. 2019;223:1066–1070. doi: 10.1111/nph.15794. [DOI] [PubMed] [Google Scholar]
- Rocha-Santos T., Duarte A.C. A critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. Trac. Trends Anal. Chem. 2015;65:47–53. [Google Scholar]
- Rodrigues M.O., Goncalves A.M.M., Goncalves F.J.M., Abrantes N. Improving cost-efficiency for MPs density separation by zinc chloride reuse. Methods (Orlando) 2020;7:100785–100791. doi: 10.1016/j.mex.2020.100785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubol S., Manzoni S., Bellin A., Porporato A. Modeling soil moisture and oxygen effects on soil biogeochemical cycles including dissimilatory nitrate reduction to ammonium (DNRA) Adv. Water Resour. 2013;62:106–124. [Google Scholar]
- Ruiz-Orejón L.F., Sardá R., Ramispujol J. Floating plastic debris in the central and western mediterranean sea. Mar. Environ. Res. 2016;120:136–144. doi: 10.1016/j.marenvres.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Salim S.Y., Kaplan G.C., Madsen K.L. Air pollution effects on the gut microbiota. Gut Microb. 2013;5:215–219. doi: 10.4161/gmic.27251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliu F., Montano S., Garavaglia M.G., Lasagni M., Seveso D., Galli P. Microplastic and charred microplastic in the Faafu Atoll, Maldives. Ma. Pollut. Bull. 2018;136:464–471. doi: 10.1016/j.marpolbul.2018.09.023. [DOI] [PubMed] [Google Scholar]
- Shim W.J., Sang H.H., Eo S. Identification methods in microplastic analysis: a review. Analytical Methods. 2017;9:1384–1391. [Google Scholar]
- Shim W.J., Song Y.K., Sang H.H., Mi J. Identification and quantification of microplastics using Nile Red staining. Mar. Pollut. Bull. 2016;113:469–476. doi: 10.1016/j.marpolbul.2016.10.049. [DOI] [PubMed] [Google Scholar]
- Song Y., Cao C., Qiu R., Hu J., Liu M., Lu S., et al. Uptake and adverse effects of polyethylene terephthalate microplastics fibers on terrestrial snails (Achatina fulica) after soil exposure. Environ. Pollut. 2019;250:447–455. doi: 10.1016/j.envpol.2019.04.066. [DOI] [PubMed] [Google Scholar]
- Studnicka M.J., Menzinger G., Drlicek M., Maruna H., Neumann M.G. Pneumoconiosis and systemic sclerosis following 10 years of exposure to polyvinyl chloride dust. Thorax. 1995;50:583–585. doi: 10.1136/thx.50.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L., Xue Y., Li L.Y., Yang D.Q., Kolandhasamy P., Li D.J., Shi H.H. Microplastics in taihu lake. China. Environ. Pollut. 2016;216:711–719. doi: 10.1016/j.envpol.2016.06.036. [DOI] [PubMed] [Google Scholar]
- Sureda A., Capó X., Busquetes-Cortés C., Tejada S. Acute exposure to sunscreen containing titanium induces an adaptive response and oxidative stress in Mytillus galloprovincialis. Ecotoxicol. Environ. Saf. 2018;149:58–63. doi: 10.1016/j.ecoenv.2017.11.014. [DOI] [PubMed] [Google Scholar]
- Tagg A.S., Harrison J.P., Ju-Nam Y.K., Sapp M., Bradley E.L., Sinclair C.J., Ojeda J.J. Fenton's reagent for the rapid and efficient isolation of microplastics from wastewater. Chem. Commun. 2017;53:372–375. doi: 10.1039/c6cc08798a. [DOI] [PubMed] [Google Scholar]
- Tang W.C., Li X., Liu H.Y., Wu S.H., Zhou Q., Du C., Teng Q., Zhong Y.Y., Yang C.P. Sequential vertical flow trickling filter and horizontal flow reactor for treatment of decentralized domestic wastewater with sodium dodecyl benzene sulfonate. Bioresour. Technol. 2020;300:122634. doi: 10.1016/j.biortech.2019.122634. [DOI] [PubMed] [Google Scholar]
- Tender D.C.A., Devriese L.I., Haegeman A., Maes S., Ruttink T., Dawyndt P. Bacterial community profiling of plastic litter in the Belgian part of the north sea. Environ. Sci. Technol. 2015;49:9629–9638. doi: 10.1021/acs.est.5b01093. [DOI] [PubMed] [Google Scholar]
- Thompson R.C., Ylva O., Mitchell R.P., Anthony D., Rowland S.J., John A.W.G., Daniel M.G., Russell A.E. Lost at sea: where is all the plastic? Science. 2004;304 doi: 10.1126/science.1094559. 838-838. [DOI] [PubMed] [Google Scholar]
- Thushari G.G.N., Senevirathna J.D.M., Yakupitiyage A., Chavanich S. Effects of microplastics on sessile invertebrates in the eastern coast of Thailand: an approach to coastal zone conservation. Mar. Pollut. Bull. 2017;124:349–355. doi: 10.1016/j.marpolbul.2017.06.010. [DOI] [PubMed] [Google Scholar]
- Tianniam S., Bamba T., Fukusaki E. Pyrolysis GC-MS-based metabolite fingerprinting for quality evaluation of commercial Angelica acutiloba roots. J. Biosci. Bioeng. 2010;109:89–93. doi: 10.1016/j.jbiosc.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Tsang Y.Y., Mak C.W., Liebich C., Lam S.W., Sze T.P., Chan K.M. Microplastic pollution in the marine waters and sediments of Hong Kong. Mar. Pollut. Bull. 2017;115:20–28. doi: 10.1016/j.marpolbul.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Turner A. In situ elemental characterisation of marine microplastics by portable XRF. Mar. Pollut. Bull. 2017;124:286–291. doi: 10.1016/j.marpolbul.2017.07.045. [DOI] [PubMed] [Google Scholar]
- Turner A., Holmes L.A. Adsorption of trace metals by microplastic pellets in fresh water. Environ. Chem. 2015;12:600–610. [Google Scholar]
- Veresoglou S.D., Halley J.M., Rillig M.C. Extinction risk of soil biota. Nat. Commun. 2015;6:9862–9871. doi: 10.1038/ncomms9862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeiren P., Muñoz C., Ikejima K. Microplastic identification and quantification from organic rich sediments: a validated laboratory protocol. Environ. Pollut. 2020;262:114298–114309. doi: 10.1016/j.envpol.2020.114298. [DOI] [PubMed] [Google Scholar]
- Vianello A., Boldrin A., Guerriero P., Moschino V., Rella R., Sturaro A., Ros L.D. Microplastic particles in sediments of Lagoon of Venice, Italy: first observations on occurrence, spatial patterns and identification. Estuar. Coast Shelf Sci. 2013;130:54–61. [Google Scholar]
- Wagner J., Wang Z.M., Ghosal S., Rochman C., Gassel M., Wall S. Novel method for the extraction and identification of microplastics in ocean trawl and fish gut matrices. Analytical Methods. 2016;9:1479–1490. [Google Scholar]
- Wang J., Coffin S., Sun C., Schlenk D., Gan J. Negligible effects of micro- plastics on animal fitness and HOC bioaccumulation in earthworm Eisenia fetida in soil. Environ. Pollut. 2019;249:776–784. doi: 10.1016/j.envpol.2019.03.102. [DOI] [PubMed] [Google Scholar]
- Wang J., Liu X.H., Li Y., Powell T., Wang X., Wang G.Y., Zhang P.P. Microplastics as contaminants in the soil environment: a mini-review. Sci. Total Environ. 2019;691:848–857. doi: 10.1016/j.scitotenv.2019.07.209. [DOI] [PubMed] [Google Scholar]
- Wang J.D., Peng J.P., Tan Z., Gao Y.F., Zhan Z.W., Chen Q.Q., Cai L.Q. Microplastics in the surface sediments from the Beijiang River littoral zone: composition, abundance, surface textures and interaction with heavy metals. Chemosphere. 2017;171:248–258. doi: 10.1016/j.chemosphere.2016.12.074. [DOI] [PubMed] [Google Scholar]
- Wang W.F., Ndungu A.W., Li Z., Wang J. Microplastics pollution in inland freshwaters of China: a case study inurban surface waters of Wuhan, China. Sci. Total Environ. 2017;575:1369–1374. doi: 10.1016/j.scitotenv.2016.09.213. [DOI] [PubMed] [Google Scholar]
- Wang W.F., Yuan W.K., Chen Y.L., Wang J. Microplastics in surface waters of Dongting Lake and hong lake, China. Sci. Total Environ. 2018;633:539–545. doi: 10.1016/j.scitotenv.2018.03.211. [DOI] [PubMed] [Google Scholar]
- Wang Z.M., Wagner J., Ghosal S., Bedi G., Wall S. SEM/EDS and optical microscopy analyses of microplastics in ocean trawl and fish guts. Sci. Total Environ. 2017;603–604:616–626. doi: 10.1016/j.scitotenv.2017.06.047. [DOI] [PubMed] [Google Scholar]
- Wright S.L., Rowe D., Thompson R.C., Galloway T.S. Microplastic ingestion decreases energy reserves in marine worms. Curr. Biol. 2013;23:R1031–R1033. doi: 10.1016/j.cub.2013.10.068. [DOI] [PubMed] [Google Scholar]
- Wright S.L., Thompson R.C., Galloway T.S. The physical impacts of microplastics on marine organisms: a review. Environ. Pollut. 2013;178:483–492. doi: 10.1016/j.envpol.2013.02.031. [DOI] [PubMed] [Google Scholar]
- Wu S.H., He H.J., Li X., Yang C.P., Zeng G.M., Wu B., He S.Y., Lu L. Insights into atrazine degradation by persulfate activation using composite of nanoscale zero-valent iron and graphene: performances and mechanisms. Chem. Eng. J. 2018;341:126–136. [Google Scholar]
- Xiong X., Wu C.X., Elser J.J., Mei Z.G., Hao Y.J. Occurrence and fate of microplastic debris in middle and lower reaches of the Yangtze River – from inland to the sea. Sci. Total Environ. 2019;659:66–73. doi: 10.1016/j.scitotenv.2018.12.313. [DOI] [PubMed] [Google Scholar]
- Xu H., Verbeken E., Vanhooren H.M., Nemery B., Hoet P.H.M. Pulmonary toxicity of polyvinyl chloride particles after a single intratracheal instillation in rats. Time course and comparison with silica. Toxicol. Appl. Pharmacol. 2004;194:111–121. doi: 10.1016/j.taap.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Xu S., Ma J., Ji R., Pan K., Miao A.J. Microplastics in aquatic environments: occurrence, accumulation, and biological effects. Sci. Total Environ. 2020;703:134699–134713. doi: 10.1016/j.scitotenv.2019.134699. [DOI] [PubMed] [Google Scholar]
- Yang C.P., Qian H., Li X., Cheng Y., He H.J., Zeng G.M., Xi J.Y. Simultaneous removal of multicomponent VOCs in biofilters. Trends Biotechnol. 2018;36:673–685. doi: 10.1016/j.tibtech.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Yang D.Q., Shi H.H., Li L., Li J.N., Jabeen K., Kolandhasamy P. Microplastic pollution in table salts from China. Environ. Sci. Technol. 2015;49:13622–13627. doi: 10.1021/acs.est.5b03163. [DOI] [PubMed] [Google Scholar]
- Yang K., Zhu L.Z., Lou B.F., Chen B.L. Correlations of nonlinear sorption of organic solutes with soil/sediment physicochemical properties. Chemosphere. 2005;61:116–128. doi: 10.1016/j.chemosphere.2005.02.095. [DOI] [PubMed] [Google Scholar]
- Yin L.S., Wen X.F., Du C.Y., Jiang J., Wu L.X., Zhang Y., Hu Z.H., Hu S.P., Feng Z.Q., Zhou Z.Y., Long Y.N., Gu Q. Comparison of the abundance of microplastics between rural and urban areas: a case study from East Dongting Lake. Chemosphere. 2020;244:125486–125492. doi: 10.1016/j.chemosphere.2019.125486. [DOI] [PubMed] [Google Scholar]
- Yu P., Liu Z.Q., Wu D.L., Chen M.H., Lv W.W., Zhao Y.L. Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquat. Toxicol. 2018;200:28–36. doi: 10.1016/j.aquatox.2018.04.015. [DOI] [PubMed] [Google Scholar]
- Yuan H., Fu S., Tan W., He J., Wu K. Study on the hydrocyclonic separation of waste plastics with different density. Waste Manag. 2015;45:108–111. doi: 10.1016/j.wasman.2015.01.037. [DOI] [PubMed] [Google Scholar]
- Yuan W.K., Liu X.N., Wang W.F., Di M.X., Wang J. Microplastic abundance, distribution and composition in water, sediments, and wild fish from Poyang Lake, China. Ecotoxicol. Environ. Saf. 2019;170:180–187. doi: 10.1016/j.ecoenv.2018.11.126. [DOI] [PubMed] [Google Scholar]
- Zada L., Leslie H.A., Vethaak A.D., Tinnevelt G.H., Jansen J.J., Boer J.F.D., Ariese F. Fast microplastics identification with stimulated Raman scattering microscopy. J. Raman Spectrosc. 2018;49:1136–1144. [Google Scholar]
- Zarfl C., Fleet D., Fries E., Galgani F., Gerdts G., Hanke G., Matthies M. Microplastics in oceans. Mar. Pollut. Bull. 2011;62:1589–1591. doi: 10.1016/j.marpolbul.2011.02.040. [DOI] [PubMed] [Google Scholar]
- Zhang K., Su J., Xiong X., Wu X., Wu C.X., Liu J.T. Microplastic pollution of lakeshore sediments from remote lakes in Tibet plateau. China. Environmental Pollution. 2016;219:450–455. doi: 10.1016/j.envpol.2016.05.048. [DOI] [PubMed] [Google Scholar]
- Zhang S.L., Yang X.M., Gertsen H., Peters P., Salánki T., Geissen V. A simple method for the extraction and identification of light density microplastics from soil. Sci. Total Environ. 2018;616–617:1056–1065. doi: 10.1016/j.scitotenv.2017.10.213. [DOI] [PubMed] [Google Scholar]
- Zhao S.Y., Danley M., Ward J.E., Li D.J., Mincer T.J. An approach for extraction, characterization and quantitation of microplastic in natural marine snow using Raman microscopy. Analytical Methods. 2017;9:1470–1478. [Google Scholar]
- Zhou Q., Lin Y., Li X., Yang C.P., Han Z.F., Zeng G.M., Lu L., He S.Y. Effect of zinc ions on nutrient removal and growth of Lemnaaequinoctialis from anaerobically digested swine wastewater. Bioresour. Technol. 2018;249:457–463. doi: 10.1016/j.biortech.2017.10.044. [DOI] [PubMed] [Google Scholar]
- Zobkov M.B., Esiukova E.E. Microplastics in a marine environment: review of methods for sampling, processing, and analyzing microplastics in water, bottom sediments, and coastal deposits. Oceanology. 2018;58:137–143. [Google Scholar]